Abstract
Six 3′R,4′R-di-O-(S)-camphanoyl-2′,2′-dimethyldihydropyrano[2,3-f]chromone (DCP) and two 3′R,4′R-di-O-(S)-camphanoyl-(+)-cis-khellactone (DCK) derivatives were designed, synthesized, and evaluated for inhibition of HIV-1NL4-3 replication in TZM-bl cells. 2-Ethyl-2′-monomethyl-1′-oxa- and -1′-thia-DCP (5a, 6a), as well as 2-ethyl-1′-thia-DCP (7a) exhibited potent anti-HIV activity with EC50 values of 30, 38 and 54 nM and therapeutic indexes of 152.6, 48.0 and 100.0, respectively, which were better than or comparable to those of the lead compound 2-ethyl-DCP in the same assay. 4-Methyl-1′-thia-DCK (8a) also showed significant inhibitory activity with an EC50 of 128 nM and TI of 237.9.
Keywords: 2′-Monomethyl-1′-oxa-DCP, 2′-Monomethyl-1′-thia-DCP, 2-Ethyl-1′-thia-DCP, 4-Methyl-1′-thia-DCK, Anti-HIV activity
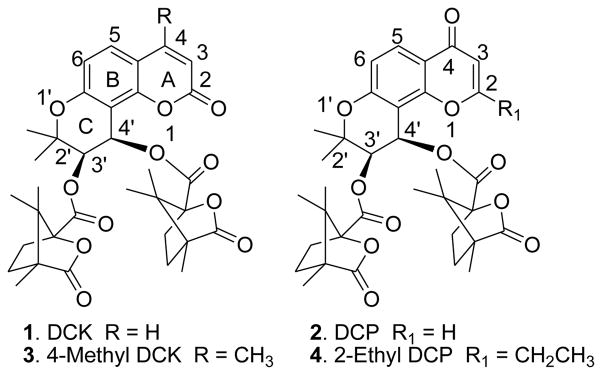
In our previous research, 3′R,4′R-di-O-(S)-camphanoyl-(+)-cis-khellactone (DCK, 1, Fig. 1) demonstrated extremely potent inhibitory activity against HIV-1 replication in H9 lymphocytic cells.1 Subsequently, hundreds of DCK and some of its ring-A positional isomer DCP (3′R,4′R-di-O-(S)-camphanoyl-2′,2′-dimethyl-dihydro-pyrano[2,3-f]chromone, 2, Fig. 1) derivatives have been designed, synthesized and screened for anti-HIV activity in H9 lymphocytes, MT-2 cell lines, and MT-4 cell lines.2–8 4-Methyl-DCK (3, Fig.1) and 2-ethyl-DCP (4, Fig.1) showed the most promising anti-HIV results in these two series. Structure-activity relationship (SAR) studies found that DCP derivatives exhibited better anti-HIV activity than the corresponding DCKs;8 2′-α-monomethyl-4-methyl DCK derivatives were more potent than 2′-gem-dimethyl DCKs;9 bio-isosteric analogues with a sulfur rather than oxygen in the ring-C of DCK exhibited remarkable inhibitory effects on HIV-1 replication;9,10 and a 3′,4′-dicamphanoyl moiety is indispensable for anti-HIV activity.11 Considering these SAR research results, we have now designed and synthesized 2′-monomethyl-DCP (5, 1′-oxa; 6, 1′-thia), 2-ethyl-1′-thia-DCP (7), and 4-methyl-1′-thia-DCK (8) analogues to further explore the pharmacophores of the 2′-position and the bioisosteric effect at the 1′-position. This paper reports their synthesis and anti-HIV bioassay data.
Figure 1.
Structures of previously synthesized DCK and DCP analogues (1–4).
