Abstract
The hypothalamic-pituitary-adrenal (HPA) axis is an important regulator of energy balance, immune function and the body’s response to stress. Signaling networks governing the initial specification of corticotropes, a major component of this axis, are not fully understood. Loss of function studies indicate that Notch signaling may be necessary to repress premature differentiation of corticotropes and to promote proliferation of pituitary progenitors. To elucidate whether Notch signaling must be suppressed in order for corticotrope differentiation to proceed and whether Notch signaling is sufficient to promote corticotrope proliferation, we examined the effects of persistent Notch expression in Pomc lineage cells. We show that constitutive activation of the Notch cascade inhibits the differentiation of both corticotropes and melanotropes and results in the suppression of transcription factors required for Pomc expression. Furthermore, persistent Notch signaling traps cells in the intermediate lobe of the pituitary in a progenitor state, but has no effect on pituitary proliferation. Undifferentiated cells are eliminated in the first two postnatal weeks in these mice, resulting in a modest increase in CRH expression in the paraventricular nucleus, hypoplastic adrenal glands and decreased stress-induced corticosterone levels. Taken together, these findings show that Notch signaling is sufficient to prevent corticotrope and melanotrope differentiation, resulting in dysregulation of the HPA axis.
Keywords: pituitary, corticotrope, melanotrope, Notch, HPA axis, SOX2
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis is critical to mediating the body’s response to stress. It also functions to regulate many important homeostatic processes, including immune response and energy balance. Stressors, either immune or environmental, activate the release of corticotropin releasing hormone (CRH) from the paraventricular nucleus (PVN) of the hypothalamus to the anterior pituitary through the hypothalamo-hypophyseal portal system. CRH release stimulates the anterior pituitary to synthesize and release adrenocorticotropic hormone (ACTH), which acts on the adrenal glands to trigger the release of cortisol (corticosterone in mice) (Garren. 1968; Vale, et al. 1981). Increased cortisol release results in a number of different physiological effects, including an increase in glucose production and mobilization and an anti-inflammatory response (Munck, et al. 1984). In addition to increased glucose release, cortisol also exerts negative feedback on both the hypothalamus and pituitary, inhibiting further release of CRH and ACTH (Swanson, Simmons. 1989). Disruption in proper development or function at any level of the HPA axis can result in multiple disorders, including Cushing’s syndrome and adrenal insufficiency, which are characterized by increased and decreased levels of cortisol, respectively (Drouin, et al. 2007).
The pituitary, a crucial component of the HPA axis, develops from an invagination of the oral ectoderm at embryonic day (e) 8.5 in mice. By e11.5, the developing pituitary pinches off from the underlying oral ectoderm, forming the structure known as Rathke’s pouch (RP). RP contains all the progenitor cells that are necessary for formation of the anterior lobe (AL) and intermediate lobe (IL) of the pituitary (Burrows, et al. 1999; Rizzoti, Lovell-Badge. 2005). The AL contains five hormone producing cell types, which include thyrotropes, lactotropes, gonadotropes, corticotropes, somatotropes, and lactotropes. The IL houses a sixth hormone cell type, the melanotropes. Current models propose that pituitary hormone producing cells are specified such that each cell type differentiates during a discrete time during embryonic development (Pope, et al. 2006). Furthermore, it is widely accepted that cells exiting the cell cycle at the same time migrate to the same location in the pituitary (Dasen, Rosenfeld. 2001; Wagner, Thomas. 2007). This synchronized cell specification and migration is thought to be coordinated by opposing gradients of bone morphogenetic proteins (BMPs) in the underlying oral ectoderm and fibroblast growth factors (FGFs) in the ventral diencephalon (Ericson, et al. 1998; Treier, et al. 1998). However, recent evidence indicates that each AL hormone cell type exits the cell cycle at the same time, with the majority of cells doing so between e11.5 and e13.5. Furthermore, there is no apparent correlation between the timing of cell specification and cell placement within the gland (Davis, et al. 2011). Based on these observations, it remains unclear how undifferentiated progenitor cells within the same region of the AL, exposed to the same chemical gradients, are specified to become different cell types.
Although all AL hormone cell types exit the cell cycle at the same time, corticotropes are the first to express hormone beginning at e12.5 (Japón, et al. 1994). Melanotropes, found in the IL, exit the cell cycle after AL hormone producing cells and express hormone beginning at e16.5 (Davis, et al. 2011). Terminal differentiation of corticotropes and melanotropes is characterized by the expression of pro-opiomelanocortin (POMC), a precursor of both ACTH, which is released by corticotropes, and melanocyte stimulating hormone (αMSH), which is produced by melanotropes.
Several studies have identified multiple transcription factors that activate or inhibit expression of POMC in the pituitary. These include TPIT (TBX19), (Lamolet, et al. 2001; Liu, et al. 2001), NEUROD1 (Poulin, et al. 1997; Lamolet, et al. 2004), Nur77 (Philips, et al. 1997a; Philips, et al. 1997b) and BMPs (Nudi, et al. 2005). While these factors are known to regulate Pomc transcription and thus the terminal differentiation of corticotropes and melanotropes, it remains unclear which signals are involved in the specification of these cells.
We hypothesize that the Notch signaling pathway, an essential player in progenitor maintenance and cell fate determination in many tissues, may play a critical role in the specification of corticotropes and melanotropes. The Notch ligands Delta-like1 and Jagged1, the Notch2 and Notch3 receptors, as well as downstream Notch effectors of the Hes and Hey families of genes, are present during development in proliferating progenitors in RP. As cells differentiate and migrate towards the developing AL, the expression of these molecules is suppressed (Raetzman, et al. 2004; Zhu, et al. 2006a). Additionally, the Notch1 and Notch2 receptors, as well as Hes and Hey transcription factors, are present in pituitary stem cells (Chen, et al. 2005; Chen, et al. 2006).
Studies have shown that loss of function manipulations of the Notch signaling pathway result in defects in corticotrope and melanotrope differentiation. In mice lacking Hes1, a canonical Notch target, Tpit mRNA expression was found to be increased (Zhu, et al. 2006b), suggesting that Notch may be necessary to prevent early expression of this transcription factor. When both Prop1, a pituitary specific Notch target, and Hes1 are lost, robust premature differentiation of corticotropes is observed (Himes, Raetzman. 2009). A similar, but more modest, acceleration of Pomc expression is seen in a pituitary-specific knockout of Rbpj, the primary mediator of Notch signaling (Zhu, et al. 2006b). Taken together, these results suggest Notch signaling is necessary to prevent differentiation of corticotropes and may act as a negative regulator of early corticotrope specification. We hypothesize that Notch signaling is sufficient to prevent the differentiation of corticotropes and melanotropes and that Notch expression needs to be extinguished for cell differentiation to proceed.
In order to address this hypothesis, we examined the effects of persistent expression of activated Notch1 in POMC-expressing cells. We find that Notch signaling is sufficient to prevent differentiation of these cells, coincident with the suppression of the transcription factors Tpit and Neurod1. Additionally, we show that these undifferentiated cells maintain progenitor qualities but are eliminated during the first two postnatal weeks. The loss of POMC expression in the pituitary results in severely hypoplastic adrenal glands and decreased stress-induced corticosterone levels. Our results indicate that Notch signaling is both necessary and sufficient to repress corticotrope differentiation and that alterations in Notch signaling could result in dysfunction of the HPA axis.
Results
NICD expression in melanotropes and corticotropes activates Notch target genes
To determine the effect of aberrant Notch activity during corticotrope and melanotrope development, we generated a mouse model with persistent expression of the activated Notch1 intracellular domain (NICD) in POMC-expressing cells. This mouse was generated from the mating of a RosaNotch floxed mouse (Murtaugh, et al. 2003) to a mouse expressing Cre recombinase under the control of the Pomc promoter(Balthasar, et al. 2004). POMC is expressed in corticotropes in the AL and in melanotropes in the IL. Additionally, POMC is produced by hypothalamic neurons in the arcuate nucleus. However, these neurons do not innervate pituitary melanotropes or corticotropes and are formed through different mechanisms than pituitary cells (McNay, et al. 2006)(Oertel, et al. 1982; Léránth, et al. 1983; Mezey, et al. 1984; Kawano, Daikoku. 1987; Goudreau, et al. 1995). In the absence of Cre recombinase, a stop codon flanked by loxP sites prevents the expression of the single-copy NICD construct. However, when Cre recombinase is active, the stop codon is excised allowing for the constitutive expression of NICD in Pomc-expressing cells (Fig. 1A). Although Notch1 is not normally expressed during pituitary development, studies have shown that the intracellular domains of Notch1 and Notch2 can have similar transcriptional activity (Kraman, McCright. 2005). Furthermore, Notch1 is expressed in the adult stem cell population (Chen, et al. 2005; Chen, et al. 2006), indicating it may play a role in cell fate choice or maintaining progenitor cells in the adult pituitary. Therefore, it is of interest to determine the effects of persistent Notch1 expression in pituitary cell specification during development and in postnatal progenitor maintenance.
