Abstract
Neurotransmitter release regulation is highly heterogeneous across the brain. The fundamental units of release, individual boutons, are difficult to access and poorly understood. Here we directly activated single boutons on mechanically isolated NTS neurons to record unitary synaptic events under voltage clamp. By scanning the cell surface with a stimulating pipette, we located unique sites that generated evoked excitatory postsynaptic currents (eEPSCs) or inhibitory events (eIPSCs). Stimulus-response relations had abrupt thresholds for all-or-none synaptic events consistent with unitary responses. Thus, irrespective of shock intensity, focal stimulation selectively evoked either eEPSCs or eIPSCs from single retained synaptic boutons and never recruited other synapses. Evoked EPSCs were only rarely encountered. Our studies thus focused primarily on the more common GABA release. At most locations, shocks often failed to release GABA even at low frequencies (0.075 Hz) and eIPSCs succeeded only on average 2.7±0.7 successful IPSCs per 10 shocks. Activation of eIPSCs decreased spontaneous IPSCs in the same neurons. The GABAA receptor antagonist gabazine (3 μM) reversibly blocked eIPSCs as did TTX (300 nM). The initial low rate of successful eIPSCs decreased further in a use-dependent manner at 0.5 Hz stimulation – depressing 70% in 2 min. The selective GABAB receptor antagonist CGP 52432 (5 μM) had three actions: tripling the initial release rate, slowing the use dependent decline without changing amplitudes, and blocking the shock related decrease in spontaneous IPSCs. The results suggest strong, surprisingly long-lasting, negative feedback by GABAB receptors within single GABA terminals that determine release probability even in isolated terminals.
Keywords: single bouton, metabotropic, release probability, synaptic failures
Introduction
Individual synaptic terminals are the final stage of the neurotransmission process. The invading action potential triggers neurotransmitter release from the presynaptic terminal following the influx of calcium that initiates the vesicle fusion (Schneggenburger and Neher, 2005). Activation of some central afferents releases a single quanta of neurotransmitter from a readily releasable pool of docked, primed vesicles and such release is often thought to reflect the intrinsic state of release probability (Gulyas et al., 1993). A common aspect of this process at many central terminals is that this activation sequence often fails to release neurotransmitter in synchrony with the stimulus shock – a synaptic transmission failure (Arancio et al., 1994; Allen and Stevens, 1994). Synaptic failures reflect a reluctance of the terminal at a given calcium level to undergo fusion and release. As part of the studies of neurotransmission since the time of Katz (Katz, 1971), neurotransmitter is spontaneously released in stochastic fusions at low rates that reflect the probabilistic nature of the release machinery (Arancio et al., 1994). These characteristics of synaptic coupling and release properties at neuronal interconnections define fundamental building blocks for integration and communication between neurons that varies greatly across the central nervous system.
Perhaps the most common approach to studies of synaptic transmission relies on activating an afferent axon with shocks that trigger action potentials which invade the synaptic terminal. Thus most often in synaptic transmission studies, the recorded postsynaptic currents are downstream events that require successful serial sequences of conduction, terminal depolarization and release to evoke transmission. For different neurons, axons rely on quite different terminal architectures that make as few as a single contact or give rise to branching processes with multiple contacts. Primary afferent axons in the solitary tract nucleus (NTS) (Bailey et al., 2006; Peters et al., 2008) always make multi-terminal contacts that evoke large compound EPSCs and assessments suggest that these unitary EPSCs arise from an average cohort of ~20 active contacts (Andresen and Peters, 2008). Variability in the amplitudes of electrically evoked responses from this population of contacts provides an estimate of the average number of release sites and the probability of neurotransmitter release (Nauen, 2011). The determinants of release properties of individual terminals are more difficult to determine and may differ across neurotransmitters, neuron phenotype and brain area.
In the present studies, we take advantage of the synaptic terminals that are retained on the cell bodies of mechanically isolated NTS neurons (Jin et al., 2004a; Jin et al., 2004b). These terminals conform to pharmacological patterns observed in slices from the same regions and individual terminal release properties have been assessed indirectly using fluorescent indicator dye incorporated into vesicle membranes (Jin et al., 2010). Here we studied neurotransmitter release from directly activated individual terminals on these neurons and measured their postsynaptic responses simultaneously to compare evoked and spontaneous excitatory (EPSCs) and inhibitory (IPSCs) synaptic currents. Principal findings include: glutamate or GABA release accounted for all single terminal responses, inter-terminal events were similar within subtype, the release of GABA was depressed in control conditions by presynaptic GABAB receptors even without stimulation, and evoked GABA from single terminals inhibited spontaneous release across the neuron.
Methods
Mechanical dissociation from NTS slices
Hindbrains of male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were prepared from 2- to 3-week-old rats for dissociated neurons as described previously (Jin et al., 2004). All of the animal procedures were conducted with the approval of the University Animal Care and Use Committee in accordance with the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals and National Institutes of Health Guide for the Care and Use of Laboratory Animals. The hindbrain was removed and placed in ice-cold artificial CSF (ACSF) composed of the following (in mM): 125 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 10 glucose, and 2 CaCl2, and bubbled medulla was trimmed to a 1 cm block (rostral-caudal) centered on the with 95% O2-5% CO2. The obex. A wedge of tissue was removed from the ventral surface to align the ST and NTS within a single cutting plane (Doyle et al., 2004) when mounted in a vibrating microtome (VT-1000S; Leica, Nussloch, Germany). Slices (150–170 μm thick) were cut with sapphire blades (Delaware Diamond Knives, Wilmington, DE).
The brainstem slices described above were preincubated (1–3 hr at 31°C) in well bubbled ACSF before mechanical dispersion. The brainstem slices were transferred to a recording chamber with a thin cover-glass bottom appropriate for an inverted microscope (see below). The chamber was filled with standard external ACSF containing the following (in mM): 150 NaCl, 5 KCl, 1 HEPES, and 10 glucose (pH was adjusted to 7.4 with Tris-base). A glass MgCl2, 2 CaCl2, 10 pipette was pulled to a fine tip and fire-polished to a final tip size of 100–120 μm (outer diameter). This polished pipette was mounted in a custom-made vibrator held by a micromanipulator (Jin et al., 2004a; Jin et al., 2010). Dispersion focused on portions of NTS medial to the visible ST identified using a stereomicroscope as the region identical to the region studied in previous caudal NTS slice studies (Jin et al., 2004a). The polished tip was lowered to the surface within this sub region and oscillated at 30 Hz horizontally with excursions of 100–300 μm. The pipette tip was moved slowly using the micromanipulator to circumscribe an area of the medial sub nucleus from the ST medial to within 50 μm of the edge of the fourth ventricle. Neurons were dissociated from the top 100 μm from the dorsal surface of the slices. After the removal of the slice, dispersed neurons were allowed to settle and adhered to the bottom of the recording chamber within 20 min.