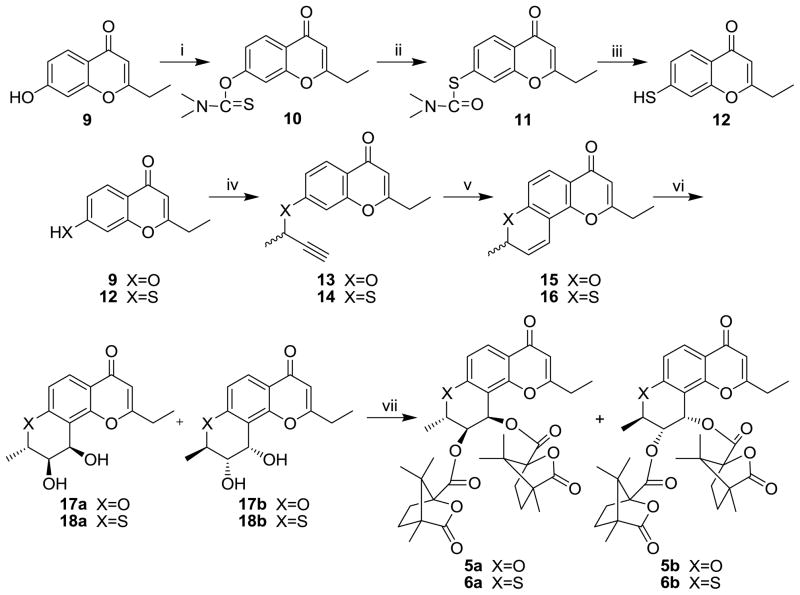
The synthetic routes to 5a, 5b, 6a and 6b are shown in Scheme 1. The intermediate 2-ethyl-7-mercapto-4H-chromen-4-one (12) was obtained by reacting 2-ethyl-7-hydroxy-4H-chromen-4-one (9) with dimethylthiocarbamoyl chloride in EtOH in the presence of anhydrous potassium carbonate, followed by a rearrangement at 240 °C, then hydrolysis with methanolic KOH and acidification with HCl. Compounds 9 and 12 were treated with 3-chloro-1-butyne in dimethyl formamide (DMF) or acetone in the presence of anhydrous potassium carbonate and potassium iodide at room temperature to produce the propargyl ethers 13 and 14, followed by thermal rearrangement in refluxing N,N-diethylaniline to form intermediates 15 and 16. Sharpless dihydroxylation (AD) of 15 and 16 afforded dihydroxy derivatives 17a/17b and 18a/18b, respectively, as diastereoisomeric mixtures. Target compounds 5a and 5b were obtained by acylation of 17a and 17b with (S)-(−)-camphanic chloride in CH2Cl2 at room temperature with 4-dimethylaminopyridine (DMAP) as acid scavenger. Compounds 6a and 6b were synthesized by the same procedure from 18a and 18b. The pure diastereoisomers 5a, 5b, 6a, and 6b were obtained by separation with column chromatography on silica gel [petroleum ether/ethyl acetate, 3:1 (v/v)].
Scheme 1.
Reagents and conditions: (i) dimethylthiocarbamoyl chloride, EtOH, K2CO3, r.t.; (ii) 240 °C, N2; (iii) KOH, CH3OH, N2, reflux; (iv) 3-chloro-1-butyne, K2CO3, KI in DMF or acetone, r.t.; (v) N,N-diethylaniline, reflux; (vi) K2OsO2(OH)4, (DHQ)2-PHAL, K3Fe(CN)6, K2CO3 in t-butanol/H2O (v/v=1:1), ice bath; (vii) (S)-camphanic chloride, DMAP in CH2Cl2, r.t.