Figure 1.
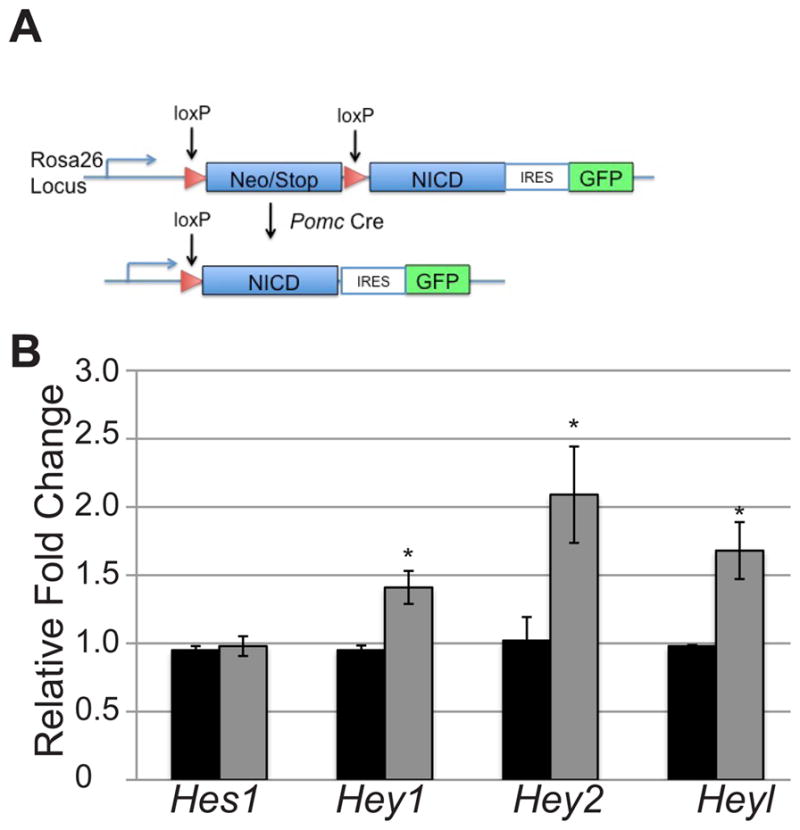
Persistent expression of NICD results in increases in mRNA levels of Notch targets. (A) Construct and mating scheme for activated NICD expression in Pomc-expressing cells. (B) Activated NICD results in modest increases in canonical target mRNA levels in Tg (gray bars) pituitaries as compared to control (black bars) at e16.5. n=3–5. *:p<0.05.
Quantitative RT-PCR for Hes1, Hey1, Hey2 and Heyl was performed on RosaNotch/+ (control) and RosaNotch/+; Pomc-Cre (Tg) pituitaries at e16.5 to determine if NICD overexpression results in an increase in any of the canonical Notch effector genes. The age e16.5 was chosen to begin analysis since it is the earliest developmental stage at which POMC expression is present in both corticotropes and melanotropes. Our results show that Hes1 mRNA levels are unchanged between the two experimental groups. However, Hey1, Hey2, and Heyl are all significantly increased in Tg pituitaries as compared to their control counterparts (Fig. 1B). These results indicate that overexpression of Notch in pituitary melanotropes and corticotropes is sufficient to produce a modest change in Notch effector mRNA levels.
Persistent expression of activated NICD is sufficient to prevent corticotrope and melanotrope terminal differentiation
Notch signaling is known to regulate the balance between proliferating progenitors and differentiated cells in many endocrine organs. Therefore, aberrant Notch signaling could possibly alter the size or morphology of the developing pituitary. To detect if any such morphological changes are present in Tg mice, control and Tg pituitary sections were stained with hematoxylin and eosin. At e16.5, the morphology and size of the control (Fig. 2A) and Tg (Fig. 2B) pituitaries appear similar, with all three lobes present in both experimental groups. The same is true at postnatal day (p) 1, when pituitaries of control (Fig. 2E) and Tg (Fig. 2F) mice are histologically indistinguishable. Since Notch signaling is also implicated in cell differentiation in many tissues, we used immunohistochemistry to examine whether POMC expression is affected by persistently activated Notch expression at e16.5 and p1. At e16.5, control mice have POMC-positive cells in the IL and scattered throughout the AL (Fig. 2C). At this same age, Tg mice have very few POMC-positive cells in either the AL or IL. (Fig. 2D), indicating their differentiation is prevented by expression of NICD. This phenotype persists through p1, when almost no cells in the IL or AL of Tg mice are POMC-positive (Fig, 2H), compared to the control (Fig. 2G). Pomc mRNA levels of control and Tg e16.5 pituitaries were measured using quantitative RT-PCR to determine if this transcript is altered by persistent NICD expression. Similar to immunohistochemical observations, Tg mice have significantly lower levels of Pomc mRNA as compared to control littermates (Fig. 2I). Taken together, these data show that constitutive NICD expression in melanotropes and corticotropes is sufficient to inhibit their differentiation and imply that it is critical that Notch signaling be suppressed in order for corticotrope and melanotrope differentiation to proceed.
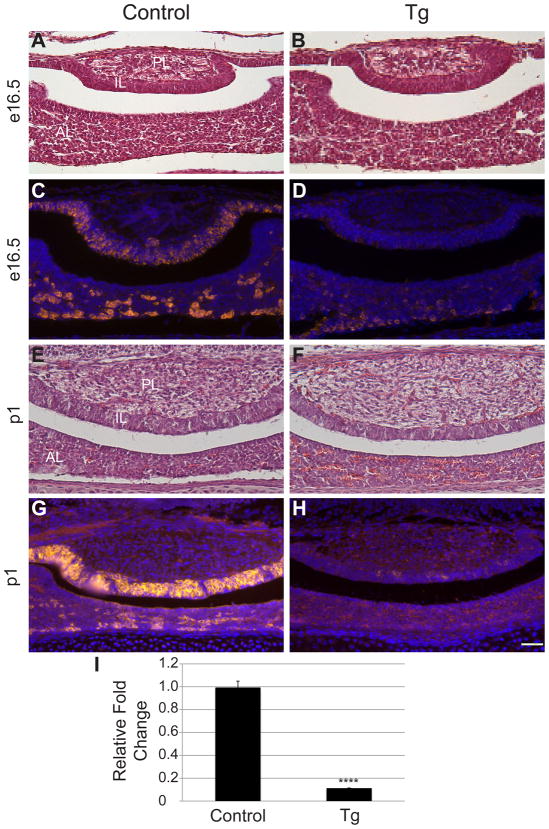
Figure 2.
Persistent NICD expression inhibits the differentiation of melanotropes and corticotropes. Coronal sections of control (A) and Tg (B) e16.5 pituitaries were stained with hematoxylin and eosin. No differences in morphology were observed between the two groups. POMC immunoreactive cells (red) are found in the IL and AL of control (C) pituitaries at e16.5 and are diminished in both lobes of Tg (D) mice. Morphology is similar at p1 in control (E) and Tg (F) pituitaries. POMC expression (red) is observed in the AL and IL of control mice (G) and is markedly decreased in Tg pituitaries (H) at p1. RT-PCR at e16.5 reveals significant decreases in Pomc mRNA in Tg mice as compared to controls (I). Photos taken at 200x; Scale bars: 50μm. ****:p<0.0001. n=5–7 (immunohistochemistry) and n=3–5 (qRT-PCR). PL=posterior lobe. AL=anterior lobe. IL=intermediate lobe.
