Summary
Cytochrome bd is a respiratory quinol:O2 oxidoreductase found in many prokaryotes, including a number of pathogens. The main bioenergetic function of the enzyme is the production of a proton motive force by the vectorial charge transfer of protons. The sequences of cytochromes bd are not homologous to those of the other respiratory oxygen reductases, i.e., the heme-copper oxygen reductases or alternative oxidases (AOX). Generally, cytochromes bd are noteworthy for their high affinity for O2 and resistance to inhibition by cyanide. In E. coli, for example, cytochrome bd (specifically, cytochrome bd-I) is expressed under O2-limited conditions. Among the members of the bd-family are the so-called cyanide-insensitive quinol oxidases (CIO) which often have a low content of the eponymous heme d but, instead, have heme b in place of heme d in at least a majority of the enzyme population. However, at this point, no sequence motif has been identified to distinguish cytochrome bd (with a stoichiometric complement of heme d) from an enzyme designated as CIO. Members of the bd-family can be subdivided into those which contain either a long or a short hydrophilic connection between transmembrane helices 6 and 7 in subunit I, designated as the Q-loop. However, it is not clear whether there is a functional consequence of this difference. This review summarizes current knowledge on the physiological functions, genetics, structural and catalytic properties of cytochromes bd. Included in this review are descriptions of the intermediates of the catalytic cycle, the proposed site for the reduction of O2, evidence for a proton channel connecting this active site to the bacterial cytoplasm, and the molecular mechanism by which a membrane potential is generated.
Keywords: metabolism, molecular bioenergetics, oxidoreduction, bacterial physiology, microbe, disease
1. Diversity of respiratory oxygen reductases
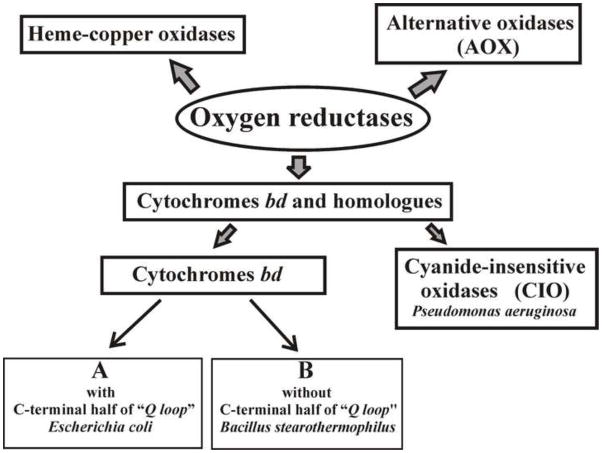
Respiratory oxygen reductases (terminal oxidases) are enzymes at the end of the respiratory chains of organisms which couple the oxidation of a respiratory substrate (one-electron donor, cytochrome c, or two-electron donor, quinol (QH2)) to the four-electron reduction of O2 to water. There are three families of oxygen reductases (Fig. 1).
Fig. 1.
Respiratory oxygen reductases. The bd-family is subdivided into the A-subfamily (long Q-loop), B-subfamily (short Q-loop) and the cyanide insensitive oxygen reductases (CIO). These are subdivisions based entirely on spectroscopic and structural observations and are not phylogentically defined clades.
1.1 Heme-copper family
The first, most extensively studied family comprises the heme-copper oxygen reductases. They have a binuclear O2-reduction site composed of a high spin heme (a3, o3, or b3) and a copper ion (CuB), and these enzymes generate a PMF via a “proton pump” mechanism [1–7]. The PMF is utilized for various biosynthetic activities (e.g., ATP production), solute active transport and mechanical movement (e.g., flagellar rotation). The heme-copper family of oxygen reductases includes both cytochrome c oxidases and quinol oxidases. Most of the heme-copper oxygen reductases are members of one of three distinct subfamilies: A, B, and C [8,9]. The A subfamily includes the mitochondrial cytochrome c oxidases as well as many prokaryotic cytochrome c oxidases and quinol oxidases. Enzymes in the A-subfamily utilize at least two proton pathways to deliver protons to the active site or for proton pumping. The B subfamily includes a number of oxygen reductases from extremophilic prokaryotes, such as the ba3-type oxygen reductase from T. thermophilus [10]. The enzymes of the C subfamily are all cbb3-type oxidases [11]. Recently, it has been shown that the enzymes from the B and C subfamilies utilize only one proton-conducting input pathway [10,12]. High-resolution x-ray crystal structures of the heme-copper oxidases from all three subfamilies have been reported [11,13–23].
1.2 Alternative oxidase (AOX) family
The second family of respiratory oxygen reductases comprises cyanide-resistant AOX found in mitochondria of higher plants, fungi and protists as well as in prokaryotes and some animal species [24]. In plants, this is a homodimeric enzyme associated with the matrix side of the inner mitochondrial membrane. AOX uses UQH2, but not cytochrome c, as the electron donor, and contains a non-heme di-iron carboxylate active site for O2 reduction.
AOX does not produce a PMF, and is not coupled to transmembrane charge transfer. However, AOX is responsible for heat generation in some tissues, and plays a role in the regulation of energy metabolism, facilitating turnover of the TCA cycle, protection against oxidative stress, and homeostasis. To date, no high-resolution AOX structure has been reported, but crystals that diffract to better than 3.0 Å have been described [25].
1.3 Cytochrome bd-family
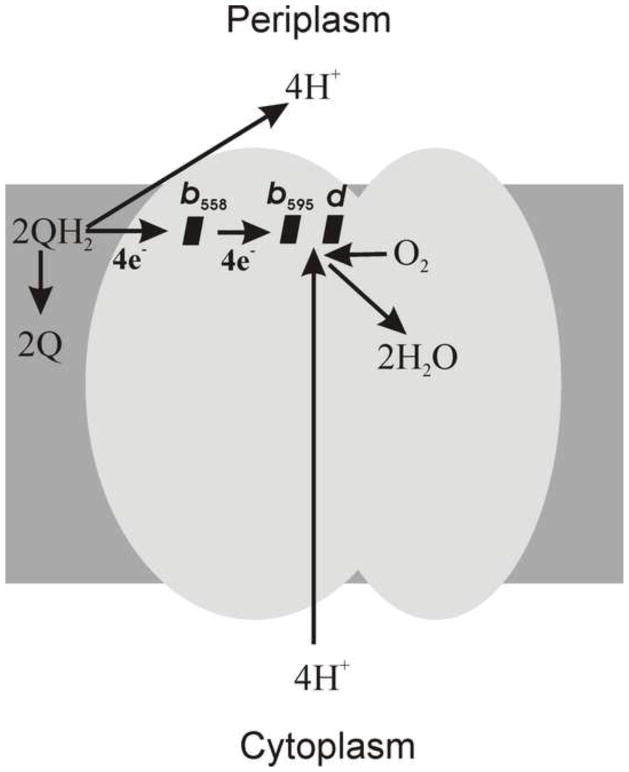
The third family of oxygen reductases comprises cytochromes bd. These are quinol oxidases found in a wide variety of prokaryotes. They show no sequence homology to any subunit of heme–copper family members or AOX and do not contain any copper or non-heme iron [26–33]. This two-subunit integral membrane protein (subunits I and II) contains three hemes, b558, b595 and d, and it is generally thought that hemes b595 and d form a di-heme site for the reduction of O2 (Fig. 2) [34–43]. Unfortunately, no X-ray structure of any bd-type oxygen reductase has been reported. Cytochrome bd generates a PMF by transmembrane charge separation, but does so without being a “proton pump” [41,44–50]. In a number of organisms, the bd oxygen reductase is induced under O2-limited conditions as well as under other growth conditions that can be considered stressful, such as Fe deficiency [51–54]. All known members of the bd-family of oxygen reductases are quinol oxidases, most commonly using ubiquinol or menaquinol as substrates.
Fig. 2.
Proposed cytochrome bd model.
Analysis of prokaryotic genomes shows that many aerobic prokaryotes do not contain any member of the bd-family, but contain only heme-copper oxygen reductases. However, there are a number of prokaryotes that encode more than one bd-family member, for example, two: E. coli [53,55], Bacillus subtilis [56]; three: Vibrio cholerae [57]; and as many as six bd-type oxygen reductases: some Acidithiobacillus strains. Organisms that express one or more bd-type oxygen reductases, usually also possess at least one heme-copper oxygen reductase. However in some cases (e.g., Lactobacillus plantarum [58], Zymomonas mobilis [59], the two Thermoplasma strains [60]) cytochrome bd is the only oxygen reductase.
1.3.1 The Q-loop
The hydrophilic region of subunit I connecting transmembrane helices 6 and 7, facing the outside of the prokaryotic cell, has been implicated as part of the quinol binding site [61–66], and this is referred to as the “Q loop”. Some of the bd-family oxygen reductases have an insert in the C-terminal portion of the Q-loop and, hence, have a “long Q-loop”, e.g., enzymes isolated from Escherichia coli and Azotobacter vinelandii [67,68]. The majority of bd-type oxygen reductases have a “short Q-loop”, e.g., the enzyme isolated from Bacillus stearothermophilus [67–69]. It is not clear what the functional consequences are, if any, from this difference in the size of the Q loop.
1.3.2 Cyanide insensitive oxidases (CIO)
An anecdotal observation is that some of the “short Q-loop” oxygen reductases appear to have an altered heme content, in which the amount of heme d is significantly reduced (or totally missing) and is replaced by a heme b. This appears to be the case for a B. subtilis cytochrome bd [70]. When these enzymes, with a low content of heme d, have been characterized in bacterial membranes, respiration continues even in the presence of 1 mM KCN [71], but the membranes do not have the spectroscopic signature of heme d (a peak in the reduced form near 630 nm) [71–75]. As a result, these enzymes have been called cyanide insensitive oxidases (CIO) [73]. Examples are P. aeruginosa [71–73,76], P. putida [77], P. pseudoalcaligenes [74], Staphylococcus carnosus [78], C. jejuni [75], Z. mobilis [59]. On the contrary, using low temperature absorption spectroscopy, EPR and mass spectrometry, Mogi et al. [79] reported that CIO in the membranes from G. oxydans has the same heme contents present in a classical cytochrome bd, although reveals unique spectroscopic and ligand-binding properties. Whether the CIO heme composition is strain- and/or growth-specific, or the heme spectral features were not detected due to a very low enzyme concentration in the tested membranes remains to be studied. It is now clear that CIOs are bd-family oxygen reductases.
cioA and cioB genes which encode CIO in P. aeruginosa and P. pseudoalcaligenes were sequenced [73,74]. They comprise the cio operon. CioA and CioB are homologous to subunits I and II of cytochrome bd-I from E. coli and the bd-oxidase from A. vinelandii [73]. Histidine and methionine residues identified in cytochrome bd-I from E. coli as the axial ligands to heme b558 and heme b595 are conserved [73]. It was proposed that the slight differences in sequence and structure of the CydB subunit are responsible for cyanide resistance [78]. It is of interest to note that cytochrome bd of the cyanobacterium Synechocystis sp. PCC 6803 appeared to be structurally related to CIO [80]. To date, no CIO has been purified and characterized, primarily because these enzymes appear to be particularly labile. At low O2 tensions, the opportunistic pathogen P. aeruginosa synthesizes HCN as a metabolic product at concentrations of up to 0.3 mM [81]. Under these conditions, the heme-copper oxidases are inhibited. CIO likely has a role in allowing aerobic respiration under cyanogenic and microaerobic growth conditions [71,73,82]. Cyanide can be found in tissues infected with P. aeruginosa [83] that is consistent with the conclusion that CIO is required for full pathogenicity of P. aeruginosa in the cyanide-mediated paralytic killing of nematodes [84]. Mutation or overexpression of the cioAB genes of P. aeruginosa leads to temperature sensitivity for growth, difficulty exiting stationary phase, abnormal cell division and multiple antibiotic sensitivity [85].
There is no distinguishing feature in the sequences of the genes that allows one to differentiate CIO from other cytochrome bd family members. It is not yet clear whether the “short Q-loop” is a requirement for having the CIO phenotype or under what conditions such enzymes may or may not contain a stoichiometric content of heme d.
2. Physiological functions
The bioenergetic function of cytochrome bd is to conserve energy in the form of ΔμH+ [41,45–50], although the H+/e− ratio is 1, half the value of the A-subfamily heme-copper oxygen reductases such as the mitochondrial cytochrome c oxidase or cytochrome bo3 from E. coli because the bd-type oxygen reductases do not pump protons [45,49,50].
Apart from PMF generation, cytochrome bd endows bacteria with a number of vitally important physiological functions. Cytochrome bd facilitates both pathogenic and commensal bacteria to colonize O2-poor environments [86–89], serves as an O2 scavenger to inhibit degradation of O2-sensitive enzymes such as nitrogenase [90–98], and support anaerobic photosynthetic growth [99]. It is of interest to note that bd-type oxygen reductases predominate in the respiratory chains of bacteria that cause such diseases as bacillary dysentery [100], brucellosis [88,101], tuberculosis [87], pneumonia, life-threatening sepsis, meningitis [102], as well as Salmonella [103,104], Bacteroides [86], and Listeria monocytogenes [105] infections. There is a positive correlation between virulence of bacterial pathogens responsible for these diseases and level of cytochrome bd expression. Cytochrome bd enhances bacterial tolerance to nitrosative stress [106–111], contributes to mechanisms of detoxification of hydrogen peroxide in E.coli [112–114], suppresses extracellular superoxide production in Enterococcus faecalis [115], and is involved in the degradation of aromatic compounds in Geobacter metallireducens [116]. The A. vinelandii cytochrome bd might be directly involved in energizing Fe-siderophore transport or in reduction of Fe(III)-chelates and, thus, metal liberation in the cytoplasm [117]. As a source of oxidizing power, cytochrome bd-I in E. coli can support disulfide bond formation upon protein folding catalyzed by the DsbA-DsbB system [118], as well as the penultimate step of heme biosynthesis, the conversion of protoporphyrinogen IX into protoporphyrin IX, catalyzed by protoporphyrinogen IX oxidase [119].
The expression and membrane content of cytochrome bd in E. coli increase not only at low O2 concentrations [120–122], but also under other stressful conditions, such as alkalization of the medium [123], high temperature [124,125], the presence of poisons in the environment (for instance, cyanide [126,127]), uncouplers-protonophores [123,128,129] and high hydrostatic pressure [130,131]. E. coli mutants defective in cytochrome bd are sensitive to H2O2 [125], zinc [127,132] and a self-produced extracellular factor that inhibits bacterial growth [133,134]. E. coli mutants that cannot synthesize cytochrome bd are also unable to exit from the stationary phase and resume aerobic growth at 37 °C [135,136].
Since cytochrome bd is found only in prokaryotes, including a number of human pathogens, the enzyme may be of interest as a drug target. A search for specific inhibitors of the bd-type oxygen reductases, which could be used in clinical practice, has been started [137,138]. An alternative, “positive” potential use of cytochrome bd might be for a therapy of respiratory chain deficiencies. It is known that mutations in genes encoding structural subunits of cytochrome bc1 complex and cytochrome c oxidase can lead to severe neuromuscular and non-neuromuscular human diseases [139,140]. At the same time, it was reported that mixing purified cytochrome bd-I from E. coli with myxothiazol-inhibited bovine heart submitochondrial particles restores up to half of the original NADH oxidase and succinate oxidase activities in the absence of exogenous ubiquinone analogs [141]. Respiration bypassing the bc1 complex is saturated at amounts of added bd-oxidase similar to that of other natural respiratory components in submitochondrial particles. Bacterial cytochrome bd-I tightly binds to the mitochondrial membrane and functions as an intrinsic component of the chimeric respiratory chain [141]. Thus, cytochrome bd, as well as AOX [142–144], might compensate for respiratory chain deficiencies in human cells.
3. Inhibitors
Table 1 shows the effect of different inhibitors on the respiratory activity of cytochrome bd from some bacteria. Quinol oxidase inhibitors can be divided into two groups: Q-like compounds acting at the Q binding site and heme ligands (e.g., cyanide, azide or NO) acting at the O2 binding/reducing site. A specific feature of cytochrome bd is that it is much less sensitive to cyanide and azide than a heme-copper oxygen reductase like cytochrome bo3 [27]. The lower sensitivity of cytochrome bd to anionic heme ligands may be a result of an elevated electron density on the central ion of iron due to breaking the conjugate π-electron structure in the d-type porphyrin ring and/or may point to a more hydrophobic environment of the O2-reducing site. It was reported that cytochrome bd-I in E. coli is a bacterial membrane target for a cationic cyclic decapeptide gramicidin S (IC50 ~5.3 μM, Table 1), although it has been generally accepted that the main target of gramicidin S is the membrane lipid bilayer rather than the protein components [145]. This finding can provide a new insight into the molecular design and development of novel gramicidin S-based antibiotics. The effect of gramicidin S on cytochrome bd-I and some other membrane-bound proteins could be the alteration of the protein structure through binding to its hydrophobic protein surface [145].
Table 1.