Electrical measurement and focal stimulation
For voltage-clamp recording, dissociated neurons were visualized using a microscope with infrared illumination and differential interference contrast optics (IR-DIC, TE2000S; Nikon, Tokyo, Japan) with a 60x objective and 10x ocular. Voltage-clamp recordings used an Axon 200A amplifier and pClamp 9 software (Axon Instruments, Molecular Devices, Foster City, CA). Electrical measurements used nystatin-perforated patch recordings (Horn and Marty, 1988) and were performed at a regulated room temperature (22°C). Recording electrodes were filled with a solution composed of the following (in mM): 50 CsCl, 100 Cs-methanesulfonate, and 10 HEPES, unless otherwise stated. The pH was adjusted to 7.2 with Tris–base. The pipette resistance was 3–5 MΩ when filled with this nystatin internal solution. The stock concentration of nystatin was 650 μg/ml. Neurons dispersed in this manner have intact presynaptic boutons as indicated by the presence of spontaneous synaptic events: IPSCs and EPSCs (Jin et al., 2004). Neurons were voltage clamped from −50 to −60 mV, and currents were sampled every 20 μ sec and saved to computer. Data were analyzed off-line using pClamp 9 software and Mini Analysis (Synaptosoft, Decatur, GA).
Glass pipettes for focal stimulation were pulled using similar methods as for the recording pipette except designed to have a lower resistance (1–2 MΩ) when filled with normal ACSF. The focal stimulating pipette was mounted on a micromanipulator (MWO-3, Narishige, Tokyo, Japan). Shocks to single boutons were negative pulses (1–34 V, 0.2–0.3 ms duration) delivered at 0.2–1 Hz and generated by a stimulator (SEN-7023, Nihon Koden, Tokyo, Japan) connected via a stimulus isolation unit (SS-202J, Nihon Koden). To locate active boutons, the focal stimulating pipette was moved so as to scan along the surface of a dissociated neuron. When a stimulating pipette was directly adjacent to a bouton, focal stimulation evoked postsynaptic responses that were synchronized to the focal shocks at invariant latencies assessed as the synaptic jitter (standard deviation of the latency for ≥10 trials).
2.4. Solutions and drugs
Neurons were constantly superfused throughout the experiments. In control conditions, the superfusate was control ACSF and, for pharmacological studies, all drugs were dissolved in ACSF and rapid switched from control. The superfusion delivery system used the Y-tube micro-perfusion configuration (Murase et al., 1989; Shirasaki et al., 1991) in which the external solution surrounding the neuron constantly flowed over the neurons but changes could be completed within 20 ms. Recovery from drug application was facilitated by returning superfusion to control ACSF (Jin et al., 2010). The GABAB receptor specific agonist baclofen and the GABAB receptor specific antagonist CGP 52432 were purchased from Tocris (Bristol, UK). NBQX (nonNMDA glutamate receptor antagonist), AP-5 (NMDA glutamate receptor antagonist), gabazine (SR-95531, GABAA receptor antagonist), tetrodotoxin (300 nM TTX, sodium channel blocker), and strychnine (1 μM, glycine receptor antagonist) were obtained from Sigma-RBI (Natick, MA).
2.5 Statistics
Data were analyzed offline using pClamp (Axon Instruments) and Mini Analysis (version 5.0, Synaptosoft Inc., Decatur, GA). Cumulative distributions of spontaneous synaptic current amplitudes and frequencies were compared using the Kolmogorov-Smirnov (K-S tests) nonparametric analysis. Differences in mean amplitude and frequency were tested by paired or unpaired t-tests or analysis of variance (ANOVA) including repeated mean measures (RM) as appropriate (SigmaStat v3.5, San Jose, CA, USA). In cases in which the data set were not normally distributed, initial comparisons used the nonparametric, Kruskal-Wallis one way ANOVA on Ranks. All data are represented as mean ± SEM and p<0.05 was considered statistically significant.
Results
Focal shocks activate either EPSCs or IPSCs on isolated NTS neurons
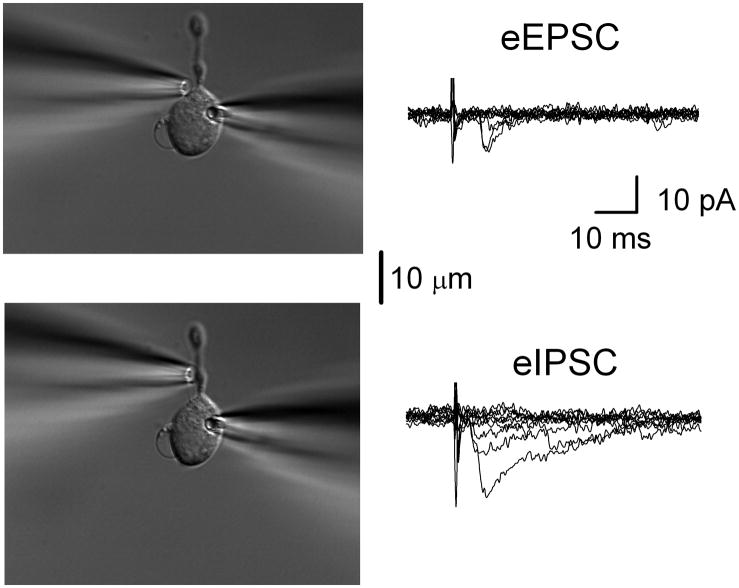
Gentle mechanical dispersion with a vibrating stylus yielded single medial NTS neurons (Figure 1) that retained spontaneously releasing glutamatergic and GABAergic boutons (Jin et al., 2010). To determine the location of single synaptic terminals and their capacity to evoke release, a focal stimulating pipette was moved along the surface of the neuron while periodically delivering focal electrical shocks of high intensity (20–30V). When this surface scan encountered a position at which shocks triggered synaptic responses, the pipette position was finely adjusted to activate the synaptic event at minimum or threshold shock intensity. Successful activation of single terminals was quite spatially limited (±2 μm) suggesting that the pipette needed to be directly over the bouton for successful activation. In practice, small movements away from this optimal site resulted in total loss of the evoked synaptic response even with 5–10 fold threshold shock intensities. Thus, all single-bouton evoked synaptic responses were highly localized with sharp spatial and intensity thresholds that discriminated all-or-none responses. Surface scanning was constrained by the position of the recording electrode which limited access to surface portions of the neurons opposite to the barrel of the recording pipette (Figure 1).
Figure 1.