Activated Notch inhibits the transcription of factors necessary for POMC expression
To better elucidate the mechanism by which Notch inhibits the differentiation of corticotropes and melanotropes, quantitative RT-PCR was used to examine the mRNA levels of the transcription factors Tpit, Neurod1 and Mash1 in control and Tg mice at e16.5. Tpit and Neurod1 have been shown to play a role in the activation of Pomc transcription, whereas Mash1 appears to be restricted to Pomc containing cells, although it is not required for Pomc transcription (Poulin, et al. 1997; Lamolet, et al. 2001; Liu, et al. 2001; Pulichino, et al. 2003a; Pulichino, et al. 2003b; Lamolet, et al. 2004; McNay, et al. 2006). Tpit, NeuroD1 and Mash1 all show significantly lower mRNA levels in Tg mice as compared to their control counterparts, with Tpit showing the strongest suppression (Fig. 3). Therefore, Notch signaling components may directly or indirectly affect the expression of factors important for terminal differentiation of corticotropes and melanotropes.
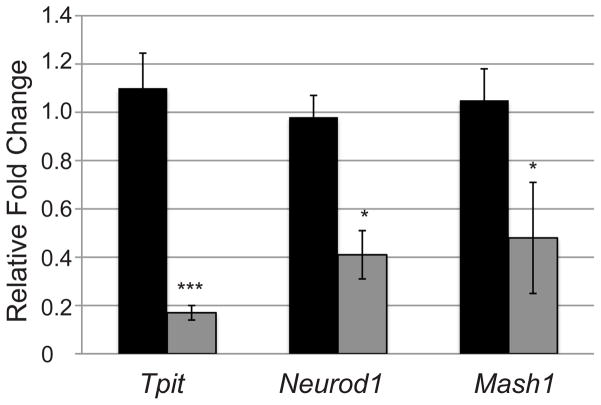
Figure 3.
Transcription factors regulating Pomc expression are decreased in pituitaries of NICD expressing mice at p1. Tpit, NeuroD1 and Mash1 mRNA levels are significantly reduced in Tg mice (gray bars) as compared to control (black bars) littermates at e16.5 determined by qRT-PCR. * p<0.05. ***: p<0.001. n=3–5.
Undifferentiated IL cells remain SOX2-positive progenitors
Given that IL cells fail to differentiate when activated NICD is constitutively expressed, we hypothesized that these cells may retain markers of pituitary progenitors, such as SOX2 (Fauquier, et al. 2008). In control mice, SOX2-positive cells are seen in the IL in a thin layer lining the lumen of the pituitary (Fig. 4, A, C, E and G). Conversely, in Tg mice, nearly every cell in the IL is positive for SOX2, indicating these cells maintain progenitor cell qualities (Fig. 4, B, D, F and H). Pax7 is a putative marker of intermediate pituitary progenitors (Hosoyama, et al. 2010) and is expressed throughout the IL in control mice at p1 (Fig 4I), however a thin layer of cells lining the pituitary cleft are mostly Pax7-negative (Fig. 4K, M, O). Based on localization, they likely constitute SOX2-positive cells. In Tg mice, Pax7 is also restricted to the IL (Fig. 4J). However, nearly every cell in the IL, including those lining the cleft, expresses Pax7 (Fig. 4, L, N, P). Taken together, these findings indicate that constitutive NICD expression results in an increased progenitor population in the IL.
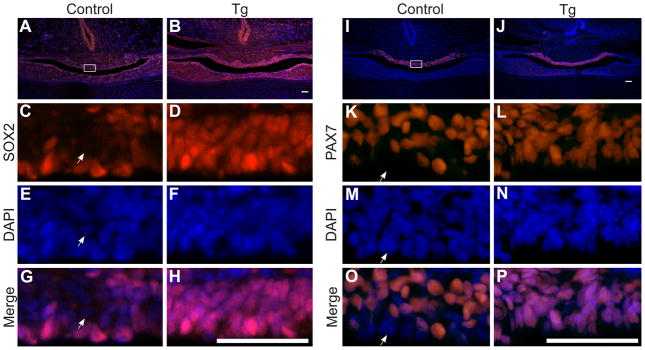
Figure 4.
IL cells containing NICD express markers of pituitary progenitors. SOX2 (red) is expressed at p1 in the pituitary of control (A) and Tg (B) mice. In control animals, SOX2 immunoreactivity (red) is mostly observed lining the cleft of the pituitary (C, G), with nuclei stained with DAPI (blue; E, G). Although there are also scattered SOX2 immunoreactive cells in the IL, many cells are not SOX2 immunoreactive (compare E (nuclei stained with DAPI) and G). In contrast, nearly every cell in the IL of Tg mice is immunoreactive for SOX2 (D, compare F (nuclei stained with DAPI) and H). Expression of Pax7, a marker of intermediate progenitors was also examined. Pax7 immunoreactive cells (red) are observed in the IL of control (I) and Tg mice (J) at p1. Many cells lining the pituitary cleft at p1 do not express Pax7 in control mice (K, O with nuclei stained with DAPI (blue; M, O), arrows show Pax7-negative cell). Nearly every cell in the IL of Tg mice is immunoreactive for Pax7 (L, compare N (nuclei stained with DAPI) and P). Photos taken at 100x (A, B, I and J), 400x (C-H, K-P). White box (A and I) indicates where higher magnification photos were taken. Scale bars (A-P): 50μm. n=4–5.
Activated Notch leads to elimination of undifferentiated melanotropes and corticotropes
Morphology and size of adolescent (p32) control and Tg pituitaries were examined using hematoxylin and eosin in order to determine if persistent Notch expression affects pituitary morphology at later ages. In control pituitaries, a prominent IL is observed surrounding the PL (Fig. 5A). In contrast, the IL of Tg mice is reduced to a single-cell thick layer surrounding the PL (Fig. 5B). The AL and PL look similar in both experimental groups. POMC expression was analyzed at p32 to determine if the remaining cells in the IL of adolescent Tg mice express terminal differentiation markers. In control mice, nearly every cell in the IL expresses POMC (Fig. 5C). However, in Tg mice, only a handful of the residual IL cells are POMC-positive (Fig. 5D). In addition, very few POMC immunoreactive cells are observed in the AL of Tg mice (Fig. 5F), as compared to control mice (Fig. 5E). The near complete loss of POMC-expressing cells was confirmed by quantitative RT-PCR for Pomc, which shows dramatically lower levels of Pomc mRNA in Tg mice than in control littermates at p32 (Fig. 5G). We also observe a reduction in Crhr1 mRNA in Tg mice, further indicating there is a loss of both melanotropes and corticotropes (data not shown). To determine if the corticotropes and melanotropes are able to regenerate in older mice, pituitary sections from four-month-old control and Tg mice were stained for POMC. Similarly, almost no POMC expression was observed in the AL or IL of Tg mice (data not shown). These results suggest that expression of activated Notch results in the elimination of undifferentiated melanotropes and corticotropes.
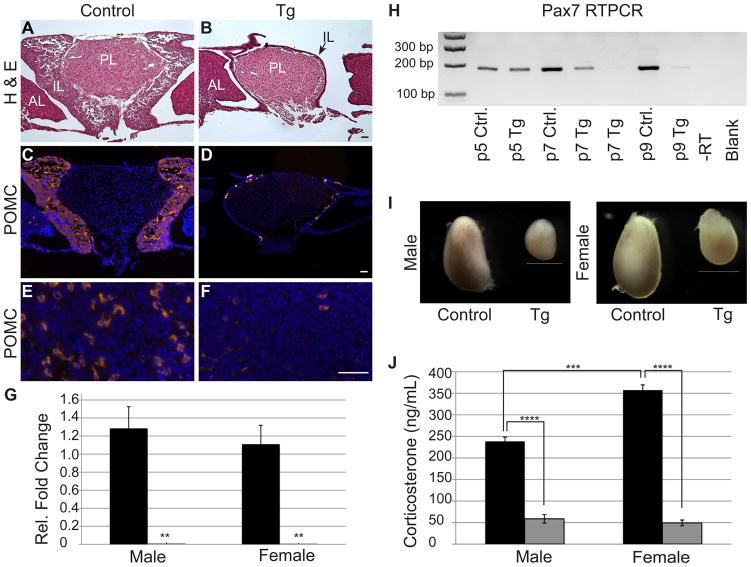
Figure 5.
