Abstract
Neurodegenerative disorders such as Alzheimer’s disease (AD) are characterized by the loss of neurotrophic factors and experimental therapeutical approaches to AD have investigated the efficacy of replacing or augmenting neurotrophic factor activity.
Cerebrolysin™, a peptide mixture with neurotrophic-like effects, has been shown to improve cognition in patients with AD and to reduce synaptic and behavioral deficits in transgenic (tg) mice over expressing the amyloid precursor protein (APP). However it is unclear how long lasting the beneficial effects of Cerebrolysin™ are and whether or not behavioral and neuropathological alterations will reappear following treatment interruption. The objective of the present study was to investigate the consequences of interrupting Cerebrolysin™ treatment (“wash-out” effect) 3 and 6 months after the completion of a 3-month treatment period in APP tg mice.
We demonstrate that in APP tg mice, Cerebrolysin™-induced amelioration of memory deficits in the water maze and reduction of neurodegenerative pathology persist for 3 months after treatment interruption, however these effects dissipate 6 months following treatment termination. Immunohistochemical analysis demonstrated that the decrease in neocortical and hippocampal amyloid plaque load observed in Cerebrolysin™ treated APP tg mice immediately following treatment was no longer apparent at 3 months after treatment interruption indicating that the beneficial effects of Cerebrolysin™ at this time point were independent of its effect on amyloid-beta deposition.
In conclusion the results demonstrate that effects of Cerebrolysin™ persist for a significant period of time following treatment termination and suggest that this prolonged effect may involve the neurotrophic factor-like activity of Cerebrolysin™.
Keywords: Amyloid precursor protein, neurodegeneration, synapses, water maze, Cerebrolysin
INTRODUCTION
Alzheimer’s Disease (AD) is among the most common neurodegenerative disorders in Europe and the US and is the leading cause of dementia in the aging population (Maslow 2010). Cognitive impairment AD patients is closely associated with synaptic damage and selective neuronal loss in the neocortex, limbic system and basal forebrain (DeKosky et al. 1996; Masliah 1995; Masliah et al. 2006; Masliah et al. 2001; Scheff et al. 1990; Terry et al. 1991; Trojanowski et al. 1995).
Nerve growth factor (NGF) has been identified as a key neurotrophic factor for the neurons that degenerate in AD (Levi-Montalcini and Hamburger 1951). In AD, NGF loss is observed in the basal forebrain (Mufson et al. 1995; Scott et al. 1995) and in the absence of NGF cholinergic neurons have been reported to exhibit shrinkage, reduced fiber density and a decrease in cholinergic transmission (Svendsen et al. 1991). NGF administration into the rat brain has been shown to prevent basal forebrain cholinergic neuron degeneration (Fischer et al. 1987; Hefti 1986; Kromer 1987; Williams et al. 1986) and NGF administration has also been reported to improve learning and memory in lesioned and aged rats (Chen and Gage 1995; Fischer et al. 1987; Markowska et al. 1994; Martinez-Serrano et al. 1996; Tuszynski and Gage 1995; Williams et al. 1991).
Experimental therapies for AD have focused on investigating the effects of neuroprotective and neurotrophic agents (Nagahara et al. 2009; Tuszynski 2007; Tuszynski et al. 2005). One such neurotrophic agent is Cerebrolysin™ (CBL). CBL is a porcine brain-derived peptide preparation, produced by standardized enzymatic breakdown, consisting of low molecular weight peptides and free amino acids; it is free of proteins, lipids and antigenic properties (EBEWENeuroPharmaGmbH 2009).
CBL has been shown to display neurotrophic activity in vitro (Chen et al. 2007; Mallory et al. 1999) and in animal models of neurodegeneration (Francis-Turner and Valouskova 1996; Francis-Turner et al. 1996; Masliah et al. 1999; Valouskova and Francis-Turmer 1996; Veinbergs et al. 2000). The effects of CBL have been shown to mimic CNTF, FGF and NGF in vitro (Chen et al. 2007) and in vivo animal models (Francis-Turner and Valouskova 1996; Francis-Turner et al. 1996; Valouskova and Francis-Turmer 1996). In patients with mild to moderate AD, CBL has been shown to improve cognitive impairment (Alvarez et al. 2006; Ruther et al. 2000; Ruther et al. 1994) and, in a recent double-blind trial, intravenous administration of CBL was demonstrated to improve activities of daily living and psychiatric deficits in patients with moderate to moderately severe AD (Alvarez et al. 2011). Several other randomized double-blind studies AD patients have shown that CBL is consistently superior to placebo at reducing cognitive alterations (Plosker and Gauthier 2009; Plosker and Gauthier 2010).
In agreement with clinical trials, experimental studies in an APP tg model of AD demonstrate that CBL ameliorates memory deficits, synaptic alterations and amyloid burden (Rockenstein et al. 2005a; Rockenstein et al. 2003a; Rockenstein et al. 2002). Moreover, we have recently shown that CBL reduces the AD-like pathology in the amyloid precursor protein (APP) tg mice by modulating the maturation and processing of APP (Rockenstein et al. 2006). However it is unclear how long-lasting the effects of CBL are and whether or not behavioral and neuropathological alterations will reappear after treatment termination. The objective of the present study was to investigate the consequences of interrupting CBL treatment in APP tg mice. The results from this study demonstrate that the neuroprotective effects of CBL are present 3 months after the cessation of treatment, however these effects are no longer apparent 6 months after the termination of CBL administration. These results support the hypothesis that the effects of CBL persist for a significant period of time after treatment interruption and provide evidence for a link between the amelioration of AD-related neuropathology and CBL therapy.
MATERIALS AND METHODS
Generation of APP Tg Mice, Cerebrolysin treatment and washout experiments
For these experiments, the APP tg mice express mutated (Swedish K670M/N671L, London V717I) human(h) APP751 under the control of the mThy-1 promoter (mThy1-hAPP751) (line 41) (Rockenstein et al. 2001) were used. We have previously shown that these mice display loss of synaptic contacts, defects in neurogenesis, high levels of Aβ 1–42 production, early amyloid deposition and behavioral deficits (Rockenstein et al. 2003a; Rockenstein et al. 2007a; Rockenstein et al. 2007c). Genomic DNA was extracted from tail biopsies and analyzed by PCR amplification, as described previously (Rockenstein et al. 1995). Transgenic lines were maintained by crossing heterozygous tg mice with non-transgenic (non tg) C57BL/6 × DBA/2 F1 breeders. All mice were heterozygous with respect to the transgene.
A total of 36 APP tg mice (3 month (m) old; n=18 CBL treated and n=18 saline) and 18 non tg mice (3 m old; saline only) were utilized. Each milliliter of CBL contains 215.2mg of the active CBL concentrate in an aqueous solution (EBEWENeuroPharmaGmbH 2009). Mass spectrometry analysis of CBL has shown that is comprised of amino acids (80%) and small (<10Da) peptides (20%) and previous work had shown that this small peptides mimic the effect of neurotrophic factors including CNTF, FGF and IGF (Chen et al. 2007).
