Abstract
Examining the neural correlates associated with processing social stimuli offers a viable option to the challenge of studying early social processing in infants at risk for autism spectrum disorders (ASDs). The present investigation included 32 12-month olds at high risk for ASD and 24 low-risk control infants, defined on the basis of family history. Infants were presented with familiar and unfamiliar faces, and three components of interest were explored for amplitude and latency differences. The anticipated developmental effects of emerging hemispheric asymmetry for face-sensitive components (the N290 and P400) were observed, as were familiarity effects for a component related to attention (the Nc). Although there were no striking group differences in the neural response to faces, there was some evidence for a developmental lag in an attentional component for the high-risk group. The infant ASD endophenotype, though elusive, may be better defined through expanding the age of study and addressing change over time in response to varied stimuli.
Keywords: Event related potentials, Infant, Autism spectrum disorders, Face processing
Introduction
The discovery that autism spectrum disorders, or ASDs—including Autistic Disorder, Asperger’s Disorder and Pervasive Developmental Disorder—not otherwise specified—are highly heritable was first made more than three decades ago (e.g., Folstein and Rutter 1977). This observation, now widely accepted, opened the doors to new avenues of investigation. One vein of research that has become the focus of great interest has been the study of infant siblings of children with autism spectrum disorders (ASD). These infants (often referred to simply as ‘infant siblings’) have the potential to elucidate the development of ASD and the characteristics that define this complex condition. Because of the shared genetic liability between these infants and their older, diagnosed siblings, the infants are at elevated risk for developing an ASD themselves. Recent estimates suggest that approximately 1 in 5 infant siblings will be diagnosed with an ASD (Elsabbagh and Johnson 2010; Tager-Flusberg 2010), while the base rate of ASD in the general population hovers around 1 in 110 (Autism and Developmental Disabilities Monitoring Network 2009). Infant siblings, therefore, represent a high-risk population of children who can easily be identified early in life. In recent years, there has been an abundance of research enrolling infant siblings in prospective, longitudinal projects, thus allowing the direct measurement of ASD symptoms as they emerge in development. Observations have been made starting in the first few months of life and have included behavioral assessment, growth measurements, recording of brain responses and infrared eye-tracking.
Despite the general supposition that ASD has its origins early in life, very few indications of the disorder have been consistently found in the first year. In fact, infants later diagnosed with ASD are indistinguishable from their typically developing peers at 6 months of age (Ozonoff et al. 2010; see Rogers 2009 for a review). By 12 months of age, however, an early ASD profile can be identified (Luyster et al. 2009; Zwaigenbaum et al. 2005). The long-term stability of these very early phenotypes has yet to be determined; nevertheless, the developmental shift to a recognizable behavioral phenotype around one year of age suggests it may be a useful point to look for the ASD-related characteristics.
The majority of infant siblings (about 80%) will not be identified with an ASD. Nevertheless, they too can provide useful information, in that they help us to define the ASD ‘endophenotype’. This construct refers to the constellation of traits that overlap with those observed in the syndrome—atypical visual attention or auditory processing, for instance—but which are not associated with a formal diagnosis. A wide range of studies have indicated that first-degree family members show evidence of an ASD endophenotype across a variety of domains, and these abnormalities are thought to reflect shared genetic risk. The fact that the familial commonalities do not necessarily equate to a diagnostic outcome suggests that there is much to learn about the significance of these features.
Early social development comprises a number of early red flags for ASD, including deficits in shared gaze, directed affect and looking towards people (Palomo et al. 2006; Wetherby et al. 2004). Consequently, this domain is also of primary interest when characterizing the high-risk endophenotype. Previous investigations using behavioral observation have noted subtle early differences in infant siblings, including decreased social smiling (Cassel et al. 2007), reduced preference for infant-directed speech (Nadig et al. 2007), and less emotional synchrony with their mothers during a still-face task (Yirmiya et al. 2006). An alternative approach to the study of early social development, and one that may shed light on underlying differences in brain function, is to use event related potentials (ERPs). ERPs represent changes in the brain’s electrical activity that can be time-locked to the presentation of a particular stimulus. They are thought to reflect the simultaneous activation of large populations of cortical neurons, and because they require no verbal capabilities on the part of the subject, they are ideal for use in infants.
ERPs have often been applied to the study of early social development in typically developing infants (Nelson and McCleery 2008), and the majority of these investigations have addressed how infants process faces. In adults, a posterior negative-going deflection around 170 ms post-stimulus (N170) has been consistently observed in response to faces; although this component is not seen in the infant brain, there are two consecutive occipito-temporal components—the first one a negative deflection called the N290, and a second, positive shift referred to as the P400—thought to be the infant precursors to the adult N170, which itself is thought to serve as a marker for the detection of faces (see Csibra et al. 2008 for a review). The P400 has been shown to be differentially responsive to faces vs. objects in infants as young as 6 months of age and is therefore considered ‘face-sensitive’ (de Haan and Nelson 1999). Furthermore, the N290/P400 complex seems to undergo a shift in hemispheric localization over early development, progressing from a medial response to more lateralized activation (similar to the adult N170) (de Haan 2008).