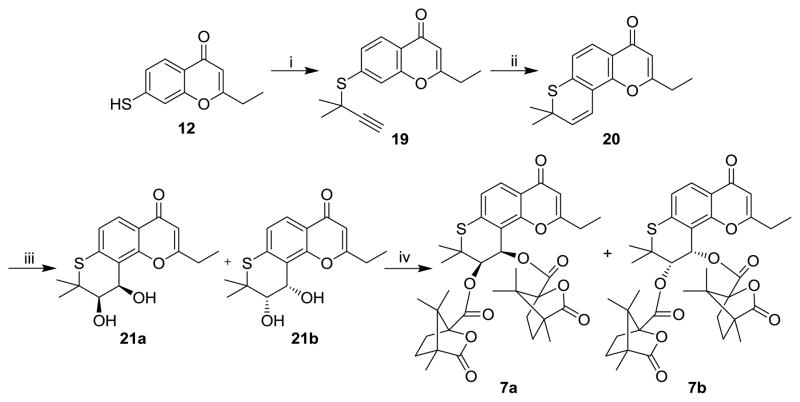
The preparation of 7a and 7b is illustrated in Scheme 2. 2-Ethyl-7-mercapto-4H-chromen-4-one (12) was treated with 3-chloro-3-methyl-1-butyne in EtOH/H2O (v/v=1:1) in the presence of potassium hydroxide at room temperature to produce the propargyl ether 19, followed by thermal rearrangement in refluxing N,N-diethylaniline to form intermediate 20. Sharpless AD of 20 afforded dihydroxy derivatives 21a and 21b. Target compounds 7a and 7b were obtained by acylation of 21a and 21b with (S)-(−)-camphanic chloride in CH2Cl2 at room temperature with DMAP as an acid scavenger. Diastereoisomers 7a and 7b could be separated by column chromatography on silica gel [petroleum ether/ethyl acetate, 3:1 (v/v)].
Scheme 2.
Reagents and conditions: (i) 3-chloro-3-methyl-1-butyne, KOH, N2, EtOH/H2O (v/v=1:1), r.t.; (ii) N,N-diethylaniline, reflux; (iii) K2OsO2(OH)4, (DHQ)2-PHAL, K3Fe(CN)6, K2CO3 in t-butanol/H2O (v/v=1:1), ice bath; (iv) (S)-camphanic chloride, DMAP in CH2Cl2, r.t.
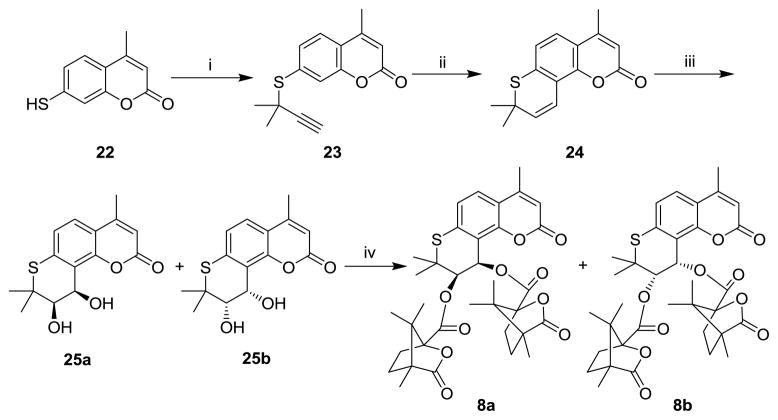
The synthesis of 8a and 8b was accomplished by a similar four-step sequence, as depicted in Scheme 3. Diastereoisomers 8a and 8b were separated by HPLC on an Alltima column (2.1 mm × 150 mm, C-18) with acetonitrile/water 70:30 (v/v) as eluant.
Scheme 3.
Reagents and conditions: (i) 3-chloro-3-methyl-1-butyne, KOH in EtOH, N2; (ii) N,N-diethylaniline, reflux; (iii) K2OsO2(OH)4, (DHQ)2-PHAL, K3Fe(CN)6, K2CO3 in t-butanol/H2O (v/v=1:1), ice bath; (iv) (S)-camphanic chloride, DMAP in CH2Cl2.
The eight newly synthesized compounds 5–812 were evaluated for anti-HIV activity in TZM-bl cells in parallel with 2-ethyl-DCP.13 The bioassay data are summarized in Table 1. Compounds 5a, 6a, and 7a showed significant anti-HIV activity with EC50 values of 30, 38 and 54 nM, which were better than the reference compound (2-ethyl-DCP, EC50: 120nM), and had good therapeutic index (TI) values of 152.6, 48.0 and 100.0, respectively. With a two-fold lower EC50 value, 2-ethyl-1′-thia-DCP (7a) was more potent than 4-methyl-1′-thia-DCK (8a). This result was coincident with the previous activity comparison between the DCP and DCK series, e.g. 2-ethyl DCP was more active than 4-methyl DCK.8 2′-Monomethyl-2-ethyl-1′-oxa- (5a) and -1′-thia-DCP derivatives (6a) exhibited better anti-HIV activity than the corresponding 2′-gem-dimethyl substituted compounds 2-ethyl-DCP and 7a. Interestingly, 5b, 6b, 7b and 8b exhibited remarkably reduced or even completely abolished anti-HIV activity, consistent with the results from prior compounds. This finding suggested that, just as in the DCK series, the spatial orientations of the 2′-methyl group and the 3′,4′-dicamphanoyls are also crucial to anti-HIV activity in DCP analogues.