The APP tg mice were divided into 3 experimental groups as follows; Group 1) APP tg mice treated with saline (n= 6) or CBL (n=6, 5 ml/kg, ip, daily) for 3 months followed by behavioral testing. As these mice were tested and euthanized immediately after the completion of the treatment, this group is designated as the time post treatment-0 months (TPT 0m) group. The mice in this group were 6 m/o at euthanization. Group 2) APP tg mice were treated with saline (n= 6) or CBL (n=6, 5 ml/kg, ip, daily) for 3 months. Three months after completion of the treatment, mice underwent behavioral testing and were euthanized (the mice were 9 m/o at euthanization). This group is designated as the time post treatment-3 months (TPT 3m) group. Group 3) APP tg mice were treated with saline (n= 6) or CBL (n=6, 5 ml/kg, ip, daily) for 3 months. Six months after completion of the treatment, mice underwent behavioral testing and were euthanized (the mice were 12 m/o at euthanization). This group is designated as the time post treatment-6 months (TPT 6m) group. For each of the 3 groups an age-matched cohort (n=6) of saline-treated non tg mice was included. All experiments described were approved by the animal subjects committee at the University of California at San Diego (UCSD) and were performed according to NIH guidelines for animal use.
Spatial learning and memory in the Morris water maze
As previously described (Rockenstein et al. 2005b), in order to evaluate the long term effects of a 3 month treatment with CBL, mice were tested in the water maze at 0, 3 or 6 months after treatment interruption. For this purpose, a pool (diameter 180 cm) was filled with opaque water (24°C) and mice were first trained to locate a visible platform (days 1–3) and then a submerged hidden platform (days 4–7) in three daily trials 2–3 min apart. Mice that failed to find the hidden platform within 90 seconds were placed on it for 30 seconds. The same platform location was used for all sessions and all mice. The starting point at which each mouse was placed into the water was changed randomly between two alternative entry points located at a similar distance from the platform. On day 8, another visible platform trial was performed to exclude differences in motivation and fatigue. Time to reach the platform (latency), path length, and swim speed were recorded with a Noldus Instruments EthoVision video tracking system (San Diego Instruments) set to analyze two samples per second.
Tissue Processing
In accordance with NIH guidelines for the humane treatment of animals, mice were anesthetized with chloral hydrate and flush-perfused transcardially with 0.9% saline. Brains were removed and divided sagitally. The left hemibrain was post-fixed in phosphate-buffered 4% paraformaldehyde (pH 7.4) at 4°C for 48 hr and sectioned at 40 µm with a Vibratome 2000 (Leica, Germany), while the right hemibrain was snap frozen and stored at −70°C for protein analysis.
Analysis of Neurodegeneration and Aβ immunoreactivity
For this purpose, blind-coded 40 µm thick vibratome sections were immunolabeled with the mouse monoclonal antibodies against synaptophysin (presynaptic terminal marker, 1:40, Chemicon, Temecula, CA) and GFAP (astroglial marker, 1:1000, Chemicon) as previously described (Rockenstein et al. 2003b; Rockenstein et al. 2006). After overnight incubation with the primary antibodies, sections were incubated with Texas red or FITC-conjugated horse anti-mouse IgG secondary antibody (1:75, Vector Laboratories, Burlingame, CA), transferred to SuperFrost slides (Fisher Scientific, Tustin, CA) and mounted under glass coverslips with anti-fading media (Vector). All sections were processed under the same standardized conditions. The immunolabeled blind-coded sections were imaged with the laser-scanning confocal microscope (LSCM, MRC1024, BioRad, Hercules, CA) and analyzed with the Image 1.43 program (NIH), as previously described (Rockenstein et al. 2003b) to determine the percent area of the neuropil covered by synaptophysin or GFAP (Glial fibrillary acidic protein) immunoreactivity. To determine neuronal density an additional set of sections were immunolabeled as previously described with an antibody against NeuN (general neuronal marker, 1:1000, Chemicon) and reacted with diamino-benzidine (DAB). Sections were analyzed with the Stereo-Investigator Software (MBF Biosciences), images collected according to the optical disector method were analyzed as previously described (Chana et al. 2006; Chana et al. 2003). Three immunolabeled sections were analyzed per mouse and the average of individual measurements was used to calculate group means. The levels of accumulation of Aβ deposits were detected as previously described (Pham et al. 2010), briefly vibratome sections were incubated overnight at 4°C with the mouse monoclonal antibody 82E1 (1:600, Immuno-Biological Laboratories), followed by incubation with a fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (Vector Laboratories). Sections were imaged with laser scanning confocal microscopy (LSCM) (MRC1024, BioRad) as described previously (Spencer et al. 2008) and digital images were analyzed with the NIH Image 1.43 program to determine the percent area occupied by Aβ deposits. Three immunolabeled sections were analyzed per mouse and the average of individual measurements was used to calculate group means. To confirm the specificity of primary antibodies, control experiments were performed where sections were incubated overnight in the absence of primary antibody (deleted) or preimmune serum and primary antibody alone.
Statistical Analysis
Analyses were carried out with the StatView 5.0 program (SAS Institute Inc., Cary, NC). Differences among means were assessed by one-way ANOVA with post-hoc Dunnett’s. Comparisons between 2 groups were assessed using the two-tailed unpaired Student's t-test. Correlation studies were carried out by simple regression analysis and the null hypothesis was rejected at the 0.05 level.