The Nc (negative central, observed primarily over frontal regions) is typically thought of as a marker for attention (Csibra et al. 2008; Nelson and Monk 2001). For instance, in some studies, the Nc has been shown to be larger to novel stimuli than to familiar ones, presumably because the infant dedicates more attentional resources when encountering an unfamiliar image. Interestingly, in the context of face processing, the Nc has been found to undergo a developmental shift. Whereas infants under a year of age exhibit a larger Nc to their mother’s face than to the face of stranger, older infants have been found to show a Nc that is larger to an unfamiliar face. A study enrolling toddlers and preschoolers reported that a shift took place after 24 months (Carver et al. 2003), but a more recent investigation of 9- to 12- month olds suggested that the transition may emerge as early as 12 months (Burden et al. 2007). This age-related change is believed to be associated with developments in social attention and interest. More specifically, whereas a very young infant’s attention might be more focused on their mother (serving critical processes such as attachment), an older child is more likely to be stimulated by the presence of new social partners. Moreover, like the N290/P400, maturational changes in the Nc have been documented, particularly in the right hemisphere (Carver et al. 2003).
These components—the N290, P400 and Nc—have been explored in young children who have already been diagnosed with ASD. When compared to typically developing peers, young children with ASD do not show pronounced differences in N290 or P400 amplitude to faces (Dawson et al. 2002; Webb et al. 2006). There is some evidence for a less lateralized and slightly delayed N290 in children with ASD (Webb et al. 2006). An atypical Nc in young children with ASD was reported elsewhere (Dawson et al. 2002), such that there were no amplitude differences in response to familiar vs. unfamiliar faces. Two investigations have extended this methodology to the study of the early ASD endophenotype in infants. In a study of 10-month olds, Elsabbagh and colleagues (2009) reported on 19 infant siblings and 17 control infants who viewed faces with direct and averted gaze. There were no group differences in N290 or P400 amplitude, nor were there group differences in the N290 latency. However, relative to the control sample, the high-risk group had a delayed P400 for the direct gaze trials; there were no group differences in the averted gaze condition. The experimenters concluded that there were differences in brain activity related to gaze-related attentional modulation.
McCleery et al. (2009) presented low- and high-risk 10-month olds with pictures of familiar and unfamiliar faces and objects; components of interest included the N290, P400 and Nc. For the N290 and P400, there were no group differences in latency to faces (although there were pronounced group differences in latency to objects, a point that will be addressed further in the Discussion section below). There were also no group effects for the amplitude of the N290/P400, but the Nc amplitude was more negative in the low-risk infants than in the high-risk group. With regards to hemispheric specialization of the N290/P400, the low-risk group showed a greater degree of hemispheric asymmetry—thought to be a marker of more advanced brain development—than the high-risk group.
The present investigation seeks to explore further the use of ERPs in improving our characterization of the early ASD endophenotype. Twelve-month old infants (at low and high risk for ASD) were shown familiar and unfamiliar faces, and the amplitude and latency of three relevant components—the N290, P400 and Nc—were explored in relation to group (high risk vs. low risk), familiarity and region (left hemisphere, midline, right hemisphere). We anticipated variability in laterality for the N290 and P400; that is, we expected a main effect of measurement location for both components, though not necessarily the same pattern of laterality for the N290 and the P400. On the other hand, we anticipated a main effect of familiarity for the Nc similar to that reported by Burden and colleagues (2007), such that amplitude would be larger for unfamiliar stimuli. Finally, based on previous findings (Elsabbagh et al. 2009; McCleery et al. 2009), we anticipated no group effects on the N290 or P400 but hypothesized that a group difference might emerge in the Nc amplitude.
Methods
Participants
Participants were enrolled in an IRB-approved longitudinal, collaborative project between Children’s Hospital Boston and Boston University. Families with a child who is either typically developing or has a diagnosis of ASD and who also have an infant under the age of 6 months of age were recruited for study participation. All parents were directly contacted by the Family Coordinator regarding involvement in the study and were screened according to a set of exclusionary criteria (gestational age of less than 36 weeks, time spent in neonatal intensive care, maternal steroid use during pregnancy, maternal diabetes or family history of genetic disorders). In the families who had an older child with ASD, the most commonly reported diagnosis was ASD or Pervasive Developmental Disorder—Not otherwise specified (n = 26), followed by Autistic Disorder (n = 5) and Asperger’s Disorder (n = 1). For the families with an older typically developing child, the Social Communication Questionnaire (SCQ; Rutter et al. 2003) was used to screen for ASD symptoms in that older child; if the total score exceeded 9, the family was excluded from participation. Similarly, if a typically developing child had any first degree relatives diagnosed with ASD, that family was excluded from participation. Informed consent was obtained at the time of the visit. Infants who completed a visit at 12 months of age were considered for inclusion in the present analyses.