Spatially discrete terminals generate unitary synaptic responses. Shocks to single boutons in dissociated NTS neurons triggered either unitary EPSCs or IPSCs. In a single representative neuron, scanning the surface of the neuron with the stimulating pipette (left) and delivering single shocks yielded eEPSCs at one position (upper panel) but eIPSCs more distally (lower panel). EPSCs and IPSCs were initially distinguished by their kinetics with IPSCs showing currents roughly 5x longer in duration than EPSC of equivalent amplitudes. Recording pipette is to the right. Traces superimpose the responses to ten consecutive shocks in each case (0.2 Hz, constant shock intensity). Note the frequent failures and the shock to shock variation in response amplitudes despite maximal intensity shocks. The latencies were eIPSC: 2.96 ms, jitter 547 μs; eEPSC: 3.06 ms, jitter 698 μs. Scale bar equals 10 μm.
In testing focal sites of each neuron, event timing was a critical criterion for causally linking shocks with evoked synaptic events (Figure 1). All isolated NTS neurons showed both spontaneous EPSCs and IPSCs. In NTS slices (Jin et al., 2004a), variability of evoked latencies measured as the synaptic jitter from the time of stimulating shock rigorously established the monosynaptic nature of a synaptic connection (Doyle et al., 2004). Those studies relied on a cutoff criterion of 200 μs synaptic jitter (SD latency) which corresponded precisely to anatomically identified second order neurons (Doyle and Andresen, 2001). In isolated NTS neurons, effective focal shocks evoked either an EPSC (eEPSC) or an eIPSC at any given location and we never observed mixed responses – again evidence of spatially restricted specific stimulation (Figure 1). Evoked events repeatedly fell within a narrow time window whereas single events falling outside of this temporal criterion were easily identified as spontaneous since they were not recurring in synchrony with the shocks and had latencies well beyond the distribution range of evoked latencies (see below). Iterative shocks that resulted in no event were considered synaptic failures and were interspersed with successful events of consistent latency (Figure 1). Single neurons displayed as many as 3 unique locales for successfully evoking synaptic events. At many successful synaptic sites, bouton structural specializations were often discernable under IR-DIC. Previously similar structures visible under IR-DIC were also observed and became marked during synaptic membrane turnover using fluorescent, activity-dependent dyes (Jin et al., 2010). The following results summarize successful studies of 28 individual terminals on 20 NTS neurons. Focal shocks activated spatially discrete responses with narrow shock intensity thresholds suggesting that terminals generate unitary synaptic responses.
Boutons releasing either glutamate or GABA on the same cell
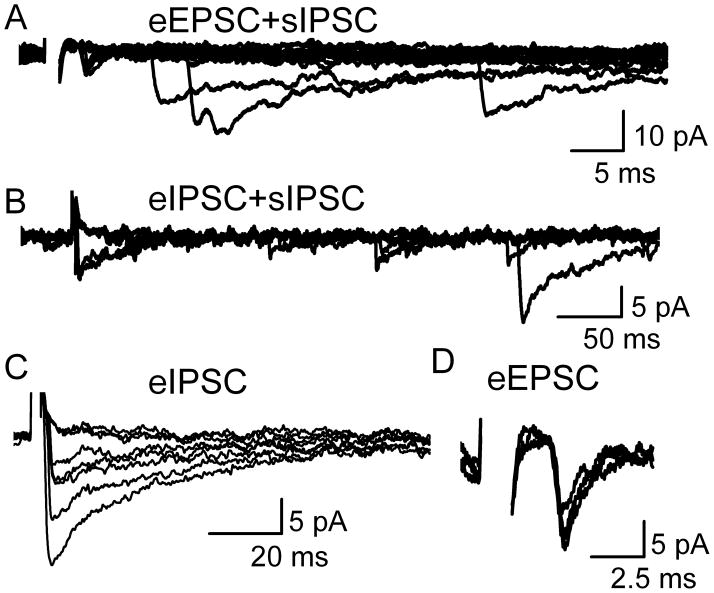
Spontaneous and evoked synaptic events (Figure 2) fell into two kinetically distinct classes, EPSCs and IPSCs. Shocked terminals (Figure 2) that responded with fast rising, slow decaying voltage-clamped current profiles resembled IPSCs as previously observed in NTS neurons in slices (Jin et al., 2004a). Other terminals responded with events with sharp rise times, rapid decays, and often lower amplitudes than the kinetically prolonged events and these resembled EPSCs (Jin et al., 2004a). The nature of these kinetically identified events was confirmed using appropriate receptor-selective antagonists for glutamate and GABA (Figure 3). In the presence of ionotropic glutamate receptor antagonists, 20 μM NBQX and 100 μM AP5, the fast kinetic events were reversibly blocked (evoked and spontaneous) identifying them as glutamatergic (n=3). The slow kinetic events were reversibly blocked by the GABAA receptor specific antagonist gabazine (n=4), identifying them as GABAergic IPSCs (Figure 3).
Figure 2.
Evoked synaptic responses were generally intermixed with spontaneous synaptic events. Shocks to single boutons in this representative dissociated NTS neuron triggered a small evoked EPSC (eEPSC) or larger, slower eIPSCs from different stimulus pipette locations along the neuron surface. A. The short latency eEPSC was accompanied by spontaneous IPSCs (sIPSCs) in 15 successive trials at constant shock intensity (5 s interval). Note evoked failures and inconsistent timing of spontaneous events indicating no synchronization of spontaneous events with bouton shocks. Expanded traces of the eEPSC are shown in D. B. Likewise, eIPSCs were activated at another location and accompanied by sIPSCs. Traces are from same neuron as A but with a different stimulating electrode position (6 successive trials). Expanded time scales show constant latency for eIPSC (C, from same case as B) and characteristically slow decay kinetics in 8 superimposed trials including 3 failures. Expanded traces for successful eEPSC trials (D, same case as A) indicate fast kinetics in three successful events (failures excluded for clarity). At each stimulation site, suprathreshold shock intensity was all-or-none but held constant throughout the displayed samples. All of the events were recorded at same holding potential (VH= −40 mV) in normal ACSF. Note that in our experimental conditions IPSCs were inward at −40 mV and ECl = −27 mV. Latency for the eIPSC: was 3.98 ms and for eEPSC was 5.39 ms. All data collected during 0.2 Hz stimulation. Shock artifacts partially blanked for clarity in all cases.