RESULTS
Cerebrolysin effects on spatial learning in the water maze persist for 3 months but are washed out after 6 months of treatment interruption
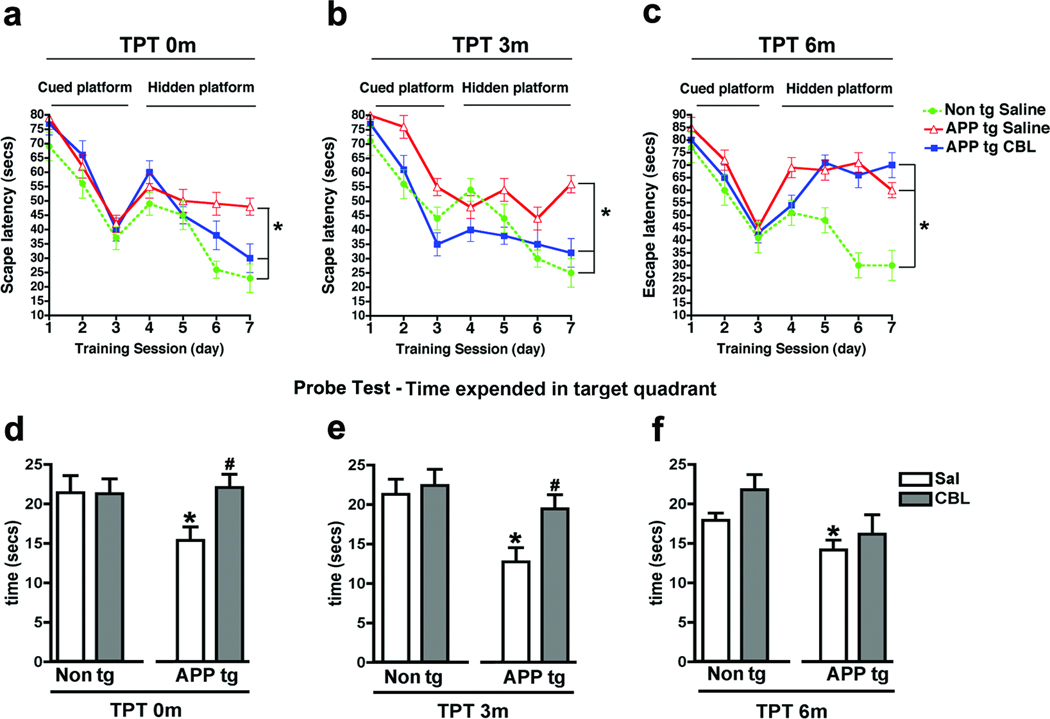
The first cohort of mice to be analyzed was the TPT 0m group, these mice were tested immediately following the 3 months of CBL treatment. During the training phase with the visible platform the non tg and APP tg mice performed similarly (Fig.1a, cued platform). When the platform was submerged, compared to non tg controls, the saline-treated APP tg mice took a significantly longer time to reach the platform, in contrast APP tg mice treated with CBL behaved similarly to the non tg saline-treated group (Fig. 1a, hidden platform). Next the TPT 3m group was tested; these mice were treated with CBL 3 months prior to the testing of behavioral performance in the water maze. As expected, during the training phase, where the platform was visible, all mice performed similarly (Fig. 1b, cued platform). When the platform was submerged, compared to non tg controls, the saline-treated APP tg mice displayed significant deficits and took longer to find the hidden platform (Fig. 1b, hidden platform). However in the TPT 3m group the behavioral performance of CBL-treated APP tg mice was comparable to that of the non tg saline-treated group (Fig. 1b, hidden platform). The final cohort of mice to be tested was the TPT 6m group (saline and CBL interrupted 6 months prior to testing). Here, once again, the performance of the saline-treated non tg and saline or CBL-treated APP tg groups was comparable with the cued platform (Fig. 1c, cued platform). In contrast, compared to non tg controls, both groups of APP tg mice displayed significant deficits during the hidden platform component of the test indicating that the effects of CBL no longer persisted at this time point (Fig. 1c, hidden platform). Analysis of the Probe Test demonstrated that saline-treated APP tg mice at TPT 0m, 3m, and 6m spend significantly less time in the target quadrant than age-matched non tg mice (Fig. 1d–f). In contrast, CBL-treated APP tg mice at TPT 0m (Fig. 1d) and TPT 3m (Fig. 1e) spend significantly longer in the target quadrant in comparison to saline-treated APP tg mice. At TPT 0m and TPT 3m the CBL-treated APP tg mice spend a length of time in the target quadrant that was comparable to the non tg mice (Fig. 1d, e). However, by TPT 6m the CBL-treated APP tg mice are no longer performing significantly differently to the saline-treated APP tg mice (Fig. 1f) indicating that by this time point the beneficial effects of CBL have been washed out.
Fig 1. Behavioral deficits in the water maze 3 and 6 months after the cessation of CBL therapy in APP tg mice.
To evaluate the effect of CBL treatment interruption on memory and spatial learning APP tg mice were tested in the water maze. (a) Escape latency in the cued and hidden portions of the water maze in saline-treated non tg mice and saline or CBL-treated APP tg mice at TPT 0m. (b) Escape latency in the cued and hidden portions of the water maze in saline-treated non tg mice and saline or CBL-treated APP tg mice at TPT 3m. (c) Escape latency in the cued and hidden portions of the water maze in saline-treated non tg mice and saline or CBL-treated APP tg mice at TPT 6m. (d) Probe Test performance in saline or CBL-treated non tg or APP tg mice at TPT 0m. (e) Probe Test performance in saline or CBL-treated non tg or APP tg mice at TPT 3m and (f) Probe Test performance in saline or CBL-treated non tg or APP tg mice at TPT 6m. Error bars represent mean ± SEM. For (a–c) (*) Indicates p<0.05 by repeated-measures two-way ANOVA and (#) indicates p<0.05 when comparing CBL-treated APP tg mice with saline-treated APP tg mice by repeated-measures two-way ANOVA. For (d–f) (*) Indicates p<0.05, when comparing APP tg mice with saline-treated non tg mice by one-way ANOVA and (#) indicates p<0.05 when comparing CBL-treated APP tg mice with saline-treated APP tg mice by one-way ANOVA.
The neuroprotective effects of Cerebrolysin continue for 3 months but are no longer apparent after 6 months of treatment interruption
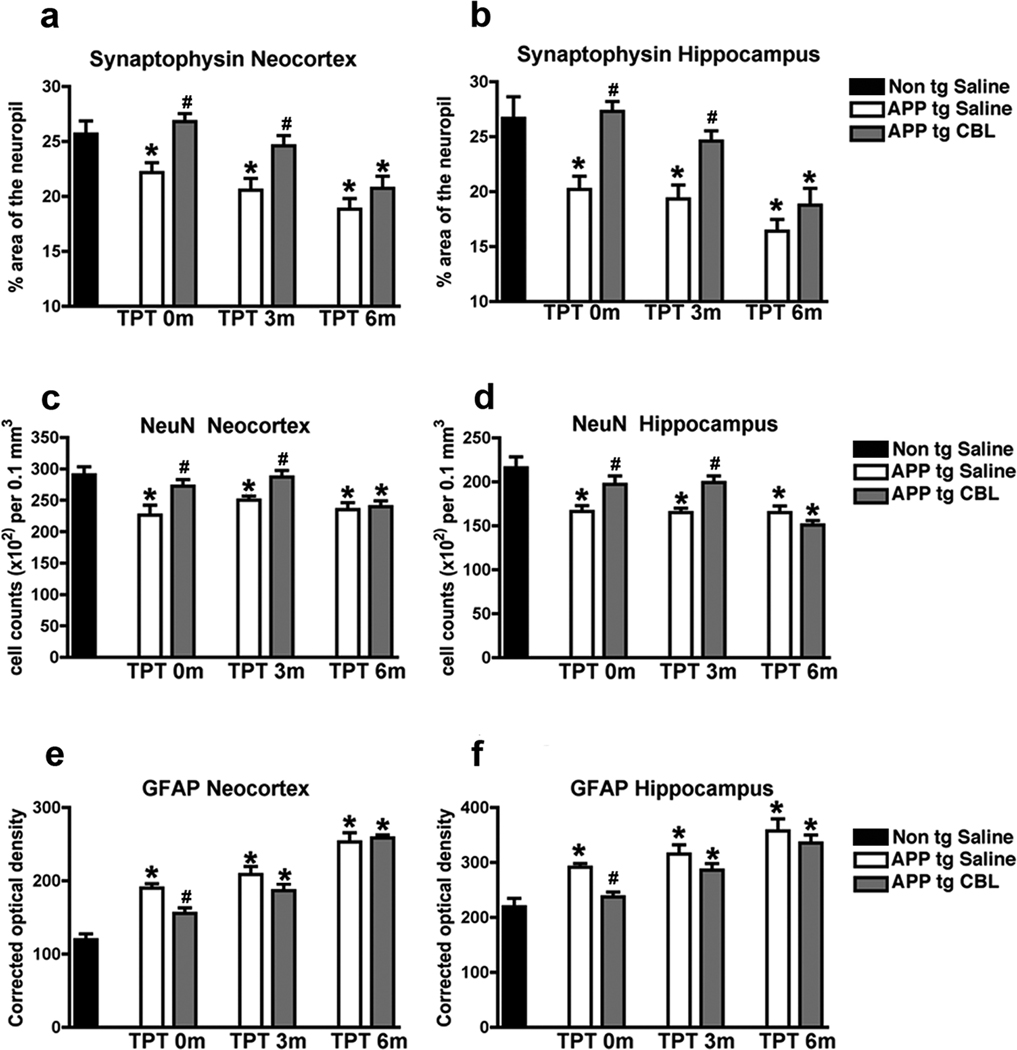