Undifferentiated cells in Tg mice are incompatible with postnatal survival, resulting in adrenal dysfunction. Adolescent (p32) pituitaries were stained with hematoxylin and eosin to visualize morphology. Control (A) animals have a prominent IL, whereas Tg (B) animals have a very thin layer of IL cells (arrow) surrounding the PL. Nearly all of the IL lobe cells are immunoreactive for POMC (red) in the control (C) mice. In contrast, very few POMC-positive cells are observed in the IL of Tg mice (D). The same is true in the AL where POMC immunoreactive corticotropes are abundant in control mice (E) and nearly absent in Tg pituitaries (F). Similarly, qRT-PCR for Pomc reveals a significant decrease in Pomc mRNA in Tg adolescent pituitaries (gray bars) as compared to control littermates (black bars) in both males and females (G). Pax7 mRNA, which is exclusive to the IL in the pituitary, is present at p5 in both experimental groups and decreases dramatically by p9 in Tg pituitaries, indicating IL cells are no longer present at this age in Tg animals. Adrenals (I) of Tg males and females are reduced in size as compared to control mice. Stress-induced corticosterone levels (J) are significantly decreased in male and female Tg mice (gray bars) when compared to control mice (black bars). Photos taken at 100x (A–D) and 400x (E & F). Scale bars: 50μm (A–F) and 1mm (I). **: p<0.01. ***: p<0.001. ****: p<0.0001. n=3–5 (RT-PCR), n=5 (immunohistochemistry) and n=4 (corticosterone assay). PL=posterior lobe, AL=anterior lobe, IL=intermediate lobe.
In order to better elucidate the age at which the elimination of these cells occurs, TUNEL staining was performed at e16.5 and p1. No differences were observed between control and Tg pituitaries at either age (data not shown). This indicates that cell death is not initiated until later postnatal development in Tg mice. In order to determine the developmental window where IL cells are eliminated, Pax7 RT-PCR was performed on p5, p7, and p9 control and Tg pituitaries (Fig. 5H). Since previous results suggest Pax7 is maintained in undifferentiated IL cells in Tg mice (see Fig. 2J), this approach was an effective method to determine the age at which IL cells are no longer present. At p5, pituitaries from both control and Tg mice express roughly equal levels of Pax7, suggesting NICD-containing cells are still present at this age. By p7, Pax7 levels in Tg mice appear to be suppressed as compared to control mice. In one p7 Tg mouse, Pax7 levels are visibly reduced as compared to control, whereas another Tg animal shows near complete loss of Pax7 pituitary mRNA. At p9, all Tg pituitaries examined contain no detectable levels of Pax7 mRNA, whereas control pituitaries still maintain Pax7 expression. These findings show that aberrant expression of NICD results in elimination of NICD-containing cells within the first two postnatal weeks.
Persistently activated NICD expression in POMC-expressing cells results in HPA axis dysfunction
The effects of NICD expression in corticotropes may be reflected in alterations throughout the HPA axis. Development and function of the adrenal cortex relies heavily on the release of ACTH from the pituitary (Estivariz, et al. 1982; Simpson, Waterman. 1988). Therefore, adrenal glands were compared between control and Tg adolescent mice at p32. Control mice have visibly larger adrenal glands than Tg counterparts in both sexes (Fig. 5I). To determine the functional capabilities of the hypoplastic adrenals in Tg mice, stress-induced corticosterone levels were measured. Significant decreases in levels of corticosterone are observed in both male and female Tg mice as compared to control counterparts (Fig. 5J).
Under normal conditions, both corticosterone released from the adrenal gland and ACTH released from the pituitary can influence CRH release at the level of the hypothalamus (Swanson, Simmons. 1989). Given that both corticosterone levels and ACTH-positive cells are decreased in Tg mice, we would expect that CRH levels in the hypothalamus would be increased as a result of positive feedback. To determine differences in CRH expression between Tg and control male mice at p32, immunohistochemical expression of CRH was examined in the PVN. Tg mice display a slight increase in CRH immunoreactivity in the medial parvocellular portion of the PVN (PaMP; Supplemental Fig. 1A, B), a region known to be highly responsive to stress (Renard, et al. 2010). As expected, without colchicine pretreatment to inhibit axonal transport of peptides, CRH-positive cell bodies in control animals are hardly detectable, with some immunopositive processes present. In contrast, Tg animals display immunoreactive CRH-positive cell bodies as well as neuronal processes, indicating an increased level of CRH in this region. These data are consistent with CRH localization in Pomc knockout mice (Yaswen, et al. 1999; Smart, et al. 2007) and, taken together with the decreased levels of corticosterone, show that Notch-induced loss of POMC expression in the pituitary leads to subsequent dysfunction of the HPA axis.
Activated NICD in melanotropes and corticotropes is not sufficient to promote their proliferation
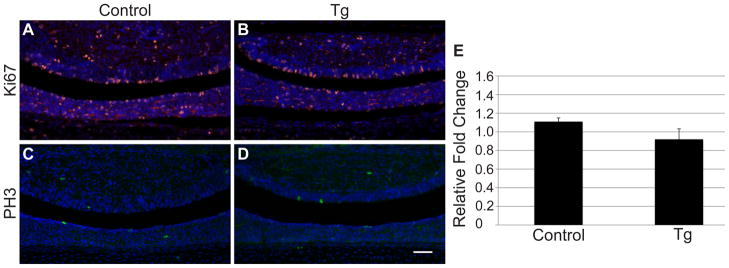
Because Notch is known to promote proliferation in many cell types (Solecki, et al. 2001; Collesi, et al. 2008; Zhang, et al. 2008), we hypothesized it may have a similar role in corticotropes and melanotropes. We, therefore, examined the expression of proliferation markers in p1 control and Tg pituitaries. Ki67, a marker of all stages of the cell cycle, is observed throughout the AL, IL and PL in control mice (Fig. 6A). No difference in expression pattern was observed in Tg mice (Fig. 6B), indicating overall proliferation in Pomc-expressing cells is unaffected by the constitutive expression of NICD. Phospho-histone-H3 staining was also performed in order to determine if mitosis is altered in cells persistently expressing NICD. Again, no visible changes in expression were observed between control (Fig. 6C) and Tg (Fig. 6D) pituitaries at p1. Histological observations were confirmed by quantitative RT-PCR analysis of control and Tg p1 pituitaries, which showed no difference in Myc mRNA levels between the two experimental groups (Fig. 6E). These results indicate that activated NICD is insufficient to promote proliferation in undifferentiated Pomc-expressing cells.
Figure 6.
Proliferation is unaffected by persistent NICD expression. Ki67 immunohistochemistry was performed on p1 control (A) and Tg pituitaries (B) and no difference in immunoreactivity (red) was observed. Similarly, no difference in Phopho-histone-H3 immunoreactivity (green) was observed between control (C) and Tg (D) pituitaries at p1. Nuclei are visualized with DAPI staining (blue; A-D). Additionally, qRT-PCR showed no change in Myc mRNA levels between control and Tg pituitaries at p1 (E). Photos taken at 200x. Scale bars: 50μm. n=4–5 (immunohistochemistry) and n=4 (RT-PCR).
Discussion
Disruption in normal corticotrope development or function can result in various disorders, including isolated ACTH deficiency and Cushing’s syndrome (Drouin, et al. 2007). Signals controlling the commitment and specification of corticotropes are not well understood, but the Notch signaling pathway has been implicated in these processes. We have previously demonstrated that global loss of Hes1 and Prop1, two Notch effector genes, results in premature differentiation of corticotropes, indicating this pathway is necessary to prevent early differentiation of these cells (Himes, Raetzman. 2009). Given this finding, we hypothesized that Notch signaling is also sufficient to inhibit the differentiation of both corticotropes and closely related melanotropes. Using a mouse model in which activated Notch is persistently expressed in these two cell types, we were able to confirm this hypothesis.