Data collection included behavioral and electrophysiological measures (described below). In addition, for families who were recruited on the basis of having an older child with an ASD, that child’s diagnosis was confirmed using standard cutoffs on the SCQ (Rutter et al. 2003). Note that because this is an ongoing longitudinal project, diagnostic outcome for the enrolled infants is not yet available for the entire sample reported here.
One hundred and 55 children completed the 12-month study visit, and 109 provided adequate data for analysis (24 refused to wear the Geodesic net, the other 22 saw an insufficient number of trials to enter in data analysis). After hand editing and averaging (described below), a total of 32 children in the high-risk for ASD (by virtue of having an older sibling with an ASD) group and 24 low-risk controls (all of whom had a typically developing older sibling) provided sufficient high-quality data to be included in the final analyses. The average age of participants was comparable across groups (t = .44, p = .663), with an average of 372 days (SD = 11.50) for the high-risk group and 371 days (SD = 9.18) for the low-risk group. Gender composition was also similar across groups, with 18 males (56.3%) in the high-risk group and 11 males (45.8%) in the low-risk group (Fisher’s exact test, p = .59).
Behavioral Data Collection
The Mullen Scales of Early Learning (Mullen 1995) was administered to children to obtain an estimate of early cognitive skills. An Early Learning Composite score (with a mean of 100 and standard deviation of 15) was calculated based on performance across four subscales: Visual Reception, Fine Motor, Receptive Language and Expressive Language. The ELC scores have previously been shown to have internal reliability of .91, with the subtest cores ranging from .75 to .83; test–retest reliability is also adequate for children under 24 months of age, with subscales coefficients between .82 and .96 (Mullen 1995).
ERP Stimuli
Stimuli included color pictures of a primary caregiver (for all infants reported here, it was their mother) and a gender-matched stranger. Both models wore a neutral expression and stood in front of a gray screen, with their neck and shoulders draped with gray cloth. For each infant, an unfamiliar face was chosen that was similar to the familiar one in ethnicity; images of mothers wearing glasses were paired with images of unfamiliar women wearing glasses. Images were cropped as needed to standardize face and image size.
ERP Recording and Data Processing
All ERP recording was completed in an electrically- and sound-shielded testing room with low lighting. Children were seated on their parent’s lap, approximately 65 cm in front of the experimental monitor. ERPs were recorded as participants viewed stimuli, presented for 500 ms each. Images were shown in random order, maintaining a 50:50 ratio. From a separate room, the examiner observed the participant’s eye movements and attentiveness through a hidden video camera mounted on top of the experimental monitor. Stimulus presentation (via ePrime software, Psychology Software Tools, Pittsburgh, PA) was initiated only when the child was attending to the screen, and any trial during which the child’s attention wavered was tagged and removed from further analysis. A maximum of 100 trials were presented. In the final sample, there was no group difference in number of trials presented (t = 1.13, p = .264), with an average of 56 trials for the high-risk participants and 51 for the low-risk participants.
Continuous electroencephalogram (EEG) was recorded using a Geodesic Sensor Net (Electrical Geodesics Inc., Eugene OR) with 64 or 128 electrodes based on the child’s head circumference and date of session (due to an equipment upgrade1), and referenced on-line to a single vertex electrode (Cz). The electrical signal was amplified with a .1–100 band-pass, digitized to 250 hz, and stored on a computer disk. The data were analyzed offline by using NetStation 4.4.1 analysis software (Electrical Geodesics Inc., Eugene OR). The continuous EEG signal was segmented to 1000 ms post-stimulus recording periods, with a baseline period beginning 100 ms before stimulus presentation. The segments were then digitally filtered by using a 30 Hz low-pass elliptical filter and baseline-corrected against the mean voltage during the 100 ms pre-stimulus period.
After excluding segments with eye movements and blinks, the remaining segments were visually scanned by an experimenter blind to study group. Bad channels and other artifacts (e.g., off-scale activity, eye movement, body movements, or high-frequency noise) were identified. If more than 10% of the channels were marked as bad, the whole segment was excluded from further analysis. Finally, average waveforms for each individual participant within each experimental condition were generated and re-referenced to the average reference. Participants with fewer than 10 good trials per condition were excluded from further analysis. The mean number of accepted trials did not differ across groups (t = 1.06, p = .292), with an average of 39 trials in the high-risk group and 34 in the low-risk group.