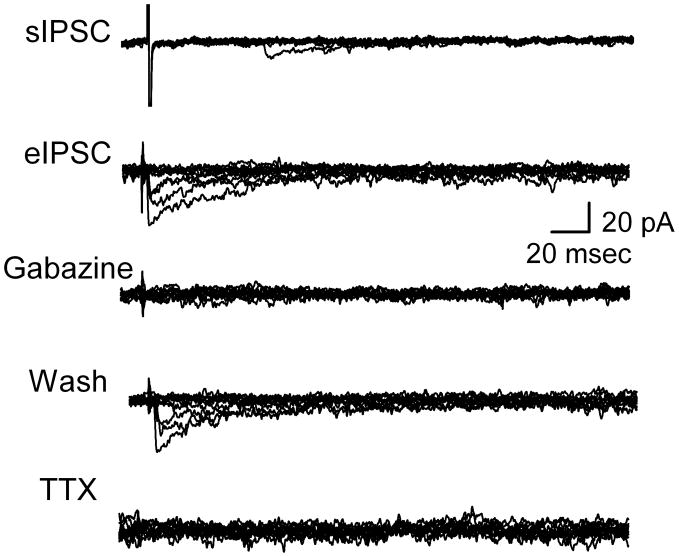
Figure 3.
Slow kinetic eIPSCs were GABAA receptor mediated. In the presence of glutamatergic blockade (20 μM NBQX and 100 μM AP5), sIPSCs were common with subthreshold shocks (artifact marks shock timing, uppermost trace). With constant, suprathreshold shocks, eIPSCs were evoked that were blocked reversibly by either gabazine (3 μM) or TTX (300 nM) and were therefore action potential evoked, GABAA events. Similar protocols for eEPSCs (traces not shown) started with gabazine showing that NBQX blocked the fast kinetic events indicating that they were mediated by non-NMDA receptors. Each panel includes ten trials superimposed. Latency for eIPSC was 2.68 ms. All the traces recorded at VH = −50 mV. All data collected during 0.2 Hz stimulation.
Focally evoked synaptic events require presynaptic Na+ channels
Generation of successful evoked single bouton synaptic events required suprathreshold intensity shocks and, yet even with adequate intensity shocks, responses were intermixed with numerous synaptic failures (Figure 3). This finding for IPSCs was consistent with low release probability (Allen and Stevens, 1994). At these single terminals (Figure 3), eIPSCs were also blocked by the voltage dependent Na+ channel antagonist 300 nM TTX (n=4). TTX block of synaptically evoked IPSCs responses was rapidly reversed (<40 s) by washing in control ACSF (data not shown). These results show that despite the close proximity of the focal electrode to the synaptic bouton, evoking IPSCs required functioning Na+ channels to promote neurotransmitter release. These evoked events had minimally variable latencies that were consistent for each site. The latencies ranged from 1.72 to 5.84 ms (3.23±0.37 ms, n=9). Latency was not different between excitatory or inhibitory transmission (p>0.18). The latencies were surprisingly long given the very limited effective proximity of focal electrodes to “hot spots” along the membrane. Latencies were however distinctly shorter than solitary tract latencies found in slices at similarly cool (room) temperatures (Peters et al., 2010). Synaptic latency jitter values ranged from 160 to 340 μs over ten or more trials – jitter values quite similar to monosynaptic values measured in slices taking into account the cooler recording conditions (Doyle and Andresen, 2001; Peters et al., 2010).
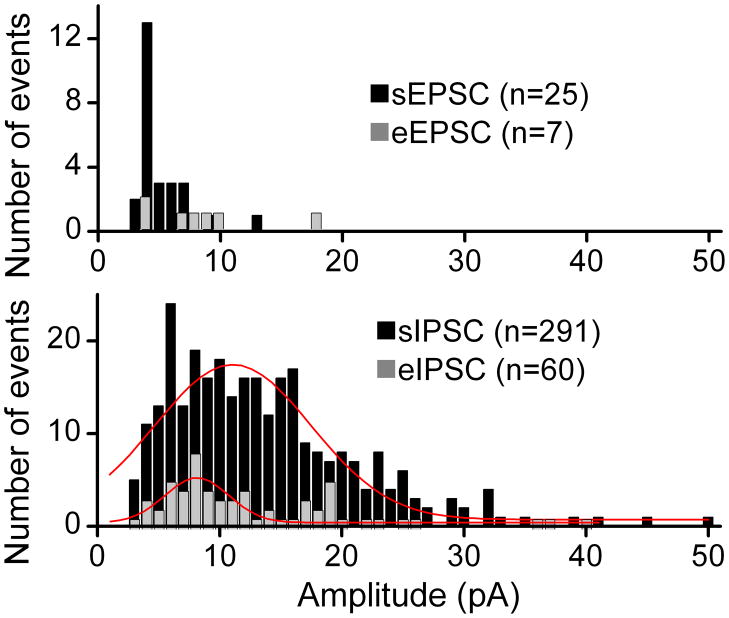
Within neurons, distributions of evoked event amplitudes were similar to distributions for spontaneous events suggesting generalized homogeneity within terminal classes – glutamatergic vs. GABAergic (Figure 4). Histograms of amplitudes of recorded eEPSCs overlapped with the amplitudes of the sEPSCs recorded simultaneously in the same neuron. The distributions showed no signs of multiple peaks and overlap between the evoked and the spontaneous amplitudes (Figure 4). Likewise, by moving the stimulating pipette to a new bouton, eIPSCs could be triggered in the same neuron. Together, these results suggest that bouton-to-bouton differences were minimal within homogenous terminal subclasses (GABA vs. glutamate) and such evidence suggests that both spontaneous and evoked single bouton events were generated from similar quantal release processes.
Figure 4.
Evoked single bouton amplitudes had similar distributions to spontaneous events within a single representative neuron. Histograms compare the amplitudes of recorded eEPSCs from stimulating a single site on this neuron with the amplitudes of sEPSCs recorded in the same neuron. Likewise, by moving the stimulating pipette across the surface of this neuron, an inhibitory site was activated and eIPSC and sIPSC amplitudes recorded. The eIPSC amplitude distribution overlapped that of the sIPSCs. Photomicrographs and original traces from this neuron are displayed in Figure 1. Latency for eIPSC was 2.96 ms and 3.6 ms for eEPSC. All data collected during 0.2 Hz stimulation. Red lines were Gaussian fits intended only to illustrate relative trends in these limited samples. Together the results suggest that evoked and spontaneous events were quite similar within subtypes and amplitudes were consistent with quantal events.