As in many developing tissues, Notch signaling components are found in RP progenitors and in the adult progenitor population but are excluded from differentiated cells (Raetzman, et al. 2004; Zhu, et al. 2006b). The presumptive pituitary progenitor population also contains the transcription factor SOX2, which is thought to be important in maintaining pluripotency and inhibiting cell differentiation (Episkopou. 2005; Masui, et al. 2007). SOX2 is expressed in isolated adult murine pituitary progenitors and is mainly localized to a single cell layer lining either side of the pituitary cleft in the adult (Rizzoti. 2010). In cochlear development, suppression of Notch signaling results in a decrease in the expression of SOX2 and in increase in differentiated cells (Dabdoub, et al. 2008). It is possible that SOX2 is a direct target of the Notch signaling cascade because luciferase reporter assays have shown that NICD has the ability to activate transcription of the SOX2 promoter (Ehm, et al. 2010). It is, therefore, not surprising that we observe an increase in SOX2 immunoreactivity in pituitary cells that contain activated Notch. This increase in SOX2 may correlate with an increase in multipotent pituitary progenitor cells, and indicates that Notch signaling acts in a similar fashion in the pituitary as it does in other developing systems.
We observed that inappropriately specified cells in the pituitary expressing NICD do not reenter the cell cycle and are eliminated during the first two postnatal weeks. Persistent expression of activated Notch has been shown to induce apoptosis in multiple cell types, including intestinal and neural progenitors (Yang, et al. 2004; Fre, et al. 2005). Although we cannot rule out a role for activated Notch in inducing apoptosis, it is likely that these cells are eliminated because they are undifferentiated. Ames dwarf mice, which lack functional Prop1, have extensive apoptosis in aberrantly undifferentiated cells in the pituitary at p1 and p8 (Ward, et al. 2005). This data supports the hypothesis that undifferentiated pituitary cells may be more prone to cell death, particularly during periods of significant postnatal proliferation (Carbajo-Pérez, Watanabe. 1990; Taniguchi, et al. 2002a; Taniguchi, et al. 2002b).
While Notch signaling in early development is known to influence the balance between proliferating progenitors and differentiated cells, it also directs cell fate determination at later stages of development in many systems. For example, Notch promotes cerebellar granule neuron precursor proliferation (Solecki, et al. 2001), but also promotes radial glia differentiation and inhibits granule neuron differentiation at later stages of development (Patten, et al. 2003). A similar dual role of Notch is seen in the intestine where Notch signaling inhibits stem cell differentiation during embryogenesis but biases cells toward an enterocyte fate and away from a secretory fate during adulthood (Stanger, et al. 2005). We found that that NICD is not sufficient to convert melanotropes and corticotropes to an alternate cell fate but instead traps them in a progenitor fate. This is especially clear in the IL where NICD-containing cells do not adopt another fate, but rather retain progenitor-like properties. However, since we are altering Notch signaling only after cells already committed to become melanotropes and corticotropes, it is possible that Notch influences cell fate at a different developmental stage as it does in other tissues discussed above.
Through both gain and loss of function studies, it is clear that Notch can influence terminal differentiation of Pomc containing cells (Zhu, et al. 2006b; Dutta, et al. 2008; Himes, Raetzman. 2009). Molecules required for Pomc transcription, and therefore for the terminal differentiation of corticotropes and melanotropes, have been well characterized. These factors include TPIT (Liu, et al. 2001; Pulichino, et al. 2003b) and NEUROD1(Poulin, et al. 1997; Lamolet, et al. 2004). In contrast, BMPs have been shown to inhibit the expression of Pomc in vitro (Nudi, et al. 2005). While these factors are known to regulate Pomc transcription and thus the terminal differentiation of corticotropes and melanotropes, it remains unclear which signals are involved in the specification of these cells. Lineage specification is likely controlled in part by extrinsic signals, such as morphogens expressed in the oral ectoderm and ventral diencephalon surrounding the developing pituitary. However, cells located in the same region during development do not necessarily develop into the same cell type (Davis, et al. 2011), suggesting intrinsic properties are also likely involved. We hypothesize that Notch signaling may be one such intrinsic factor. We show that persistently expressing NICD in corticotropes and melanotropes results in suppression of Tpit and Neurod1, indicating their expression may be under the control of the Notch signaling cascade. It is also possible that the suppression of these transcription factors is a result of the progenitor-like nature of the cells, which are ectopically expressing SOX2, and is not a result of their direct regulation by NICD. Future studies will be necessary in order to elucidate if and how Notch signaling directly regulates these molecules. Taken together with the fact that Prop1, a direct transcriptional target of Notch signaling, is required for PIT1 expression and the subsequent differentiation of lactotropes, somatotropes, and thyrotropes (Gage, et al. 1996a; Gage, et al. 1996b; Zhu, et al. 2006a), we propose a model by which the Notch signaling pathway regulates the choice between precursors of the corticotrope and PIT1 lineages during early pituitary cell specification (Fig. 7)
Figure 7.
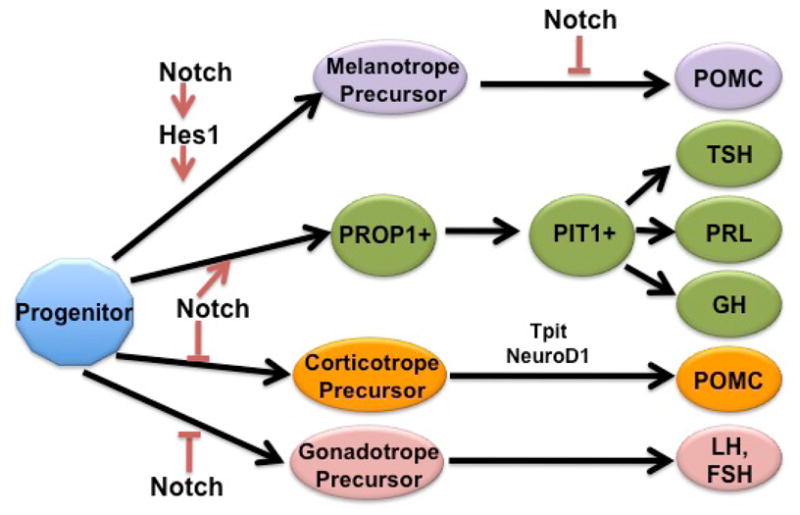
A proposed model for the role of Notch signaling in pituitary cell specification. Notch signaling must be repressed for corticotrope, melanotrope and gonadotrope differentiation to occur. In addition to repressing differentiation, Notch signaling is also necessary for the activation of PROP1, which is required for the emergence of the PIT1 lineage. Hes1, another Notch effector molecule, is necessary for the specification of melanotropes.
This study is the first to show that Notch signaling is sufficient to inhibit melanotrope and corticotrope differentiation, resulting in loss of these cells and subsequent HPA axis dysfunction. This mouse model physiologically resembles a Pomc knockout mouse (Yaswen, et al. 1999) and highlights the importance of Notch signaling in the regulation of corticotrope ontogeny during HPA axis development. Our study indicates that subtle alterations in Notch signaling within POMC lineage cells during early embryonic development can lead to disruption of the HPA axis at the level of the hypothalamus, as well as the adrenal gland. Disruption of the HPA axis during development has significant physiological consequences on the body’s response to stress, and can result in multiple disorders, including Cushing’s syndrome and adrenal insufficiency (Drouin, et al. 2007). Our findings are not only important to the study of normal pituitary development, but also demonstrate how alterations in signaling within corticotropes can contribute to dysfunction along the entire HPA axis.
Materials and Methods
Mice
RosaNotch floxed mice (Murtaugh, et al. 2003) were purchased from Jackson Laboratories (Bar Harbor, ME). A breeding colony was established and progeny were bred to Pomc Cre mice (Balthasar, et al. 2004), also purchased from Jackson Laboratories (Bar Harbor, ME). The resulting progeny all possess one RosaNotch floxed allele, with half also expressing Cre recombinase. To genotype the mice, tail biopsies were obtained and DNA was extracted using a salt-out method. PCR for the Pomc and Cre alleles was performed as previously described (Himes, Raetzman. 2009). PCR for the RosaNotch floxed allele was performed in a similar manner with the following exceptions. The primers used to detect this allele were 5′-AAA GTC GCT CTG AGT TGT TAT-3′, 5′-TAA GCC TGC CCA GAA GAC T-3′ and 5′-GAA AGA CCG CGA AGA GTT T G-3′. The samples underwent 35 cycles of denaturing at 95 C for 30 sec, annealing at 55 C for 30 sec, and elongation at 72 C for 60 sec, followed by 72 C for 5 min. All animals were housed in a facility with a 12 hour light-dark cycle and were maintained in accordance with the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee.