Effect of inhibitors on respiratory activity of cytochrome bd
| Inhibitor | Bacterium
|
|||
|---|---|---|---|---|
| E. coli(a) | B. stearo-thermophilus | A. vinelandii | Photobacterium phosphoreum | |
| KCN or NaCN | 2 mM (b) [27] | 0.5 mM (e) [68] | - | 62 μM (b) [288] |
| NaN3 | 400 mM (b) [27] | 8.2 mM (e) [68] | - | 40 mM (b) [288] |
| H2O2 | 120 mM (b) [27] | - | - | - |
| 2-n-heptyl-4-hydroxyquinoline N-oxide (HOQNO) | 7 μM (b) [27] | - | 5–20 μM (d) [249] | 8.2 μM (b) [288] |
| ZnSO4 or ZnCl2 | 60 μM (b) [27] | 200 μM (e) [68] | - | 2.7 μM (b) [288] |
| Piericidin A | 15 μM (b) [27] | - | - | - |
| Antimicin A | 50 μM, 80% (c) [285] | - | 11 μM (d) [279,286] | - |
| Undecylhydroxydioxobenzothiazole (UHDBT) | 20 μM, 18% (c) [285] | - | 20 μM (d) [279,286] | - |
| (1,5-Dimethylhexyl)quinazolinamide | 100 μM, 88% (c) [285] | - | - | - |
| (1-Methyldecyl)quinazolinamide | 100 μM, 85% (c) [285] | - | - | - |
| Stigmatellin | 200 μM, 14% (c) [285] | - | - | - |
| Nigericin | 100 μM, 44% (c) [285] | - | - | - |
| Dibromothymoquinone | 100 μM, 38% (c) [285] | - | - | - |
| Aurachin A | 700 μM, 27% (c) [285] | - | - | - |
| Aurachin C | 214 nM, 90% (c) [285] | - | - | - |
| Aurachin D | 400 nM, 93% (c) [285] | - | - | - |
| decyl-aurachin D | - | - | 13 nM (d) [249] | - |
| p-benzoquinone | - | 120 μM (e) [68] | - | - |
| 2,6-Dimethyl-p-benzoquinone | - | 65 μM (e) [68] | - | - |
| Nitric oxide (NO) | 100 nM (d) [106] | - | 100 nM (d) [106] | - |
| Carbon monoxide (CO) | - | - | 0.5–1 mM, 80% (g) [287] | - |
| Pentachlorophenol (PCP) | 200 μM (d) [32] | - | - | - |
| 2-Thenoyl trifluoroacetone (TTFA) | 1 mM, 35% (f) [26] | - | - | - |
| Gramicidin S | 5.3 μM (b) [145] | - | - | - |
Data are referred to cytochrome bd-I.
IC50 for ubiquinol-1 oxidase activity of the purified enzyme.
Concentration and % inhibition of duroquinol oxidase activity of cytochrome bd-containing membranes.
Inhibition constant (Ki) for ubiquinol-1 oxidase activity of the purified enzyme.
IC50 for duroquinol oxidase activity of the purified enzyme.
Concentration and % inhibition of ubiquinol-1 oxidase activity of the purified enzyme.
Concentration and % inhibition of ascorbate-2,6-dichlorophenolindophenol oxidase activity of cytochrome bd-containing particles.
4. Genetics
4.1. Genes in E. coli encoding the protein subunits and assembly factors
Of the bd family, the best studied oxidase is cytochrome bd-I from E. coli. The two subunits of cytochrome bd-I are encoded by the cydAB operon [28,146,147] located at 16.6 min on the E. coli genetic map [146,148]. It was cloned [149] and sequenced [28]. The molecular weights of subunit I (CydA) – 57 kDa, and subunit II (CydB) - 43 kDa, determined by sodium dodecyl sulfate-polyacrylamide-gel electrophoresis [26], are consistent with those of 58 and 42.5 kDa based on DNA sequence [28]. The enzyme subunits carry three hemes: b558, b595, and d [34,150]. Heme b558 is located on subunit I (CydA), whereas hemes b595 and d are likely to be in the area of the subunit contact [151]. CydA can be expressed and purified without CydB using mutant strains defective in cydB [152]. The purified CydA retains heme b558 but lacks hemes b595 and d [152]. In addition to the cydAB operon, the two other genes, cydC and cydD of the cydCD operon located at 19 min on the E. coli genetic map [132,153,154], are essential for the assembly of cytochrome bd-I [153–156]. CydC and CydD however are not subunits of cytochrome bd-I. It was shown that cydCD encodes a heterodimeric ATP-binding cassette-type transporter that is a glutathione transport system [157]. An orphan protein, YhcB, was proposed to be a third subunit of cytochrome bd-I [158], but this was later shown not to be the case [159].
In E. coli, a second cytochrome bd (bd-II) encoded by cyxAB genes (also named appBC or cbdAB) was identified [160]. The cyxAB genes, located at 22 min on the E. coli genetic map, are upstream from pH 2.5 acid phosphatase (appA) gene [160]. The cyxAB and appA genes constitute the complex operon. The cyxA and cyxB genes encode 58.1 kDa and 42.4 kDa integral membrane proteins, respectively. The deduced amino acid sequences of cyxA and cyxB genes reveal homologies of 60 and 57%, respectively, to subunit I (CydA) and subunit II (CydB) of cytochrome bd-I [160].
4.2. Regulation of gene expression in E. coli and other bacteria
Cytochrome bd-I is expressed by E. coli when the O2 tension is low [120–122,161,162]. The expression of the cydAB operon is controlled by the two global transcriptional regulators, Arc and Fnr [121,161,163–169]. Arc is a two-component regulatory system that includes ArcA, a cytosolic response regulator, and ArcB, a transmembrane histidine kinase sensor. ArcA controls several hundred genes [170] and responds to the oxidation state of the Q pool which is sensed by ArcB [171]. ArcB is activated in response to the transition from aerobic to microaerobic growth and remains active during anaerobic growth. Upon stimulation, ArcB autophosphorylates and then transphosphorylates ArcA [171,172]. Under microaerobic conditions (i.e., O2 tension of 2 to 15% of air saturation), the increased level of phosphorylated ArcA activates the cydAB operon [173]. Another global regulator, Fnr (an O2-labile transcription factor regulating hundreds of genes), controls induction of anaerobic processes in E. coli [174,175]. The Fnr protein has a Fe-S cluster which serves as a redox sensor. The levels of the Fnr protein are similar under both aerobic and anaerobic conditions [165,176], but the protein is active only during anaerobic growth. The active Fnr protein represses cydAB operon during the transition to anaerobic conditions (i.e., O2 tension of less than 2% of air saturation) [167,168,176].
Expression of cyxAB-appA operon (coding for cytochrome bd-II in E. coli) is induced by phosphate starvation and entry into a stationary phase [177]. The cyxAB genes can also be induced by anaerobic growth and this induction is controlled by transcriptional regulators AppY and ArcA but independent of Fnr, in contrast to cyd operon [177,178]. Cytochrome bd-II is likely to function under even more-O2-limiting conditions than cytochrome bd-I [178]. Cytochrome bd-II has been partially purified [179], and contains two subunits by SDS-PAGE with apparent molecular weights 43 kDa (subunit I) and 27 kDa (subunit II). These subunits show no cross-reactivity to subunit-specific polyclonal antibodies directed against the subunits of cytochrome bd-I [179]. The spectral properties of cytochrome bd-II closely resemble those of cytochrome bd-I. Of the quinols tested as substrates, cytochrome bd-II utilizes menadiol as the preferred substrate (although ubiquinol-1, the most efficient in vitro substrate for cytochrome bd-I, was not tested). TMPD oxidase activity of cytochrome bd-II is much more sensitive to cyanide than that of cytochrome bd-I [179]. It was reported that though the electron flux through cytochrome bd-II can be significant, the enzyme does not contribute to the generation of the PMF [180]. Shepherd et al. [181] proposed that under conditions of an apparently fully uncoupled mode, E. coli can create PMF by means of consumption of intracellular protons in synthesis of γ-aminobutyric acid (GABA) and the generation of a pH gradient via uptake of glutamate and export of GABA by glutamate/GABA antiport.
In A. vinelandii, regulation of cytochrome bd expression is achieved by CydR (an Fnr homologue), which represses transcription of the cydAB genes [182]. The cydABCD operon coding for cytochrome bd in B. subtilis was reported to be activated by ResD and repressed by YdiH (Rex) and CcpA regulators [183–185]. Rex is also a repressor for the cydABCD operon in Streptomyces coelicolor [127]. ResD may activate the cydA gene in L. monocytogenes [105]. In Rhodobacter capsulatus, expression of cytochrome bd is likely controlled by RegA regulator [186].
5. Distribution and Evolution
The bd-family of oxygen reductases has a wide phylogenetic distribution with homologs found in at least one sequenced member of 18 bacterial phyla: Acidobacteria, Actinobacteria, Aquificae, Bacteroidetes, Chlamydiae, Caldithrix, Chlorobi, Chloroflexi, Chrysiogenetes, Cyanobacteria, Deferribacteres, Firmicutes, Nitrospirae, Planctomycetes, Proteobacteria, Thermi, Thermodesulfobacteria and Verrucomicrobia. To date no bd-family homologues have been detected in the following 12 bacterial phyla: Dictyoglomi, Elusimicrobia, Fibrobacteres, Fusobacteria, Gemmatimonadetes, Lentisphaerae, Poribacteria, Synergistetes, Thermotogales, and candidate phyla NC10, TM7 and WWE1. A number of Archaea also encode bd-family homologues, with members of the family found in Crenarchaeota, Euryarchaeota [60] and Korarchaeota. Cytochrome bd-type oxygen reductases are very common is some phyla, such as the Proteobacteria and Actinobacteria, and sporadically distributed in others. Interesingly, bd-family homologues have been detected in many species described as strict anaerobes such as Methanosarcina barkeri, Methanosarcina acetivorans [60], Bacteroides fragilis [86], Desulfovibrio gigas [187–189], Desulfovibrio vulgaris Hildenborough [190], Geobacter metallireducens [116], Moorella thermoacetica [191] and Chlorobaculum tepidum [192].
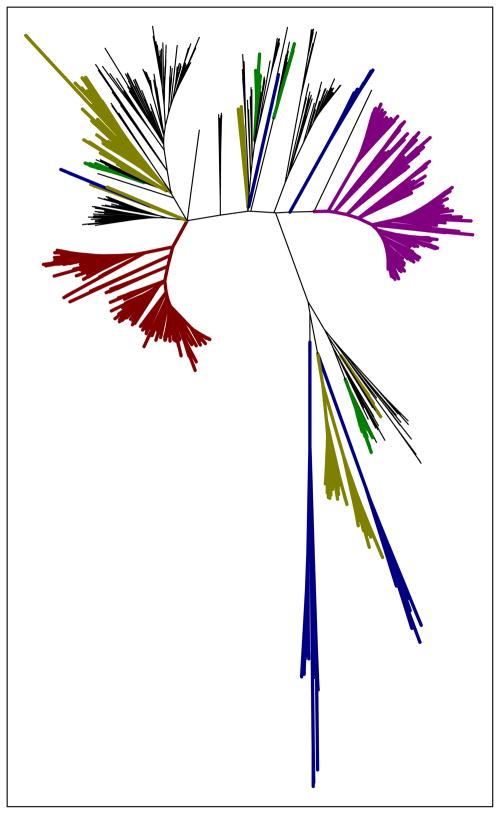
Early work suggested that the bd-family of oxygen reductases is an ancient innovation, already present in the ancestor of both Bacteria and Archaea [193]. However it was recently reported that the family may have originated in Bacteria and was later acquired by Archaea via horizontal gene transfer [60,194]. Phylogenetic analysis of the bd-family showed that horizontal gene transfer plays a significant role in the distribution of the family, with many phyla acquiring cytochrome bd genes multiple times independently (Fig. 3).
Fig. 3.
The bd-family of oxygen reductases. An unrooted phylogenetic tree showing the relationships between 815 sequences of cytochrome bd oxidases. Members with the Q-loop insertion (long Q-loop) are shown in red. All other members of the family have the “short Q-loop”. A number of members from the purple clade have been classified as cyanide insensitive oxidases (CIO) with a low content of heme d. Cytochromes bd from Archaea are shown in blue and form two related clades. In contrast, cytochrome bd-type oxygen reductases from the Firmicutes (yellow) and Bacteroidetes (green) are highlighted to demonstrate the sporadic distribution of enzymes within these phyla which resulted from horizontal gene transfer.
Sequence analysis has demonstrated that subunits I and II have different rates of evolution, with subunit II evolving 1.2 times faster than subunit I [194]. The biological relevance of this asymmetrical evolution is currently unknown.
6. Membrane localization
Cytochrome bd is embedded in the prokaryotic cytoplasmic (plasma) membrane. It was reported that in E. coli, cytochrome bd-I is not evenly distributed within the plasma membrane, being concentrated in mobile (on the subsecond time scale) patches, of the order of 100 nm in diameter [195,196]. These clusters contain variable numbers of cytochrome bd-I tetramers [196]. Cytochrome bd in cyanobacteria [197–203] has been reported to also be located in the thylakoid membrane [200,201,203–207], though this has been disputed [208–211]. The presence of a bd-type PQH2 oxidase in cyanobacterial thylakoid and/or cytoplasmic membranes may depend on culturing conditions and the light regime [201,206].
7. Cofactors and Substrates
7.1. Quinones
The nature of the quinols used by cytochrome bd as an electron donor is species-specific. For instance, in A. vinelandii and E. coli the cytochrome bd enzyme can oxidize ubiquinol (UQH2), in B. stearothermophilus, the substrate is menaquinol (MQH2). In E. coli, cytochrome bd-I can also oxidize MQH2 [212,213], which replaces UQH2 upon change of growth conditions from aerobic to anaerobic [166]. There is evidence that in cyanobacteria cytochrome bd is active as a plastoquinol (PQH2) oxidase [200,201,203–206], although some reports have questioned this conclusion [208–210]. The presence or absence of bound Q in solubilized cytochrome bd-I from E. coli depends on the purification protocol. In some preparations of the purified enzyme, there is no apparently bound quinone [26,27,46,214] whereas others clearly contain bound quinone [41,215]. A stable semiquinone radical has been observed in the E. coli cytochrome bd-I [216,217].
7.2. Hemes
The two subunits of E. coli cytochrome bd-I carry three metal-containing redox-centers, two protoheme IX groups (hemes b558 and b595) and a chlorin molecule (heme d) which are in 1:1:1 stoichiometry per the enzyme complex. The enzyme contains no Fe-S cluster and no copper ion [218–222]. Heme b558 is clearly located within subunit I. Both subunits are required for the assembly of heme b595 and heme d, suggesting that these two hemes may reside at the subunit interface [151]. Heme b595 appears to be oriented with its heme plane at ~55° to the plane of the membrane [223]. The millimolar extinction coefficients used commonly for the determination of the cytochrome bd concentration in E. coli and A. vinelandii are listed in Table 2.
Table 2.
Extinction coefficients used for determination of cytochrome bd concentration in E. coli and A. vinelandii.
| Absorption spectrum | Heme | Wavelength pair (nm) | Δε (mM−1·cm−1) | Reference |
|---|---|---|---|---|
| E. coli (cytochrome bd-I) | ||||
| Difference: | d | 628–607 | 10.8 | [37] |
| Reduced minus ‘as prepared’ | d | 628–651 a | 27.9 | [36] |
| d | 628–649 a | 18.8 | [27] | |
| b558 | 561–580 | 21 | [36] | |
| b595 | 595–606.5 | 1.9 | [36] | |
| all | 429–700 b | 303 | [36] | |
| CO/reduced minus reduced | d | 642–622 | 12.6 | [27] |
| d | 643–623 | 13.2 | [48] | |
| Absolute: | ||||
| Reduced | d | 628–670 | 25 | [41] |
| ‘As prepared’ | all | 414–700 b | 223 | [36] |
| A. vinelandii | ||||
| Difference: | ||||
| Reduced minus ‘as prepared’ | d | 628–605 | 9.5 | [241] |
| d | 629–608 | 12 | [257] | |
| d | 629–650 a | 27 | [257] | |
| CO/reduced minus reduced | d | 622–642 | 18 | [257] |
These values cannot be recommended for determination of cytochrome bd concentration since
the ‘as prepared’ enzyme contains varying amounts of the ferrous heme d-oxy complex that absorbs at 649–651 nm, and
the intensity of the Soret band is variable depending on the purity of the preparation.
7.2.1. Heme b558
Heme b558 has been shown to be located within subunit I by expressing subunit I (cydA) in the absence of subunit II (cydB) and showing that the isolated subunit I contains heme b558 [152]. Antibodies directed against subunit I [61,63], as well as selective proteolysis of this subunit [62,64], inhibit UQH2 oxidase activity of cytochrome bd-I. These findings suggest that heme b558 is associated with subunit I and is involved in QH2 oxidation. The α- and β-bands of the reduced heme b558 at room temperature reveal maxima at 560–562 and 531–532 nm, respectively (Table 3) [150,224,225]. The maximum and minimum of the γ-band in the “reduced minus oxidized” difference absorption spectrum are 429.5 and 413 nm, respectively (Table 3) [225]. Heme b558 is low-spin hexacoordinate [37], and amino acid residues His186** and Met393 of subunit I (E. coli cytochrome bd-I) have been identified as its axial ligands [226–228]. The location of heme b558 is predicted to be near the periplasmic surface [67,229].