ERP Statistical Analysis
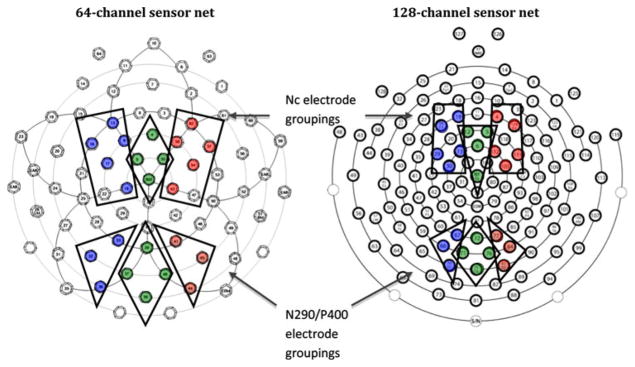
Statistical analysis of the ERP data addressed three components sensitive to face processing, including the occipital-temporal N290 (150–450 ms post-stimulus) and P400 (260–600 ms post-stimulus), and the frontal Nc (400–850 ms post-stimulus). Peak amplitudes and latencieswere calculated for the N290 and P400 in the chosen time windows; this was not done for the Nc because of the diffuse nature of the deflection. Mean amplitude within the selected window was calculated for all three components. Ten posterior electrodes were chosen for the occipital-temporal components (N290 and P400), and fourteen frontal electrodes for the frontal component of interest (Nc); these electrodes were selected within equivalent scalp locations across the 64- and 128-channel sensor nets. Regions of interest were chosen based on prior studies in this laboratory addressing face processing in infants and are shown in Fig. 1.
Fig. 1.
Electrode groupings for 64- and 128-channel Geodesic Sensor Nets
Data Analyses
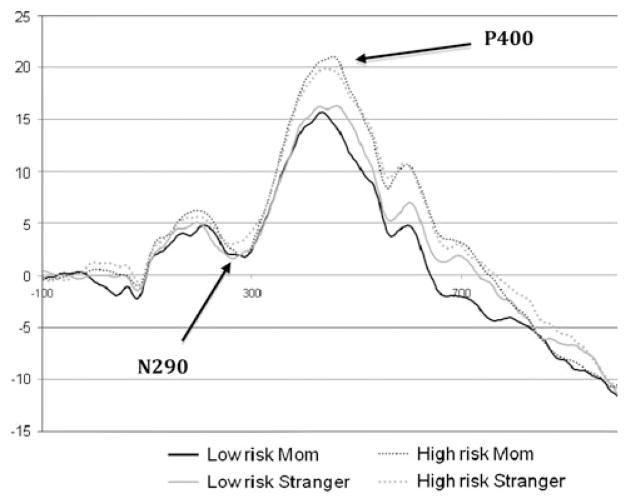
A set of 2 × 2 × 3 repeated measures analyses of variance (ANOVA; subject group [high-risk vs. low-risk, between subject] × familiarity [familiar vs. unfamiliar, within subject] × region [left hemisphere, midline, right hemisphere; within subject]) were completed on peak amplitude, mean amplitude and latency for three ERP components: N290, P400 and Nc (see Figs. 2, 3 for grand averaged waveforms). Omnibus ANOVAs were conducted using Greenhouse-Geisser adjusted degrees of freedom, and when significant main effects were revealed, post hoc comparisons were carried out using t-tests with a Bonferroni correction for multiple comparisons. Preliminary analyses indicated a main effect of electrode net type on the N290 (64ch: 283 ms; 128ch: 264 ms; t = 2.47, p = .017) and P400 (64ch: 437 ms; 128ch: 420 ms; t = 2.15, p = .036) latencies. However, there were no interactions with any of the other factors, and because the number of children using 64- vs. 128-channel systems was not different across groups, the final analyses collapsed across net type.
Fig. 2.
Grand averaged waveform for N290/P400. Note: See Fig. 1 for N290/P400 electrode grouping
Fig. 3.
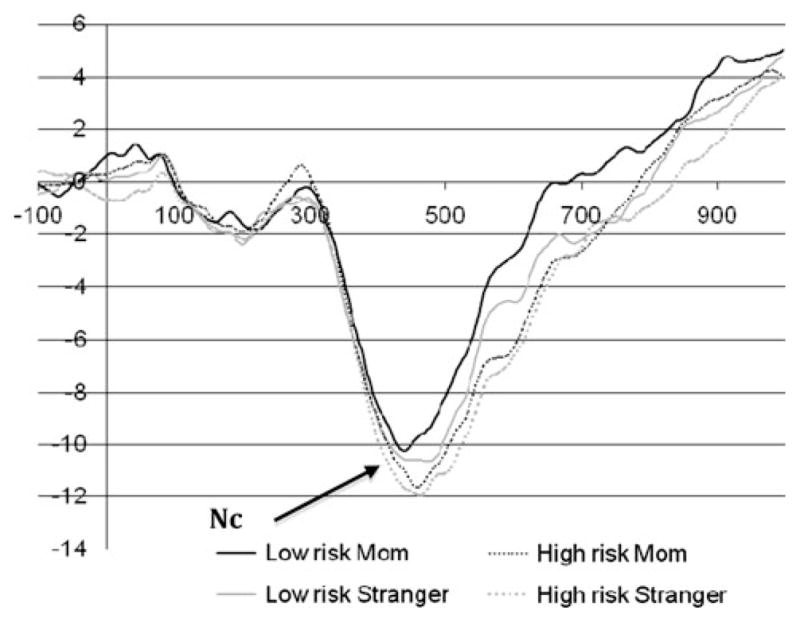
Grand averaged waveform for Nc. Note: See Fig. 1 for Nc electrode grouping
Results
Behavioral Data
Early Learning Composite standard scores from the Mullen Scales of Early Learning were compared across groups. An independent samples t-test revealed that the low-risk group scored significantly higher (M = 110.79, SD = 10.27) than the high-risk group (M = 101.94, SD = 13.44) [t(54) = 2.69, p<.01]; however, both groups’ scores were within the normal range.