Immunohistochemistry
Mice were sacrificed at e16.5, p1, and p32 and embryos and pituitaries were fixed in 3.7% formaldehyde diluted in phosphate buffered saline (PBS). Samples were dehydrated through a series of graded ethanol, embedded coronally in paraffin, sectioned to a thickness of 6 microns and mounted on charged slides. For frozen section preparation used to detect CRH in the hypothalamus, p32 control and Tg brains were snap frozen in 2-methylbutane, cryoprotected overnight at 4°C in 30% sucrose diluted in PBS, and sectioned at 14μm.
Paraffin sections were deparaffinized, rehydrated and washed in PBS. Samples were then boiled in hot citrate solution (10uM citrate, pH6) for 10 minutes and cooled for 10 minutes (for all antibodies except POMC and CRH). Following antigen retrieval, samples were blocked in a solution containing 5% normal donkey serum diluted in immunohistochemical block which contains 3% bovine serum albumin and 0.5% TritonX-100 diluted in PBS. Frozen sections were fixed in 4% PFA for 30 mins, washed in PBS prior to application of blocking solution. For all immunohistochemistry, blocking was followed by an overnight incubation at 4°C with primary antibody diluted in immunohistochemical block. Primary antibodies used were raised against the following peptides: POMC (1:300, Dako, Carpinteria, CA), Sox2 (1:750, Millipore, Billerica, MA), Pax7 (1:500, Developmental Studies Hybridoma Bank, Iowa City, IA), ki67 (1:100, Dako, Carpinteria, CA), phospho-histone-H3 (1:500 Upstate Cell Signaling Solutions, Lake Placid, NY) and CRH (1:1000, Millipore, Billerica, MA, USA). Slides were then incubated with biotin-conjugated rat (ki67), mouse (Pax7) or rabbit (POMC, Sox2, phospho-histone-H3) secondary antibody diluted in immunohistochemical block for one half hour at room temperature. This was followed by a series of washes and a thirty-minute incubation with a streptavidin-conjugated cy3 or dylight488 fluorophore. Secondary and streptavidin-conjugated antibodies were purchased from Jackson ImmunoResearch (West Grove, PA) and were used at a concentration of 1:200. Slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, 1:1000, Sigma, St. Louis, MO) and visualized at 100x, 200x, or 400x magnification using a Leica DM2560 microscope. Photographs were taken using a Retiga 2000R color camera (Q-Imaging, Surrey, Canada) and acquired using Q-Capture Pro software (Q-Imaging). Images were processed using Adobe Photoshop CS4 (San José, CA).
Quantitative Reverse Transcriptase PCR
RNA was isolated from embryonic and postnatal whole pituitaries using an RNAqueous micro kit (Ambion, Austin, TX) as per manufacturers protocol. For p32 pituitaries, 0.5μg of RNA was synthesized into cDNA using the ProtoScript M-MuLV First Strand cDNA Synthesis Kit (New England Biolabs, Ipswich, MA). For e16.5 and p1 pituitaries, RNA was isolated from individual pituitaries of each genotype and the total RNA from each pituitary was converted into cDNA. A no enzyme control was also prepared and used as a negative control. For quantitative RT-PCR 0.2μL of cDNA from p32 and p1 pituitaries and 0.5μL from e16.5 pituitaries was amplified using gene-specific primers and SYBR green mix (Bio-Rad Laboratories, Hercules, CA) on a Bio-Rad MyIQ real-time PCR machine. Data were analyzed with the change in cycle threshold (ΔCt) value method. Specifically, for each sample, the mean Ct of the gene of interest was calculated as a average of the duplicates of that sample. This normalized mean was subtracted from the mean Ct for Gapdh to obtain the ΔCt. The ΔΔCt was then calculated as the difference between the ΔCt for each Tg and each control mouse. The relative fold change of Tg mice as compared to control mice was calculated as 2−ΔΔCt. The error bars represent the standard error of the mean of the relative fold change. Statistical significance was determined using Student’s t test.
Gene specific primers for real time RT-PCR include: Gapdh forward, GGT GAG GCC GGT GCT GAG TAT G; Gapdh reverse, GAC CCG TTT GGC TCC ACC CTT C; Hes1 forward, CTC GCT CAC TTC GGA CTC; Hes1 reverse, GTG GGC TAG GGA CTT TAC; Hey1 forward, CAC GCC ACT ATG CTC AAT; Hey1 reverse, CCT TCA CCT CAC TGC TCT G; Hey2 forward, GAT TCC GAG AGT GCT TGA C; Hey2 reverse, AGG TGC TGA GAT GAG AGA C; Heyl forward, GGA ACA ACA GAG AAT GAA C; Heyl reverse, CAG CAG TAG TGA GTA ACC; Pomc forward, GTT ACG GTG GCT TCA TGA CCT C; Pomc reverse, CGC GTT CTT GAT GAT GGC GTT C; Tpit forward, GATGCC AAG GAG AGA AAC C; Tpit reverse, AGC TTT TCT ATC AAA TTC ACT GA; Neurod1 forward, GCCCAGCTTAATGCCATCTTTC; Neurod1 reverese, AGC CAC AGT GGA TTC GTT TCC C; Mash1 forward, TGG ACT TTG GAA GCA GGA TGG; Mash1 reverse, TGA CGT CGT TGG CGA GAA ACA; Myc forward, TGA CCT AAC TCG AGG AGG AGC TGG AAT C; Myc reverse, AAG TTT GAG GCA GTT AAA ATT ATG GCT GAA GC.
Reverse Transcriptase PCR
RNA and cDNA form p5, p7 and p9 pituitaries were prepared in the same way as described above with the exception that 0.33μL of cDNA was amplified. Amplification was performed using Pax7 specific primers on a C1000 Thermal Cycler (Bio-Rad Laboratories, Hercules, CA). Amplification was visualized using an ethidium bromide stained gel. Sequences of primers used: Pax7 forward, GCA CAG AGG ACC AAG CTC AC; Pax7 Reverse TGG TGG TGG GGT AGG TAG AG.
Corticosterone Assay and Adrenal Collection
p32 mice were subjected to one hour of restraint stress in a ventilated 50mL conical tube beginning at 1500h. At 1600h, mice were sacrificed using CO2. Blood was collected by cardiac puncture and allowed to clot for 30 minutes in EDTA-coated tubes. Blood was spun down and plasma was collected and stored at −20°C until needed. A corticosterone Enzyme Immunoassay kit (Assay Designs, Ann Arbor, MI) was used to measure plasma corticosterone levels as per manufacturers protocol. All samples were run in duplicate and statistical significance was determined using Student’s t test. Adrenals were dissected from p32 male and female mice. They were fixed overnight in 3.7% formaldehyde diluted in PBS, washed in PBS and photographed.
Supplementary Material
Corticotropin releasing hormone (CRH) expression in the paraventricular (PVN) hypothalamus of male mice at P32. CRH immunoreactivity appears lower in control mice (A) compared to Tg mice (B) in the medial parvocellular portion of the PVN (PaMP). In other regions of the PVN, including the ventral PVN (PaV), CRH immunoreactivity is comparable between controls (C) and Tg mice (D). Scale bar: 50μm. n=3.
Highlights.
Persistent expression of activated Notch inhibits the differentiation of corticotropes and melanotropes.
Notch activation induces the expression of progenitor markers in undifferentiated corticotropes and melanotropes.
Melanotropes and corticotropes fail to regenerate.
Alterations of Notch signaling in corticotropes leads to the dysfunction of the hypothalamic-pituitary-adrenal axis.
Acknowledgments
We are grateful to Drs. Jodi Flaws, Ann Nardulli and Phil Newmark for use of equipment, to Dr. Jeff Huang, Bonnie Zeigler, Jackye Peretz and Dr. David Forsthoefel for technical assistance and to Dr. Megan Mahoney for use of CRH antibody. The Pax7 antibody was kindly provided by the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). Financial support was received from the National Institute of Health grant R01DK076647A (LTR) and T32 HD007333 (PKA).