Table 3.
Spectral properties of cytochrome bd-I from E. coli. Shown are wavelengths (nm) and extinction coefficients (in parentheses, mM−1·cm−1) for “reduced-minus-oxidized” difference absorption spectra. Data are taken from reference [225].
| Heme b558 | Heme b595 | Heme d | |
|---|---|---|---|
| Maxima | 429.5 (90), 531.5 (5.8), 561 (17.2) | 439 (113), 561.5 (8.2), 594 (5.3) | 430 (30), 629 (18) |
| Minima | 413 (−40), 497 (−4.3), 545 (~0) | 400 (−37), 500 (−3.6), 643 (−1.18) | 405 (−23), 468 (−6.3), 657.5 (−2.7), 739±2 (−2.4) |
| Isosbestic points | 421, 450, 518, 573 | 422, 457, 535, 613 | 418.5, 449, 602, 648 |
7.2.2. Heme b595
The spectrum of heme b595 is similar to that of catalases and peroxidases containing pentacoordinate (high-spin) protoheme IX [150]. Heme b595 has an α-band at 594–595 nm and β-band at 560–562 nm in the difference absorption spectrum (Table 3) [150,224,225]. A trough at 643–645 nm in the difference spectrum of heme b595 is indicative of the disappearance in the reduced heme b595 of an absorption feature due to charge transfer from the Fe to the ligand, characteristic of oxidized high-spin heme b, as in the case of peroxidases. The γ-band of ferrous heme b595 is characterized by a maximum at ~440 nm as clearly revealed by femtosecond spectroscopy [38]. The maximum and minimum of the γ-band in the difference “reduced minus oxidized” absorption spectrum are 439 and 400 nm, respectively (Table 3) [225]. Heme b595 is high-spin pentacoordinate [37], ligated by His19 of subunit I [230] and located near the periplasmic surface [67,229]. The role of heme b595 remains obscure. It is proposed that heme b595 participates in the reduction of O2 forming, together with heme d, a di-heme O2-reducing site, somewhat similar to the heme/Cu O2-reducing site in heme-copper oxidases [35–41,43,231]. In favor of this hypothesis is the finding that the CD spectrum of the reduced wild type cytochrome bd in the Soret band shows strong excitonic interaction between ferrous hemes d and b595 [42]. Modeling the excitonic interactions in the absorption and CD spectra yields an estimate of the Fe-to-Fe distance between heme d and heme b595 to be about 10 Å [42]. In the opinion of some, the function of heme b595 is limited to transferring an electron from heme b558 to heme d [232,233], whereas others have postulated that heme b595 can form a second site capable of reacting with O2 [218,234].
7.2.3. Heme d
Heme d is a chlorin-type molecule [235]. The α-band of the reduced heme d in the absolute absorption spectrum of E. coli cytochrome bd-I shows a peak at 628–630 nm. However, upon isolation of the enzyme, heme d is in the stable oxygenated (O2-ligated ferrous) form, which is characterized by an absorption band with a maximum at 647–650 nm in the absolute absorption spectrum [236–239]. The affinity of ferrous heme d for O2 is indeed high, showing the Kd(O2) values of 0.28 μM and 0.5 μM for the enzymes from E. coli and A. vinelandii, respectively [240,241]. The maximum and minimum of the γ-band in the difference “reduced minus oxidized” absorption spectrum are 430 and 405 nm, respectively (Table 3) [225].
Remarkably, the spectral contribution of heme d to the complex Soret band is much smaller than those of either hemes b [225]. Heme d is predicted to be located near the periplasmic surface [67,229], and is the site for capturing and, subsequently, reducing O2 to H2O. In the absence of external ligands, heme d is in the high-spin state with an open coordination site for binding O2. The nature of the axial ligation of heme d to the protein, or even whether there is an axial ligand provided by the protein, is unclear. It has been claimed that the reduction of cytochrome bd is associated with binding of an endogenous protein ligand to heme d [242]. The oxidized heme d may or may not be ligated to an endogenous protein substituent. Resonance Raman and ENDOR studies indicate that the ligand is not histidine, cysteine or tyrosinate, but that the single axial ligand is either a weakly coordinating protein donor or a water molecule [230,243,244]. In contrast, EPR studies indicated that the heme d axial ligand is histidine in an anomalous condition or some other nitrogenous amino acid residue [245]. Finally, it has been suggested that Glu99 of subunit I is a prime candidate for such a role [214,246].
7.3. Heme redox potentials
The apparent values for the midpoint redox potentials of hemes b558, b595 and d for the bd enzymes solubilized in n-dodecyl-β-D-maltoside at pH 7.0 (Em) are respectively +176, +168, +258 mV (E. coli bd-I) and +166, +251, +310 mV (A. vinelandii) [241]. These are within the range of the values reported earlier for E. coli [219,220,224,247,248] and A. vinelandii [249]. Notably, the Em value of heme b558 can depend on the detergent used for solubilization [248]. In particular, octylglucoside and cholate cause a large decrease in the Em value of heme b558, and this correlates with the reversible inactivation of the enzyme [248]. The Em values of all three heme components of cytochrome bd are sensitive to pH between pH 5.8 and 8.3 with a Em/pH of −61 mV for heme d and −40 mV for hemes b558 and b595, indicating that reduction of cytochrome bd is accompanied by enzyme protonation [248]. A recent study [225] revealed a significant redox interaction between heme b558 and heme b595, whereas the interaction between heme d and either both hemes b appears to be rather weak. However, the presence of heme d itself decreases the much larger interaction between the two hemes b [225].
8. Proposed structure
The X-ray structure of cytochrome bd has not been determined. Conventional studies of the protein topology in the membrane suggest that all three hemes are located near the periplasmic side of the membrane [67,229], although an alternative view also exists [250,251]. Fig. 4 shows topological models of subunits I (CydA) and II (CydB) of cytochrome bd-I from E. coli [213]. Both subunits are integral membrane proteins. Subunit I consists of nine transmembrane helices with the N-terminus in the periplasm and the C-terminus in the cytoplasm [67]. Subunit II is composed of eight transmembrane helices with both N- and C-termini in the cytoplasm [67]. The Q-loop in subunit I connects transmembrane helices 6 and 7, and is directly involved in QH2 binding and oxidation [61–66]. Thus the QH2-oxidizing site in cytochrome bd is located on the periplasmic side of the membrane. Cytochrome bd-I from E. coli is proposed to contain a single site for the binding and oxidation of quinol [65,66,252]. However, evidence for a second quinone binding site in cytochrome bd from Corynebacterium glutamicum has also reported [69].
Fig. 4.
Proposed topology of subunits I and II of cytochrome bd-I from E. coli. The axial ligands of heme b595 (H19) and heme b558 (H186 and M393) in subunit I are highlighted. The model is based on the data reported in [67,213,229].
Using a set of 815 sequences of genes encoding cytochrome bd, a number of residues in subunit I are totally (>99%) conserved [213]. These residues include those which are identified as ligands to the heme components of the enzyme. In addition, since the active site of O2 reduction is located near the periplasmic surface and protons for H2O production are taken from the bacterial cytoplasm, there must be at least one transmembrane proton-conducting pathway to convey protons from the cytoplasm to the heme b595/heme d site [41,46,48,67] (Fig. 5). Several polar or ionizable residues that are highly conserved in the bd-family have been postulated to be a part of this putative proton channel.
Fig. 5.
Scheme for electron and proton transfer pathways in cytochrome bd-I from E. coli. There are two protonatable groups, XP and XN redox-coupled to the heme b595/heme d active site. A highly conserved E445 was proposed to be either the XP group or the gateway in a channel that connects XP with the cytoplasm or the periplasm [41]. A strictly conserved E107 is a part of the channel mediating proton transfer to XN from the cytoplasm [48].
The residues that are totally conserved within the entire bd-family include His19 (the heme b595 axial ligand [230]), His186 and Met393 (the heme b558 axial ligands [226–228]), Lys252 and Glu257 (involved in QH2 binding [66]), Arg448 (unknown function), and Glu99, Glu107, and Ser140 (proposed to be components of a proton channel [48,67] and important for heme binding in the heme d/heme b595 di-heme site [213,214]). Slightly less conserved (95–99%) are Glu445 (required for charge compensation of the b595/d O2-reducing site upon its full reduction by two electrons [41]), Asn148 (plausible component of a proton channel), and Arg9 (unknown function) [213]. Somewhat less conserved (~ 85%) are Arg391 (stabilizes the reduced form of heme b558 [253]) and Asp239 (unknown function), however these residues are totally conserved within the A subfamily of cytochromes bd [213]. Other conserved residues are glycines, prolines, phenylalanines, or tryptophans, which may play structural roles. There is only one totally (>99%) conserved residue (Trp57) in subunit II [213]. Within the subfamily of bd-type oxygen reductases which have the “long Q-loop”, Arg100, Asp29, and Asp120 of subunit II are totally conserved and Asp58 (subunit II of E. coli cytochrome bd-I) is either an aspartate or glutamate [213]. The N-terminal portion of subunit II has been suggested to be involved in the binding of heme d/heme b595 [213,254].
Fig. 3 shows an unrooted tree showing the relative sequence relationships of 815 sequences of cytochrome bd from the genomes of Bacteria and Archaea. It is seen in Fig. 3 that the “long Q-loop” members form a phylogenetic clade distinct from the other members of the family. This is most likely due to an insertional event within the Q-loop. This subfamily contains many, but not all, of the cytochrome bd oxygen reductases from Proteobacteria (including E. coli). Also shown in Fig. 3 are two clades that define the bd-family members found in Archaea. In contrast, the bd-family oxygen reductases found in Firmicutes or Bacteroides are distributed widely among the phylogenetic groups shown in Fig. 3. This illustrates the large role played by horizontal gene transfer in the distribution of the bd-type oxygen reductases.
9. Binding of ligands (other than O2)
Since hemes d and b595 in cytochrome bd are in the high-spin pentacoordinate state, they could potentially bind ligands. One may anticipate that the enzyme in the reduced state binds electroneutral molecules like O2, CO, and NO, whereas the oxidized cytochrome bd prefers ligands in the anionic form such as cyanide and azide. Heme d binds ligands readily whereas the ligand reactivity of heme b595 is minor despite the fact that this is a high spin heme [37,39,255]. Heme b558, although a low-spin hexacoordinate, may also bind ligands to some extent (e.g., CO or cyanide) [37,255]. Such a marginal reactivity is possibly due to weakening the bond of the methionine axial ligand (Met393) to heme b558 iron caused by the isolation procedure and/or protein denaturation [255].
9.1. Carbon monoxide
Addition of CO to the three-electron reduced form of cytochrome bd, denoted as R3, causes a red shift of the 628 nm heme d band and the increased absorption around 540 nm in the visible, as well as a distinctive W-shaped difference spectrum in the Soret region [37,39,150,255–257]. The W-shaped feature is due to a small bandshift of unligated heme b595 induced by CO interaction with the nearby heme d [38,40,43]. Only a small fraction of heme b595 (<5%) in cytochrome bd binds CO at room or low temperature [37,39]. The apparent Kd for the CO-heme d complex with the fully reduced (R3) cytochrome bd-I from E. coli was determined to be ~80 nM [255]. The R3 cytochrome bd can form a photosensitive heme d-CO complex [258]. Flash photolysis of CO bound to heme d at cryogenic temperatures results in a redistribution of CO such that as much 15% of heme b595 is bound to CO, showing the proximity of these two hemes [35]. Following flash-photolysis of the heme d-CO complex in the fully reduced enzyme (R3) at room temperature, CO recombines with ferrous heme d proportionally to the external CO concentration with a second order bimolecular rate constant of 108 M−1 s−1 (Table 4) [43,222,249,259].
Table 4.
Kinetic and thermodynamic parameters for reaction of cytochrome bd with gaseous ligands at room temperature.
The one-electron reduced form of the enzyme (R1) can also be examined. Since heme d has a substantially higher midpoint potential than the other two heme components, heme d is the only heme reduced in the R1, or mixed-valence, state of the ‘as prepared’ enzyme. Upon reaction with CO, one gets the CO-heme d adduct (b5583+b5953+d2+-CO) [38,40,43,48,107,249]. After flash photolysis of the R1-CO complex, a substantial fraction of the CO flashed off heme d2+ gets trapped inside the protein and undergoes geminate recombination with heme d2+ on the pico- and nanosecond time scale [38,43]. The data indicate that the redox state of heme b595 controls the pathway for ligand (CO) transfer between heme d and the bulk phase, which is open when heme b595 is reduced but closed when heme b595 is oxidized [38,43,107].
9.2. Nitric oxide and other nitrogen-containing ligands
A number of small nitrogen-containing molecules can react with R3 cytochrome bd from E. coli and A. vinelandii. NO3−, NO2−, N2O32− (trioxodinitrate), NH2OH and NO, when added to membranes containing cytochrome bd or the purified enzyme, give rise to decrease in amplitude and shift of the 630 nm peak of ferrous heme d to 641–645 nm [31,37,106,107,218,245,257,260–264]. It appears that all of these ligands result in chemical reactions, forming the same or a very similar heme-nitrosyl compound [31], e.g., heme d2 +-NO adduct. It has also been suggested that a heme b5952+-NO adduct can be observed upon adding nitrite to cytochorme bd in membranes [218].
Cytochrome bd can also produce a stable complex with NO in the R1 state, in which ligand bound heme d is reduced while the b hemes are oxidized [107,245]. The rates of NO dissociation from heme d2+ in both R3 and R1 states of cytochrome bd were determined [107]. In the R3 state, NO dissociates from heme d2+ at an unusually high rate, koff = 0.133 s−1 [107], which is ~30-fold higher than the off-rate measured for the ferrous heme a3 of the mitochondrial cytochrome c oxidase (koff = 0.004 s−1 [265]). These data are consistent with the proposal that, in the heme–copper oxidases, CuB acts as a gate controlling ligand binding to the heme in the active site [266]. Another remarkable feature of NO dissociation from cytochrome bd is that the koff value in the R1 state (0.036 s−1), although still quite high, is significantly lower than that measured with the R3 enzyme [107] (Table 4). These data show that the redox state of heme b595 controls the kinetic barrier for ligand dissociation from the active site of cytochrome bd, similar to the observations with CO dissociation from ferrous heme d [38,43,107]. The unusually high NO dissociation rate from cytochrome bd may explain the observation [106] that the NO-poisoned cytochrome bd recovers respiratory function much more rapidly than a heme–copper oxygen reductase. It is postulated that expression of bd-type, instead of heme–copper-type oxygen reductase, enhances bacterial tolerance to nitrosative stress, thus promoting colonization of host intestine or other microaerobic environments [107,108]. It was reported that, apart from ferrous heme d, NO can also react with the oxoferryl and ferric state of heme d, yielding the oxidized nitrite-bound heme d and the nitrosyl adduct, respectively [110,111].
9.3. Cyanide
Reaction of ‘air-oxidized” cytochrome bd with KCN causes the decay of the ferrous heme d oxy-complex [267–273]. Cyanide-induced changes to the EPR-spectrum include a low-spin signal and, after prolonged incubation, a second weak low-spin signal that may indicate some interaction of cyanide with heme b595 [220,257,274]. A simple and fast method for conversion of the oxygenated enzyme into the O form with the use of lipophilic electron acceptors [239] allowed us to study the interaction of cyanide with the homogenous oxidized preparation of cytochrome bd [37]. The MCD spectrum of the O cytochrome bd-I from E. coli is dominated by an asymmetric signal in the Soret. Submillimolar cyanide has no effect on the initial MCD spectrum. 50 mM KCN induces minor changes of the MCD signal in the Soret band, which can be modeled as transition of a part of the low-spin heme b558 (15–20%) to its low-spin cyano-complex [37]. There is no evidence of the interaction of high-spin ferric heme b595 with the ligand [37]. On the contrary, based on the EPR spectra, Tsubaki et al. [36] proposed that the treatment of ‘air-oxidized’ cytochrome bd with cyanide results in a cyanide-bridging species with a “heme d3+–C=N–heme b5953+” structure. However the authors [36] did not account for the electron released from heme d upon cyanide binding to ‘as prepared’ cytochrome bd. Resonance Raman studies suggest that heme d is in the high-spin pentacoordinate state when it is compounded with cyanide [230,275]. This would require either that the endogenous axial ligand to heme d is displaced by cyanide, maintaining a high-spin pentacoordinate state, or that there is no endogenous axial ligand to heme d in the fully oxidized form of the enzyme.