N290
The repeated measures ANOVA on peak amplitude of the N290 revealed no significant main effects or interactions. For N290 mean amplitude, there was a significant main effect of region (F = 5.16, p = .014), and pairwise comparisons indicated that activity measured from the left hemisphere was significantly more negative than that observed in midline leads (by 2.08 μV, p = .002; see Table 1).
Table 1.
Topographical differences for N290 and P400 amplitude (SD in parentheses)
| Left hemisphere | Midline | Right hemisphere | Differences | |
|---|---|---|---|---|
| N290a mean amplitude | 6.86 (6.49) | 8.94 (7.35) | 8.11 (7.78) | Midline>Left, p = .002 |
| P400 peak amplitude | 19.70 (10.01) | 22.75 (10.16) | 21.34 (10.29) | Midline>Left, p = .001 |
| P400 mean amplitude | 10.12 (7.98) | 11.99 (8.34) | 11.06 (8.56) | Midline>Left, p = .017 |
Note that because this is a negative going deflection, a lower value indicates a larger response
For the N290 latency, there was a marginally significant 3-way interaction of condition by region by group (F = 3.19, p = .06). Because this interaction was only marginally significant, further analyses were not done.
P400
For P400 peak amplitude, a significant main effect of region (F = 7.07, p = .002) was found. Post hoc pairwise comparisons indicated that the peak amplitude over midline leads was more positive than that recorded in the left hemisphere (by 3.05 μV, p = .001; see Table 1). Additionally, a marginal main effect of group (F = 3.35, p = .073) was found for P400 peak amplitude, with the high-risk group amplitude exceeding that of the low-risk group (by 4.62 μV). Similarly, the P400 mean amplitude showed a significant main effect of region (F = 3.58, p = .042) in the direction reported for peak amplitude (midline more positive than left hemisphere by 1.87 μV, p = .017; see Table 1). For the P400 latency, a condition by region interaction was significant (F = 3.64, p = .034), but the direct pairwise comparisons were not.
Nc
Nc mean amplitude exhibited a significant main effect of condition (F = 4.17, p = .046), with a more negative mean amplitude (by 1.1 μV) to the unfamiliar face than to the familiar one. Moreover, a main effect of region (F = 3.55, p = .037) was found, but follow-up tests were not significant.
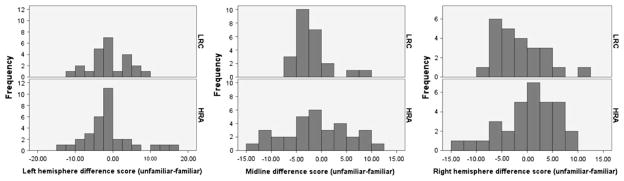
In order to explore within-subject differences in the Nc, the degree to which children in the low- and high-risk groups were differentiating across conditions was explored by calculating a difference score for each infant, in which mean amplitude in the familiar condition was subtracted from mean amplitude in the unfamiliar condition. The Nc is a negative deflection; therefore, in this calculation, a negative difference score indicates that the child exhibited the anticipated main effect of condition. That is, they exhibited larger mean negative amplitude to unfamiliar faces than familiar ones. Conversely, a positive difference score indicates that the mean negative amplitude was larger to familiar than to unfamiliar faces, a finding that is contrary to the expected result. Furthermore, because of the main effect of region found for Nc mean amplitude, this was done for each region (left hemisphere, midline, right hemisphere) separately. See Fig. 4 for distributions of difference scores by group and region.
Fig. 4.