Table 1.
Anti-HIV-1 NL4-3 data of analogues 5–8 in TZM-bl cellsa
| Compound | CC50 (μM) | EC50 (μM) | TI |
|---|---|---|---|
| 5a | 4.55 | 0.030 | 153 |
| 5b | - | - | NS |
| 6a | 1.84 | 0.038 | 48.0 |
| 6b | 3.83 | 0.184 | 20.8 |
| 7a | 5.4 | 0.054 | 100 |
| 7b | - | - | NS |
| 8a | >30.6 | 0.128 | >238 |
| 8b | >30.6 | 8.59 | >3.6 |
| 2-Ethyl-DCP | 14.3 | 0.12 | 119 |
All data presented in this table were averaged from at least three independent experiments. EC50: concentration that inhibits NL4-3 replication by 50%. CC50: concentration that inhibits uninfected TZM-bl cell growth by 50%. TI = CC50/EC50. NS: there was no inhibition at concentrations below the CC50.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China awarded to Y. Chen (No. 30200348 and 30873164) and P. Xia (No. 20272010), respectively, and Grant AI-33066 from the National Institute of Allergies and Infectious Diseases awarded to K.H.L. Thanks are also due to the National Drug Innovative Program 2009ZX09301-011) for partial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang L, Kashiwada Y, Cosentino LM, Fan S, Chen CH, McPhail AT, Fujioka T, Mihashi K, Lee KH. J Med Chem. 1994;37:3947. doi: 10.1021/jm00049a014. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi Y, Xie L, Cosentino LM, Lee KH. Bioorg Med Chem Lett. 1997;7:2573. doi: 10.1016/s0960-894x(98)00367-9. [DOI] [PubMed] [Google Scholar]
- 3.Xie L, Takeuchi Y, Cosentino LM, Lee KH. Bioorg Med Chem Lett. 1998;8:2151. doi: 10.1016/s0960-894x(98)00367-9. [DOI] [PubMed] [Google Scholar]
- 4.Xie L, Takeuchi Y, Concentino LM, McPhail AT, Lee KH. J Med Chem. 2001;44:664. doi: 10.1021/jm000070g. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, Chen Y, Xia P, Xia Y, Yang ZY, Yu DL, Morris-Natschke SL, Lee KH. Bioorg Med Chem Lett. 2004;14:5855. doi: 10.1016/j.bmcl.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Huang SX, Xia P, et al. Bioorg Med Chem Lett. 2007;17:4316. doi: 10.1016/j.bmcl.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu DL, Brossi A, Kilgore N, Wild C, Allaway G, Lee KH. Bioorg Med Chem Lett. 2003;13:1575. doi: 10.1016/s0960-894x(03)00201-4. [DOI] [PubMed] [Google Scholar]
- 8.Yu DL, Chen CH, Brossi A, Lee KH. J Med Chem. 2004;47:4072. doi: 10.1021/jm0400505. [DOI] [PubMed] [Google Scholar]