9.4. Hydrogen peroxide
Addition of excess H2O2 to E. coli membranes containing cytochrome bd-I [276] and the purified enzyme in the ‘as prepared’ [231,237] or the O [46,231,277] states gives rise to an absorption band at ~680 nm. The reaction of H2O2 with the O cytochrome bd also induces a red shift of the γ-band [231,277]. H2O2 binds to ferric heme d with an apparent Kd value of 30 μM, but it seems not to interact with heme b595 [231,277]. The O cytochrome bd reacts with H2O2 with a second order rate constant of 600 M−1 s−1. The decay of the H2O2-induced spectral changes upon addition of catalase (k ~ 10−3 s−1) is about 20-fold slower than expected for dissociation of H2O2 from the complex with heme d assuming a simple reversible binding of peroxide [277]. This suggests that the interaction of H2O2 with cytochrome bd is essentially irreversible, giving rise to the F state of heme d [277]. The assignment of the compound 680 to the F state of heme d is confirmed by resonance Raman spectroscopy data [221]. Heme d in the F state is suggested to be high-spin pentacoordinate [275].
10. Proposed catalytic mechanism
As discussed above, under physiological conditions cytochrome bd from different prokaryotes likely oxidizes UQH2, MQH2 or PQH2. In vitro a bd-type oxygen reductase can also utilize short chain ubiquinols, menadiol, duroquinol, and artificial electron donors such as TMPD. Of the in vitro substrates, ubiquinol-1 (plus excess dithiothreitol) shows the highest turnover numbers [248,278]. The activity of the purified oxidase depends on the nature of the detergent in which the enzyme is solubilized. Cytochrome bd-I from E. coli is inactive in octylglucoside or cholate but shows high activity in Tween-20, Triton X-100 [248] or N-lauroyl-sarcosine [106]. The ubiquinol-1 oxidase activity of cytochrome bd-I has a broad optimum above pH 7.5 but decreases at more acidic pH values [248]. Cytochrome bd possesses three distinct active sites - for QH2 oxidation, TMPD oxidation and O2 reduction. All the three sites seem to be located at or close to the periplasmic surface of the membrane. Electrons donated from QH2 transfer to heme b558 and then to the b595/d di-heme site, whereas electrons donated from TMPD transfer directly to the b595/d site bypassing the QH2-binding site and heme b558 [62,279].
10.1. Mechanism of generation of the proton motive force
Cytochrome bd from E. coli and A. vinelandii was reported to generate a transmembrane electric potential both in single turnover [41,46–48] and under multiple turnover [27,44,280] conditions (H+/e− ~ 1 [34,45,49,50]; q/e− ~ 1 [281]). When reconstituted into liposomes, cytochrome bd generates an uncoupler-sensitive transmembrane voltage difference with a value of 160–180 mV (negative inside) [27,44]. The QH2 molecule generated by the dehydrogenases of the respiratory chain can diffuse laterally within the bilayer, finding its way into the QH2 oxidizing site located near the outer side of the membrane. Upon oxidation of QH2, two protons are released into the periplasmic space, and two electrons are transferred through heme b558 to the b595/d O2-reducing site, also located near the periplasmic surface of the membrane. The four protons used for O2 reduction are taken up from the cytoplasm. Single-turnover electrometric experiments show that the generation of the membrane potential is associated with electron transfer from heme b558 to the b595/d active site [41,46–48]. However, since all of the three hemes are likely located close to the periplasmic side of the membrane [67,229], the electron transfer itself is expected to be parallel to the membrane surface and, therefore, cannot be electrogenic [46]. Rather, it is proposed that electron transfer from heme b558 to the b595/d active site is coupled to vectorial proton transfer from the cytoplasm towards the active site on the opposite (periplasmic) side of the membrane [41,46–48]. The latter implies that there must be a proton-conducting channel connecting the cytoplasm to the b595/d active site [41,46,48] (Fig. 5). The transmembrane potential originates primarily from protons moving from the cytoplasm to the O2-reducing site on the opposite side of the membrane, and this accompanies electron transfer from heme b558 to the b595/d active site. As shown in Fig. 5, it is proposed that near the b595/d active site there are two protonatable sites (XP and XN) that are accessible to the cytoplasm via a proton-conducting channel.
10.2. Reaction of the fully reduced enzyme (R3) with O2
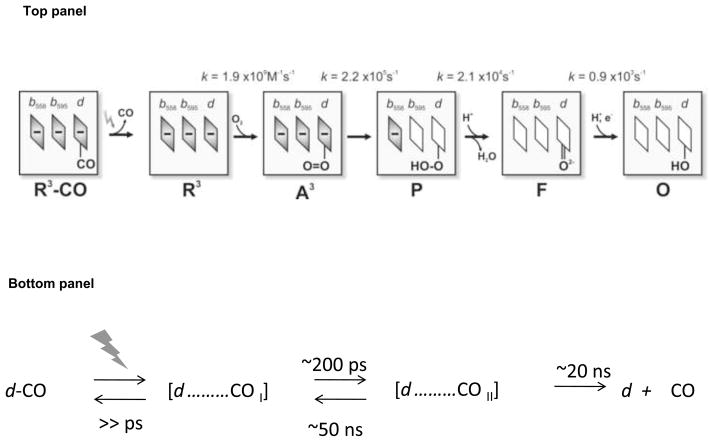
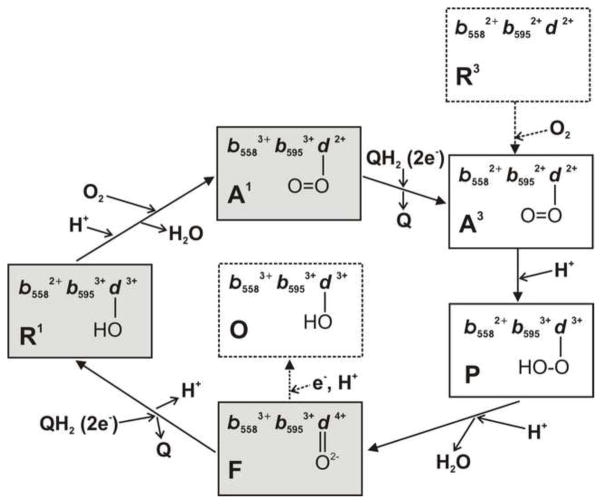
The reaction of the R3 cytochrome bd with O2 has been studied using the flow-flash method [282] by means of spectroscopic and electrometric techniques [41,46–48,222]. Recording absorption spectra and membrane potential development with 1 μs time resolution resolves the sequence of the catalytic intermediates and establishes which catalytic steps are linked to electric potential generation [47]. The scheme for this reaction is presented in Fig. 6 (top panel). The initial complex of R3 cytochrome bd with CO (R3-CO) is photolyzed (the photolysis details are shown in Fig. 6, bottom panel) in the presence of O2. The unliganded R3 enzyme, generated by the CO-photolysis, binds O2 very rapidly, forming the ferrous heme d oxy species (A3). The R3→A3 transition is not electrogenic and its rate is proportional to [O2] (kon = 1.9 × 109 M−1·s−1 [47,222]). The A3 formation is followed by electron transfer from heme b595 to form state P. The A3→P transition occurs with τ = 4.5 μs and is also nonelectrogenic [47]. Thus, electron transfer from heme b595 to heme d is not coupled to membrane potential generation [41,47]. It is proposed that P is a peroxy complex of ferric heme d [47]. If this is the case, the bound peroxide is likely not to be in the anionic form but at least singly protonated. The proton may come from one of two postulated protonatable groups, XP and XN, near the b595/d di-heme active site upon oxidation of the hemes [41]. P is further converted into F upon electron transfer from heme b558 with τ = 48 μs. Formation of F is coupled to generation of a membrane potential [41,46–48] due to the accompanying proton transfer through the proposed proton channel (Fig. 5). At the F stage, the b-type hemes are in a ferric state and heme d in an oxoferryl state. When cytochrome bd contains bound QH2, the reaction proceeds further to form the O enzyme. The F→O transition occurs with τ = 1.1 ms and is electrogenic as well [41,47] since this also involves electron transfer from heme b558 to the b595/d active site with the accompanying proton transfer.
Fig. 6.
Top: Scheme for reaction of fully reduced cytochrome bd with O2. The three rhombuses represent hemes b558, b595, and d, respectively. The minus sign denotes that the heme is in the ferrous state. Bottom: Photolysis of CO from heme d in the fully reduced enzyme. Two different configurations of dissociated CO in the enzyme (d……COi, i=I, II) are proposed [43]. The state (d + CO) denotes a state where CO escaped from the enzyme.
Cytochrome bd can bind O2 being in the R1 state. Remarkably, in this reaction, the dependence of the rate of O2 binding on [O2] is hyperbolic thus revealing a saturation behavior. This is not observed for O2 binding to the R3 enzyme [241]. It is speculated that the R1 enzyme exists in the two different conformations in equilibrium, but only one of these forms binds to O2. When in the “closed” conformation, cytochrome bd provides no access for O2 to heme d2+, whereas in the “open” conformation, O2 binds easily. The R3 enzyme is always in the open conformation [241].
10.3. Catalytic cycle
Several relatively stable forms of cytochrome bd corresponding to the intermediates of the catalytic cycle have been identified. Under aerobic conditions, cytochrome bd is predominantly in the one-electron-reduced state bound to O2 (A1), with lesser amounts of the F and O forms. Under anaerobic conditions, the reduced forms of the enzyme lacking an O2 ligand with one (R1) and three (R3) electrons can be generated and examined. A short-lived complex of the three-electron reduced cytochrome bd with O2 (A3) [46,47,222,241], an “peroxide” intermediate P [47] and an oxoferryl compound F [46,47,222] can be sequentially formed (Fig. 6). Turnover intermediates of E. coli cytochrome bd-I detected at steady-state are A1 and F species (~40% each) and, to a lesser extent (~20%), a species with ferric heme d and possibly one electron on heme b558 (R1) [283]. These data differ from those obtained with mammalian cytochrome c oxidase, in which oxygenous intermediates were not found to be populated at detectable levels under similar conditions [284]. A plausible scheme of the catalytic cycle of cytochrome bd is shown in Fig. 7.
Fig. 7.
Cytochrome bd catalytic cycle. The scheme is based on the reports of Junemann et al. [278], Kavanagh et al. [289], Matsumoto et al. [252], Belevich et al. [47], Yang et al. [290], and Borisov et al. [283]. Solid arrows show the natural catalytic reaction pathway. Dotted arrows indicate transitions that are not being part of the catalytic cycle can be observed experimentally. The O form of the enzyme is most likely not to be an intermediate of the catalytic cycle [290]. Intermediates populated at steady-state [283] are highlighted in grey.
10.4. Role of heme b595
Exogenous ligands added to cytochrome bd bind to heme d but do not bind to a majority of the heme b595 population [31,37,39,255]. Heme b595, although in the high-spin pentacoordinate state, is resistant to interaction with the classical ligands of high-spin iron-porphyrin complexes. It cannot be ruled out that despite the high-spin pentacoordinate state of the iron-porphyrin group, the specific features of the protein environment are such that this redox cofactor is protected from interaction with ligands. In such case, the participation of heme b595 in O2 reduction in cooperation with heme d is unlikely and its role would be limited to the transfer of an electron to heme d. A more likely explanation is the following: (1) both heme b595 and heme d potentially can bind ligands; (2) the hemes are located close to each other forming a di-heme active site; (3) the spatial proximity of hemes b595 and d results in steric restrictions allowing the di-heme site to bind only one ligand molecule; (4) heme d has a higher affinity for ligands than heme b595, in which case the final result observed upon addition of a ligand will always be the ligand binding to heme d, whereas heme b595 will remain mainly in the unliganded state [37,39,231,255]. The data on the redox coupling of the two hemes to the same ionizable groups [41], and the migration of CO within the protein from heme d to heme b595 at cryogenic temperatures [35] are in agreement with this proposal. Modeling the excitonic interactions in absorption and CD spectra of cytochrome bd yields an estimate of the Fed-to-Feb595 distance of about 10 Å [42]. This is markedly larger than that for the Fe/CuB pair in heme-copper oxidases (4–5 Å). If this is the case, heme b595 cannot be a functional analogue of CuB. A possible role of heme b595, apart from electron delivery to heme d and/or to an oxygenated intermediate form of heme d, would be as a binding site for hydroxide produced from heme d-bound O2 upon reductive cleavage of the O-O bond [42].
11. Conclusion
There are at least two reasons why cytochromes bd may be of interest. First, they are found in many pathogenic bacteria and there is growing evidence for a positive correlation between the virulence and the level of cytochrome bd expression. We hope that our knowledge on the structure and function of the bd enzymes will provide new tools to combat diseases caused by pathogens, for instance, by using a bacterial bd-type respiratory oxygen reductases as a drug target. Second, it would be useful to know what are the common features and the differences between the mechanisms of O2 reduction to H2O by cytochromes bd and heme-copper oxidases. Such a comparison could allow us to gain further insight into the elements essential for proton pumping coupled to the redox reaction inherent in heme-copper oxidases.
Research highlights.
Physiological functions and genetics of cytochrome bd terminal oxidases reviewed.
Structural and catalytic properties of cytochromes bd discussed.
Phylogenetic analysis of cytochromes bd and their homologues presented.
Acknowledgments
Studies in our groups were supported by the Russian Foundation for Basic Research, grant 11-04-00031-a (to V.B.B.), the National Institutes of Health, grant HL16101 (to R.B.G.), and the Biocentrum Helsinki, the Sigrid Jusélius Foundation, the Academy of Finland (to M.I.V.).