Threshold and single terminal release
Surface scanning established the location of sites at which stimulation evoked synaptic release. Our expectation was that graded stimulation would produce all-or-none responses based on a single biophysical threshold for activation of each terminal or pre-terminal axon. To test whether our focal shocks were activating single release sites or perhaps alternatively whether excitation could spread or was conducted to additional release sites, we applied graded intensity shocks to look for evidence of added release. Intensity profiling of each test site was coupled to surface scans (moving the focal stimulating pipette) to determine the optimum placement of the pipette. The location was fixed in this manner for more detailed studies to the single optimized site which required the minimum shock intensity to evoke a synaptic event. Using sites selected on the basis of minimal intensity shocks, increased shock intensity well above threshold at these optimal locations failed to change synaptic response amplitudes or failure rates (Figure 5). Once threshold intensity was exceeded, successful events were intermixed with numerous failed trials in which shocks evoked no synchronous event. Thresholds were marked by abrupt reductions in failure rates to a steady level of between 30 and 60% failures. Note that substantial increases in shock intensity well above threshold failed to alter the response likelihood at these low frequencies of stimulation. The amplitudes for successfully evoked events fluctuated greatly although the waveforms and mean values did not change when stimulus intensity was increased substantially (Figure 5). Increases in shock intensity did not alter the amplitude distributions or the shapes of the events (p>0.7, n = 4 neurons, Figure 5).
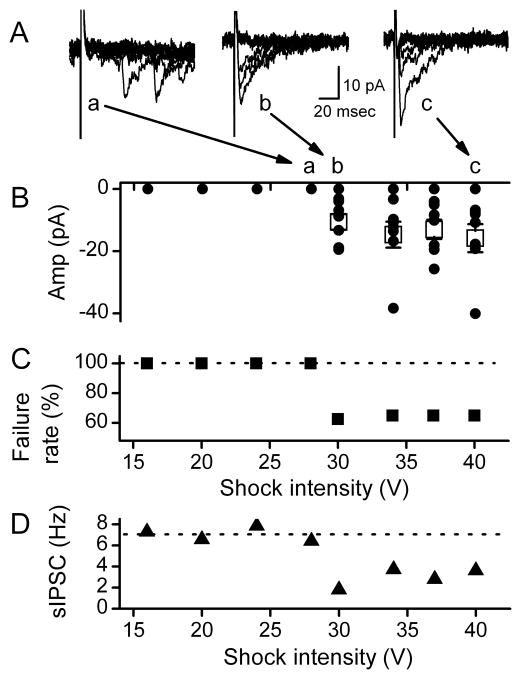
Figure 5.
Evoked responses from single GABA boutons were all-or-none eIPSCs. A. In a representative neuron, shocks of subthreshold intensities (Aa., <20V for this terminal) did not evoke events (B. zero amplitudes and C. 100% failure rate) and only spontaneous events are evident at random times. Once threshold intensity was exceeded (Ab.), both failures and evoked eIPSCs at fixed latency were evident from this single bouton. All data collected at 0.2 Hz stimulation rate. However even at very high intensities (1.5x threshold, Ac), failures and eIPSCs of varying amplitudes prevailed. B. Over 20 trials, eIPSC amplitudes varied within a similar overall average (open squares). C. Likewise, failure rates did not change with intensity. Failure rates even with suprathreshold shocks remained at about 60%. D. Shocks at suprathreshold intensities decreased the rate of sIPSCs with an intensity recruitment profile that paralleled that of the eIPSC (C.) and both were consistent with an all-or-nothing, unitary response-intensity recruitment profile. Event collected across 20 trials. All data were from same representative cell in 100 μM AP5 and 20 μM NBQX at VH −50mV.
Another notable and consistent result was the close coincidence of successful triggering of eIPSCs (Figure 5C) and decreases in the frequency of spontaneous sIPSCs recorded at the same time (Figure 5D). Both the onset of consistent eIPSCs and the decrease in sIPSC rate occurred at a common threshold stimulating intensity. This identity of thresholds for eIPSC successes and sIPSC suppression suggested that focal bouton activation affected at least some of the sIPSC release sites. The lack of changes in these responses (eIPSC and sIPSC) with greatly increased shock intensity (Figure 5C, D) indicates that added intensity failed to access additional terminals on the recorded cell despite the likelihood that depolarizing current spread further from the stimulating pipette tip. We conclude that all-or-none eIPSCs reflect the release properties of single synaptic terminals directly under the stimulus pipette. Both the stimulating intensity profiling and the focal pipette scanning results indicate that each terminal is quite isolated from other terminals.
Sites at which focal shocks evoked EPSCs were less common than eIPSCs (4 of 28 terminals). A shock interval of 5 seconds evoked consistent amplitudes of eEPSCs or eIPSCs at suprathreshold shock intensities. However across neurons with sEPSCs (n=4), these evoked events were difficult to maintain and with 0.2 Hz shock rates, responses fully failed after an average of 7.1 ± 1.0 min. Increases in the stimulation strength failed to restore eEPSCs. This limited period of time together with the limited detection of glutamatergic terminals prevented comprehensive studies of glutamate release. Within this successful period, eEPSC amplitudes varied substantially and were not related to the number of times the terminal had been stimulated and therefore the disappearance of responses likely represented a depletion of the terminal or a long lasting depression of release (Figure 2C).
GABAB receptor antagonism reduces failures of evoked release at single inhibitory terminals
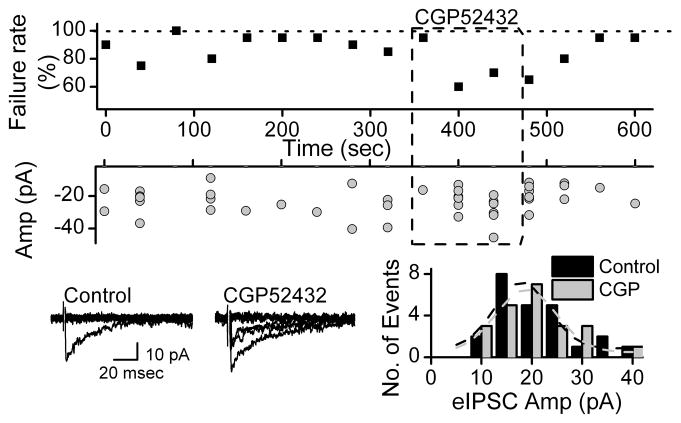
Most stimulating shocks failed to generate synchronous eIPSCs and failure rates were generally 70–80% at 0.5 Hz (Figure 6). Blockade of GABAB receptors substantially improved the success of GABA release and washing rapidly reversed this facilitated release (Figure 6). Although the number of successful events increased during GABAB receptor block, the eIPSC amplitude distribution was similar and overlapping (Figure 6). Thus, presynaptic GABAB receptors limit the likelihood of release at even these low shock frequencies but do not affect the quantal content released during successful activations. Although GABAB receptors importantly modulate GABA release at 0.5 Hz stimulation rates, vesicle depletion might be correlated with depressed release rate. Single synaptic boutons of CNS neurons have limited number of synaptic vesicles therefore repeated release of synaptic vesicles from the same terminal may deplete the readily releasable pool of vesicles. Antagonizing GABAB receptors increased the response rate suggesting that 300 seconds of continuous stimulation at 0.5Hz strongly decreased response rate by the synaptic terminal in control conditions (Figure 6). GABAB receptor antagonist CGP 52432 (5 μM) restored the depressed success rate during stimulation to near control levels from 6.6 ± 2.0 to 22 ± 5.7% without changing amplitudes (n=4). At the same time, CGP 52432 did not affect the amplitude distribution. The mean amplitude before and during CGP 52432 were 24.6±2.0 and 21.6±1.8 pA, respectively. GABAB receptor antagonists decreased the failure rate of eIPSCs (Figure 6).
