Abstract
The cholinergic cardiac vagal neurons (CVNs), located in the nucleus ambiguus, are the origin of cardioinhibitory parasympathetic activity. Catecholaminergic neurons in nearby regions of the brainstem, including the C1 and C2 cell groups, are thought to play a key role in both arousing from sleep and maintaining wakefulness. Since norepinephrine (NE) could play an important role in influencing the activity of CVNs, particularly in response to sleeping/waking and arousal states, the present study investigated the contribution of α1-adrenergic receptor activation to augment inhibitory and/or blunt excitatory neurotransmission to CVNs. To test the effects of α1-adrenergic receptor activation, CVNs were labeled in rats by retrograde tracing and synaptic events were recorded by whole cell voltage clamp techniques in vitro. Prazosin, an inverse agonist of α1-adrenergic receptor, significantly decreased the frequency of both GABAergic and glycinergic neurotransmission to CVNs. Activation of α1-adrenergic receptors by the α1-adrenergic receptor agonists NE or phenylephrine (PE) both significantly increased GABAergic and glycinergic inhibitory event frequency. This effect was prevented by the sodium channel blocker tetrodotoxin (TTX). Activation of α1-adrenergic receptors did not alter glutamatergic neurotransmission to CVNs. This study indicates that α1-adrenergic receptor activation in the brainstem can facilitate inhibitory GABAergic and glycinergic neurotransmission to reduce CVN activity; this synaptic modulation may play a role in the tachycardia seen during NE-dependent behavioral arousal.
Keywords: autonomic, parasympathetic, adrenergic, cardiovascular, heart, norepinephrine
Parasympathetic output to the heart is mediated though changes in the activity of cardiac vagal neurons (CVNs) in the nucleus ambiguus (NA) which are intrinsically silent and receive inputs from GABAergic, glycinergic, and glutamatergic pathways (Wang et al., 2001). Modulation of these synaptic pathways changes parasympathetic activity to the heart during different conditions and challenges. One possible, yet poorly explored, neurotransmitter that may alter neurotransmission to CVNs is norepinephrine (NE). Previous work has shown tryosine-hydroxylase (an essential enzyme in the generation of NE) immuno-reactive synapses innervate CVNs (Massari et al., 1998) and α1-adrenergic receptors are also present within the NA (Day et al., 1997).
Modulation of neurotransmission to CVNs by NE could be involved in the state-dependent increases in heart rate and reductions in cardioinhibitory parasympathetic activity that occur during arousals and wakefulness. Although many important sleep/wake systems have been proposed throughout the hypothalamus and brainstem, NE appears critical to the stimulation of behavioral arousal and wakefulness (McCarley and Hobson, 1975, Aston-Jones and Bloom, 1981, Carter et al., 2010). Moreover, high concentrations of NE occur within certain brain regions during wakefulness and steadily decline during sleep (Shouse et al., 2000). Arousal and wakefulness are characterized by tachycardias, increases in blood pressure, and decreases in parasympathetic output compared to sleep states (del Bo et al., 1982, Kuo et al., 2008). Mice with a deficiency in NE production demonstrate significantly lower baseline heart rates and altered circadian rhythms. During specific stressors, these NE deficient mice do not produce appropriate heart rate responses seen in normal mice. (Swoap et al., 2004). Moreover both tachycardia and increased blood pressure have been demonstrated following microinjections of specific α1-adrenergic receptor agonists in the critical sleep/wake and cardiovascular regulatory center, the nucleus tractus solitarius (NTS) (Bhuiyan et al., 2009), while bradycardia is induced by an intracisternal administration of an α1-adrenergic receptor antagonist (Huchet et al., 1983). It is possible that NE, in addition to directly inducing cortical arousal, is capable of orchestrating changes in autonomic function during these changes in states of attention. Modulation of neurotransmission by NE is therefore likely involved in sleep, emotional, and attention related changes in heart rate and other autonomic functions.
Although it is possible NE directly activates post-synaptic receptors in CVNs, it is more likely NE acts via changes in the activity of preceding neurons or presynaptic terminals that synapse upon CVNs. Recent studies demonstrate that other state-related brain nuclei and neuropeptides modulate CVNs through changes in all three input types, GABA, glycine and glutamate. For example, the REM regulatory center, the lateral paragigantocellular nucleus, mediates inhibitory neurotransmission to CVNs through GABAergic neurotransmission, while the wake promoting neuropeptide, orexin, alters both inhibitory and excitatory inputs (Dergacheva et al., 2005, Dergacheva et al., 2010, Dergacheva et al., 2011). Previous work from our laboratory also demonstrated that α2-adrenergic receptor activation decreased the frequency of GABAergic events to CVNs (Philbin et al., 2009). The present study was undertaken to test the hypothesis that α1 receptor activation increases inhibitory GABAergic or glycinergic activity and/or diminishes excitatory neurotransmission to CVNs.