Abbreviations
- AOX
alternative oxidase
- CIO
cyanide-insensitive quinol oxidase
- Em
apparent midpoint redox potential
- IC50
the half maximal inhibitory concentration
- PMF
proton motive force
- TMPD
N,N,N′,N′-tetramethyl-p-phenylendiamine
- Q
quinone
- QH2
quinol
- UQH2
ubiquinol
- MQH2
menaquinol
- PQH2
plastoquinol
- A1
one electron-reduced O2-bound species
- A3
fully reduced O2-bound species
- R1
one electron-reduced species
- R3
fully reduced species
- O
fully oxidized species
- F
oxoferryl species
- P
peroxide-bound species
- ΔμH+
transmembrane difference in the electrochemical H+ potentials
- τ
time constant reciprocal of rate constant (t1/e)
Footnotes
Here and below – amino acid numbering refers to cytochrome bd-I from E. coli
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brunori M, Giuffre A, Sarti P. Cytochrome c oxidase, ligands and electrons. J Inorg Biochem. 2005;99:324–336. doi: 10.1016/j.jinorgbio.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Hosler JP, Ferguson-Miller S, Mills DA. Energy transduction: proton transfer through the respiratory complexes. Annu Rev Biochem. 2006;75:165–187. doi: 10.1146/annurev.biochem.75.062003.101730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branden G, Gennis RB, Brzezinski P. Transmembrane proton translocation by cytochrome c oxidase. Biochim Biophys Acta. 2006;1757:1052–1063. doi: 10.1016/j.bbabio.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Wikstrom M, Verkhovsky MI. Mechanism and energetics of proton translocation by the respiratory heme-copper oxidases. Biochim Biophys Acta. 2007;1767:1200–1214. doi: 10.1016/j.bbabio.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Belevich I, Verkhovsky MI. Molecular mechanism of proton translocation by cytochrome c oxidase. Antioxid Redox Signal. 2008;10:1–29. doi: 10.1089/ars.2007.1705. [DOI] [PubMed] [Google Scholar]
- 6.Richter OM, Ludwig B. Electron transfer and energy transduction in the terminal part of the respiratory chain - Lessons from bacterial model systems. Biochim Biophys Acta. 2009;1787:626–634. doi: 10.1016/j.bbabio.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Brzezinski P, Johansson AL. Variable proton-pumping stoichiometry in structural variants of cytochrome c oxidase. Biochim Biophys Acta. 2010;1797:710–723. doi: 10.1016/j.bbabio.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Pereira MM, Santana M, Teixeira M. A novel scenario for the evolution of haem-copper oxygen reductases. Biochim Biophys Acta. 2001;1505:185–208. doi: 10.1016/s0005-2728(01)00169-4. [DOI] [PubMed] [Google Scholar]
- 9.Pereira MM, Sousa FL, Verissimo AF, Teixeira M. Looking for the minimum common denominator in haem-copper oxygen reductases: towards a unified catalytic mechanism. Biochim Biophys Acta. 2008;1777:929–934. doi: 10.1016/j.bbabio.2008.05.441. [DOI] [PubMed] [Google Scholar]
- 10.Chang HY, Hemp J, Chen Y, Fee JA, Gennis RB. The cytochrome ba3 oxygen reductase from Thermus thermophilus uses a single input channel for proton delivery to the active site and for proton pumping. Proc Natl Acad Sci USA. 2009;106:16169–16173. doi: 10.1073/pnas.0905264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buschmann S, Warkentin E, Xie H, Langer JD, Ermler U, Michel H. The structure of cbb3 cytochrome oxidase provides insights into proton pumping. Science. 2010;329:327–330. doi: 10.1126/science.1187303. [DOI] [PubMed] [Google Scholar]
- 12.Hemp J, Han H, Roh JH, Kaplan S, Martinez TJ, Gennis RB. Comparative genomics and site-directed mutagenesis support the existence of only one input channel for protons in the C-family (cbb3 oxidase) of heme-copper oxygen reductases. Biochemistry. 2007;46:9963–9972. doi: 10.1021/bi700659y. [DOI] [PubMed] [Google Scholar]
- 13.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima T, Yaono R, Yoshikawa S. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science. 1995;269:1069–1074. doi: 10.1126/science.7652554. [DOI] [PubMed] [Google Scholar]
- 14.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996;272:1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- 15.Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Fei MJ, Libeu CP, Mizushima T, Yamaguchi H, Tomizaki T, Tsukihara T. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 16.Iwata S, Ostermeier C, Ludwig B, Michel H. Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- 17.Abramson J, Svensson-Ek M, Byrne B, Iwata S. Structure of cytochrome c oxidase: a comparison of the bacterial and mitochondrial enzymes. Biochim Biophys Acta. 2001;1544:1–9. doi: 10.1016/s0167-4838(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 18.Svensson-Ek M, Abramson J, Larsson G, Tornroth S, Brzezinski P, Iwata S. The X-ray crystal structures of wild-type and EQ(I-286) mutant cytochrome c oxidases from Rhodobacter sphaeroides. J Mol Biol. 2002;321:329–339. doi: 10.1016/s0022-2836(02)00619-8. [DOI] [PubMed] [Google Scholar]
- 19.Soulimane T, Buse G, Bourenkov GP, Bartunik HD, Huber R, Than ME. Structure and mechanism of the aberrant ba3-cytochrome c oxidase from Thermus thermophilus. EMBO J. 2000;19:1766–1776. doi: 10.1093/emboj/19.8.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abramson J, Riistama S, Larsson G, Jasaitis A, Svensson-Ek M, Laakkonen L, Puustinen A, Iwata S, Wikstrom M. The structure of the ubiquinol oxidase from Escherichia coli and its ubiquinone binding site. Nat Struct Biol. 2000;7:910–917. doi: 10.1038/82824. [DOI] [PubMed] [Google Scholar]
- 21.Ostermeier C, Harrenga A, Ermler U, Michel H. Structure at 2.7 A resolution of the Paracoccus denitrificans two-subunit cytochrome c oxidase complexed with an antibody Fv fragment. Proc Natl Acad Sci USA. 1997;94:10547–10553. doi: 10.1073/pnas.94.20.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin L, Hiser C, Mulichak A, Garavito RM, Ferguson-Miller S. Identification of conserved lipid/detergent-binding sites in a high-resolution structure of the membrane protein cytochrome c oxidase. Proc Natl Acad Sci USA. 2006;103:16117–16122. doi: 10.1073/pnas.0606149103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koepke J, Olkhova E, Angerer H, Muller H, Peng G, Michel H. High resolution crystal structure of Paracoccus denitrificans cytochrome c oxidase: new insights into the active site and the proton transfer pathways. Biochim Biophys Acta. 2009;1787:635–645. doi: 10.1016/j.bbabio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Albury MS, Elliott C, Moore AL. Towards a structural elucidation of the alternative oxidase in plants. Physiol Plant. 2009;137:316–327. doi: 10.1111/j.1399-3054.2009.01270.x. [DOI] [PubMed] [Google Scholar]
- 25.Kido Y, Shiba T, Inaoka DK, Sakamoto K, Nara T, Aoki T, Honma T, Tanaka A, Inoue M, Matsuoka S, Moore A, Harada S, Kita K. Crystallization and preliminary crystallographic analysis of cyanide-insensitive alternative oxidase from Trypanosoma brucei brucei. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66:275–278. doi: 10.1107/S1744309109054062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller MJ, Gennis RB. The purification and characterization of the cytochrome d terminal oxidase complex of the Escherichia coli aerobic respiratory chain. J Biol Chem. 1983;258:9159–9165. [PubMed] [Google Scholar]
- 27.Kita K, Konishi K, Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J Biol Chem. 1984;259:3375–3381. [PubMed] [Google Scholar]
- 28.Green GN, Fang H, Lin R-J, Newton G, Mather M, Georgiou CD, Gennis RB. The nucleotide sequence of the cyd locus encoding the two subunits of the cytochrome d terminal oxidase complex of Escherichia coli. J Biol Chem. 1988;263:13138–13143. [PubMed] [Google Scholar]
- 29.Poole RK. Oxygen reactions with bacterial oxidases and globins: binding, reduction and regulation. Anthonie van Leeuwenhoek. 1994;65:289–310. doi: 10.1007/BF00872215. [DOI] [PubMed] [Google Scholar]
- 30.Trumpower BL, Gennis RB. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: The enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu Rev Biochem. 1994;63:675–716. doi: 10.1146/annurev.bi.63.070194.003331. [DOI] [PubMed] [Google Scholar]
- 31.Junemann S. Cytochrome bd terminal oxidase. Biochim Biophys Acta. 1997;1321:107–127. doi: 10.1016/s0005-2728(97)00046-7. [DOI] [PubMed] [Google Scholar]
- 32.Borisov VB. Cytochrome bd: structure and properties. Biochemistry (Moscow) 1996;61:565–574. (translated from Biokhimiya (in Russian) (1996), 61, 786–799) [PubMed] [Google Scholar]
- 33.Tsubaki M, Hori H, Mogi T. Probing molecular structure of dioxygen reduction site of bacterial quinol oxidases through ligand binding to the redox metal centers. J Inorg Biochem. 2000;82:19–25. doi: 10.1016/s0162-0134(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 34.Miller MJ, Hermodson M, Gennis RB. The active form of the cytochrome d terminal oxidase complex of Escherichia coli is a heterodimer containing one copy of each of the two subunits. J Biol Chem. 1988;263:5235–5240. [PubMed] [Google Scholar]
- 35.Hill JJ, Alben JO, Gennis RB. Spectroscopic evidence for a heme-heme binuclear center in the cytochrome bd ubiquinol oxidase from Escherichia coli. Proc Natl Acad Sci USA. 1993;90:5863–5867. doi: 10.1073/pnas.90.12.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsubaki M, Hori H, Mogi T, Anraku Y. Cyanide-binding site of bd-type ubiquinol oxidase from Escherichia coli. J Biol Chem. 1995;270:28565–28569. doi: 10.1074/jbc.270.48.28565. [DOI] [PubMed] [Google Scholar]
- 37.Borisov V, Arutyunyan AM, Osborne JP, Gennis RB, Konstantinov AA. Magnetic circular dichroism used to examine the interaction of Escherichia coli cytochrome bd with ligands. Biochemistry. 1999;38:740–750. doi: 10.1021/bi981908t. [DOI] [PubMed] [Google Scholar]
- 38.Vos MH, Borisov VB, Liebl U, Martin J-L, Konstantinov AA. Femtosecond resolution of ligand-heme interactions in the high-affinity quinol oxidase bd: A di-heme active site? Proc Natl Acad Sci USA. 2000;97:1554–1559. doi: 10.1073/pnas.030528197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borisov VB, Sedelnikova SE, Poole RK, Konstantinov AA. Interaction of cytochrome bd with carbon monoxide at low and room temperatures: evidence that only a small fraction of heme b595 reacts with CO. J Biol Chem. 2001;276:22095–22099. doi: 10.1074/jbc.M011542200. [DOI] [PubMed] [Google Scholar]
- 40.Borisov VB, Liebl U, Rappaport F, Martin J-L, Zhang J, Gennis RB, Konstantinov AA, Vos MH. Interactions between heme d and heme b595 in quinol oxidase bd from Escherichia coli: a photoselection study using femtosecond spectroscopy. Biochemistry. 2002;41:1654–1662. doi: 10.1021/bi0158019. [DOI] [PubMed] [Google Scholar]
- 41.Belevich I, Borisov VB, Zhang J, Yang K, Konstantinov AA, Gennis RB, Verkhovsky MI. Time-resolved electrometric and optical studies on cytochrome bd suggest a mechanism of electron-proton coupling in the di-heme active site. Proc Natl Acad Sci USA. 2005;102:3657–3662. doi: 10.1073/pnas.0405683102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arutyunyan AM, Borisov VB, Novoderezhkin VI, Ghaim J, Zhang J, Gennis RB, Konstantinov AA. Strong excitonic interactions in the oxygen-reducing site of bd-type oxidase: the Fe-to-Fe distance between hemes d and b595 is 10 A. Biochemistry. 2008;47:1752–1759. doi: 10.1021/bi701884g. [DOI] [PubMed] [Google Scholar]
- 43.Rappaport F, Zhang J, Vos MH, Gennis RB, Borisov VB. Heme-heme and heme-ligand interactions in the di-heme oxygen-reducing site of cytochrome bd from Escherichia coli revealed by nanosecond absorption spectroscopy. Biochim Biophys Acta. 2010;1797:1657–1664. doi: 10.1016/j.bbabio.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller MJ, Gennis RB. The cytochrome d complex is a coupling site in the aerobic respiratory chain of Escherichia coli. J Biol Chem. 1985;260:14003–14008. [PubMed] [Google Scholar]
- 45.Puustinen A, Finel M, Haltia T, Gennis RB, Wikstrom M. Properties of the two terminal oxidases of Escherichia coli. Biochemistry. 1991;30:3936–3942. doi: 10.1021/bi00230a019. [DOI] [PubMed] [Google Scholar]
- 46.Jasaitis A, Borisov VB, Belevich NP, Morgan JE, Konstantinov AA, Verkhovsky MI. Electrogenic reactions of cytochrome bd. Biochemistry. 2000;39:13800–13809. doi: 10.1021/bi001165n. [DOI] [PubMed] [Google Scholar]
- 47.Belevich I, Borisov VB, Verkhovsky MI. Discovery of the true peroxy intermediate in the catalytic cycle of terminal oxidases by real-time measurement. J Biol Chem. 2007;282:28514–28519. doi: 10.1074/jbc.M705562200. [DOI] [PubMed] [Google Scholar]
- 48.Borisov VB, Belevich I, Bloch DA, Mogi T, Verkhovsky MI. Glutamate 107 in subunit I of cytochrome bd from Escherichia coli is part of a transmembrane intraprotein pathway conducting protons from the cytoplasm to the heme b595/heme d active site. Biochemistry. 2008;47:7907–7914. doi: 10.1021/bi800435a. [DOI] [PubMed] [Google Scholar]
- 49.Kolonay JF, Jr, Maier RJ. Formation of pH and potential gradients by the reconstituted Azotobacter vinelandii cytochrome bd respiratory protection oxidase. J Bacteriol. 1997;179:3813–3817. doi: 10.1128/jb.179.11.3813-3817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertsova YV, Bogachev AV, Skulachev VP. Generation of protonic potential by the bd-type quinol oxidase of Azotobacter vinelandii. FEBS Lett. 1997;414:369–372. doi: 10.1016/s0014-5793(97)01047-8. [DOI] [PubMed] [Google Scholar]
- 51.Ingledew WJ, Poole RK. The respiratory chains of Escherichia coli. Microbiol Rev. 1984;48:222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poole RK, Cook GM. Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv Microb Physiol. 2000;43:165–224. doi: 10.1016/s0065-2911(00)43005-5. [DOI] [PubMed] [Google Scholar]
- 53.Borisov VB, Verkhovsky MI. In: EcoSal - Escherichia coli and Salmonella: cellular and molecular biology. Bock A, RCI, Kaper JB, Karp PD, Neidhardt FC, Nystrom T, Slauch JM, Squires CL, Ussery D, editors. ASM Press; Washington, DC: 2009. http://www.ecosal.org. [Google Scholar]
- 54.Trutko SM, Evtushenko LI, Dorofeeva LV, Shlyapnikov MG, Gavrish EY, Suzina NE, Akimenko VK. Terminal oxidases in representatives of different genera of the family Microbacteriaceae. Microbiology (Moscow) 2003;72:301–307. [PubMed] [Google Scholar]
- 55.Blattner FR, Plunkett G, 3rd, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 56.Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell SC, Bron S, Brouillet S, Bruschi CV, Caldwell B, Capuano V, Carter NM, Choi SK, Codani JJ, Connerton IF, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 57.Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Umayam L, Gill SR, Nelson KE, Read TD, Tettelin H, Richardson D, Ermolaeva MD, Vamathevan J, Bass S, Qin H, Dragoi I, Sellers P, McDonald L, Utterback T, Fleishmann RD, Nierman WC, White O, Salzberg SL, Smith HO, Colwell RR, Mekalanos JJ, Venter JC, Fraser CM. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brooijmans RJ, de Vos WM, Hugenholtz J. Lactobacillus plantarum WCFS1 electron transport chains. Appl Environ Microbiol. 2009;75:3580–3585. doi: 10.1128/AEM.00147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sootsuwan K, Lertwattanasakul N, Thanonkeo P, Matsushita K, Yamada M. Analysis of the respiratory chain in Ethanologenic Zymomonas mobilis with a cyanide-resistant bd-type ubiquinol oxidase as the only terminal oxidase and its possible physiological roles. J Mol Microbiol Biotechnol. 2008;14:163–175. doi: 10.1159/000112598. [DOI] [PubMed] [Google Scholar]
- 60.Brochier-Armanet C, Talla E, Gribaldo S. The multiple evolutionary histories of dioxygen reductases: Implications for the origin and evolution of aerobic respiration. Mol Biol Evol. 2009;26:285–297. doi: 10.1093/molbev/msn246. [DOI] [PubMed] [Google Scholar]
- 61.Kranz RG, Gennis RB. Characterization of the cytochrome d terminal oxidase complex of Escherichia coli using polyclonal and monoclonal antibodies. J Biol Chem. 1984;259:7998–8003. [PubMed] [Google Scholar]