- 9.Xu SQ, Yan X, Chen Y, Xia P, Qian KD, Yu DL, Xia Y, Yang ZY, Morris-Natschke SL, Lee KH. Bioorg Med Chem. 2010;18:7203. doi: 10.1016/j.bmc.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Zhang Q, Zhang BN, Xia P, Xia Y, Yang ZY, Kilgore N, Wild C, Morris-Natschke SL, Lee KH. Bioorg Med Chem. 2004;12:6383. doi: 10.1016/j.bmc.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 11.Xie L, Takeuchi Y, Cosentino LM, Lee KH. J Med Chem. 1999;42:2662. doi: 10.1021/jm9900624. [DOI] [PubMed] [Google Scholar]
- 12.Analytical data of target compounds 5–8: Configuration assignments of isomeric compound pairs were based on prior data in reference 9.5a. mp 138–141°C; 1H NMR (CDCl3, 400 MHz) δ 0.95–1.12 (18H, m, 6×-CH3 in camphanoyl), 1.67–2.50 (8H, m, 4×-CH2 in camphanoyl), 1.24 (3H, t, -CH3 in ethyl), 1.48 (3H, d, J = 6.3 Hz, 2′-CH3), 2.56 (2H, m, -CH2 in ethyl), 4.55 (1H, m, 2′-CH), 5.17 (1H, m, 3′-H), 6.14 (1H, s, 3-H), 6.80 (1H, d, J = 3.1 Hz, 4′-H), 6.93 (1H, d, J = 9.0 Hz, 6-H), 8.12 (1H, d, J = 9.0 Hz, 5-H). [α]D −37 (c 0.1, CHCl3). HRMS(MALDI-DHB): Calcd. for C35H40O11: 636.2571; Found: 637.2643 [M+H+]. 5b. mp 203–206°C; 1H NMR (CDCl3, 400 MHz) δ 0.85 (3H, s, -CH3 in camphanoyl), 1.02–1.13 (15H, m, -CH3×5 in camphanoyl), 1.68–2.41 (8H, m, 4×-CH2 in camphanoyl), 1.29 (3H, t, -CH3 in ethyl), 1.47 (3H, d, J = 6.3 Hz, 2′-CH3), 2.47 (2H, m, -CH2 in ethyl), 4.66 (1H, m, 2′-CH), 5.29 (1H, m, 3′-H), 6.14 (1H, s, 3-H), 6.81 (1H, d, J = 3.5 Hz, 4′-H), 6.92 (1H, d, J = 8.6 Hz, 6-H), 8.11 (1H, d, J = 9.0 Hz, 5-H). [α]D +81 (c 0.1, CHCl3). HRMS(MALDI-DHB): Calcd. for C35H40O11: 636.2571; Found: 637.2643 [M+H+]. 6a. mp 175–178°C; 1H NMR (CDCl3, 400 MHz) δ 0.98–1.13 (18H, m, 6×-CH3 in camphanoyl), 1.68–2.59 (8H, m, 4×-CH2 in camphanoyl), 1.25 (3H, t, -CH3 in ethyl), 1.37 (3H, d, J = 6.7 Hz, 2′-CH3), 2.47 (2H, m, -CH2 in ethyl), 3.87 (1H, m, 2′-CH), 5.32 (1H, m, 3′-H), 6.15 (1H, s, 3-H), 6.97 (1H, d, J = 2.7 Hz, 4′-H), 7.12 (1H, d, J = 8.6 Hz, 6-H), 8.05 (1H, d, J = 8.6 Hz, 5-H). [α]D −272 (c 0.1, CHCl3). HRMS(MALDI-DHB): Calcd. for C35H40O10S: 675.2240 [M+Na+]; Found: 675.2234 [M+Na+]. 6b. mp 152–155°C; 1H NMR (CDCl3, 400 MHz) 0.82–1.13 (18H, m, -CH3×6 in camphanoyl), 1.68–2.66 (8H, m, 4×-CH2 in camphanoyl), 1.29 (3H, t, -CH3 in ethyl), 1.38 (3H, d, J = 6.7 Hz, 2′-CH3), 2.44 (2H, m, -CH2 in ethyl), 3.99 (1H, m, 2′-CH), 5.44 (1H, m, 3′-H), 6.16 (1H, s, 3-H), 6.97 (1H, d, J = 2.4 Hz, 4′-H), 7.12 (1H, d, J = 8.7 Hz, 6-H), 8.05 (1H, d, J = 8.3 Hz, 5-H). [α]D +135 (c 0.1, CHCl3). HRMS(MALDI-DHB): Calcd. for C35H40O10S: 675.2240 [M+Na+]; Found: 675.2234 [M+Na+]. 