This research was funded by the National Institutes of Health grants R01DK076647 to L.T.R and T32 HD007333 to P.K.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Elmquist JK, Lowell BB. Leptin Receptor Signaling in POMC Neurons is Required for Normal Body Weight Homeostasis. Neuron. 2004;6:983–91. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Burrows HL, Douglas KR, Seasholtz AF, Camper SA. Genealogy of the Anterior Pituitary Gland: Tracing a Family Tree. Trends in endocrinology and metabolism: TEM. 1999;9:343–352. doi: 10.1016/s1043-2760(99)00189-7. [DOI] [PubMed] [Google Scholar]
- Carbajo-Pérez E, Watanabe YG. Cellular Proliferation in the Anterior Pituitary of the Rat during the Postnatal Period. Cell and tissue research. 1990;2:333–8. doi: 10.1007/BF00318674. [DOI] [PubMed] [Google Scholar]
- Chen J, Crabbe A, Van Duppen V, Vankelecom H. The Notch Signaling System is Present in the Postnatal Pituitary: Marked Expression and Regulatory Activity in the Newly Discovered Side Population. Molecular endocrinology (Baltimore, Md) 2006;12:3293–307. doi: 10.1210/me.2006-0293. [DOI] [PubMed] [Google Scholar]
- Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. The Adult Pituitary Contains a Cell Population Displaying stem/progenitor Cell and Early Embryonic Characteristics. Endocrinology. 2005;9:3985–98. doi: 10.1210/en.2005-0185. [DOI] [PubMed] [Google Scholar]
- Collesi C, Zentilin L, Sinagra G, Giacca M. Notch1 Signaling Stimulates Proliferation of Immature Cardiomyocytes. The Journal of cell biology. 2008;1:117–28. doi: 10.1083/jcb.200806091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KSE, Pevny LH, Kelley MW. Sox2 Signaling in Prosensory Domain Specification and Subsequent Hair Cell Differentiation in the Developing Cochlea. Proceedings of the National Academy of Sciences of the United States of America. 2008;47:18396–401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, Rosenfeld MG. Signaling and Transcriptional Mechanisms in Pituitary Development. Annual review of neuroscience. 2001:327–55. doi: 10.1146/annurev.neuro.24.1.327. [DOI] [PubMed] [Google Scholar]
- Davis SW, Mortensen AH, Camper SA. Birthdating Studies Reshape Models for Pituitary Gland Cell Specification. Developmental biology. 2011 doi: 10.1016/j.ydbio.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin J, Bilodeau S, Vallette S. Of Old and New Diseases: Genetics of Pituitary ACTH Excess (Cushing) and Deficiency. Clinical genetics. 2007;3:175–82. doi: 10.1111/j.1399-0004.2007.00877.x. [DOI] [PubMed] [Google Scholar]
- Dutta S, Dietrich J, Westerfield M, Varga ZM. Notch Signaling Regulates Endocrine Cell Specification in the Zebrafish Anterior Pituitary. Developmental biology. 2008;2:248–57. doi: 10.1016/j.ydbio.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehm O, Göritz C, Covic M, Schäffner I, Schwarz TJ, Karaca E, Kempkes B, Kremmer E, Pfrieger FW, Espinosa L, Bigas A, Giachino C, Taylor V, Frisén J, Lie DC. RBPJkappa-Dependent Signaling is Essential for Long-Term Maintenance of Neural Stem Cells in the Adult Hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;41:13794–807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Episkopou V. SOX2 Functions in Adult Neural Stem Cells. Trends in neurosciences. 2005;5:219–21. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP Signaling Controls the Progression of Progenitor Cell Differentiation and the Emergence of Pattern in the Embryonic Anterior Pituitary. Development (Cambridge, England) 1998;6:1005–15. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Estivariz FE, Iturriza F, McLean C, Hope J, Lowry PJ. Stimulation of Adrenal Mitogenesis by N-Terminal Proopiocortin Peptides. Nature. 1982;5865:419–22. doi: 10.1038/297419a0. [DOI] [PubMed] [Google Scholar]
- Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson ICAF. SOX2-Expressing Progenitor Cells Generate all of the Major Cell Types in the Adult Mouse Pituitary Gland. Proceedings of the National Academy of Sciences of the United States of America. 2008;8:2907–12. doi: 10.1073/pnas.0707886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch Signals Control the Fate of Immature Progenitor Cells in the Intestine. Nature. 2005;7044:964–8. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Brinkmeier ML, Scarlett LM, Knapp LT, Camper SA, Mahon KA. The Ames Dwarf Gene, Df, is Required Early in Pituitary Ontogeny for the Extinction of Rpx Transcription and Initiation of Lineage-Specific Cell Proliferation. Molecular endocrinology (Baltimore, Md) 1996a;12:1570–81. doi: 10.1210/mend.10.12.8961267. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Roller ML, Saunders TL, Scarlett LM, Camper SA. Anterior Pituitary Cells Defective in the Cell-Autonomous Factor, Df, Undergo Cell Lineage Specification but Not Expansion. Development (Cambridge, England) 1996b;1:151–60. doi: 10.1242/dev.122.1.151. [DOI] [PubMed] [Google Scholar]
- Garren LD. The Mechanism of Action of Adrenocorticotropic Hormone. Vitamins and hormones. 1968:119–45. doi: 10.1016/s0083-6729(08)60753-0. [DOI] [PubMed] [Google Scholar]
- Goudreau JL, Falls WM, Lookingland KJ, Moore KE. Periventricular-Hypophysial Dopaminergic Neurons Innervate the Intermediate but Not the Neural Lobe of the Rat Pituitary Gland. Neuroendocrinology. 1995;2:147–54. doi: 10.1159/000126999. [DOI] [PubMed] [Google Scholar]
- Himes AD, Raetzman LT. Premature Differentiation and Aberrant Movement of Pituitary Cells Lacking both Hes1 and Prop1. Developmental biology. 2009;1:151–61. doi: 10.1016/j.ydbio.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoyama T, Nishijo K, Garcia MM, Schaffer BS, Ohshima-Hosoyama S, Prajapati SI, Davis MD, Grant WF, Scheithauer BW, Marks DL, Rubin BP, Keller C. A Postnatal Pax7 Progenitor Gives Rise to Pituitary Adenomas. Genes & cancer. 2010;4:388–402. doi: 10.1177/1947601910370979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japón MA, Rubinstein M, Low MJ. In Situ Hybridization Analysis of Anterior Pituitary Hormone Gene Expression during Fetal Mouse Development. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1994;8:1117–25. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- Kawano H, Daikoku S. Functional Topography of the Rat Hypothalamic Dopamine Neuron Systems: Retrograde Tracing and Immunohistochemical Study. The Journal of comparative neurology. 1987;2:242–53. doi: 10.1002/cne.902650208. [DOI] [PubMed] [Google Scholar]
- Kraman M, McCright B. Functional Conservation of Notch1 and Notch2 Intracellular Domains. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;10:1311–3. doi: 10.1096/fj.04-3407fje. [DOI] [PubMed] [Google Scholar]
- Lamolet B, Pulichino AM, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, Drouin J. A Pituitary Cell-Restricted T Box Factor, Tpit, Activates POMC Transcription in Cooperation with Pitx Homeoproteins. Cell. 2001;6:849–59. doi: 10.1016/s0092-8674(01)00282-3. [DOI] [PubMed] [Google Scholar]
- Lamolet B, Poulin G, Chu K, Guillemot F, Tsai M, Drouin J. Tpit-Independent Function of NeuroD1(BETA2) in Pituitary Corticotroph Differentiation. Molecular endocrinology (Baltimore, Md) 2004;4:995–1003. doi: 10.1210/me.2003-0127. [DOI] [PubMed] [Google Scholar]
- Léránth C, Palkovits M, Krieger DT. Serotonin Immunoreactive Nerve Fibers and Terminals in the Rat Pituitary--Light- and Electron-Microscopic Studies. Neuroscience. 1983;2:289–96. doi: 10.1016/0306-4522(83)90294-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Lin C, Gleiberman A, Ohgi KA, Herman T, Huang HP, Tsai MJ, Rosenfeld MG. Tbx19, a Tissue-Selective Regulator of POMC Gene Expression. Proceedings of the National Academy of Sciences of the United States of America. 2001;15:8674–9. doi: 10.1073/pnas.141234898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, Ko MSH, Niwa H. Pluripotency Governed by Sox2 Via Regulation of Oct3/4 Expression in Mouse Embryonic Stem Cells. Nature cell biology. 2007;6:625–35. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- McNay DEG, Pelling M, Claxton S, Guillemot F, Ang S. Mash1 is Required for Generic and Subtype Differentiation of Hypothalamic Neuroendocrine Cells. Molecular endocrinology (Baltimore, Md) 2006;7:1623–32. doi: 10.1210/me.2005-0518. [DOI] [PubMed] [Google Scholar]