To evaluate how long the neuroprotective effects of CBL administration persisted after the cessation of treatment, the mice that were analyzed in the water maze were euthanized and analyzed for markers of neurodegeneration including the synaptic marker, synaptophysin, the neuronal marker, NeuN and the astroglial marker GFAP. The first cohort of mice to be analyzed was the TPT 0m group. Compared to the saline-treated non tg controls, the saline-treated APP tg mice displayed significantly lower levels of synaptophysin immunoreactive terminals and a reduction in NeuN-positive neuronal counts estimated by the disector method in the frontal cortex (Fig. 2a, c) and hippocampus (Fig. 2b, d). Concomitantly, levels of astrogliosis were increased in the saline-treated APP tg mice in comparison to saline-treated non tg mice (Fig. 2e, f). In contrast, TPT 0m CBL-treated APP tg mice displayed neocortical and hippocampal levels of synaptophysin (Fig. 2a, b), NeuN (Fig. 2c, d) and GFAP (Fig. 2e, f) comparable to those observed in the saline-treated non tg controls. The TPT 3m group was the next to be analyzed. Compared to saline-treated non tg age matched controls, the saline-treated APP tg mice continued to display a significant decrease in synaptophysin and NeuN and an increase in GFAP immunoreactivity in the frontal cortex (Fig. 2a, c, e) and hippocampus (Fig. 2b, d, f). However the TPT 3m CBL-treated APP tg mice (those that had been treated with CBL for 3 months and analyzed 3 months after the termination of treatment) showed neocortical and hippocampal levels of synaptophysin and NeuN comparable to the age-matched saline-treated non tg group (Fig. 2a–d). Levels of GFAP immunoreactivity were increased in this cohort and comparable to the saline-treated APP tg mice (Fig. 2e, f). The final cohort of mice to be analyzed was the TPT 6m group (APP tg mice that had been treated with CBL for 3 months and analyzed 6 months after the termination of treatment). Here once more, compared to age-matched saline-treated non tg controls, the saline-treated APP tg mice display significant deficits in synaptophysin and NeuN and increased in GFAP levels in the frontal cortex (Fig. 2a, c, e) and hippocampus (Fig. 2b, d, f). However, at this time point the TPT 6m CBL-treated APP tg mice displayed significant deficits in the neocortical and hippocampal levels of synaptophysin immunoreactive terminals (Fig. 2a, b) and NeuN-positive neuronal cell counts (Fig. 2c, d) and an increase in astrogliosis (Fig. 2e, f) in comparison to the age-matched saline-treated non tg group. In summary, these studies demonstrate that the neuroprotective effects of CBL on synaptophysin and NeuN levels persist for 3 months following the termination of CBL treatment but are washed out after 6 months of treatment interruption.
Fig 2. Effects of CBL in the markers of neurodegeneration 3 and 6 months after therapeutical interruption in APP tg mice.
Immunohistochemical analysis was performed to evaluate the effect of CBL treatment interruption on neurodegeneration in APP tg mice. (a) Levels of the synaptic marker, synaptophysin, in the neocortex of saline-treated non tg and saline or CBL-treated APP tg mice at TPT 0m, TPT 3m and TPT 6m. (b) Levels of synaptophysin, in the hippocampus of saline-treated non tg and saline or CBL-treated APP tg mice at TPT 0m, TPT 3m and TPT 6m. (c) Levels of the neuronal marker, NeuN, in the neocortex of saline-treated non tg and saline or CBL-treated APP tg mice at TPT 0m, TPT 3m and TPT6m. (d) Levels of NeuN, in the hippocampus of saline-treated non tg and saline or CBL-treated APP tg mice at TPT 0m, TPT 3m and TPT 6m. (e) Levels of the astroglial marker, GFAP, in the neocortex of saline-treated non tg and saline or CBL-treated APP tg mice at TPT 0m, TPT 3m and TPT 6m. (f) Levels of GFAP, in the hippocampus of saline-treated non tg and saline or CBL-treated APP tg mice at TPT 0m, TPT 3m and TPT 6m. Error bars represent mean ± SEM. (*)Indicates p<0.05, when comparing APP tg mice with saline-treated non tg mice assessed by one-way ANOVA with post-hoc Dunnett’s and (#) indicates p<0.05 when comparing CBL-treated APP tg mice with saline-treated APP tg mice assessed by one-way ANOVA with post-hoc Dunnett’s.