Figure 6.
GABAB receptor antagonists decrease the failure rate of eIPSCs. The failure rates and amplitude distribution were collected in time bins of 40 sec. The bottom left panel shows examples of original, expanded currents from this experiment before and during a GABAB receptor antagonist 5 μM CGP 52432. The traces superimpose 10 consecutive trials. The bottom right panel shows the distribution of amplitudes for both conditions using bins of 5 pA. The responses were evoked by 0.5 Hz stimulation in the presence of the NBQX (20 μM) and AP5 (100 μM). Latency for the eIPSC was 1.98 ms. All data was taken from same cell (VH −50mV). Shock artifacts partially blanked for clarity in all cases. All data collected during 0.2 Hz stimulation.
Frequency dependent depression of GABA release
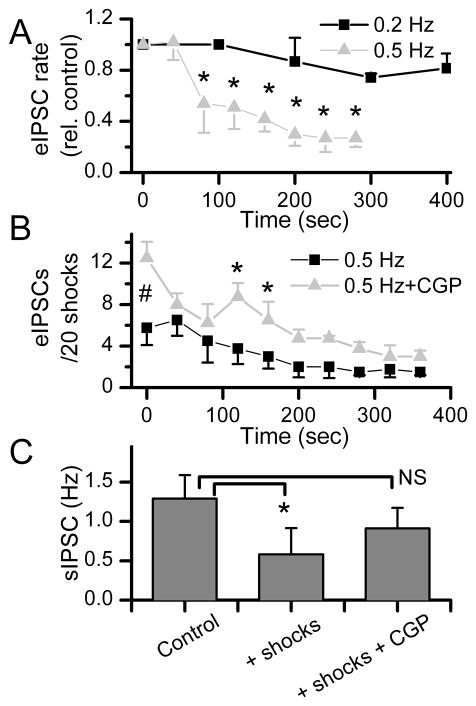
Use dependent changes in synaptic transmission are common in NTS (Miles, 1986) as elsewhere in the CNS (Deisz and Prince, 1989; Brager et al., 2003). Successes in responses to focal activation of single inhibitory terminals at a 5 second intervals (0.2 Hz) did not change over time (p>0.15, n=4, Figure 7A) showing that a low frequency of activation produced reliably consistent rates of GABA release that were well sustained. However when shocks were repeated at shorter intervals (every 2 seconds), the relative success rate for eIPSCs decreased substantially within 1–3 min (Figure 7A) – evidence of single terminal frequency dependent depression of transmission. At 0.5 Hz stimulation, the average eIPSC success rate rapidly decreased as a result of increased failures of shocks to evoke IPSCs (n=4 neurons). In the control condition, the success rate for evoked IPSCs averaged ~25% of trials and then declined to ~10% of trials after 3 min of stimulation (Figure 7B).
Figure 7.
The response rate to repeated shocks triggered substantial, frequency dependent depression of successful eIPSCs from single terminals as well as spontaneous IPSCs out of synchrony with the shocks. A. On average, GABA releasing boutons shocked at 0.2 Hz (n = 4, A) had relative success rates for triggering IPSCs that did not change over many minutes of stimulation (black squares). However, increasing the bouton activation rate of these terminals to 0.5 Hz markedly decreased the eIPSC relative response rate (n = 4). The number of responses initially were 8.5 (n=4) and 5.8 (n=4) at 0.2 and 0.5 Hz, respectively. B. At 0.5 Hz stimulation, the number of eIPSC responses declined in control conditions with repeated trials (200 trials total, n=4 neurons). Application of the GABAB antagonist CGP 52432 (5 μM) substantially increased the initial success rate for eIPSCs at Time 0 (#, p<0.0003). Continued stimulation at 0.5 Hz resulted in a decline in successful eIPSCs (*p<0.04). C. Activation of single GABAergic boutons substantially decreased the frequency of sIPSCs recorded during sustained stimulation (*p<0.05, n=5 neurons). Application of CGP 52432 increased sIPSC rates well above control levels between 200 and 300 sec of stimulation. Thus, despite continued bouton stimulation, CGP eliminated a GABAB mediated suppression of spontaneous GABA release. All points are means±SEM. Differences tested with RM-ANOVA.
To test whether autoreceptor feedback contributed to this frequency dependent depression of evoked GABA release, we applied the GABAB receptor antagonist CGP 52432 (5 μM) and repeated the 0.5 Hz stimulation. Interestingly, CGP 52432 immediately and substantially increased (Time 0, p<0.0003) the success rate (Figure 7B). However with continued stimulation, the response eIPSC success rate fell to near control levels after 200 s of stimulation (100 shocks). This temporal relationship suggests that the release from “resting” terminals (pre-stimulus) was strongly restrained by active GABAB receptors in a time period of no synaptic activation of that terminal. Release was generally more successful during GABAB receptor blockade (Figure 6B, p<0.04, RM ANOVA) but then declined to very low (~10%) success rates similar to control. In all cases of frequency dependent depression, eIPSC amplitudes were unaltered even at times of maximally depressed success rates (p>0.27, results not shown).
The readiness for synchronous release in these neurons is under GABAB receptor influence at single terminals. However in addition to the synchronous eIPSCs, all neurons showed evidence of stochastic or spontaneous GABA release (Figure 2A). This sIPSC activity decreased substantially during focal activation of single GABAergic boutons (Figure 6C). Overall, sIPSC frequency averaged a >50% reduction after 3 min of stimulation (p<0.017, n=5 neurons). In these same neurons, CGP 52432 blocked this depression of sIPSC rates by focal bouton stimulation (Figure 7C). The amplitudes of sIPSC were unaltered even at times of maximally depressed eIPSC success rates (p>0.15, results not shown). The shock intensity response profile for evoking eIPSCs precisely predicted shock-induced depression of the rate of sIPSCs. Together, the findings indicated that presynaptic GABAB receptors were responsible for the depression of both directly evoked release (eIPSCs) as well as spontaneous GABA release onto these cells.