Experimental Procedure
Experiments were performed on Sprague Dawley rats (Hilltop Lab Animals Inc, Scottdale, PA) housed in the George Washington University (GWU) animal care facility. Procedures were approved by the GWU Institutional Animal Care and Use Committee prior to use. Rats were maintained under standard environmental conditions (12:12h light:dark cycle) with free access to food and water.
After birth, pups at postnatal days 3-5 were anesthetized with hypothermia by cooling to ~4°C. Once heart rate had significantly slowed and no pain reflex was observed, a right thoracotomy was performed, and the retrograde tracer X-rhodamine-5-(and-6)-isothiocynate (Molecular Probes, Eugene, OR) was injected into the fat pads at the base of the heart as previously described in detail (Mendelowitz and Kunze, 1991). Following surgery, buprenorphine was administered and pups were monitored for 30 min and every 20 min thereafter until ambulatory.
After 24-48h of recovery, animals were overdosed with isoflurane and euthanized by cervical dislocation. Brains were collected and the tissue was placed in 4°C physiological saline buffer solution with the following composition: NaCl (140mM), KCl (5mM), CaCl2 (2mM), glucose (5mM) and HEPES (10mM). A single thick (440-550μm) section that included CVNs was cut and submerged in a recording chamber with room-temperature perfusate with the following composition: NaCl (125mM), KCl (3mM), CaCl2 (2mM), NaHCO3 (26mM), glucose (5mM), HEPES (5mM) in equilibrium with 95% O2-5%CO2. The osmolarity of all solutions was 285-290 mOsm, and the pH was maintained between 7.35 and 7.4.
CVNs were identified by the presence of the fluorescent tracer and differential interference contrast optics along with infrared illumination and infrared-sensitive video detection cameras were used to gain better spatial resolution. Patch pipettes were filled with a solution at the pH of 7.3 consisting of either KCl (150 mM), MgCl2 (4mM), EGTA (10mM), Na-ATP (2mM), HEPES (10mM) or K-gluconic acid (150mM), HEPES (10mM), EGTA (10mM), MgCl2 (1mM), CaCl2 (1mM) to isolate for inhibitory or excitatory currents respectively. Identified CVNs were voltage-clamped at a holding potential of −80mV. To prevent any effect of α2 receptor activation, atipamezole (1μM) was included in all the perfusates when NE was applied. To determine the effect of α1 receptors on GABAergic and glycinergic neurotransmission, strychnine (1μM) and gabazine (25μM), respectively, were included in the perfusate. Glutamatergic events were isolated by adding both gabazine and strychnine to the perfusate. Tetrodotoxin (TTX; 1μM) was added to the perfusate to block action potential generation and isolate spontaneous miniature postsynaptic events. All the drugs used in the experiment were applied by inclusion in the perfusate and only one experiment was conducted per slice. Atipamezole (1μM), NE (20μM), gabazine (25μM), phenylephrine (PE; 50μM), prazosin (3μM), propranolol (10μM), strychnine (1μM) and TTX (1μM) were all received from Sigma-Aldrich (St. Louis, MO).