Cerebrolysin continues to partially reduce amyloid deposition 3 months after treatment interruption but the effects disappear after 6 months
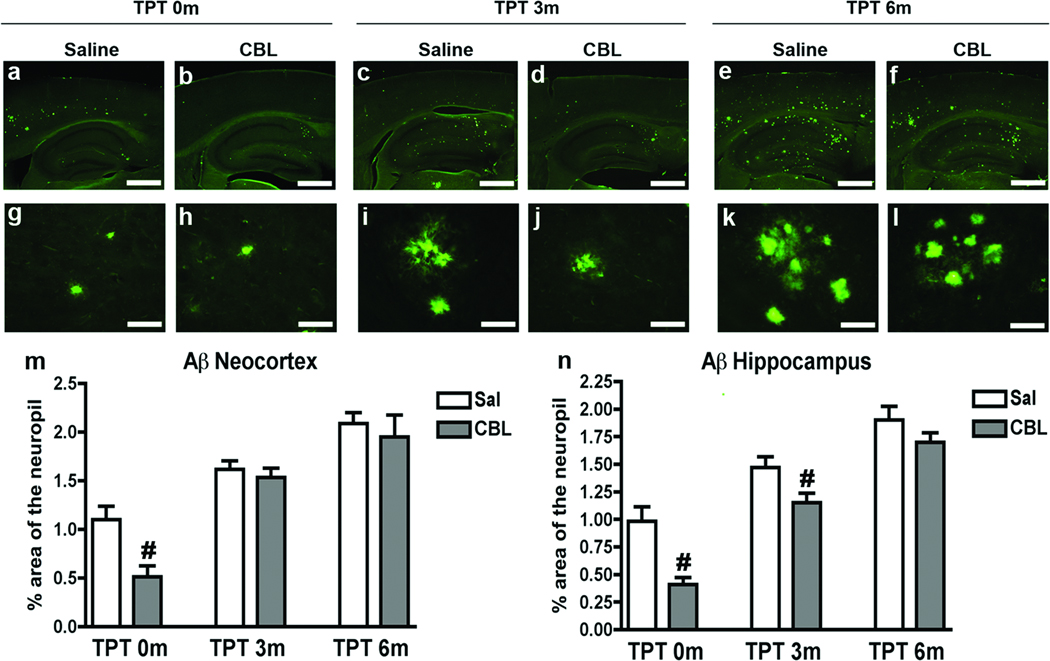
In order to the investigate to length of time CBL was able to reduce amyloid deposition following the termination of administration, mice were immunolabeled with a monoclonal antibody against Aβ (clone 82E1) and evaluated by LCSM/image analysis. The first cohort of mice to be analyzed was the TPT 0m group. Consistent with previous studies (Rockenstein et al. 2003a; Rockenstein et al. 2002; Rockenstein et al. 2006), compared to the saline-treated APP tg mice, the CBL-treated APP tg mice displayed a significant 55–65% reduction in the area of the neuropil occupied by Aβ immunoreactive deposits in the frontal cortex (Fig. 3a, b, m) and hippocampus (Fig. 3a, b, g, h, n). Upon analysis of the TPT 3m group, the saline and CBL-treated APP tg mice showed comparable levels of Aβ immunoreactive deposits in the frontal cortex (Fig. 3c, d, m). In contrast, the CBL-treated APP tg mice continued to exhibit a significant 35% reduction in Aβ immunoreactive deposits in the hippocampus in comparison to the saline-treated APP tg mice (Fig. 3c, d, i, j, n). Analysis of the TPT 6m group demonstrated comparable levels of Aβ immunoreactive deposits in the saline and CBL-treated groups in the frontal cortex (Fig. 3e, f, m) and hippocampus (Fig. 3e, f, k, l, n). When compared over time, in the saline-treated APP tg mice the levels of Aβ immunoreactive deposits progressively increased in the frontal cortex and hippocampus (Fig. 3m, n), consistent with the age-dependent increased in AD-related neuropathology. Taken together, these results indicate that 3 months after the termination of CBL administration there remains a partial effect at suppressing Aβ, most notably in the hippocampus, that ceases to exist at 6 months after treatment interruption.
Fig 3. Patterns of Aβ immunoreactivity 3 and 6 months following the interruption of CBL administration in APP tg mice.
Immunohistochemical analysis of Aβ levels was performed using the monoclonal antibody against Aβ (clone 82E1) in order to evaluate the effect of CBL treatment interruption on amyloid deposition in the APP tg mice. (a, b) Aβ immunoreactivity in the saline and CBL-treated TPT 0m APP tg mice, respectively. (c, d) Aβ immunoreactivity in the saline and CBL-treated TPT 3m APP tg mice, respectively. (e, f) Aβ immunoreactivity in the saline and CBL-treated TPT 6m APP tg mice, respectively. (g, h) High magnification of Aβ plaques in the hippocampus of saline and CBL-treated TPT 0m APP tg mice, respectively. (i, j) High magnification of Aβ plaques in the hippocampus of saline and CBL-treated TPT 3m APP tg mice, respectively. (k, l) High magnification of Aβ plaques in the hippocampus of saline and CBL-treated TPT 6m APP tg mice, respectively. (m) Analysis of the % of Aβ immunoreactive neocortical neuropil in saline and CBL-treated APP tg mice at TPT 0m, 3m and 6m. (n) Analysis of the % of Aβ immunoreactive hippocampal neuropil in saline and CBL-treated APP tg mice at TPT 0m, 3m and 6m. Error bars represent mean ± SEM. (#) Indicates p<0.05, when comparing saline and CBL-treated APP tg mice assessed by one-way ANOVA with post-hoc Dunnett’s. Scale bars (a–f) = 200uM, (g–l) = 20uM
DISCUSSION
The present study showed that CBL-induced amelioration of memory deficits and neurodegenerative pathology, including synapse formation, persist for 3 months following treatment interruption.
Although the precise mechanisms underlying the long-lasting effects of CBL remain unclear, based on the persistence of behavioral improvements over and above the reduction in Aβ plaque load, we propose that the neurotrophic-like effects of CBL, rather than its effects on Aβ deposition, may be involved the observed sustained beneficial following the cessation of treatment.
CBL has previously been reported to decrease Aβ production in APP tg mice by regulating the maturation of the amyloid protein precursor (Rockenstein et al. 2006) and to decrease amyloid deposition around the cerebrovasculature (Rockenstein et al. 2005a) however our results indicate that whilst behavior analysis in the water maze test demonstrated a sustained beneficial effect of CBL up to 3 months after the cessation of treatment, neocortical amyloid plaque load in the TPT 3m CBL-treated APP tg mice was comparable to that observed in the saline-treated APP tg mice at this age indicating that,at TPT 3m, the beneficial effect of CBL, whilst still leading to improved behavioral performance, did not extend to a continued reduction in Aβ plaques. Although there was a slight, but significant, decrease in hippocampal amyloid plaques at TPT 3m in the CBL-treated APP tg mice, the reduction in Aβ deposition at this time point was nowhere near as dramatic as that observed at TPT 0m.
These results, in conjunction with the immunohistochemical analysis of synaptic structure and neuronal density, which showed an increase in neocortical and hippocampal synaptic and neuronal number at TPT 3m, consistent with the sustained behavioral improvement at this time, suggest that whatever mechanism underlie the long-term effect of CBL, they are independent of the effects of CBL on Aβ plaques. CBL has been widely reported to have neurotrophic factor-like activity (Chen et al. 2007; Francis-Turner and Valouskova 1996; Nagahara et al. 2009; Rockenstein et al. 2005a; Satou et al. 2000; Svendsen et al. 1991; Veinbergs et al. 2000; Windisch et al. 1998) and we propose that it is this facet of CBL that may underlie the observed long-term effects of CBL following treatment reduction, although the precise neurotrophic factor-like activity (CNTF, NGF, BDNF - or a combination) sub-served by CBL and the receptors involved in this activity remain to be further examined.