- Mezey E, Léránth C, Brownstein MJ, Friedman E, Krieger DT, Palkovits M. On the Origin of the Serotonergic Input to the Intermediate Lobe of the Rat Pituitary. Brain research. 1984;2:231–7. doi: 10.1016/0006-8993(84)91034-5. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological Functions of Glucocorticoids in Stress and their Relation to Pharmacological Actions. Endocr Rev. 1984;1:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch Signaling Controls Multiple Steps of Pancreatic Differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2003;25:14920–5. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudi M, Ouimette J, Drouin J. Bone Morphogenic Protein (Smad)-Mediated Repression of Proopiomelanocortin Transcription by Interference with Pitx/Tpit Activity. Molecular endocrinology (Baltimore, Md) 2005;5:1329–42. doi: 10.1210/me.2004-0425. [DOI] [PubMed] [Google Scholar]
- Oertel WH, Mugnaini E, Tappaz ML, Weise VK, Dahl AL, Schmechel DE, Kopin IJ. Central GABAergic Innervation of Neurointermediate Pituitary Lobe: Biochemical and Immunocytochemical Study in the Rat. Proceedings of the National Academy of Sciences of the United States of America. 1982;2:675–9. doi: 10.1073/pnas.79.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten BA, Peyrin JM, Weinmaster G, Corfas G. Sequential Signaling through Notch1 and erbB Receptors Mediates Radial Glia Differentiation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;14:6132–40. doi: 10.1523/JNEUROSCI.23-14-06132.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips A, Lesage S, Gingras R, Maira MH, Gauthier Y, Hugo P, Drouin J. Novel Dimeric Nur77 Signaling Mechanism in Endocrine and Lymphoid Cells. Molecular and cellular biology. 1997a;10:5946–51. doi: 10.1128/mcb.17.10.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips A, Maira M, Mullick A, Chamberland M, Lesage S, Hugo P, Drouin J. Antagonism between Nur77 and Glucocorticoid Receptor for Control of Transcription. Molecular and cellular biology. 1997b;10:5952–9. doi: 10.1128/mcb.17.10.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C, McNeilly JR, Coutts S, Millar M, Anderson RA, McNeilly AS. Gonadotrope and Thyrotrope Development in the Human and Mouse Anterior Pituitary Gland. Developmental biology. 2006;1:172–81. doi: 10.1016/j.ydbio.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Poulin G, Turgeon B, Drouin J. NeuroD1/beta2 Contributes to Cell-Specific Transcription of the Proopiomelanocortin Gene. Molecular and cellular biology. 1997;11:6673–82. doi: 10.1128/mcb.17.11.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulichino A, Vallette-Kasic S, Couture C, Gauthier Y, Brue T, David M, Malpuech G, Deal C, Van Vliet G, De Vroede M, Riepe FG, Partsch C, Sippell WG, Berberoglu M, Atasay B, Drouin J. Human and Mouse TPIT Gene Mutations Cause Early Onset Pituitary ACTH Deficiency. Genes & development. 2003a;6:711–6. doi: 10.1101/gad.1065603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulichino A, Vallette-Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J. Tpit Determines Alternate Fates during Pituitary Cell Differentiation. Genes & development. 2003b;6:738–47. doi: 10.1101/gad.1065703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental Regulation of Notch Signaling Genes in the Embryonic Pituitary: Prop1 Deficiency Affects Notch2 Expression. Developmental biology. 2004;2:329–40. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Renard GM, Rivarola MA, Suarez MM. Gender-Dependent Effects of Early Maternal Separation and Variable Chronic Stress on Vasopressinergic Activity and Glucocorticoid Receptor Expression in Adult Rats. Dev Neurosci. 2010;1:71–80. doi: 10.1159/000280102. [DOI] [PubMed] [Google Scholar]
- Rizzoti K. Adult Pituitary progenitors/stem Cells: From in Vitro Characterization to in Vivo Function. The European journal of neuroscience. 2010;12:2053–62. doi: 10.1111/j.1460-9568.2010.07524.x. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Lovell-Badge R. Early Development of the Pituitary Gland: Induction and Shaping of Rathke’s Pouch. Reviews in endocrine & metabolic disorders. 2005;3:161–72. doi: 10.1007/s11154-005-3047-7. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Waterman MR. Regulation of the Synthesis of Steroidogenic Enzymes in Adrenal Cortical Cells by ACTH. Annual review of physiology. 1988:427–40. doi: 10.1146/annurev.ph.50.030188.002235. [DOI] [PubMed] [Google Scholar]
- Smart JL, Tolle V, Otero-Corchon V, Low MJ. Central Dysregulation of the Hypothalamic-Pituitary-Adrenal Axis in Neuron-Specific Proopiomelanocortin-Deficient Mice. Endocrinology. 2007;2:647–59. doi: 10.1210/en.2006-0990. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME. Activated Notch2 Signaling Inhibits Differentiation of Cerebellar Granule Neuron Precursors by Maintaining Proliferation. Neuron. 2001;4:557–68. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct Regulation of Intestinal Fate by Notch. Proceedings of the National Academy of Sciences of the United States of America. 2005;35:12443–8. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM. Differential Steroid Hormone and Neural Influences on Peptide mRNA Levels in CRH Cells of the Paraventricular Nucleus: A Hybridization Histochemical Study in the Rat. The Journal of comparative neurology. 1989;4:413–35. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Mitoses of Thyrotrophs Contribute to the Proliferation of the Rat Pituitary Gland during the Early Postnatal Period. Anatomy and embryology. 2002a;1–2:67–72. doi: 10.1007/s00429-002-0283-4. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Proliferation and Differentiation of Rat Anterior Pituitary Cells. Anatomy and embryology. 2002b;1–2:1–11. doi: 10.1007/s00429-002-0271-8. [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep Signaling Requirements for Pituitary Organogenesis in Vivo. Genes & development. 1998;11:1691–704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-Residue Ovine Hypothalamic Peptide that Stimulates Secretion of Corticotropin and Beta-Endorphin. Science (New York, N Y) 1981;4514:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Wagner J, Thomas P. Genetic Determinants of Mammalian Pituitary Morphogenesis. Frontiers in bioscience: a journal and virtual library. 2007:125–34. doi: 10.2741/2053. [DOI] [PubMed] [Google Scholar]
- Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of PROP1 in Pituitary Gland Growth. Molecular endocrinology (Baltimore, Md) 2005;3:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- Yang X, Klein R, Tian X, Cheng H, Kopan R, Shen J. Notch Activation Induces Apoptosis in Neural Progenitor Cells through a p53-Dependent Pathway. Developmental biology. 2004;1:81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the Mouse Model of Pro-Opiomelanocortin Deficiency Responds to Peripheral Melanocortin. Nature medicine. 1999;9:1066–70. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zheng G, Zou L, Liu H, Hou L, Zhou P, Yin D, Zheng Q, Liang L, Zhang S, Feng L, Yao L, Yang A, Han H, Chen J. Notch Activation Promotes Cell Proliferation and the Formation of Neural Stem Cell-Like Colonies in Human Glioma Cells. Molecular and cellular biochemistry. 2008;1–2:101–8. doi: 10.1007/s11010-007-9589-0. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG. Sustained Notch Signaling in Progenitors is Required for Sequential Emergence of Distinct Cell Lineages during Organogenesis. Genes & development. 2006a;19:2739–53. doi: 10.1101/gad.1444706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG. Sustained Notch Signaling in Progenitors is Required for Sequential Emergence of Distinct Cell Lineages during Organogenesis. Genes & development. 2006b;19:2739–53. doi: 10.1101/gad.1444706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Corticotropin releasing hormone (CRH) expression in the paraventricular (PVN) hypothalamus of male mice at P32. CRH immunoreactivity appears lower in control mice (A) compared to Tg mice (B) in the medial parvocellular portion of the PVN (PaMP). In other regions of the PVN, including the ventral PVN (PaV), CRH immunoreactivity is comparable between controls (C) and Tg mice (D). Scale bar: 50μm. n=3.