Nc amplitude difference scores (unfamiliar—familiar) by group and region. Note: LRC = low risk controls; HRA = high risk for ASD
Each group was divided according to whether or not the anticipated response (greater Nc to unfamiliar than familiar faces) was demonstrated, and chi-square tests were used to explore group differences within region. There were no group differences in the left hemisphere, with 16 (66.7%) low-risk children and 24 (75.0%) high-risk children showing the anticipated response (Fisher’s exact test, p = .558). However, a marginal effect (Fisher’s exact test, p = .079) was found for the midline leads, such that a greater proportion of the low-risk group (20 out of 24 children, or 83.3%) showed the expected response than the high-risk group (19 out of 32 children, or 59.4%). Similar results were found for the right hemisphere electrode grouping, where a marginal effect was again found (Fisher’s exact test, p = .064) in a similar direction. Two-thirds of the low-risk sample (16 out of 24 children, or 66.7%) showed the expected amplitude effect, whereas only slightly more than one-third (13 out of 32, or 40.6%) of the high-risk group showed a greater response to unfamiliar faces.
Discussion
By one year of age or shortly thereafter, infants at high risk for ASD have been found to show behavioral deficits in social development at increased rates, relative to their low-risk peers (Cassel et al. 2007; Nadig et al. 2007; Yirmiya et al. 2006). The present investigation addressed whether high-risk infants might also exhibit atypical neural responses to social stimuli. Components of interest included the face-sensitive N290/P400 complex and the Nc, associated with the allocation of attention.
As anticipated, there was a main effect of location for the N290 and P400. However, the two components showed slightly different patterns of spatial distribution, consistent with prior investigations (Halit et al. 2004). The N290 was most negative over left hemisphere leads, a finding that is similar to a previous report of larger left activation in semi-medial electrodes in 12-month olds (Halit et al. 2003). The P400 was smaller over the left hemisphere than over midline leads, while there was no difference between midline and right hemisphere electrode groupings. These results are partially consistent with previous findings: on the one hand, it has been reported elsewhere that the P400 amplitude is reduced over the left hemisphere (Halit et al. 2003). On the other hand, it is generally thought that the P400 amplitude is maximal over the right hemisphere (de Haan and Nelson 1997; de Haan et al. 2003; Taylor et al. 2004), a finding that was not replicated here. It is worth noting that this study and the one reported by Halit et al. (2003) employed an average reference (recommended practice in the field, see Picton et al. 2000), whereas other references have been used elsewhere (e.g., linked mastoids in de Haan and Nelson 1997). Some of the variation in component characteristics across these studies may be associated with differing reference selection (for example, see Joyce and Rossion 2005).
For the most part, the two groups showed similar responses in face-sensitive components. The N290 showed no differences in amplitude or latency associated with ASD risk, and the latency of the P400 was similar across groups. There was one unexpected finding: a trend towards a larger P400 peak amplitude in the high-risk group than in the controls. Interestingly, this finding did not carry through to the mean amplitude measurement. This finding is difficult to interpret, because it is not entirely consistent with previous reports that have noted group differences in P400 latency, but not amplitude (Elsabbagh et al. 2009; McCleery et al. 2009). It is critical to note, moreover, that previously reported differences in P400 responses have been in the context of slightly different tasks, which used images of objects or faces with direct vs. averted gaze.
What is more consistent with previous literature is the overall apparent normality of early social processing children at risk for ASD, at least at this age; as discussed earlier, McCleery and colleagues (2009) reported no amplitude or latency differences in response to social stimuli (i.e., faces) between low- and high-risk infants. Another recent investigation reported that high-risk 10-month olds showed no differences from a control sample in social working memory (Noland et al. 2010), measured through the presentation of an examiner’s face.
Interestingly, an emerging characterization of the early ASD endophenotype points to the importance of non-social processing in distinguishing these infants from their low-risk peers. More specifically, it has been surmised that children at risk for ASD may in fact show a strength in non-social processing: McCleery and colleagues (2009) reported an ‘object advantage’ in the components of interest, such that 10-month old infants at high risk showed faster and stronger neural responses to objects than the comparison infants. Furthermore, Noland and colleagues (2010) observed that 9-month old high-risk infants surpassed their low-risk counterparts in the accuracy of their non-social working memory (also measured through the presentation of colorful objects). In sum, then, there is accumulating evidence across the current and previous investigations that the early ASD endophenotype may have less to do with social processing than originally supposed and more to do with non-social or object processing (see Tager-Flusberg 2010 for a discussion).
Results for the Nc generally confirmed our hypotheses. First, we found that the Nc was more negative to unfamiliar faces than to familiar ones (similar to the 12 month olds reported by Burden et al. 2007) across both of our groups, suggesting that the infants were recruiting greater attentional resources for an unfamiliar face than for a familiar one. Our expectation that there would be a group effect in Nc amplitude was only partly supported. Although there was not a main effect of high- vs. low-risk group, the two samples of infants differed somewhat on the degree to which their neural response differentiated familiar from unfamiliar social stimuli. There was a trend for the low-risk group to have a more pronounced differential response to familiar and unfamiliar faces (in the anticipated direction) than the high-risk infants. The group difference emerged in the midline and right hemisphere leads but not in the left hemisphere electrodes. This diminished differentiation between familiar and unfamiliar stimuli in high-risk infants is reminiscent of the results reported by McCleery et al. (2009), although the unanticipated lack of differentiation in their comparison sample makes the interpretation of these previous findings challenging.