These results provide experimental evidence to support previous clinical trials that have shown intravenous administration of CBL in patients with AD and vascular dementia has statistically significant beneficial effects on clinical global outcome measures (Plosker and Gauthier 2009; Plosker and Gauthier 2010). Moreover, the benefits of CBL appeared to last for several months after stopping treatment in these studies (Plosker and Gauthier 2009; Plosker and Gauthier 2010). Behavioral and other functional benefits, as assessed by neuro-psychiatric tests and activities of daily living, respectively, were also noted to persist several months after stopping the treatment in patients with AD or vascular dementia. Given that CBL has been reported to display neurotrophic-like effects (Chen et al. 2007; Francis-Turner and Valouskova 1996; Francis-Turner et al. 1996; Valouskova and Francis-Turmer 1996; Veinbergs et al. 2000), promote neurogenesis (Chen et al. 2007; Onose et al. 2009; Rockenstein et al. 2007b; Zhang et al. 2010) and synaptogenesis (Mallory et al. 1999; Rockenstein et al. 2003a) it is probable that the persistent nature of the effects of CBL following termination of the treatment might be related to the ability of this peptide mixture at promoting the long term remodeling of neuronal circuitries and enhancing synaptic connectivity. In support of this hypothesis clinical studies have shown that CBL treatment stabilizes EEG activity in patients with AD, the elderly or those with brain injury (Alvarez et al. 2000; Alvarez et al. 2008; Alvarez et al. 2003; Funke et al. 1998; Muresanu et al. 2008).
The continued beneficial effects of CBL in the spatial learning and memory 3 months after treatment interruption were consistent with the effects on synaptophysin-immunoreactive terminals but did not follow the patterns of Aβ deposition and astrogliosis, which were elevated in the CBL-treated APP tg mice 3 months after the termination of CBL administration. These results are consistent with previous studies showing that the improvement in behavioral parameters in aged rats (Reinprecht et al. 1999) and APP tg mice correlate best with the levels of synaptophysin-immunoreactive terminals and not with the amyloid plaques (Rockenstein et al. 2003a; Rockenstein et al. 2006). Moreover this outcome is in agreement with studies showing that loss of synapses is the best correlate of behavioral deficits in AD (DeKosky et al. 1996; Scheff et al. 1990; Terry et al. 1991) patients and that synaptic damage in AD (Pham et al. 2010) and in APP tg mice is independent of amyloid deposits (Mucke et al. 2000).
The results from this study also suggest that the mechanisms through which CBL promotes synaptogenesis and reduces Aβ deposition might be somewhat different. We have previously shown that in APP tg mice CBL reduces Aβ production by modulating APP phosphorylation and maturation and that this involves the regulation of CDK5 and GSK3β (Rockenstein et al. 2006); CBL also reduces TAU phosphorylation and related neurodegenerative pathology through this pathway (Ubhi et al. 2009). Thus the CDK5 and GSK3β signaling pathways might be only partially involved in modulating the synaptogenic effects of CBL and other pathways such as those mediated by calpain I (Wronski et al. 2000) or anti-apoptotic factors (eg: bcl2) might play a more substantial role (Deigner et al. 2000). Taken together, these findings support the possibility that CBL might act at the site of a neurotrophic factor-like receptor that couples with the Akt/GSK3β and CDK5 signaling pathways. The effects of modulating such cascade might exert pleotropic effects including, neuroprotection, anti-apoptosis, neurogenesis, and anti-amyloid and TAU pathology. The identity of such receptor or sites and more detailed dissection of the signaling pathways involved await further investigation.
Another interesting finding of the present study was that effects of CBL on synapses and behavior are washed out 6 months after suspending the treatment. This provides evidence for a close relationship between the amelioration of the AD-related neuropathology and treatment with CBL. To our knowledge this is one of the first studies in APP tg mice to demonstrate the time course for reversal of therapeutic effects upon interruption of treatment. The results from this study not only provide important information for the management of CBL administration and its duration but may also be relevant for any therapy aimed at the amelioration of AD-related deficits.
ACKNOWLEDGEMENTS
This work was partially supported by NIH grant AG05131 and by a grant from EVER Pharma.
REFERENCES
- Alvarez XA, Cacabelos R, Laredo M, Couceiro V, Sampedro C, Varela M, Corzo L, Fernandez-Novoa L, Vargas M, Aleixandre M, Linares C, Granizo E, Muresanu D, Moessler H. A 24-week, double-blind, placebo-controlled study of three dosages of Cerebrolysin in patients with mild to moderate Alzheimer's disease. Eur J Neurol. 2006;13(1):43–54. doi: 10.1111/j.1468-1331.2006.01222.x. [DOI] [PubMed] [Google Scholar]
- Alvarez XA, Cacabelos R, Sampedro C, Aleixandre M, Linares C, Granizo E, Doppler E, Moessler H. Efficacy and safety of Cerebrolysin in moderate to moderately severe Alzheimer's disease: results of a randomized, double-blind, controlled trial investigating three dosages of Cerebrolysin. Eur J Neurol. 2011;18(1):59–68. doi: 10.1111/j.1468-1331.2010.03092.x. [DOI] [PubMed] [Google Scholar]
- Alvarez XA, Lombardi VR, Corzo L, Perez P, Pichel V, Laredo M, Hernandez A, Freixeiro F, Sampedro C, Lorenzo R, Alcaraz M, Windisch M, Cacabelos R. Oral Cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315–328. doi: 10.1007/978-3-7091-6781-6_33. [DOI] [PubMed] [Google Scholar]
- Alvarez XA, Sampedro C, Figueroa J, Tellado I, Gonzalez A, Garcia-Fantini M, Cacabelos R, Muresanu D, Moessler H. Reductions in qEEG slowing over 1 year and after treatment with Cerebrolysin in patients with moderate-severe traumatic brain injury. J Neural Transm. 2008;115(5):683–692. doi: 10.1007/s00702-008-0024-9. [DOI] [PubMed] [Google Scholar]
- Alvarez XA, Sampedro C, Perez P, Laredo M, Couceiro V, Hernandez A, Figueroa J, Varela M, Arias D, Corzo L, Zas R, Lombardi V, Fernandez-Novoa L, Pichel V, Cacabelos R, Windisch M, Aleixandre M, Moessler H. Positive effects of cerebrolysin on electroencephalogram slowing, cognition and clinical outcome in patients with postacute traumatic brain injury: an exploratory study. Int Clin Psychopharmacol. 2003;18(5):271–278. doi: 10.1097/00004850-200309000-00003. [DOI] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67(8):1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- Chana G, Landau S, Beasley C, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry. 2003;53(12):1086–1098. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- Chen H, Tung YC, Li B, Iqbal K, Grundke-Iqbal I. Trophic factors counteract elevated FGF-2-induced inhibition of adult neurogenesis. Neurobiol Aging. 2007;28(8):1148–1162. doi: 10.1016/j.neurobiolaging.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Chen KS, Gage FH. Somatic gene transfer of NGF to the aged brain: behavioral and morphological amelioration. J Neurosci. 1995;15(4):2819–2825. doi: 10.1523/JNEUROSCI.15-04-02819.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deigner HP, Haberkorn U, Kinscherf R. Apoptosis modulators in the therapy of neurodegenerative diseases. Expert Opin Investig Drugs. 2000;9(4):747–764. doi: 10.1517/13543784.9.4.747. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration. 1996;5(4):417–421. doi: 10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- EBEWENeuroPharmaGmbH. Cerebrolysin ® solution for injection: summary of product characteristics. 2009 [Google Scholar]