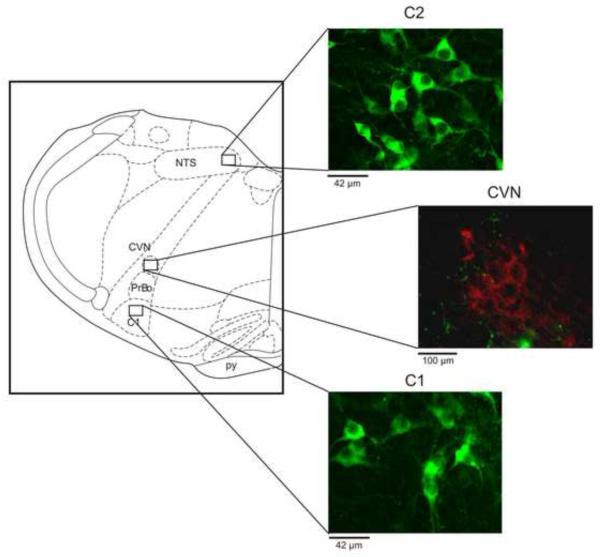
To determine the possible location of catecholaminergic neurons, the tissue that contains the neurons and nuclei used for electrophysiological recordings were stained for tyrosine hydroxylase (TH) using immunohistrochemistry (Figure 1). Slices were soaked in 4% paraformaldehyde and processed for TH-positive neuronal identification using a primary rabbit anti-TH antibody (1:1,000 dilution; Chemicon., Billerica, MA) with a secondary Alexa-Flour 488 (1:100 dilution; Invitrogen, Carlsbad, CA) and imaged by confocal microscopy (Ziess LSM 7.10, Thornwood, NY). Figure 1 shows the schematic used to identify brain regions of interest (Paxinos, 1998). Neurons were identified as TH-positive or CVNs by the presence of green or red fluorescence within the cell soma respectively.
Figure 1. Representative images of tyrosine-hydoxylase (TH) positive neurons (green) in the C1 and C2 regions, with fluorescent localization of CVNs in the NA (red, by the presence of the fluorescent tracer rhodamine (X491)).
Schematic figure was adapted from Paxinos and Watson. (CVN: cardiac vagal neuron; NTS: nucleus of the solitary tract; pr: pyramidal complex; PrBo: pre-Bötzinger complex)
MiniAnalysis (Synaptosoft version 4.3.1) was used to analyze all experimental traces. The threshold for GABAergic, glycinergic, and glutamatergic events was set to 5 times the root mean square of noise. All data is represented by mean±SEM. Student paired t-test were used to determine statistical significance using a P-value of 0.05.
Results
As shown in Figure 1, tyrosine-hydoxylase (TH) positive neurons were located in the same coronal section as CVNs in the NA. The TH-positive neurons were predominately localized to the anatomical groups known as A and C groups, including the C1 and C2 cell groups, which are ventral and dorso-medial to CVNs in the Nucleus Ambiguus, respectively.
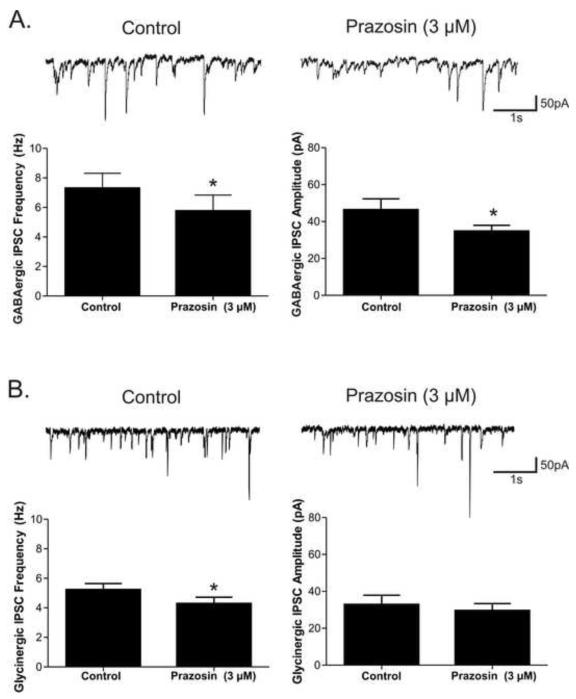
Application of the α1-adrenergic receptor inverse agonist prazosin (3 μM) significantly decreased the frequency of both GABAergic (7.3 ± 1.0 to 5.8 ± 1.1; p<0.05, n=7) and glycinergic (5.2 ± 0.4 to 4.3 ± 0.4; p<0.05, n=11) neurotransmission to CVNs, see Figure 2. Prazosin had no significant effect on glycinergic IPSC amplitudes or baseline currents (Figure 2). Prazosin did, however, additionally depress GABAergic amplitude (46.3±6.0 to 34.7±3.2, p<0.05), without any effect on baseline current in these CVNs. As prazosin is an inverse agonist for α1-adrenergic receptors, these results suggest prazosin acts via reducing the endogenous α1-adrenergic receptor facilitation of inhibitory neurotransmission to CVNs.
Figure 2. The α1-adrenergic receptor inverse agonist prazosin (3μM) significantly decreased the spontaneous inhibitory neurotransmission to cardiac vagal neurons (CVNs).