Discussion
Diverse architectures of nerve terminals support neurotransmission across CNS neurons, but aside from highly specialized super-terminals such as the Calyx of Held (Borst and Sakmann, 1996), most single axons terminate in more modest contacts ranging from single contacts up to clustered structures containing multi-bouton contact arrays (Rollenhagen and Lubke, 2006; Kochubey et al., 2011). In all cases, the fundamental building block of transmission is the active zone within each terminal. Frequency dependent depression is common in NTS (Miles, 1986) as elsewhere in the CNS (Deisz and Prince, 1989; Brager et al., 2003) but has largely been studied using aggregate measures averaged across terminals. For many parts of the nervous system, only compound events are available for study. In the present work, we have taken advantage of the terminals retained on isolated NTS neuron cell bodies using gentle mechanical dispersion with a vibrating stylus but in the absence of enzymes (Jin et al., 2010). Direct activation of terminals on these neurons yielded the following principal findings: all single terminal responses required intact Na+ channels for effective excitation-release coupling, very high intensities failed to activate the underlying neuron or recruit added events beyond the unitary, low threshold EPSC or IPSC, inter-terminal events were similar within subtype (glutamate or GABA), activated GABAB receptors depressed the release of GABA even in the absence of terminal activation, and release of GABA from single terminals suppressed spontaneous GABA release. Together these findings suggest that the two major amino acid neurotransmitters (glutamate and GABA) rely on relatively low release probability processes at single terminals and importantly contrast to transmission performance by terminal processes arising from single axons in caudal NTS slices.
High failure rates at single terminals
The terminals retained on these dispersed NTS neurons exhibit vesicle turnover during specific pharmacological or ionic challenges that is similar across all boutons (Jin et al., 2010). In our present studies, we used direct stimulation of terminals to evoke release and monitored events postsynaptically. This approach offers the advantage of a millisecond time scale resolution that is much closer to the release/transmission time course. Electrical synaptic responses were also far more consistent and reproducible in NTS neurons than the anomalies observed using conventional high potassium methods and dye imaging of vesicle turnover (Jin et al., 2010). For single bouton activation, a very consistent observation for evoked responses at all terminals on these NTS neurons was the high proportion of synaptic failures even under basal conditions. These failures occurred despite lengthy pauses without stimulation or under conditions that increased release probability by elevating external calcium concentration (2 mM) (Bailey et al., 2006). Calcium sensitivity varies across transmitters and maximal release of inhibitory transmitter requires much higher concentrations (Balakrishnan et al., 2009) than found for glutamate at NTS neurons (Bailey et al., 2006). Most terminals in our studies failed to evoke synchronized synaptic events to 70–80% of shocks using very low shock repetition rates (<0.01 Hz). Such shocks were not successful in TTX as reported in other, non-cultured neurons (Akaike et al., 2002). This presumably reflects the necessity to generate an action potential for success and also likely contributes to the latency to release (1–3 ms) at these room temperatures. The present experiments were performed at room temperature and evoked release in NTS slices substantially increases latency and jitter (Peters et al., 2010) but not failures and has very little effect on sIPSCs (Shoudai et al., 2010). Given that the stimulating pipettes were close to but not in intimate contact with the terminal membranes, it is most likely that Na+ channels represented the most voltage-sensitive target detected by our intensity profiling and selection process. Even when we raised the stimulus intensity quite substantially, we never detected the sort of near zero latency responses encountered by others (Fedulova et al., 1999). This finding suggests that our shock effects were all-or-none and even intense currents failed to spread to additional activation sites beyond the initial, most sensitive site. Combined with the high sensitivity to movements away from the optimal location, the results suggest that the stimuli were highly localized and spatially damped likely by shunting.
Synaptic transmission at mechanically dispersed neurons
The present experiments focused on dissociated neurons and offered unique and direct access to these terminals but under conditions that were clearly not physiological. Among the potential qualifiers for our experiments was room temperature which would be expected to slow many important biological processes. In NTS slices, cooling substantially increased latency and jitter of evoked EPSCs (Peters et al., 2010) but not their failures and had remarkably little effect on sIPSCs (Shoudai et al., 2010). Our calcium concentration was nearly twice normal which had the pragmatic experimental benefit of increasing the probability of release. However, lower temperature and potential physical disruption of the relationship between these neurons and glia during dissociation may importantly impair GABA uptake and this could contribute to the observed activation of GABAB receptors. The absence of the GABA synthesizing neurons and their activity should remove any activity-driven GABA release as well. The net contributions of each of these potentially confounding factors are difficult to know with confidence. Overall, GABAB receptors acted to constrain GABA release probability in these isolated neurons: both in the unstimulated state as well as in the early stages of sustained activation during which success rate for eIPSCs becomes progressively depressed. Ultimately however, GABAB receptor blockade was not restorative after prolonged stimulation suggesting that other processes dominate and this might reflect depletion of readily releasable vesicles.
Low probability of release
Using FM dyes to follow the fate of indicator dye and incorporated vesicles at NTS neurons, we previously found that overall vesicle turnover was not distinctly different across terminals (Jin et al., 2010). However, this optical technique of tracking synaptic release from single terminals is quite indirect compared with our current electrophysiological approach. High failure rates together with high amplitude variability were consistent with relatively low release probabilities (Silver, 2003). Such results contrast substantially with slice studies of inhibitory transmission to neurons from medial NTS region, the source region for the dissociated neurons of the present study. In medial NTS, afferent glutamate transmission is extraordinarily reliable with failure rates approaching zero in all neurons (Andresen and Peters, 2008; McDougall et al., 2009). GABAergic transmission is activated polysynaptically from the ST to neurons in this same medial region of NTS, but also has surprisingly low (<5%) failure rates (Doyle and Andresen, 2001). Thus, a minimal polysynaptic path would consist of ST activating a GABAergic neuron that then synapses on the recorded neuron. The single GABA terminals of the present study likely belong to the final segment of this pathway. Thus although the initial ST step rarely fails to generate an action potential (Bailey et al., 2007; Appleyard et al., 2007), it is harder to understand how the next stage – a highly failing GABA release – could generate such consistent IPSCs to short bursts of ST shocks. One possible explanation to reconcile these apparently disparate results is suggested in recruitment studies in CA1 pyramidal cells in which the recruitment of multiple inputs reaches 100% (i.e. reliable) transmission by activating multiple axons that were quantal and individually unreliable (30–40% successful) (Allen and Stevens, 1994). Thus, in this view, reliable inhibitory synaptic transmission might result from redundant, multiple synaptic contacts that collectively convey reliable IPSCs despite a unitary intermittent release process at each single terminal. Our present results are the first to show that GABA transmission in NTS is intrinsically unreliable. This finding needs to be compared with activation of axons that generate direct monosynaptic IPSCs in intact brain slices and coupled with approaches such as variance-mean analysis to determine the number of release sites typically arising from single inhibitory axons. Presumably in the present study, presynaptic activity is minimal but our results suggest that GABAB receptors nonetheless actively maintain a brake on GABA release. Future work will be needed to determine whether this GABAB receptor activation represents constitutive activity or persistent ambient local GABA, but certainly in vivo, activity dependent GABA release will only serve to further restrain and enhance the GABAB receptor mechanism.