7a. mp 249–252°C; 1H NMR (CDCl3, 400 MHz) δ 0.96–1.76 (24H, 6×-CH3 in camphanoyl, 2×2′-CH3), 1.25 (3H, t, -CH3 in ethyl), 1.70–2.60 (10H, m, -CH2 in ethyl, 4×-CH2 in camphanoyl), 5.62 (1H, d, J = 4.3 Hz, 3′-CH), 6.16 (1H, s, 3-H), 6.96 (1H, d, J = 4.3 Hz, 4′-H), 7.11 (1H, d, J = 8.2 Hz, 6-H), 8.06 (1H, d, J = 8.6 Hz, 5-H). [α]D −130 (c 0.1, CHCl3). HRMS(MALDI-DHB): Calcd. for C36H42O10S: 689.2396 [M+Na+]; Found: 689.2391 [M+Na+]. 7b. mp 188–189°C; 1H NMR (CDCl3, 400 MHz) δ 0.88–1.76 (24H, 6×-CH3 in camphanoyl, 2×2′-CH3), 1.26 (3H, t, -CH3 in ethyl), 1.60–2.66 (10H, m, -CH2 in ethyl, 4×-CH2 in camphanoyl), 5.71 (1H, d, J = 4.3 Hz, 3′-CH), 6.15 (1H, s, 3-H), 6.92 (1H, d, J = 4.3 Hz, 4′-H), 7.10 (1H, d, J = 8.6 Hz, 6-H), 8.04 (1H, d, J = 8.2 Hz, 5-H). [α]D +27 (c 0.1, CHCl3). HRMS(MALDI-DHB): Calcd. for C36H42O10S: 689.2396 [M+Na+]; Found: 689.2391 [M+Na+]. 8a. mp 135–137°C; 1H NMR (CDCl3, 300 MHz) δ 1.11–1.13 (18H, 6×-CH3 in camphanoyl), 1.56–2.53 (8H, m, 4×-CH2 in camphanoyl), 1.38 (3H, s, 2′-CH3), 1.66 (3H, s, 2′-CH3), 2.40 (3H, s, 4-CH3), 5.63 (1H, d, J = 4.5 Hz, 3′-H), 6.18 (1H, d, J = 0.9 Hz, 3-H), 6.76 (1H, d, J = 4.5 Hz, 4′-H), 7.04 (1H, d, J = 8.7 Hz, 6-H), 7.48 (1H, d, J = 8.4 Hz, 5-H). HRMS (MALDI-DHB) calcd mass for C35H40O10S [M+-H] 651.2269, found 651.2270. 8b. mp 151–153°C; 1H NMR (CDCl3, 300 MHz) δ 0.87–1.15 (18H, 6×-CH3 in camphanoyl), 1.64–2.64 (8H, m, 4×-CH2 in camphanoyl), 1.38 (3H, s, 2′-CH3), 1.73 (3H, s, 2′-CH3), 2.40 (3H, d, J = 1.5 Hz, 4-CH3), 5.69 (1H, d, J = 4.5 Hz, 3′-CH), 6.17 (1H, d, J = 1.5 Hz, 3-H), 6.92 (1H, d, J = 4.5 Hz, 4′-H), 7.04 (1H, d, J = 8.1 Hz, 6-H), 7.48 (1H, d, J = 8.4 Hz, 5-H). HRMS (MALDI-DHB) calcd mass for C35H40O10S [M+-H] 651.2269, found 651.2273.
- 13.HIV-1 infectivity assay:Anti-HIV-1 activity was measured as reductions in Luc reporter gene expression after a single round of virus infection of TZM-bl cells. HIV-1 at 200 TCID50 and various dilutions of test samples (eight dilutions, four-fold stepwise) were mixed in a total volume of 100 μL growth medium in 96-well black solid plates (Corning-Costar). After 48-h incubation, culture medium was removed from each well and 100 μL of Bright Glo luciferase reagent was added to each culture well. The luciferase activity in the assay wells was measured using a Victor 2 luminometer. The 50% inhibitory dose (EC50) was defined as the sample concentration that caused a 50% reduction in Relative Luminescence Units (RLU) compared to virus control wells after subtraction of background RLU.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.