A. The mean frequency and amplitude averages for GABAergic IPSCs (n=7). B. The mean frequency and amplitude averages for glycinergic IPSCs (n=11).* indicates a significant difference (p<0.05) between groups.
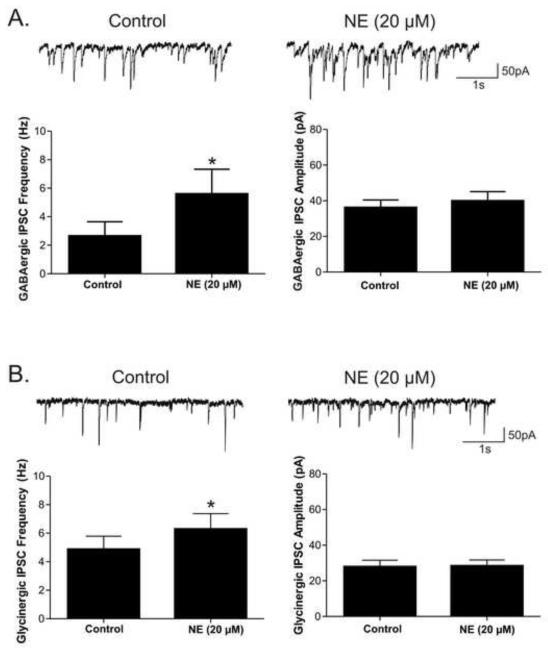
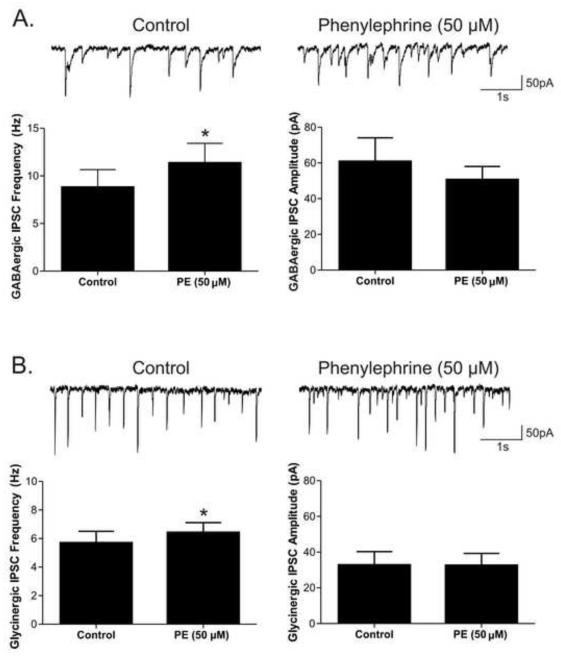
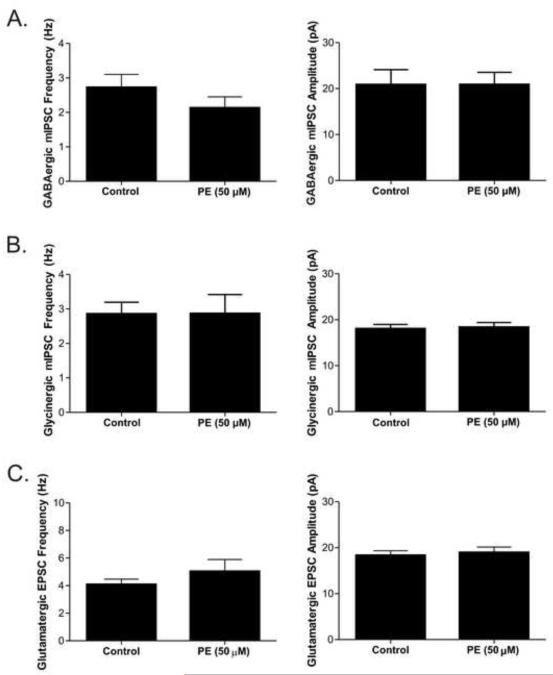
To further test the α1-adrenergic receptor’s facilitation of inhibitory neurotransmission to CVNs, the β-antagonist propranolol (10 μM) and α2-adrenergic antagonist atipamezole (1 μM) were included in the perfusate and non-selective neurotransmitter NE was applied. NE (20 μM) evoked a significant (p<0.05) increase in frequency of both GABAergic (3.3 ± 0.8 to 5.6 ± 1.2; p<0.05, n=7) and glycinergic (4.9± 0.9 to 6.3 ± 1.1; p<0.05, n=9) inhibitory neurotransmission to CVNs, see Figure 3. NE did not significantly change either GABAergic or glycinergic IPSC amplitudes or baseline currents. Similar to the effects with NE, phenylephrine (PE; 50 μM), a selective α1-adrenergic receptor agonist, also increased the frequency of both GABAergic (8.8 ± 1.8 to 11.4 ± 2.1; p<0.05, n=7) and glycinergic (5.7 ± 0.8 to 6.4 ± 0.69; p<0.05, n=7) events, but had no effect on inhibitory IPSC amplitudes or baseline currents, see Figure 4.