- 62.Lorence RM, Carter K, Gennis RB, Matsushita K, Kaback HR. Trypsin proteolysis of the cytochrome d complex of Escherichia coli selectively inhibits ubiquinol oxidase activity while not affecting N,N,N′,N′-tetramethyl-p-phenylenediamine oxidase activity. J Biol Chem. 1988;11:5271–5276. [PubMed] [Google Scholar]
- 63.Dueweke TJ, Gennis RB. Epitopes of monoclonal antibodies which inhibit ubiquinol oxidase activity of Escherichia coli cytochrome d complex localize a functional domain. J Biol Chem. 1990;265:4273–4277. [PubMed] [Google Scholar]
- 64.Dueweke TJ, Gennis RB. Proteolysis of the cytochrome d complex with trypsin and chymotrypsin localizes a quinol oxidase domain. Biochemistry. 1991;30:3401–3406. doi: 10.1021/bi00228a007. [DOI] [PubMed] [Google Scholar]
- 65.Matsumoto Y, Murai M, Fujita D, Sakamoto K, Miyoshi H, Yoshida M, Mogi T. Mass spectrometric analysis of the ubiquinol-binding site in cytochrome bd from Escherichia coli. J Biol Chem. 2006;281:1905–1912. doi: 10.1074/jbc.M508206200. [DOI] [PubMed] [Google Scholar]
- 66.Mogi T, Akimoto S, Endou S, Watanabe-Nakayama T, Mizuochi-Asai E, Miyoshi H. Probing the ubiquinol-binding site in cytochrome bd by site-directed mutagenesis. Biochemistry. 2006;45:7924–7930. doi: 10.1021/bi060192w. [DOI] [PubMed] [Google Scholar]
- 67.Osborne JP, Gennis RB. Sequence analysis of cytochrome bd oxidase suggests a revised topology for subunits I. Biochim Biophys Acta. 1999;1410:32–50. doi: 10.1016/s0005-2728(98)00171-6. [DOI] [PubMed] [Google Scholar]
- 68.Sakamoto J, Koga E, Mizuta T, Sato C, Noguchi S, Sone N. Gene structure and quinol oxidase activity of a cytochrome bd-type oxidase from Bacillus stearothermophilus. Biochim Biophys Acta. 1999;1411:147–158. doi: 10.1016/s0005-2728(99)00012-2. [DOI] [PubMed] [Google Scholar]
- 69.Kusumoto K, Sakiyama M, Sakamoto J, Noguchi S, Sone N. Menaquinol oxidase activity and primary structure of cytochrome bd from the amino-acid fermenting bacterium Corynebacterium glutamicum. Arch Microbiol. 2000;173:390–397. doi: 10.1007/s002030000161. [DOI] [PubMed] [Google Scholar]
- 70.Azarkina N, Siletsky S, Borisov V, von Wachenfeldt C, Hederstedt L, Konstantinov AA. A cytochrome bb′-type quinol oxidase in Bacillus subtilis strain 168. J Biol Chem. 1999;274:32810–32817. doi: 10.1074/jbc.274.46.32810. [DOI] [PubMed] [Google Scholar]
- 71.Cunningham L, Williams HD. Isolation and characterization of mutants defective in the cyanide-insensitive respiratory pathway of Pseudomonas aeruginosa. J Bacteriol. 1995;177:432–438. doi: 10.1128/jb.177.2.432-438.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zannoni D. The respiratory chains of pathogenic pseudomonads. Biochim Biophys Acta. 1989;975:299–316. doi: 10.1016/s0005-2728(89)80337-8. [DOI] [PubMed] [Google Scholar]
- 73.Cunningham L, Pitt M, Williams HD. The cioAB genes from Pseudomonas aeruginosa code for a novel cyanide-insensitive terminal oxidase related to the cytochrome bd quinol oxidases. Mol Microbiol. 1997;24:579–591. doi: 10.1046/j.1365-2958.1997.3561728.x. [DOI] [PubMed] [Google Scholar]
- 74.Quesada A, Guijo MI, Merchan F, Blazquez B, Igeno MI, Blasco R. Essential role of cytochrome bd-related oxidase in cyanide resistance of Pseudomonas pseudoalcaligenes CECT5344. Appl Environ Microbiol. 2007;73:5118–5124. doi: 10.1128/AEM.00503-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jackson RJ, Elvers KT, Lee LJ, Gidley MD, Wainwright LM, Lightfoot J, Park SF, Poole RK. Oxygen reactivity of both respiratory oxidases in Campylobacter jejuni: the cydAB genes encode a cyanide-resistant, low-affinity oxidase that is not of the cytochrome bd type. J Bacteriol. 2007;189:1604–1615. doi: 10.1128/JB.00897-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matsushita K, Yamada M, Shinagawa E, Adachi O, Ameyama M. Membrane-bound respiratory chain of Pseudomonas aeruginosa grown aerobically. A KCN-insensitive alternate oxidase chain and its energetics. J Biochem. 1983;93:1137–1144. doi: 10.1093/oxfordjournals.jbchem.a134239. [DOI] [PubMed] [Google Scholar]
- 77.Morales G, Ugidos A, Rojo F. Inactivation of the Pseudomonas putida cytochrome o ubiquinol oxidase leads to a significant change in the transcriptome and to increased expression of the CIO and cbb3–1 terminal oxidases. Environ Microbiol. 2006;8:1764–1774. doi: 10.1111/j.1462-2920.2006.01061.x. [DOI] [PubMed] [Google Scholar]
- 78.Voggu L, Schlag S, Biswas R, Rosenstein R, Rausch C, Gotz F. Microevolution of cytochrome bd oxidase in Staphylococci and its implication in resistance to respiratory toxins released by Pseudomonas. J Bacteriol. 2006;188:8079–8086. doi: 10.1128/JB.00858-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mogi T, Ano Y, Nakatsuka T, Toyama H, Muroi A, Miyoshi H, Migita CT, Ui H, Shiomi K, Omura S, Kita K, Matsushita K. Biochemical and spectroscopic properties of cyanide-insensitive quinol oxidase from Gluconobacter oxydans. J Biochem. 2009;146:263–271. doi: 10.1093/jb/mvp067. [DOI] [PubMed] [Google Scholar]
- 80.Mogi T, Miyoshi H. Properties of cytochrome bd plastoquinol oxidase from the cyanobacterium Synechocystis sp. PCC 6803. J Biochem. 2009;145:395–401. doi: 10.1093/jb/mvn179. [DOI] [PubMed] [Google Scholar]
- 81.Blumer C, Haas D. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch Microbiol. 2000;173:170–177. doi: 10.1007/s002039900127. [DOI] [PubMed] [Google Scholar]
- 82.Castric PA. Hydrogen cyanide production by Pseudomonas aeruginosa at reduced oxygen levels. Can J Microbiol. 1983;29:1344–1349. doi: 10.1139/m83-209. [DOI] [PubMed] [Google Scholar]
- 83.Goldfarb WB, Margraf H. Cyanide production by Pseudomonas aeruginosa. Ann Surg. 1967;165:104–110. doi: 10.1097/00000658-196701000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zlosnik JEA, Tavankar GR, Bundy JG, Mossialos D, O’Toole R, Williams HD. Investigation of the physiological relationship between the cyanide-insensitive oxidase and cyanide production in Pseudomonas aeruginosa. Microbiology. 2006;152:1407–1415. doi: 10.1099/mic.0.28396-0. [DOI] [PubMed] [Google Scholar]
- 85.Tavankar GR, Mossialos D, Williams HD. Mutation or overexpression of a terminal oxidase leads to a cell division defect and multiple antibiotic sensitivity in Pseudomonas aeruginosa. J Biol Chem. 2003;278:4524–4530. doi: 10.1074/jbc.M210355200. [DOI] [PubMed] [Google Scholar]
- 86.Baughn AD, Malamy MH. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature. 2004;427:441–444. doi: 10.1038/nature02285. [DOI] [PubMed] [Google Scholar]
- 87.Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, Gennaro ML. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc Natl Acad Sci USA. 2005;102:15629–15634. doi: 10.1073/pnas.0507850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loisel-Meyer S, Jimenez de Bagues MP, Kohler S, Liautard JP, Jubier-Maurin V. Differential use of the two high-oxygen-affinity terminal oxidases of Brucella suis for in vitro and intramacrophagic multiplication. Infect Immun. 2005;73:7768–7771. doi: 10.1128/IAI.73.11.7768-7771.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jones SA, Chowdhury FZ, Fabich AJ, Anderson A, Schreiner DM, House AL, Autieri SM, Leatham MP, Lins JJ, Jorgensen M, Cohen PS, Conway T. Respiration of Escherichia coli in the mouse intestine. Infect Immun. 2007;75:4891–4899. doi: 10.1128/IAI.00484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kelly MJS, Poole RK, Yates MG, Kennedy C. Cloning and mutagenesis of genes encoding the cytochrome bd terminal oxidase complex in Azotobacter vinelandii: Mutants deficient in the cytochrome d complex are unable to fix nitrogen in air. J Bacteriol. 1990;172:6010–6019. doi: 10.1128/jb.172.10.6010-6019.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hill S, Viollet S, Smith AT, Anthony C. Roles for enteric d-type cytochrome oxidase in N2 fixation and microaerobiosis. J Bacteriol. 1990;172:2071–2078. doi: 10.1128/jb.172.4.2071-2078.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith A, Hill S, Anthony C. The purification, characterization and role of the d-type cytochrome oxidase of Klebsiella pneumoniae during nitrogen fixation. J Gen Microbiol. 1990;136:171–180. doi: 10.1099/00221287-136-1-171. [DOI] [PubMed] [Google Scholar]
- 93.D’Mello R, Hill S, Poole RK. Determination of the oxygen affinities of terminal oxidases in Azotobacter vinelandii using the deoxygenation of oxyleghaemoglobin and oxymyoglobin: Cytochrome bd is a low-affinity oxidase. Microbiology. 1994;140:1395–1402. [Google Scholar]
- 94.Kaminski PA, Kitts CL, Zimmerman Z, Ludwig RA. Azorhizobium caulinodans uses both cytochrome bd (quinol) and cytochrome cbb3 (cytochrome c) terminal oxidases for symbiotic N2 fixation. JBacteriol. 1996;178:5989–5994. doi: 10.1128/jb.178.20.5989-5994.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Juty NS, Moshiri F, Merrick M, Anthony C, Hill S. The Klebsiella pneumoniae cytochrome bd′ terminal oxidase complex and its role in microaerobic nitrogen fixation. Microbiology. 1997;143:2673–2683. doi: 10.1099/00221287-143-8-2673. [DOI] [PubMed] [Google Scholar]
- 96.Poole RK, Hill S. Respiratory protection of nitrogenase activity in Azotobacter vinelandii - roles of the terminal oxidases. Bioscience Reports. 1997;17:307–317. doi: 10.1023/a:1027336712748. [DOI] [PubMed] [Google Scholar]
- 97.Bertsova YV, Demin OV, Bogachev AV. Respiratory protection of nitrogenase complex in Azotobacter vinelandii. Uspekhi biologicheskoj khimii (in Russian) 2005;45:205–234. [Google Scholar]
- 98.Dincturk HB, Demir V, Aykanat T. Bd oxidase homologue of photosynthetic purple sulfur bacterium Allochromatium vinosum is co-transcribed with a nitrogen fixation related gene. Antonie van Leeuwenhoek. 2011;99:211–220. doi: 10.1007/s10482-010-9478-5. [DOI] [PubMed] [Google Scholar]
- 99.Hassani BK, Steunou AS, Liotenberg S, Reiss-Husson F, Astier C, Ouchane S. Adaptation to oxygen: role of terminal oxidases in photosynthesis initiation in the purple photosynthetic bacterium, Rubrivivax gelatinosus. J Biol Chem. 2010;285:19891–19899. doi: 10.1074/jbc.M109.086066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Way SS, Sallustio S, Magliozzo RS, Goldberg MB. Impact of either elevated or decreased levels of cytochrome bd expression on Shigella flexneri virulence. J Bacteriol. 1999;181:1229–1237. doi: 10.1128/jb.181.4.1229-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Endley S, McMurray D, Ficht TA. Interruption of the cydB locus in Brucella abortus attenuates intracellular survival and virulence in the mouse model of infection. J Bacteriol. 2001;183:2454–2462. doi: 10.1128/JB.183.8.2454-2462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamamoto Y, Poyart C, Trieu-Cuot P, Lamberet G, Gruss A, Gaudu P. Respiration metabolism of Group B Streptococcus is activated by environmental haem and quinone and contributes to virulence. Mol Microbiol. 2005;56:525–534. doi: 10.1111/j.1365-2958.2005.04555.x. [DOI] [PubMed] [Google Scholar]
- 103.Zhang-Barber L, Turner AK, Martin G, Frankel G, Dougan G, Barrow PA. Influence of genes encoding proton-translocating enzymes on suppression of Salmonella typhimurium growth and colonization. J Bacteriol. 1997;179:7186–7190. doi: 10.1128/jb.179.22.7186-7190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turner AK, Barber LZ, Wigley P, Muhammad S, Jones MA, Lovell MA, Hulme S, Barrow PA. Contribution of proton-translocating proteins to the virulence of Salmonella enterica Serovars Typhimurium, Gallinarum, and Dublin in chickens and mice. Infect Immun. 2003;71:3392–3401. doi: 10.1128/IAI.71.6.3392-3401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Larsen MH, Kallipolitis BH, Christiansen JK, Olsen JE, Ingmer H. The response regulator ResD modulates virulence gene expression in response to carbohydrates in Listeria monocytogenes. Mol Microbiol. 2006;61:1622–1635. doi: 10.1111/j.1365-2958.2006.05328.x. [DOI] [PubMed] [Google Scholar]
- 106.Borisov VB, Forte E, Konstantinov AA, Poole RK, Sarti P, Giuffre A. Interaction of the bacterial terminal oxidase cytochrome bd with nitric oxide. FEBS Lett. 2004;576:201–204. doi: 10.1016/j.febslet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 107.Borisov VB, Forte E, Sarti P, Brunori M, Konstantinov AA, Giuffre A. Redox control of fast ligand dissociation from Escherichia coli cytochrome bd. Biochem Biophys Res Commun. 2007;355:97–102. doi: 10.1016/j.bbrc.2007.01.118. [DOI] [PubMed] [Google Scholar]
- 108.Mason MG, Shepherd M, Nicholls P, Dobbin PS, Dodsworth KS, Poole RK, Cooper CE. Cytochrome bd confers nitric oxide resistance to Escherichia coli. Nat Chem Biol. 2009;5:94–96. doi: 10.1038/nchembio.135. [DOI] [PubMed] [Google Scholar]
- 109.Forte E, Borisov VB, Konstantinov AA, Brunori M, Giuffre A, Sarti P. Cytochrome bd,a key oxidase in bacterial survival and tolerance to nitrosative stress. Ital J Biochem. 2007;56:265–269. [PubMed] [Google Scholar]
- 110.Borisov VB, Forte E, Sarti P, Brunori M, Konstantinov AA, Giuffre A. Nitric oxide reacts with the ferryl-oxo catalytic intermediate of the CuB-lacking cytochrome bd terminal oxidase. FEBS Lett. 2006;580:4823–4826. doi: 10.1016/j.febslet.2006.07.072. [DOI] [PubMed] [Google Scholar]
- 111.Borisov VB, Forte E, Giuffre A, Konstantinov A, Sarti P. Reaction of nitric oxide with the oxidized di-heme and heme-copper oxygen-reducing centers of terminal oxidases: Different reaction pathways and end-products. J Inorg Biochem. 2009;103:1185–1187. doi: 10.1016/j.jinorgbio.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 112.Lindqvist A, Membrillo-Hernandez J, Poole RK, Cook GM. Roles of respiratory oxidases in protecting Escherichia coli K12 from oxidative stress. Antonie Van Leeuwenhoek. 2000;78:23–31. doi: 10.1023/a:1002779201379. [DOI] [PubMed] [Google Scholar]
- 113.Borisov VB, Davletshin AI, Konstantinov AA. Peroxidase activity of cytochrome bd from Escherichia coli. Biochemistry (Moscow) 2010;75:428–436. doi: 10.1134/s000629791004005x. (translated from Biokhimiya (in Russian) (2010), 75, 520–530) [DOI] [PubMed] [Google Scholar]
- 114.Korshunov S, Imlay JA. Two sources of endogenous hydrogen peroxide in Escherichia coli. Mol Microbiol. 2010;75:1389–1401. doi: 10.1111/j.1365-2958.2010.07059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huycke MM, Moore D, Joyce W, Wise P, Shepard L, Kotake Y, Gilmore MS. Extracellular superoxide production by Enterococcus faecalis requires demethylmenaquinone and is attenuated by functional terminal quinol oxidases. Mol Microbiol. 2001;42:729–740. doi: 10.1046/j.1365-2958.2001.02638.x. [DOI] [PubMed] [Google Scholar]
- 116.Heintz D, Gallien S, Wischgoll S, Ullmann AK, Schaeffer C, Kretzschmar AK, van Dorsselaer A, Boll M. Differential membrane proteome analysis reveals novel proteins involved in the degradation of aromatic compounds in Geobacter metallireducens. Mol Cell Proteomics. 2009;8:2159–2169. doi: 10.1074/mcp.M900061-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Edwards SE, Loder CS, Wu G, Corker H, Bainbridge BW, Hill S, Poole RK. Mutation of cytochrome bd quinol oxidase results in reduced stationary phase survival, iron deprivation, metal toxicity and oxidative stress in Azotobacter vinelandii. FEMS Microbiol Lett. 2000;185:71–77. doi: 10.1111/j.1574-6968.2000.tb09042.x. [DOI] [PubMed] [Google Scholar]
- 118.Bader M, Muse W, Ballou DP, Gassner C, Bardwell JCA. Oxidative protein folding is driven by the electron transport system. Cell. 1999;98:217–227. doi: 10.1016/s0092-8674(00)81016-8. [DOI] [PubMed] [Google Scholar]