- Fischer W, Wictorin K, Bjorklund A, Williams LR, Varon S, Gage FH. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature. 1987;329(6134):65–68. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- Francis-Turner L, Valouskova V. Nerve growth factor and nootropic drug Cerebrolysin but not fibroblast growth factor can reduce spatial memory impairment elicited by fimbria-fornix transection: short-term study. Neurosci Lett. 1996;202(3):193–196. doi: 10.1016/0304-3940(95)12240-0. [DOI] [PubMed] [Google Scholar]
- Francis-Turner L, Valouskova V, Mokry J. The long-term effect of NGF, b-FGF and Cerebrolysin on the spatial memory after fimbria-fornix lesion in rats. J Neural Transm Suppl. 1996;47:277. doi: 10.1007/978-3-7091-6892-9_22. [DOI] [PubMed] [Google Scholar]
- Funke M, Fiehler J, Mewes I, Eiselt M, Rother I, Windisch M. Dose-dependent effects of Cerebrolysin on EEG and short-term memory of healthy volunteers during control and hyperventilation induced cerebral ischemia. J Neural Transm Suppl. 1998;53:385–398. doi: 10.1007/978-3-7091-6467-9_34. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986;6(8):2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer LF. Nerve growth factor treatment after brain injury prevents neuronal death. Science. 1987;235(4785):214–216. doi: 10.1126/science.3798108. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116(2):321–361. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- Mallory M, Honer W, Hsu L, Johnson R, Rockenstein E, Masliah E. In vitro synaptotrophic effects of Cerebrolysin in NT2N cells. Acta Neuropathol. 1999;97(5):437–446. doi: 10.1007/s004010051012. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Koliatsos VE, Breckler SJ, Price DL, Olton DS. Human nerve growth factor improves spatial memory in aged but not in young rats. J Neurosci. 1994;14(8):4815–4824. doi: 10.1523/JNEUROSCI.14-08-04815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Serrano A, Fischer W, Soderstrom S, Ebendal T, Bjorklund A. Long-term functional recovery from age-induced spatial memory impairments by nerve growth factor gene transfer to the rat basal forebrain. Proc Natl Acad Sci U S A. 1996;93(13):6355–6360. doi: 10.1073/pnas.93.13.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E. Mechanisms of synaptic dysfunction in Alzheimer's disease. HistolHistopathol. 1995;10:509–519. [PubMed] [Google Scholar]
- Masliah E, Armasolo F, Veinbergs I, Mallory M, Samuel W. Cerebrolysin ameliorates performance deficits, and neuronal damage in apolipoprotein E-deficient mice. Pharmacol Biochem Behav. 1999;62(2):239–245. doi: 10.1016/s0091-3057(98)00144-0. [DOI] [PubMed] [Google Scholar]
- Masliah E, Crews L, Hansen L. Synaptic remodeling during aging and in Alzheimer's disease. J Alzheimers Dis. 2006;9(3 Suppl):91–99. doi: 10.3233/jad-2006-9s311. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56(1):127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- Maslow K. 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6(2):158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20(11):4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Conner JM, Kordower JH. Nerve growth factor in Alzheimer's disease: defective retrograde transport to nucleus basalis. Neuroreport. 1995;6(7):1063–1066. doi: 10.1097/00001756-199505090-00028. [DOI] [PubMed] [Google Scholar]
- Muresanu DF, Alvarez XA, Moessler H, Buia M, Stan A, Pintea D, Moldovan F, Popescu BO. A pilot study to evaluate the effects of Cerebrolysin on cognition and qEEG in vascular dementia: cognitive improvement correlates with qEEG acceleration. J Neurol Sci. 2008;267(1–2):112–119. doi: 10.1016/j.jns.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15(3):331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onose G, Muresanu DF, Ciurea AV, Daia Chendreanu C, Mihaescu AS, Mardare DC, Andone I, Spanu A, Popescu C, Dumitrescu A, Popescu M, Grigorean V, Ungur B, Marinescu F, Colibbeanu I, Onose L, Haras M, Sandu A, Spircu T. Neuroprotective and consequent neurorehabilitative clinical outcomes, in patients treated with the pleiotropic drug cerebrolysin. J Med Life. 2009;2(4):350–360. [PMC free article] [PubMed] [Google Scholar]
- Pham E, Crews L, Ubhi K, Hansen L, Adame A, Cartier A, Salmon D, Galasko D, Michael S, Savas JN, Yates JR, Glabe C, Masliah E. Progressive accumulation of amyloid-beta oligomers in Alzheimer's disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J. 2010;277(14):3051–3067. doi: 10.1111/j.1742-4658.2010.07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plosker GL, Gauthier S. Cerebrolysin: a review of its use in dementia. Drugs Aging. 2009;26(11):893–915. doi: 10.2165/11203320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Plosker GL, Gauthier S. Spotlight on cerebrolysin in dementia. CNS Drugs. 2010;24(3):263–266. doi: 10.2165/11204820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Reinprecht I, Gschanes A, Windisch M, Fachbach G. Two peptidergic drugs increase the synaptophysin immunoreactivity in brains of 24-month-old rats. Histochem J. 1999;31(6):395–401. doi: 10.1023/a:1003752208971. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Adame A, Mante M, Larrea G, Crews L, Windisch M, Moessler H, Masliah E. Amelioration of the cerebrovascular amyloidosis in a transgenic model of Alzheimer's disease with the neurotrophic compound cerebrolysin. J Neural Transm. 2005a;112(2):269–282. doi: 10.1007/s00702-004-0181-4. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Adame A, Mante M, Moessler H, Windisch M, Masliah E. The neuroprotective effects of Cerebrolysin in a transgenic model of Alzheimer's disease are associated with improved behavioral performance. J Neural Transm. 2003a;110(11):1313–1327. doi: 10.1007/s00702-003-0025-7. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Adame A, Mante M, Moessler H, Windisch M, Masliah E. The neuroprotective effects of Cerebrolysin trade mark in a transgenic model of Alzheimer's disease are associated with improved behavioral performance. J Neural Transm. 2003b;110(11):1313–1327. doi: 10.1007/s00702-003-0025-7. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Crews L, Masliah E. Transgenic animal models of neurodegenerative diseases and their application to treatment development. Adv Drug Deliv Rev. 2007a;59(11):1093–1102. doi: 10.1016/j.addr.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Mante M, Alford M, Windisch M, Moessler H, Masliah E. Effects of Cerebrolysin on amyloid-beta deposition in a transgenic model of Alzheimer's disease. J Neural Transm Suppl. 2002;62:327–336. [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Mante M, Sisk A, Masliaha E. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta(1–42) J Neurosci Res. 2001;66(4):573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mante M, Adame A, Crews L, Moessler H, Masliah E. Effects of Cerebrolysin on neurogenesis in an APP transgenic model of Alzheimer's disease. Acta Neuropathol. 2007b;113(3):265–275. doi: 10.1007/s00401-006-0166-5. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mante M, Alford M, Adame A, Crews L, Hashimoto M, Esposito L, Mucke L, Masliah E. High beta-secretase activity elicits neurodegeneration in transgenic mice despite reductions in amyloid-beta levels: implications for the treatment of Alzheimer disease. J Biol Chem. 2005b;280(38):32957–32967. doi: 10.1074/jbc.M507016200. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Torrance M, Adame A, Mante M, Bar-on P, Rose JB, Crews L, Masliah E. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer's disease are associated with reduced amyloid precursor protein phosphorylation. J Neurosci. 2007c;27(8):1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Torrance M, Mante M, Adame A, Paulino A, Rose JB, Crews L, Moessler H, Masliah E. Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer's disease. J Neurosci Res. 2006;83(7):1252–1261. doi: 10.1002/jnr.20818. [DOI] [PubMed] [Google Scholar]
- Rockenstein EM, McConlogue L, Tan H, Power M, Masliah E, Mucke L. Levels and alternative splicing of amyloid beta protein precursor (APP) transcripts in brains of APP transgenic mice and humans with Alzheimer's disease. J Biol Chem. 1995;270(47):28257–28267. doi: 10.1074/jbc.270.47.28257. [DOI] [PubMed] [Google Scholar]
- Ruther E, Ritter R, Apecechea M, Freytag S, Gmeinbauer R, Windisch M. Sustained improvements in patients with dementia of Alzheimer's type (DAT) 6 months after termination of Cerebrolysin therapy. J Neural Transm. 2000;107(7):815–829. doi: 10.1007/s007020070061. [DOI] [PubMed] [Google Scholar]
- Ruther E, Ritter R, Apecechea M, Freytag S, Windisch M. Efficacy of the peptidergic nootropic drug cerebrolysin in patients with senile dementia of the Alzheimer type (SDAT) Pharmacopsychiatry. 1994;27(1):32–40. doi: 10.1055/s-2007-1014271. [DOI] [PubMed] [Google Scholar]
- Satou T, Itoh T, Tamai Y, Ohde H, Anderson AJ, Hashimoto S. Neurotrophic effects of FPF-1070 (Cerebrolysin) on cultured neurons from chicken embryo dorsal root ganglia, ciliary ganglia, and sympathetic trunks. J Neural Transm. 2000;107(11):1253–1262. doi: 10.1007/s007020070015. [DOI] [PubMed] [Google Scholar]
- Scheff S, DeKosky S, Price D. Quantitative assessment of cortical synaptic density in Alzheimer's disease. NeurobiolAging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- Scott SA, Mufson EJ, Weingartner JA, Skau KA, Crutcher KA. Nerve growth factor in Alzheimer's disease: increased levels throughout the brain coupled with declines in nucleus basalis. J Neurosci. 1995;15(9):6213–6221. doi: 10.1523/JNEUROSCI.15-09-06213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, Marr RA, Rockenstein E, Crews L, Adame A, Potkar R, Patrick C, Gage FH, Verma IM, Masliah E. Long-term neprilysin gene transfer is associated with reduced levels of intracellular Abeta and behavioral improvement in APP transgenic mice. BMC Neurosci. 2008;9:109. doi: 10.1186/1471-2202-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen CN, Cooper JD, Sofroniew MV. Trophic factor effects on septal cholinergic neurons. Ann N Y Acad Sci. 1991;640:91–94. doi: 10.1111/j.1749-6632.1991.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Shin RW, Schmidt ML, Lee VM. Relationship between plaques, tangles, and dystrophic processes in Alzheimer's disease. Neurobiol Aging. 1995;16(3):335–340. doi: 10.1016/0197-4580(94)00176-2. discussion 341-335. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH. Nerve growth factor gene therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21(2):179–189. doi: 10.1097/WAD.0b013e318068d6d2. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Gage FH. Bridging grafts and transient nerve growth factor infusions promote long-term central nervous system neuronal rescue and partial functional recovery. Proc Natl Acad Sci U S A. 1995;92(10):4621–4625. doi: 10.1073/pnas.92.10.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11(5):551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- Ubhi K, Rockenstein E, Doppler E, Mante M, Adame A, Patrick C, Trejo M, Crews L, Paulino A, Moessler H, Masliah E. Neurofibrillary and neurodegenerative pathology in APP-transgenic mice injected with AAV2-mutant TAU: neuroprotective effects of Cerebrolysin. Acta Neuropathol. 2009;117(6):699–712. doi: 10.1007/s00401-009-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valouskova V, Francis-Turmer L. The short-term influence of b-FGF, NGF and Cerebrolysin on the memory impaired after fimbria-fornix lesion. J Neural Transm Suppl. 1996;47:280. doi: 10.1007/978-3-7091-6892-9_25. [DOI] [PubMed] [Google Scholar]
- Veinbergs I, Mante M, Mallory M, Masliah E. Neurotrophic effects of Cerebrolysin in animal models of excitotoxicity. J Neural Transm Suppl. 2000;59:273–280. doi: 10.1007/978-3-7091-6781-6_29. [DOI] [PubMed] [Google Scholar]
- Williams LR, Rylett RJ, Moises HC, Tang AH. Exogenous NGF affects cholinergic transmitter function and Y-maze behavior in aged Fischer 344 male rats. Can J Neurol Sci. 1991;18(3 Suppl):403–407. doi: 10.1017/s0317167100032546. [DOI] [PubMed] [Google Scholar]
- Williams LR, Varon S, Peterson GM, Wictorin K, Fischer W, Bjorklund A, Gage FH. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci U S A. 1986;83(23):9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch M, Gschanes A, Hutter-Paier B. Neurotrophic activities and therapeutic experience with a brain derived peptide preparation. J Neural Transm Suppl. 1998;53:289–298. doi: 10.1007/978-3-7091-6467-9_25. [DOI] [PubMed] [Google Scholar]
- Wronski R, Tompa P, Hutter-Paier B, Crailsheim K, Friedrich P, Windisch M. Inhibitory effect of a brain derived peptide preparation on the Ca++-dependent protease, calpain. J Neural Transm. 2000;107(2):145–157. doi: 10.1007/s007020050013. [DOI] [PubMed] [Google Scholar]
- Zhang C, Chopp M, Cui Y, Wang L, Zhang R, Zhang L, Lu M, Szalad A, Doppler E, Hitzl M, Zhang ZG. Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J Neurosci Res. 2010;88(15):3275–3281. doi: 10.1002/jnr.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]