Figure 3. Norepinephrine (NE) was applied in the presence of the α2-adrenergic antagonist atipamezole (1 μM) and β-adrenergic receptor antagonist propanolol (10 μM) to examine the effect of α1-adrenergic receptor activation on inhibitory pathways to CVNs.
A. NE significantly augmented both GABAergic and glycinergic neurotransmission to CVNs. The mean frequency and amplitude averages for GABAergic IPSCs (n=7). B. The mean frequency and amplitude averages for glycinergic IPSCs (n=9).* indicates a significant (p<0.05) difference between groups.
Figure 4. The selective α1-adrenergic receptor agonist phenylephrine (PE; 50 μM) was applied to examine the effect of α1-adrenergic receptor activation on spontaneous inhibitory events in cardiac vagal neurons (CVNs).
PE increased both GABAergic and glycinergic IPSC frequency in CVNs. A. The mean frequency and amplitude averages for GABAergic IPSCs (n=7). B. The mean frequency and amplitude averages for glycinergic IPSCs (n=7).* indicates a significant difference (p<0.05) between groups.
Tetrodotoxin (TTX, 1μM), a voltage-gated sodium channel blocker, was included in the perfusate to investigate the sites of action of the α1-adrenergic receptor activation responsible for the augmented inhibitory neurotransmission to CVNs. TTX prevented the significant increase in frequency for both GABAergic (2.7 ± 0.3 to 2.1 ± 0.3; n=7) and glycinergic (2.9 ± 0.3 to 2.9 ± 0.5; n=8) events that occurred with PE (see Figure 5). Additionally, after TTX application, there were no significant changes in inhibitory event amplitudes with PE.
Figure 5. The selective α1-adrenergic receptor agonist phenylephrine (PE; 50 μM) was applied in the presence of the sodium channel blocker TTX (1 μM) to examine the potential mechanisms responsible for the increase in inhibitory neurotransmission to cardiac vagal neurons (CVNs) after α1-adrenergic receptors activation.
Control bath condition in A and B includes TTX which prevented the inhibition that occurred with α1-adrenergic receptor activation. A. The mean frequency and amplitude averages for GABAergic IPSCs (n=7). B. The mean frequency and amplitude averages for glycinergic IPSCs (n=8). C. The mean frequency and amplitude averages for glutamatergic EPSC after PE (n=9).
To further characterize the NE-induced changes in neurotransmission to CVNs, excitatory glutamatergic neurotransmission was examined by inclusion of both strychnine and gabazine in the perfusate to block glycinergic and GABAergic receptors, respectively. Prazosin application alone had no effect on glutamatergic EPSC frequency, amplitude, or baseline currents (n=5). Application of PE (n=9) also did not significantly change glutamatergic synaptic EPSC frequency, amplitude, or baseline currents (Figure 5).
Discussion
Although, the interaction between autonomic parasympathetic activity and the sleep/wake regulatory circuits is clinically important, little is known about the role of the brainstem wake-promoting pathways and neurotransmitters, particularly catecholamines, in the modulation of parasympathetic neurons that control heart rate. The present data demonstrate three novel findings that address these gaps in our knowledge. The results indicate that α1-adrenergic receptors are endogenously activated in the brainstem, and via facilitation of both GABAergic and glycinergic neurotransmission provide increased inhibitory neurotransmission to CVNs. Secondly, this modulation of inhibitory neurotransmission can be augmented by application of the neurotransmitter norepinephrine acting through α1-adrenergic receptors. Lastly, the site of action of α1 receptor induced facilitation of inhibitory neurotransmission is unlikely to occur at the inhibitory presynaptic terminals on CVNs, as α1 receptor activation had no effect on miniature IPSCs (mIPSC) in the presence of TTX. Instead α1 receptor activation likely alters inhibitory neurotransmission via an action-potential dependent mechanism, involving alterations in preceding neurons within the brainstem network. Collectively, these data demonstrate a potential brainstem mechanism whereby NE activation could decrease parasympathetic activity. It is expected that this decrease in parasympathetic activity would cause an increase in heart rate during arousal and wakefulness.