The current results should be interpreted within the developmental framework of the Nc. It is well-established that the features of the Nc shift over time (although the details of this shift remain under debate), starting with a larger response to familiar stimuli than to unfamiliar stimuli and transitioning to the reverse (Carver et al. 2003). The distribution of difference scores across the low-risk and high-risk group illustrates the predominance of a more pronounced unfamiliar response in the low-risk group (that is, a negative difference score), whereas the high-risk group is clustered more evenly around a null difference score. A possible interpretation is that the low-risk group has transitioned more completely from the developmentally earlier Nc response to the later one, while the infants at risk are still approaching this transition or have only just entered it. Such an interpretation is supported by the group difference in Mullen scores, as well as the observation of this effect most strongly in the right hemisphere, where Carver and colleagues (2003) also reported the strongest relationship between age and the amplitude difference of the Nc. Future research approaching this question from a longitudinal perspective will be critical in clarifying the significance of these group differences. Moreover, other metrics for visual attention—infra-red eye tracking, for instance—might be useful in confirming or refuting this conclusion.
In sum, the N290/P400 complex—thought to be developmentally associated with the emergence of holistic face processing (e.g., Csibra et al. 2008)—seems to be generally intact in 12-month old infants at high risk for ASD. On the other hand, results suggest a possible developmental lag in the allocation of attention to familiar vs. unfamiliar faces. Future research will help to clarify the robustness of these findings and embed them within a larger developmental framework of ASD risk.
Acknowledgments
We would like to thank the Infant Sibling Project staff, past and present, for their hard work in collecting these data. We are very grateful for Alexandra Libby’s assistance with data processing. Finally, we would like to acknowledge the very dedicated families who committed years of their lives to the Infant Sibling Project and who made this work possible. Funding was provided by NIH (R21DC08637) to H.T-F., NIDCD (1R01DC010290-01) to C.A.N. and H.T-F., the Simons Foundation (137186) to C.A.N. and the Autism Speaks Pilot Grants Program to H.T-F.
Footnotes
This is one of several papers published together in Brain Topography on the “Special Issue: Brain Imaging across the Lifespan”.
The EGI electrode nets used in this project underwent an upgrade approximately 2 years after the project commenced, and all 64-channel sensor nets were replaced with 128-channel sensor nets. Out of the current sample, the number of children on whom data was collected on the 64- and 128-channel nets did not differ across group (Fisher’s exact test, p = .79), with 16 (66.7%) low-risk children and 20 (62.5%) high-risk children on the original 64-channel system.
Contributor Information
Rhiannon J. Luyster, Email: rhiannon.luyster@childrens.harvard.edu, Laboratories of Cognitive Neuroscience, Division of Developmental Medicine, Children’s Hospital Boston, 1 Autumn St., 6th Fl, Boston, MA 02215, USA. Harvard Medical School, Boston, MA, USA
Jennifer B. Wagner, Laboratories of Cognitive Neuroscience, Division of Developmental Medicine, Children’s Hospital Boston, 1 Autumn St., 6th Fl, Boston, MA 02215, USA. Harvard Medical School, Boston, MA, USA
Vanessa Vogel-Farley, Laboratories of Cognitive Neuroscience, Division of Developmental Medicine, Children’s Hospital Boston, 1 Autumn St., 6th Fl, Boston, MA 02215, USA.
Helen Tager-Flusberg, Department of Psychology, Boston University, 64 Cummington St., Boston, MA 02215, USA.
Charles A. Nelson, III, Laboratories of Cognitive Neuroscience, Division of Developmental Medicine, Children’s Hospital Boston, 1 Autumn St., 6th Fl, Boston, MA 02215, USA. Harvard Medical School, Boston, MA, USA. Harvard Center on the Developing Child, Harvard University, Cambridge, MA, USA.