Highlights.
Activation of single terminals on solitary tract neurons was spatially distinct with sharp fixed, all-or-none thresholds:
Yielded either glutamate or GABA release only with GABA terminals more common
Inter-terminal events were homogeneous within subtype
GABA release was low in probability but depressed in control by presynaptic GABAB receptors even without stimulation
Evoked GABA from single terminals inhibited spontaneous release across the neuron
Acknowledgments
This work was supported by grants from the National Institutes of Health, HL-41119 (MCA) and HL-105703 (MCA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Akaike N, Murakami N, Katsurabayashi S, Jin Y-H, Imazawa T. Focal stimulation of single GABAergic presynaptic boutons on the rat hippocampal neuron. Neurosci Res. 2002;42:187–195. doi: 10.1016/s0168-0102(01)00320-0. [DOI] [PubMed] [Google Scholar]
- Allen C, Stevens CF. An evaluation of causes for unreliability of synaptic transmission. Proc Natl Acad Sci U S A. 1994;91:10380–10383. doi: 10.1073/pnas.91.22.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC, Peters JH. Comparison of baroreceptive to other afferent synaptic transmission to the solitary tract nucleus. Am J Physiol Heart Circ Physiol. 2008;295:H2032–H2042. doi: 10.1152/ajpheart.00568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci. 2007;27:13292–13302. doi: 10.1523/JNEUROSCI.3502-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancio O, Korn H, Gulyas A, Freund T, Miles R. Excitatory synaptic connections onto rat hippocampal inhibitory cells may involve a single transmitter release site. J Physiol. 1994;481:395–405. doi: 10.1113/jphysiol.1994.sp020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Aicher SA, Andresen MC. Target-specific, dynamic pathway tuning by A-type potassium channels in solitary tract nucleus: cranial visceral afferent pathways to caudal ventrolateral medulla or paraventricular hypothalamus. J Physiol. 2007;582:613–628. doi: 10.1113/jphysiol.2007.132365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Jin Y-H, Doyle MW, Smith SM, Andresen MC. Vasopressin inhibits glutamate release via two distinct modes in the brainstem. J Neurosci. 2006;26:6131–6142. doi: 10.1523/JNEUROSCI.5176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan V, Kuo SP, Roberts PD, Trussell LO. Slow glycinergic transmission mediated by transmitter pooling. Nat Neurosci. 2009;12:286–294. doi: 10.1038/nn.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Brager DH, Luther PW, Erdelyi F, Szabo G, Alger BE. Regulation of exocytosis from single visualized GABAergic boutons in hippocampal slices. J Neurosci. 2003;23:10475–10486. doi: 10.1523/JNEUROSCI.23-33-10475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz RA, Prince DA. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic transmission in brain stem neurons in vitro. J Neurophysiol. 2001;85:2213–2223. doi: 10.1152/jn.2001.85.5.2213. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin Y-H, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular identification in nucleus tractus solitarius slices. J Neurosci Methods. 2004;37:37–48. doi: 10.1016/j.jneumeth.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Fedulova SA, Vasilyev DV, Isaeva EV, Romanyuk SG, Veselovsky NS. Possibility of multiquantal transmission at single inhibitory synapse in cultured rat hippocampal neurons. Neurosci. 1999;92:1217–1230. doi: 10.1016/s0306-4522(99)00084-6. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Miles R, Sik A, Toth K, Tamamaki N, Freund TF. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993;366:683–687. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- Jin YH, Cahill EA, Fernandes LG, Wang X, Chen W, Smith SM, Andresen MC. Optical tracking of phenotypically diverse individual synapses on solitary tract nucleus neurons. Brain Res. 2010;1312:54–66. doi: 10.1016/j.brainres.2009.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y-H, Bailey TW, Andresen MC. Cranial afferent glutamate heterosynaptically modulates GABA release onto second order neurons via distinctly segregated mGluRs. J Neurosci. 2004a;24:9332–9340. doi: 10.1523/JNEUROSCI.1991-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y-H, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals. J Neurosci. 2004b;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. Quantal mechanism of neural transmitter release. Science. 1971;173:123–126. doi: 10.1126/science.173.3992.123. [DOI] [PubMed] [Google Scholar]
- Kochubey O, Lou X, Schneggenburger R. Regulation of transmitter release by Ca(2+) and synaptotagmin: insights from a large CNS synapse. Trends Neurosci. 2011 doi: 10.1016/j.tins.2011.02.006. Ref Type: In Press. [DOI] [PubMed] [Google Scholar]
- McDougall SJ, Peters JH, Andresen MC. Convergence of cranial visceral afferents within the solitary tract nucleus. J Neurosci. 2009;29:12886–12895. doi: 10.1523/JNEUROSCI.3491-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R. Frequency dependence of synaptic transmission in nucleus of the solitary tract in vitro. J Neurophysiol. 1986;55:1076–1090. doi: 10.1152/jn.1986.55.5.1076. [DOI] [PubMed] [Google Scholar]
- Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989;103:56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- Nauen DW. Methods of measuring activity at individual synapses: A review of techniques and the findings they have made possible. J Neurosci Methods. 2011;194:195–205. doi: 10.1016/j.jneumeth.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron. 2010;65:657–669. doi: 10.1016/j.neuron.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci. 2008;28:11731–11740. doi: 10.1523/JNEUROSCI.3419-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollenhagen A, Lubke JH. The morphology of excitatory central synapses: from structure to function. Cell Tissue Res. 2006 doi: 10.1007/s00441-006-0288-z. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Presynaptic calcium and control of vesicle fusion. Curr Opin Neurobiol. 2005;15:266–274. doi: 10.1016/j.conb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Shirasaki T, Klee MR, Nakaye T, Akaike N. Differential blockade of bicuculline and strychnine on GABA- and glycine-induced responses in dissociated rat hippocampal pyramidal cells. Brain Res. 1991;561:77–83. doi: 10.1016/0006-8993(91)90751-g. [DOI] [PubMed] [Google Scholar]
- Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J Neurosci. 2010;30:14470–14475. doi: 10.1523/JNEUROSCI.2557-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RA. Estimation of nonuniform quantal parameters with multiple-probability fluctuation analysis: theory, application and limitations. J Neurosci Methods. 2003;130:127–141. doi: 10.1016/j.jneumeth.2003.09.030. [DOI] [PubMed] [Google Scholar]