As the α1 receptor inverse agonist prazosin decreased both GABAergic and glycinergic inhibitory neurotransmission to CVNs, α1-adrenergic receptor activation likely acts to maintain some of the ongoing tonic inhibitory activity to CVNs. As decreased inhibition to CVNs would result in a decrease in heart rate, the present results agree with previous research indicating that an injection of an α1 receptor antagonist into the intracisternal space evokes bradycardias (Huchet et al., 1983). The origin and mechanisms of this tonic adrenergic activity however requires further investigation. The presence of adrenergic neurons at the same coronal level as CVNs indicates one likely effect of prazosin may be to inhibit the effect of activating these nearby adrenergic neurons via decreased activation of α1 receptors. Since prazosin is an inverse agonist, an alternative possibility that prazosin is blocking the constitutive activity of these adrenergic receptors and thereby reducing their activity independent of ligand-binding. Regardless of mechanism of action, the present results suggest that there is a basal activity of adrenergic receptors which facilitate inhibitory neurotransmission to CVNs.
The source of the adrenergic neurons responsible for this α1-adrenergic receptor dependent tonic facilitation of GABAergic and glycinergic neurotransmission requires further investigation. The adrenergic C1 and C2 groups are present at the same coronal section as CVNs in the NA, and it is possible that these groups are responsible for the endogenous catecholaminergic release observed in this study. Indeed, it is well established that the C1 cluster in the rostral ventral lateral medulla (RVLM) are involved in central presympathetic modulation of overall autonomic output (Schreihofer and Guyenet, 1997, Schreihofer et al., 2000). Moreover, both the NTS and RVLM send inhibitory projections to CVNs (Frank et al., 2009). It is possible that either C1 and/or C2 neurons are modulating inhibitory projections from the NTS and RVLM and may be contributing to the tonic GABAergic and glycinergic inhibitory neurotransmission to CVNs examined in this study.
Another potential source of the adrenergic activity examined in this study is the locus coeruleus (LC). Since the NE containing cells within this nucleus are thought to be responsible for stimulating behavioral arousal and maintaining wakefulness (McCarley and Hobson, 1975, Aston-Jones and Bloom, 1981), it is possible their activation could facilitate the activity of descending cardiovascular centers which may be responsible for the tachycardia seen during arousals and/or wakefulness. Indeed, ablation of the anterior region of the LC induces behavioral state-dependent bradycardias, and significantly reduces the phasic tachycardia seen during REM sleep (Liesiene et al., 1981). Although the largest projections from the LC are to ascending arousal-related cortical structures, the LC does project to caudal cardiorespiratory brainstem regions including the NA, raphe and pre-Botzinger complex (Westlund and Coulter, 1980, Standish et al., 1995, Samuels and Szabadi, 2008). It has also been suggested that LC may activate brainstem regions through volume transmission, rather than direct synaptic connections (Haxhiu et al., 2003) similar to its mode of transmission to several cortical nuclei (Fuxe et al., 2007). Regardless of signaling mechanism, electrical stimulation of LC increases respiratory frequency by endogenous release of NE into the respiratory pacemaking center, the pre-Bötzinger complex (Doi and Ramirez, 2010), an area known to send functionally important GABAergic projections to CVNs (Frank et al., 2009). Additionally, chemical stimulation of the LC evokes significantly elevated levels of NE within the rostral NA (Haxhiu et al., 2003). While it is unlikely that the LC contributes to the tonic inhibitory drive to CVNs because it is not present within the brain slices used in this study, the activation of α1-adrenergic receptors by the release of NE through LC stimulation may act through α1-adrenergic receptors to facilitate increases in inhibitory neurotransmission onto CVNs.