- 119.Mobius K, Arias-Cartin R, Breckau D, Hannig AL, Riedmann K, Biedendieck R, Schroder S, Becher D, Magalon A, Moser J, Jahn M, Jahn D. Heme biosynthesis is coupled to electron transport chains for energy generation. Proc Natl Acad Sci USA. 2010;107:10436–10441. doi: 10.1073/pnas.1000956107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rice CW, Hempfling WP. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978;134:115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cotter PA, Chepuri V, Gennis RB, Gunsalus RP. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. J Bacteriol. 1990;172:6333–6338. doi: 10.1128/jb.172.11.6333-6338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fu H-A, Iuchi S, Lin ECC. The requirement of ArcA and Fnr for peak expression of the cyd operon in Escherichia coli under microaerobic conditions. Mol Gen Genet. 1991;226:209–213. doi: 10.1007/BF00273605. [DOI] [PubMed] [Google Scholar]
- 123.Avetisyan AV, Bogachev AV, Murtasina RA, Skulachev VP. Involvement of a d-type oxidase in the Na+-motive respiratory chain of Escherichia coli growing under low ΔμH+ conditions. FEBS Lett. 1992;306:199–202. doi: 10.1016/0014-5793(92)80999-w. [DOI] [PubMed] [Google Scholar]
- 124.Wall D, Delaney JM, Fayet O, Lipinska B, Yamamoto T, Georgopoulos C. arc-Dependent thermal regulation and extragenic suppression of the Escherichia coli cytochrome d operon. J Bacteriol. 1992;174:6554–6562. doi: 10.1128/jb.174.20.6554-6562.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Delaney JM, Wall D, Georgopoulos C. Molecular characterization of the Escherichia coli htrD gene: Cloning, sequence, regulation, and involvement with cytochrome d oxidase. J Bacteriol. 1993;175:166–175. doi: 10.1128/jb.175.1.166-175.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ashcroft JR, Haddock BA. Synthesis of alternative membrane-bound redox carriers during aerobic growth of Escherichia coli in the presence of potassium cyanide. Biochem J. 1975;148:349–352. doi: 10.1042/bj1480349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brekasis D, Paget MS. A novel sensor of NADH/NAD+ redox poise in Streptomyces coelicolor A3(2) EMBO J. 2003;22:4856–4865. doi: 10.1093/emboj/cdg453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bogachev AV, Murtazina RA, Skulachev VP. Cytochrome d induction in Escherichia coli growing under unfavorable conditions. FEBS Lett. 1993;336:75–78. doi: 10.1016/0014-5793(93)81612-4. [DOI] [PubMed] [Google Scholar]
- 129.Bogachev AV, Murtazine RA, Shestopalov AI, Skulachev VP. Induction of the Escherichia coli cytochrome d by low ΔμH+ and by sodium ions. Eur J Biochem. 1995;232:304–308. doi: 10.1111/j.1432-1033.1995.tb20812.x. [DOI] [PubMed] [Google Scholar]
- 130.Tamegai H, Kato C, Horikoshi K. Pressure-regulated respiratory system in barotolerant bacterium, Shewanella sp. strain DSS12. J Biochem Mol Biol Biophys. 1998;1:213–220. [Google Scholar]
- 131.Tamegai H, Kawano H, Ishii A, Chikuma S, Nakasone K, Kato C. Pressure-regulated biosynthesis of cytochrome bd in piezo- and psychrophilic deep-sea bacterium Shewanella violacea DSS12. Extremophiles. 2005;9:247–253. doi: 10.1007/s00792-005-0439-2. [DOI] [PubMed] [Google Scholar]
- 132.Poole RK, Williams HD, Downie JA, Gibson F. Mutations affecting the cytochrome d-containing oxidase complex of Escherichia coli K12: Identification and mapping of a fourth locus,cydD. J Gen Microbiol. 1989;135:1865–1874. doi: 10.1099/00221287-135-7-1865. [DOI] [PubMed] [Google Scholar]
- 133.Macinga DR, Rather PN. aarD, a Providencia stuartii homologue of cydD: role in 2′-N-acetyltransferase expression, cell morphology and growth in the presence of an extracellular factor. Mol Microbiol. 1996;19:511–520. doi: 10.1046/j.1365-2958.1996.385912.x. [DOI] [PubMed] [Google Scholar]
- 134.Cook GM, Loder C, Soballe B, Stafford GP, Membrillo-Hernandez J, Poole RK. A factor produced by Escherichia coli K-12 inhibits the growth of E. coli mutants defective in the cytochrome bd quinol oxidase complex: enterochelin rediscovered. Microbiology. 1998;144:3297–3308. doi: 10.1099/00221287-144-12-3297. [DOI] [PubMed] [Google Scholar]
- 135.Siegele DA, Kolter R. Isolation and characterization of an Escherichia coli mutant defective in resuming growth after starvation. Genes Dev. 1993;7:2629–2640. doi: 10.1101/gad.7.12b.2629. [DOI] [PubMed] [Google Scholar]
- 136.Siegele DA, Imlay KR, Imlay JA. The stationary-phase-exit defect of cydC (surB) mutants is due to the lack of a functional terminal cytochrome oxidase. J Bacteriol. 1996;178:6091–6096. doi: 10.1128/jb.178.21.6091-6096.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mogi T, Ui H, Shiomi K, Omura S, Miyoshi H, Kita K. Antibiotics LL-Z1272 identified as novel inhibitors discriminating bacterial and mitochondrial quinol oxidases. Biochim Biophys Acta. 2009;1787:129–133. doi: 10.1016/j.bbabio.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 138.Mogi T, Kita K. Gramicidin S and polymyxins: the revival of cationic cyclic peptide antibiotics. Cell Mol Life Sci. 2009;66:3821–3826. doi: 10.1007/s00018-009-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borisov VB. Defects in mitochondrial respiratory complexes III, IV, and human pathologies. Mol Aspects Med. 2002;23:385–412. doi: 10.1016/s0098-2997(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 140.Borisov VB. Mutations in respiratory chain complexes and human diseases. Ital J Biochem. 2004;53:34–40. [PubMed] [Google Scholar]
- 141.Gavrikova EV, Grivennikova VG, Borisov VB, Cecchini G, Vinogradov AD. Assembly of a chimeric respiratory chain from bovine heart submitochondrial particles and cytochrome bd terminal oxidase of Escherichia coli. FEBS Lett. 2009;583:1287–1291. doi: 10.1016/j.febslet.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Perales-Clemente E, Bayona-Bafaluy MP, Perez-Martos A, Barrientos A, Fernandez-Silva P, Enriquez JA. Restoration of electron transport without proton pumping in mammalian mitochondria. Proc Natl Acad Sci USA. 2008;105:18735–18739. doi: 10.1073/pnas.0810518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dassa EP, Dufour E, Goncalves S, Paupe V, Hakkaart GA, Jacobs HT, Rustin P. Expression of the alternative oxidase complements cytochrome c oxidase deficiency in human cells. EMBO Mol Med. 2009;1:30–36. doi: 10.1002/emmm.200900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dassa EP, Dufour E, Goncalves S, Jacobs HT, Rustin P. The alternative oxidase, a tool for compensating cytochrome c oxidase deficiency in human cells. Physiol Plant. 2009;137:427–434. doi: 10.1111/j.1399-3054.2009.01248.x. [DOI] [PubMed] [Google Scholar]
- 145.Mogi T, Ui H, Shiomi K, Omura S, Kita K. Gramicidin S identified as a potent inhibitor for cytochrome bd-type quinol oxidase. FEBS Lett. 2008;582:2299–2302. doi: 10.1016/j.febslet.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 146.Calhoun MW, Newton G, Gennis RB. E. coli map. Physical map locations of genes encoding components of the aerobic respiratory chain of Escherichia coli. J Bacteriol. 1991;173:1569–1570. doi: 10.1128/jb.173.5.1569-1570.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kranz RG, Barassi CA, Miller MJ, Green GN, Gennis RB. Immunological characterization of an E. coli strain which is lacking cytochrome d. J Bacteriol. 1983;156:115–121. doi: 10.1128/jb.156.1.115-121.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bachmann BJ. Linkage map of Escherichia coli K-12, Edition 8. Microbiol Rev. 1990;54:130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Green GN, Kranz JE, Gennis RB. Cloning the cyd gene locus coding for the cytochrome d complex of Escherichia coli. Gene. 1984;32:99–106. doi: 10.1016/0378-1119(84)90037-4. [DOI] [PubMed] [Google Scholar]
- 150.Lorence RM, Koland JG, Gennis RB. Coulometric and spectroscopic analysis of the purified cytochrome d complex of Escherichia coli: Evidence for the identification of “cytochrome a1” as cytochrome b595. Biochemistry. 1986;25:2314–2321. doi: 10.1021/bi00357a003. [DOI] [PubMed] [Google Scholar]
- 151.Newton G, Gennis RB. In vivo assembly of the cytochrome d terminal oxidase complex of Escherichia coli from genes encoding the two subunits expressed on separate plasmids. Biochim Biophys Acta. 1991;1089:8–12. doi: 10.1016/0167-4781(91)90077-y. [DOI] [PubMed] [Google Scholar]
- 152.Green GN, Lorence RM, Gennis RB. Specific overproduction and purification of the cytochrome b558 component of the cytochrome d complex from Escherichia coli. Biochemistry. 1986;25:2309–2314. doi: 10.1021/bi00357a002. [DOI] [PubMed] [Google Scholar]
- 153.Georgiou CD, Fang H, Gennis RB. Identification of the cydC locus required for the expression of the functional form of the cytochrome d terminal oxidase complex in Escherichia coli. J Bacteriol. 1987;169:2107–2112. doi: 10.1128/jb.169.5.2107-2112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Poole RK, Hatch L, Cleeter MWJ, Gibson F, Cox GB, Wu G. Cytochrome bd biosynthesis in Escherichia coli: The sequences of the cydC and cydD genes suggest that they encode the components of an ABC membrane transporter. Mol Microbiol. 1993;10:421–430. [PubMed] [Google Scholar]
- 155.Bebbington KJ, Williams HD. Investigation of the role of the cydD gene product in production of a functional cytochrome d oxidase in Escherichia coli. FEMS Microbiol Lett. 1993;112:19–24. doi: 10.1111/j.1574-6968.1993.tb06417.x. [DOI] [PubMed] [Google Scholar]
- 156.Poole RK, Gibson F, Wu G. The cydD gene product, component of a heterodimeric ABC transporter, is required for assembly of periplasmic cytochrome c and of cytochrome bd in Escherichia coli. FEMS Microbiol Lett. 1994;117:217–224. doi: 10.1111/j.1574-6968.1994.tb06768.x. [DOI] [PubMed] [Google Scholar]
- 157.Pittman MS, Robinson HC, Poole RK. A bacterial glutathione transporter (Escherichia coli CydDC) exports reductant to the periplasm. J Biol Chem. 2005;280:32254–32261. doi: 10.1074/jbc.M503075200. [DOI] [PubMed] [Google Scholar]
- 158.Stenberg F, Chovanec P, Maslen SL, Robinson CV, Ilag LL, von Heijne G, Daley DO. Protein complexes of the Escherichia coli cell envelope. J Biol Chem. 2005;280:34409–34419. doi: 10.1074/jbc.M506479200. [DOI] [PubMed] [Google Scholar]
- 159.Mogi T, Mizuochi-Asai E, Endou S, Akimoto S, Nakamura H. Role of a putative third subunit YhcB on the assembly and function of cytochrome bd-type ubiquinol oxidase from Escherichia coli. Biochim Biophys Acta. 2006;1757:860–864. doi: 10.1016/j.bbabio.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 160.Dassa J, Fsihi H, Marck C, Dion M, Kieffer-Bontemps M, Boquet PL. A new oxygen-regulated operon in Escherichia coli comprises the genes for a putative third cytochrome oxidase and for pH 2.5 acid phosphatase (appA) Mol Gen Genet. 1991;229:341–352. doi: 10.1007/BF00267454. [DOI] [PubMed] [Google Scholar]
- 161.Tseng C-P, Albrecht J, Gunsalus RP. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J Bacteriol. 1996;178:1094–1098. doi: 10.1128/jb.178.4.1094-1098.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Alexeeva S, Hellingwerf K, Teixeira de Mattos MJ. Quantitative assessment of oxygen availability: Perceived aerobiosis and its effect on flux distribution in the respiratory chain of Escherichia coli. J Bacteriol. 2002;184:1402–1406. doi: 10.1128/JB.184.5.1402-1406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Iuchi S, Chepuri V, Fu HA, Gennis RB, Lin EC. Requirement for terminal cytochromes in generation of the aerobic signal for the arc regulatory system in Escherichia coli: Study utilizing deletions and lac fusions of cyo and cyd. J Bacteriol. 1990;172:6020–6025. doi: 10.1128/jb.172.10.6020-6025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cotter PA, Gunsalus RP. Contribution of the fnr and arcA gene products in coordinate regulation of cytochrome o and d oxidase (cyoABCDE and cydAB) genes in Escherichia coli. FEMS Microbiol Lett. 1992;91:31–36. doi: 10.1016/0378-1097(92)90558-6. [DOI] [PubMed] [Google Scholar]
- 165.Gunsalus RP. Control of electron flow in Escherichia coli: Coordinated transcription of respiratory pathway genes. J Bacteriol. 1992;174:7069–7074. doi: 10.1128/jb.174.22.7069-7074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Unden G, Bongaerts J. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta. 1997;1320:217–234. doi: 10.1016/s0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 167.Cotter PA, Melville SB, Albrecht JA, Gunsalus RP. Aerobic regulation of cytochrome d oxidase (cydAB) operon expression in Escherichia coli: roles of Fnr and ArcA in repression and activation. Mol Microbiol. 1997;25:605–615. doi: 10.1046/j.1365-2958.1997.5031860.x. [DOI] [PubMed] [Google Scholar]
- 168.Govantes F, Albrecht JA, Gunsalus RP. Oxygen regulation of the Escherichia coli cytochrome d oxidase (cydAB) operon: roles of multiple promoters and the Fnr-1 and Fnr-2 binding sites. Mol Microbiol. 2000;37:1456–1469. doi: 10.1046/j.1365-2958.2000.02100.x. [DOI] [PubMed] [Google Scholar]
- 169.Shalel-Levanon S, San KY, Bennett GN. Effect of oxygen, and ArcA and FNR regulators on the expression of genes related to the electron transfer chain and the TCA cycle in Escherichia coli. Metabolic Engineering. 2005;7:364–374. doi: 10.1016/j.ymben.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 170.Lynch AS, Lin ECC. In: Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. 2. Neidhardt FCea., editor. ASM Press; Washington, D.C: 1996. pp. 1526–1538. [Google Scholar]
- 171.Georgellis D, Kwon O, Lin EC. Quinones as the redox signal for the arc two-component system of bacteria. Science. 2001;292:2314–2316. doi: 10.1126/science.1059361. [DOI] [PubMed] [Google Scholar]
- 172.Georgellis D, Kwon O, Lin EC. Amplification of signaling activity of the arc two-component system of Escherichia coli by anaerobic metabolites. An in vitro study with different protein modules. J Biol Chem. 1999;274:35950–35954. doi: 10.1074/jbc.274.50.35950. [DOI] [PubMed] [Google Scholar]
- 173.Alexeeva S, Hellingwerf KJ, Teixeira de Mattos MJ. Requirement of ArcA for redox regulation in Escherichia coli under microaerobic but not anaerobic or aerobic conditions. J Bacteriol. 2003;185:204–209. doi: 10.1128/JB.185.1.204-209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kiley PJ, Beinert H. Oxygen sensing by the global regulator, FNR: the role of the iron-sulfur cluster. FEMS Microbiol Rev. 1998;22:341–352. doi: 10.1111/j.1574-6976.1998.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 175.Overton TW, Griffiths L, Patel MD, Hobman JL, Penn CW, Cole JA, Constantinidou C. Microarray analysis of gene regulation by oxygen, nitrate, nitrite, FNR, NarL and NarP during anaerobic growth of Escherichia coli: new insights into microbial physiology. Biochem Soc Trans. 2006;34:104–107. doi: 10.1042/BST0340104. [DOI] [PubMed] [Google Scholar]
- 176.Becker S, Holighaus G, Gabrielczyk T, Unden G. O2 as the regulatory signal for FNR-dependent gene regulation in Escherichia coli. J Bacteriol. 1996;178:4515–4521. doi: 10.1128/jb.178.15.4515-4521.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Atlung T, Brondsted L. Role of the transcriptional activator AppY in regulation of the cyx appA operon of Escherichia coli by anaerobiosis, phosphate starvation, and growth phase. J Bacteriol. 1994;176:5414–5422. doi: 10.1128/jb.176.17.5414-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brondsted L, Atlung T. Effect of growth conditions on expression of the acid phosphatase (cyx-appA) operon and the appY gene,which encodes a transcriptional activator of Escherichia coli. J Bacteriol. 1996;178:1556–1564. doi: 10.1128/jb.178.6.1556-1564.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sturr MG, Krulwich TA, Hicks DB. Purification of a cytochrome bd terminal oxidase encoded by the Escherichia coli app locus from a Δcyo Δcyd strain complemented by genes from Bacillus firmus OF4. J Bacteriol. 1996;176:1742–1749. doi: 10.1128/jb.178.6.1742-1749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bekker M, de Vries S, Ter Beek A, Hellingwerf KJ, de Mattos MJ. Respiration of Escherichia coli can be fully uncoupled via the nonelectrogenic terminal cytochrome bd-II oxidase. J Bacteriol. 2009;191:5510–5517. doi: 10.1128/JB.00562-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shepherd M, Sanguinetti G, Cook GM, Poole RK. Compensations for diminished terminal oxidase activity in Escherichia coli: cytochrome bd-II-mediated respiration and glutamate metabolism. J Biol Chem. 2010;285:18464–18472. doi: 10.1074/jbc.M110.118448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu G, Cruz-Ramos H, Hill S, Green J, Sawers G, Poole RK. Regulation of cytochrome bd expression in the obligate aerobe Azotobacter vinelandii by CydR (Fnr). Sensitivity to oxygen, reactive oxygen species, and nitric oxide. J Biol Chem. 2000;275:4679–4686. doi: 10.1074/jbc.275.7.4679. [DOI] [PubMed] [Google Scholar]