References
- Autism and Developmental Disabilities Monitoring Network . Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58:1–20. [PubMed] [Google Scholar]
- Burden MJ, Westerlund AJ, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, Angelilli ML, Jacobson JL. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120:e336–e345. doi: 10.1542/peds.2006-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver LJ, Dawson G, Panagiotides H, Meltzoff AN, McPartland J, Gray J, Munson J. Age-related differences in neural correlates of face recognition during the toddler and preschool years. Dev Psychobiol. 2003;42:148–159. doi: 10.1002/dev.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel TD, Messinger DS, Ibanez LV, Haltigan JD, Acosta SI, Buchman AC. Early social and emotional communication in the infant siblings of children with autism spectrum disorders: an examination of the broad phenotype. J Autism Dev Disord. 2007;37:122–132. doi: 10.1007/s10803-006-0337-1. [DOI] [PubMed] [Google Scholar]
- Csibra G, Kushnerenko E, Grossmann T. Electrophysiological methods in studying infant cognitive development. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. MIT Press; Cambridge: 2008. pp. 247–262. [Google Scholar]
- Dawson G, Carver L, Meltzoff A, Panagiotides H, McPartland J, Webb S. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay and typical development. Child Dev. 2002;73:700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M. Neurocognitive mechanisms for the development of face processing. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. MIT Press; Cambridge: 2008. pp. 509–520. [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother’s face by six-month-old infants: a neurobehavioral study. Child Dev. 1997;68:187–210. [PubMed] [Google Scholar]
- de Haan M, Nelson C. Brain activity differentiates face and object processing in 6-month-old infants. Develop Psychol. 1999;35(4):1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- de Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: a review. Int J Psychophysiol. 2003;51:45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Johnson MH. Getting answers from babies about autism. Trends Cogn Sci. 2010;14:81–87. doi: 10.1016/j.tics.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Csibra G, Holmboe K, Garwood H, Tucker L, et al. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biol Psychiatry. 2009;65(1):31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1977;18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Halit H, de Haan M, Johnson MH. Cortical specialisation for face processing: face-sensitive event-related potential components in 3- and 12-month-old infants. Neuroimage. 2003;19:1180–1193. doi: 10.1016/s1053-8119(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Halit H, Csibra G, Volein A, Johnson MH. Face-sensitive cortical processing in early infancy. J Child Psychol Psychiatry. 2004;45:1228–1234. doi: 10.1111/j.1469-7610.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- Joyce C, Rossion B. The face-sensitive N170 and VPP components manifest the same brain processes: the effect of reference selection site. Clin Neurophysiol. 2005;116:2613–2631. doi: 10.1016/j.clinph.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K, Bishop S, Esler A, Hus V, Oti R, Richler J, Risi S, Lord C. The Autism Diagnostic Observation Schedule-toddler module: a new module of a standardized diagnostic measure for autism spectrum disorders. J Autism Dev Disord. 2009;39:1305–1320. doi: 10.1007/s10803-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery J, Akshoomoff N, Dobkins K, Carver L. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biol Psychiatry. 2009;66:950–957. doi: 10.1016/j.biopsych.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. Mullen scales of early learning. American Guidance Service, Inc; Circle Pines: 1995. [Google Scholar]
- Nadig AS, Ozonoff S, Young GS, Rozga A, Sigman M, Rogers SJ. A prospective study of response to name in infants at risk for autism. Arch Pediatr Adolesc Med. 2007;161:378–383. doi: 10.1001/archpedi.161.4.378. [DOI] [PubMed] [Google Scholar]
- Nelson CA, McCleery JP. Use of event-related potentials in the study of typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2008;47:1252–1261. doi: 10.1097/CHI.0b013e318185a6d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Monk C. The use of event-related potentials in the study of cognitive development. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. MIT Press; Cambridge: 2001. pp. 125–136. [Google Scholar]
- Noland JS, Reznick S, Stone W, Walden T, Sheridan EH. Better working memory for non-social targets in infant siblings of children with Autism Spectrum Disorder. Develop Sci. 2010;13(1):244–251. doi: 10.1111/j.1467-7687.2009.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, Rogers SJ, Rozga A, Sangha S, Sigman M, Steinfeld MB, Young GS. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49:256, 66.e1–2. [PMC free article] [PubMed] [Google Scholar]
- Palomo R, Belinchon M, Ozonoff S. Autism and family home movies: a comprehensive review. J Dev Behav Pediatr. 2006;27:S59–S68. doi: 10.1097/00004703-200604002-00003. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophys. 2000;37:127–152. [PubMed] [Google Scholar]
- Rogers S. What are infant siblings teaching us about autism in infancy? Autism Res. 2009;2:125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social communication questionnaire. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- Tager-Flusberg H. The origins of social impairments in autism spectrum disorder: studies of infants at risk. Neural Netw. 2010;23:1072–1076. doi: 10.1016/j.neunet.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Batty M, Itier RJ. The faces of development: a review of early face processing over childhood. J Cogn Neurosci. 2004;16:1426–1442. doi: 10.1162/0898929042304732. [DOI] [PubMed] [Google Scholar]
- Webb S, Dawson G, Bernier R, Panagiotides H. ERP evidence of atypical face processing in young children with autism. J Autism Dev Disord. 2006;36:881–890. doi: 10.1007/s10803-006-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby AM, Woods J, Allen L, Cleary J, Dickinson H, Lord C. Early indicators of autism spectrum disorders in the second year of life. J Autism Dev Disord. 2004;34:473–493. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-Cohen S, Sigman M. The development of siblings of children with autism at 4 and 14 months: social engagement, communication, and cognition. J Child Psychol Psychiatry. 2006;47:511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]