The effects of α1 receptor activation shown in this study are similar to studies performed in other brain regions. For example, α1-adrenergic receptor activation increased both GABAergic and glycinergic currents within the substantia gelatinosa (Baba et al., 2000). and was specific to GABAergic neurotransmission to parvocellular neurons within the hypothalamus (Han et al., 2002). Moreover, an increase in inhibitory neurotransmission may cause decreased firing activity in neurons, and indeed in gastric-projecting neurons within the dorsal motor nucleus α1-adrenergic receptor activation caused a decreasing in firing activity (Martinez-Pena y Valenzuela et al., 2004). Interestingly, unlike α1 receptors, α2-adrenergic receptors augmented the firing patterns in these gastric neurons. These results fit both our previous work demonstrating that clondine, an α2-adrenergic receptor agonist, decreases GABAergic neurotransmission to CVNs, as well as the results from the present study. The often opposing relationship of α2- and α1-adrenergic receptors may be a common theme within the nervous system as these receptors have often been shown to have antagonizing actions on neurotransmission in many locations and synaptic pathways involving cardiovascular responses (Bhuiyan et al., 2009) and the control of sleep and wakefulness (Nishino and Mignot, 1997, Nelson et al., 2003).
Conclusions
In conclusion, the present study demonstrates that there is a basal activation of α1 adrenergic receptors in the brainstem that facilitates GABAergic and glycinergic inhibitory neurotransmission to CVNs and the arousal related/wake-promoting neurotransmitter, NE, can facilitate this inhibitory neurotransmission to CVNs. This increase in inhibitory neurotransmission would act to decrease parasympathetic output and result in tachycardia. These data constitute a potential cellular mechanism for an increase in heart rate during increased activation of adrenergic neurons and pathways within the brainstem and NE-mediated arousals and/or wakefulness. Further work is necessary to identify the origin and mechanisms responsible for this modulation of the cardiorespiratory network and CVNs in particular.
Highlights.
α1-adrenergic receptor activity facilitates a tonic inhibitory postsynaptic current to cardiac vagal neurons.
α1-adrenergic receptor activation augments both GABAergic and glycinergic neurotransmission to cardiac vagal neurons.
α1-adrenergic receptors in the present study are being activated at a brain region upstream from cardiac vagal neurons.
Acknowledgements
Supported by NIH grants HL49965, 59895 and 72006 to DM. Images were taken from equipment purchased with a shared instrumention grant (SLORR025565).
Abbreviations
- CVN
cardiac vagal neurons
- EPSC
excitatory post-synaptic currents
- IPSC
inhibitory post-synaptic currents
- NA
nucleus ambiguus
- NE
norepinephrine
- NTS
nucleus of the solitary tract
- PE
phenylephrine
- REM
rapid eye movement
- RVLM
rostral ventral lateral medulla
- TH
tyrosine hydroxylase
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92:473–484. doi: 10.1097/00000542-200002000-00030. [DOI] [PubMed] [Google Scholar]
- Bhuiyan ME, Waki H, Gouraud SS, Takagishi M, Cui H, Yamazaki T, Kohsaka A, Maeda M. Complex cardiovascular actions of alpha-adrenergic receptors expressed in the nucleus tractus solitarii of rats. Exp Physiol. 2009;94:773–784. doi: 10.1113/expphysiol.2008.046490. [DOI] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr., Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- del Bo A, Ledoux JE, Tucker LW, Harshkfield GA, Reis DJ. Arterial pressure and heart rate changes during natural sleep in rat. Physiol Behav. 1982;28:425–429. doi: 10.1016/0031-9384(82)90135-4. [DOI] [PubMed] [Google Scholar]
- Dergacheva O, Philbin K, Bateman R, Mendelowitz D. Hypocretin-1 (orexin A) prevents the+ effects of hypoxia/hypercapnia and enhances the GABAergic pathway from the lateral paragigantocellular nucleus to cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2010.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Wang X, Huang ZG, Bouairi E, Stephens C, Gorini C, Mendelowitz D. Hypocretin-1 (orexin-A) facilitates inhibitory and diminishes excitatory synaptic pathways to cardiac vagal neurons in the nucleus ambiguus. J Pharmacol Exp Ther. 2005;314:1322–1327. doi: 10.1124/jpet.105.086421. [DOI] [PubMed] [Google Scholar]
- Dergacheva O, Wang X, Lovett-Barr MR, Jameson H, Mendelowitz D. The lateral paragigantocellular nucleus modulates parasympathetic cardiac neurons: a mechanism for rapid eye movement sleep-dependent changes in heart rate. J Neurophysiol. 2010;104:685–694. doi: 10.1152/jn.00228.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank JG, Jameson HS, Gorini C, Mendelowitz D. Mapping and identification of GABAergic neurons in transgenic mice projecting to cardiac vagal neurons in the nucleus ambiguus using photo-uncaging. J Neurophysiol. 2009;101:1755–1760. doi: 10.1152/jn.91134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Dahlstrom A, Hoistad M, Marcellino D, Jansson A, Rivera A, Diaz-Cabiale Z, Jacobsen K, Tinner-Staines B, Hagman B, Leo G, Staines W, Guidolin D, Kehr J, Genedani S, Belluardo N, Agnati LF. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res Rev. 2007;55:17–54. doi: 10.1016/j.brainresrev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD. Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol. 2002;87:2287–2296. doi: 10.1152/jn.2002.87.5.2287. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Kc P, Neziri B, Yamamoto BK, Ferguson DG, Massari VJ. Catecholaminergic microcircuitry controlling the output of airway-related vagal preganglionic neurons. J Appl Physiol. 2003;94:1999–2009. doi: 10.1152/japplphysiol.01066.2002. [DOI] [PubMed] [Google Scholar]
- Huchet AM, Huguet F, Ostermann G, Bakri-Logeais F, Schmitt H, Narcisse G. Central alpha 1-adrenoceptors and cardiovascular control in normotensive and spontaneously hypertensive rats. Eur J Pharmacol. 1983;95:207–213. doi: 10.1016/0014-2999(83)90636-2. [DOI] [PubMed] [Google Scholar]
- Kuo TB, Shaw FZ, Lai CJ, Yang CC. Asymmetry in sympathetic and vagal activities during sleep-wake transitions. Sleep. 2008;31:311–320. doi: 10.1093/sleep/31.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesiene V, Adrien J, Benoit O. Effects of locus coeruleus lesions on heart rate during sleep in the cat. Arch Ital Biol. 1981;119:125–138. [PubMed] [Google Scholar]
- Martinez-Pena y Valenzuela I, Rogers RC, Hermann GE, Travagli RA. Norepinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol. 2004;286:G333–339. doi: 10.1152/ajpgi.00289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari VJ, Dickerson LW, Gray AL, Lauenstein JM, Blinder KJ, Newsome JT, Rodak DJ, Fleming TJ, Gatti PJ, Gillis RA. Neural control of left ventricular contractility in the dog heart: synaptic interactions of negative inotropic vagal preganglionic neurons in the nucleus ambiguus with tyrosine hydroxylase immunoreactive terminals. Brain Res. 1998;802:205–220. doi: 10.1016/s0006-8993(98)00613-1. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 1975;189:58–60. doi: 10.1126/science.1135627. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D, Kunze DL. Identification and dissociation of cardiovascular neurons from the medulla for patch clamp analysis. Neurosci Lett. 1991;132:217–221. doi: 10.1016/0304-3940(91)90305-d. [DOI] [PubMed] [Google Scholar]
- Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- Paxinos GW, Charles . The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Philbin KE, Bateman RJ, Mendelowitz D. Clonidine, an alpha2-receptor agonist, diminishes GABAergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res. 2009;1347:65–70. doi: 10.1016/j.brainres.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol. 2008;6:254–285. doi: 10.2174/157015908785777193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG. Regulation of sympathetic tone and arterial pressure by rostral ventrolateral medulla after depletion of C1 cells in rat. J Physiol. 2000;529(Pt 1):221–236. doi: 10.1111/j.1469-7793.2000.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouse MN, Staba RJ, Saquib SF, Farber PR. Monoamines and sleep: microdialysis findings in pons and amygdala. Brain Res. 2000;860:181–189. doi: 10.1016/s0006-8993(00)02013-8. [DOI] [PubMed] [Google Scholar]
- Standish A, Enquist LW, Escardo JA, Schwaber JS. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. J Neurosci. 1995;15:1998–2012. doi: 10.1523/JNEUROSCI.15-03-01998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, Weinshenker D, Palmiter RD, Garber G. Dbh(−/−) mice are hypotensive, have altered circadian rhythms, and have abnormal responses to dieting and stress. Am J Physiol Regul Integr Comp Physiol. 2004;286:R108–113. doi: 10.1152/ajpregu.00405.2003. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann N Y Acad Sci. 2001;940:237–246. doi: 10.1111/j.1749-6632.2001.tb03680.x. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Coulter JD. Descending projections of the locus coeruleus and subcoeruleus/medial parabrachial nuclei in monkey: axonal transport studies and dopamine-beta-hydroxylase immunocytochemistry. Brain Res. 1980;2:235–264. doi: 10.1016/0165-0173(80)90009-0. [DOI] [PubMed] [Google Scholar]