- 183.Schau M, Chen Y, Hulett FM. Bacillus subtilis YdiH is a direct negative regulator of the cydABCD operon. J Bacteriol. 2004;186:4585–4595. doi: 10.1128/JB.186.14.4585-4595.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Larsson JT, Rogstam A, von Wachenfeldt C. Coordinated patterns of cytochrome bd and lactate dehydrogenase expression in Bacillus subtilis. Microbiology. 2005;151:3323–3335. doi: 10.1099/mic.0.28124-0. [DOI] [PubMed] [Google Scholar]
- 185.Puri-Taneja A, Schau M, Chen Y, Hulett FM. Regulators of the Bacillus subtilis cydABCD operon: identification of a negative regulator, CcpA, and a positive regulator, ResD. J Bacteriol. 2007;189:3348–3358. doi: 10.1128/JB.00050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Swem LR, Elsen S, Bird TH, Swem DL, Koch HG, Myllykallio H, Daldal F, Bauer CE. The RegB/RegA two-component regulatory system controls synthesis of photosynthesis and respiratory electron transfer components in Rhodobacter capsulatus. J Mol Biol. 2001;309:121–138. doi: 10.1006/jmbi.2001.4652. [DOI] [PubMed] [Google Scholar]
- 187.Lemos RS, Gomes CM, Santana M, LeGall J, Xavier AV, Teixeira M. The ‘strict’ anaerobe Desulfovibrio gigas contains a membrane-bound oxygen-reducing respiratory chain. FEBS Lett. 2001;496:40–43. doi: 10.1016/s0014-5793(01)02399-7. [DOI] [PubMed] [Google Scholar]
- 188.Lemos RS, Gomes CM, LeGall J, Xavier AV, Teixeira M. The quinol:fumarate oxidoreductase from the sulphate reducing bacterium Desulfovibrio gigas: spectroscopic and redox studies. J Bioenerg Biomembr. 2002;34:21–30. doi: 10.1023/a:1013814619023. [DOI] [PubMed] [Google Scholar]
- 189.Machado P, Felix R, Rodrigues R, Oliveira S, Rodrigues-Pousada C. Characterization and expression analysis of the cytochrome bd oxidase operon from Desulfovibrio gigas. Curr Microbiol. 2006;52:274–281. doi: 10.1007/s00284-005-0165-0. [DOI] [PubMed] [Google Scholar]
- 190.Santana M. Presence and expression of terminal oxygen reductases in strictly anaerobic sulfate-reducing bacteria isolated from salt-marsh sediments. Anaerobe. 2008;14:145–156. doi: 10.1016/j.anaerobe.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 191.Das A, Silaghi-Dumitrescu R, Ljungdahl LG, Kurtz DM., Jr Cytochrome bd oxidase, oxidative stress, and dioxygen tolerance of the strictly anaerobic bacterium Moorella thermoacetica. J Bacteriol. 2005;187:2020–2029. doi: 10.1128/JB.187.6.2020-2029.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li H, Jubelirer S, Garcia Costas AM, Frigaard NU, Bryant DA. Multiple antioxidant proteins protect Chlorobaculum tepidum against oxygen and reactive oxygen species. Arch Microbiol. 2009;191:853–867. doi: 10.1007/s00203-009-0514-7. [DOI] [PubMed] [Google Scholar]
- 193.Castresana J. Comparative genomics and bioenergetics. Biochim Biophys Acta. 2001;1506:147–162. doi: 10.1016/s0005-2728(01)00227-4. [DOI] [PubMed] [Google Scholar]
- 194.Hao W, Golding GB. Asymmetrical evolution of cytochrome bd subunits. J Mol Evol. 2006;62:132–142. doi: 10.1007/s00239-005-0005-7. [DOI] [PubMed] [Google Scholar]
- 195.Lenn T, Leake MC, Mullineaux CW. Clustering and dynamics of cytochrome bd-I complexes in the Escherichia coli plasma membrane in vivo. Mol Microbiol. 2008;70:1397–1407. doi: 10.1111/j.1365-2958.2008.06486.x. [DOI] [PubMed] [Google Scholar]
- 196.Lenn T, Leake MC, Mullineaux CW. Are Escherichia coli OXPHOS complexes concentrated in specialized zones within the plasma membrane? Biochem Soc Trans. 2008;36:1032–1036. doi: 10.1042/BST0361032. [DOI] [PubMed] [Google Scholar]
- 197.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 198.Howitt CA, Vermaas WF. Quinol and cytochrome oxidases in the cyanobacterium Synechocystis sp. PCC 6803. Biochemistry. 1998;37:17944–17951. doi: 10.1021/bi981486n. [DOI] [PubMed] [Google Scholar]
- 199.Schneider D, Berry S, Rich P, Seidler A, Rogner M. A regulatory role of the PetM subunit in a cyanobacterial cytochrome b6f complex. J Biol Chem. 2001;276:16780–16785. doi: 10.1074/jbc.M009503200. [DOI] [PubMed] [Google Scholar]
- 200.Pils D, Schmetterer G. Characterization of three bioenergetically active respiratory terminal oxidases in the cyanobacterium Synechocystis sp. strain PCC 6803. FEMS Microbiol Lett. 2001;203:217–222. doi: 10.1111/j.1574-6968.2001.tb10844.x. [DOI] [PubMed] [Google Scholar]
- 201.Berry S, Schneider D, Vermaas WF, Rogner M. Electron transport routes in whole cells of Synechocystis sp. strain PCC 6803: the role of the cytochrome bd-type oxidase. Biochemistry. 2002;41:3422–3429. doi: 10.1021/bi011683d. [DOI] [PubMed] [Google Scholar]
- 202.Pils D, Wilken C, Valladares A, Flores E, Schmetterer G. Respiratory terminal oxidases in the facultative chemoheterotrophic and dinitrogen fixing cyanobacterium Anabaena variabilis strain ATCC 29413: characterization of the cox2 locus. Biochim Biophys Acta. 2004;1659:32–45. doi: 10.1016/j.bbabio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 203.Schultze M, Forberich B, Rexroth S, Dyczmons NG, Roegner M, Appel J. Localization of cytochrome b6f complexes implies an incomplete respiratory chain in cytoplasmic membranes of the cyanobacterium Synechocystis sp. PCC 6803. Biochim Biophys Acta. 2009;1787:1479–1485. doi: 10.1016/j.bbabio.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 204.Berry S, Bolychevtseva YV, Rogner M, Karapetyan NV. Photosynthetic and respiratory electron transport in the alkaliphilic cyanobacterium Arthrospira (Spirulina) platensis. Photosynth Res. 2003;78:67–76. doi: 10.1023/A:1026012719612. [DOI] [PubMed] [Google Scholar]
- 205.Kufryk GI, Vermaas WF. Sll1717 affects the redox state of the plastoquinone pool by modulating quinol oxidase activity in thylakoids. J Bacteriol. 2006;188:1286–1294. doi: 10.1128/JB.188.4.1286-1294.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gutthann F, Egert M, Marques A, Appel J. Inhibition of respiration and nitrate assimilation enhances photohydrogen evolution under low oxygen concentrations in Synechocystis sp. PCC 6803. Biochim Biophys Acta. 2007;1767:161–169. doi: 10.1016/j.bbabio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 207.Tsunoyama Y, Bernat G, Dyczmons NG, Schneider D, Rogner M. Multiple Rieske proteins enable short- and long-term light adaptation of Synechocystis sp. PCC 6803. J Biol Chem. 2009;284:27875–27883. doi: 10.1074/jbc.M109.011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Peschek GA, Wastyn M, Fromwald S, Mayer B. Occurrence of heme O in photoheterotrophically growing, semi-anaerobic cyanobacterium Synechocystis sp. PCC6803. FEBS Lett. 1995;371:89–93. doi: 10.1016/0014-5793(95)00821-p. [DOI] [PubMed] [Google Scholar]
- 209.Fromwald S, Zoder R, Wastyn M, Lubben M, Peschek GA. Extended heme promiscuity in the cyanobacterial cytochrome c oxidase: characterization of native complexes containing hemes A, O, and D, respectively. Arch Biochem Biophys. 1999;367:122–128. doi: 10.1006/abbi.1999.1236. [DOI] [PubMed] [Google Scholar]
- 210.Peschek GA, Obinger C, Paumann M. The respiratory chain of blue-green algae (cyanobacteria) Physiol Plant. 2004;120:358–369. doi: 10.1111/j.1399-3054.2004.00274.x. [DOI] [PubMed] [Google Scholar]
- 211.Hart SE, Schlarb-Ridley BG, Bendall DS, Howe CJ. Terminal oxidases of cyanobacteria. Biochem Soc Trans. 2005;33:832–835. doi: 10.1042/BST0330832. [DOI] [PubMed] [Google Scholar]
- 212.Zhang J, Oettmeier W, Gennis RB, Hellwig P. FTIR spectroscopic evidence for the involvement of an acidic residue in quinone binding in cytochrome bd from Escherichia coli. Biochemistry. 2002;41:4612–4617. doi: 10.1021/bi011784b. [DOI] [PubMed] [Google Scholar]
- 213.Yang K, Zhang J, Vakkasoglu AS, Hielscher R, Osborne JP, Hemp J, Miyoshi H, Hellwig P, Gennis RB. Glutamate 107 in subunit I of the cytochrome bd quinol oxidase from Escherichia coli is protonated and near the heme d/heme b595 binuclear center. Biochemistry. 2007;46:3270–3278. doi: 10.1021/bi061946+. [DOI] [PubMed] [Google Scholar]
- 214.Mogi T, Endou S, Akimoto S, Morimoto-Tadokoro M, Miyoshi H. Glutamates 99 and 107 in transmembrane helix III of subunit I of cytochrome bd are critical for binding of the heme b595-d binuclear center and enzyme activity. Biochemistry. 2006;45:15785–15792. doi: 10.1021/bi0615792. [DOI] [PubMed] [Google Scholar]
- 215.Belevich I, Bloch DA, Belevich N, Wikstrom M, Verkhovsky MI. Exploring the proton pump mechanism of cytochrome c oxidase in real time. Proc Natl Acad Sci USA. 2007;104:2685–2690. doi: 10.1073/pnas.0608794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hastings SF, Ingledew WJ. A study of the stabilization of semiquinones by the Escherichia coli quinol oxidase cytochrome bd. Biochem Soc Trans. 1996;24:131–132. doi: 10.1042/bst0240131. [DOI] [PubMed] [Google Scholar]
- 217.Hastings SF, Kaysser TM, Jiang F, Salerno JC, Gennis RB, Ingledew WJ. Identification of a stable semiquinone intermediate in the purified and membrane bound ubiquinol oxidase-cytochrome bd from Escherichia coli. Eur J Biochem. 1998;255:317–323. doi: 10.1046/j.1432-1327.1998.2550317.x. [DOI] [PubMed] [Google Scholar]
- 218.Rothery RA, Houston AM, Ingledew WJ. The respiratory chain of anaerobically grown Escherichia coli: Reactions with nitrite and oxygen. J Gen Microbiol. 1987;133:3247–3255. doi: 10.1099/00221287-133-11-3247. [DOI] [PubMed] [Google Scholar]
- 219.Meinhardt SW, Gennis RB, Ohnishi T. EPR studies of the cytochrome-d complex of Escherichia coli. Biochim Biophys Acta. 1989;975:175–184. doi: 10.1016/s0005-2728(89)80216-6. [DOI] [PubMed] [Google Scholar]
- 220.Rothery R, Ingledew WJ. The cytochromes of anaerobically grown Escherichia coli. Biochem J. 1989;262:437–443. doi: 10.1042/bj2610437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kahlow MA, Zuberi TM, Gennis RB, Loehr TM. Identification of a ferryl intermediate of Escherichia coli cytochrome d terminal oxidase by Resonance Raman spectrosopy. Biochemistry. 1991;30:11485–11489. doi: 10.1021/bi00113a001. [DOI] [PubMed] [Google Scholar]
- 222.Hill BC, Hill JJ, Gennis RB. The room temperature reaction of carbon monoxide and oxygen with the cytochrome bd quinol oxidase from Escherichia coli. Biochemistry. 1994;33:15110–15115. doi: 10.1021/bi00254a021. [DOI] [PubMed] [Google Scholar]
- 223.Ingledew WJ, Rothery RA, Gennis RB, Salerno JC. The orientation of the three haems of the in situ ubiquinol oxidase, cytochrome bd, of Escherichia coli. Biochem J. 1992;282:255–259. doi: 10.1042/bj2820255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Koland JG, Miller MJ, Gennis RB. Potentiometric analysis of the purified cytochrome d terminal oxidase complex from Escherichia coli. Biochemistry. 1984;23:1051–1056. [Google Scholar]
- 225.Bloch DA, Borisov VB, Mogi T, Verkhovsky MI. Heme/heme redox interaction and resolution of individual optical absorption spectra of the hemes in cytochrome bd from Escherichia coli. Biochim Biophys Acta. 2009;1787:1246–1253. doi: 10.1016/j.bbabio.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 226.Fang H, Lin R-J, Gennis RB. Location of heme axial ligands in the cytochrome d terminal oxidase complex of Escherichia coli determined by site-directed mutagenesis. J Biol Chem. 1989;264:8026–8032. [PubMed] [Google Scholar]
- 227.Spinner F, Cheesman MR, Thomson AJ, Kaysser T, Gennis RB, Peng Q, Peterson J. The haem b558 component of the cytochrome bd quinol oxidase complex from Escherichia coli has histidine-methionine axial ligation. Biochem J. 1995;308:641–644. doi: 10.1042/bj3080641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kaysser TM, Ghaim JB, Georgiou C, Gennis RB. Methionine-393 is an axial ligand of the heme b558 component of the cytochrome bd ubiquinol oxidase from Escherichia coli. Biochemistry. 1995;34:13491–13501. doi: 10.1021/bi00041a029. [DOI] [PubMed] [Google Scholar]
- 229.Zhang J, Barquera B, Gennis RB. Gene fusions with β-lactamase show that subunit I of the cytochrome bd quinol oxidase from E. coli has nine transmembrane helices with the O2 reactive site near the periplasmic surface. FEBS Lett. 2004;561:58–62. doi: 10.1016/S0014-5793(04)00125-5. [DOI] [PubMed] [Google Scholar]
- 230.Sun J, Kahlow MA, Kaysser TM, Osborne JP, Hill JJ, Rohlfs RJ, Hille R, Gennis RB, Loehr TM. Resonance Raman spectroscopic identification of a histidine ligand of b595 and the nature of the ligation of chlorin d in the fully reduced Escherichia coli cytochrome bd oxidase. Biochemistry. 1996;35:2403–2412. doi: 10.1021/bi9518252. [DOI] [PubMed] [Google Scholar]
- 231.Borisov VB, Gennis RB, Konstantinov AA. Interaction of cytochrome bd from Escherichia coli with hydrogen peroxide. Biochemistry (Moscow) 1995;60:231–239. (translated from Biokhimiya (in Russian) (1995), 60, 315–327) [PubMed] [Google Scholar]
- 232.Poole RK, Williams HD. Proposal that the function of the membrane-bound cytochrome a1-like haemoprotein (cytochrome b-595) in Escherichia coli is a direct electron donation to cytochrome d. FEBS Lett. 1987;217:49–52. doi: 10.1016/0014-5793(87)81240-1. [DOI] [PubMed] [Google Scholar]
- 233.Hata-Tanaka A, Matsuura K, Itoh S, Anraku Y. Electron flow and heme-heme interaction between cytochromes b-558, b-595 and d in a terminal oxidase of Escherichia coli. Biochim Biophys Acta. 1987;893:289–295. doi: 10.1016/0005-2728(87)90050-8. [DOI] [PubMed] [Google Scholar]
- 234.D’mello R, Hill S, Poole RK. The Cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two-oxygen-binding haems: implicaitons for regulation of activity in vivo by oxygen inihibition. Microbiology. 1996;142:755–763. doi: 10.1099/00221287-142-4-755. [DOI] [PubMed] [Google Scholar]
- 235.Timkovich R, Cork MS, Gennis RB, Johnson PY. Proposed structure of heme d, a prosthetic group of bacterial terminal oxidases. J Am Chem Soc. 1985;107:6069–6075. [Google Scholar]
- 236.Poole RK, Kumar C, Salmon I, Chance B. The 650 nm chromophore in Escherichia coli is an ‘Oxy-’ or oxygenated compound, not the oxidized form of cytochrome oxidase d: A hypothesis. J Gen Microbiol. 1983;129:1335–1344. doi: 10.1099/00221287-129-5-1335. [DOI] [PubMed] [Google Scholar]
- 237.Lorence RM, Gennis RB. Spectroscopic and quantitative analysis of the oxygenated and peroxy states of the purified cytochrome d complex of Escherichia coli. J Biol Chem. 1989;264:7135–7140. [PubMed] [Google Scholar]
- 238.Kahlow MA, Loehr TM, Zuberi TM, Gennis RB. The oxygenated complex of cytochrome d terminal oxidase: direct evidence for Fe-O2 coordination in a chlorin-containing enzyme by Resonance Raman spectroscopy. J Am Chem Soc. 1993;115:5845–5846. [Google Scholar]
- 239.Borisov VB, Smirnova IA, Krasnosel’skaya IA, Konstantinov AA. Oxygenated cytochrome bd from Escherichia coli can be converted into the oxidized form by lipophilic electron acceptors. Biochemistry (Moscow) 1994;59:437–443. (translated from Biokhimiya (in Russian) (1994), 59, 598–606) [PubMed] [Google Scholar]
- 240.Belevich I, Borisov VB, Konstantinov AA, Verkhovsky MI. Oxygenated complex of cytochrome bd from Escherichia coli: stability and photolability. FEBS Lett. 2005;579:4567–4570. doi: 10.1016/j.febslet.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 241.Belevich I, Borisov VB, Bloch DA, Konstantinov AA, Verkhovsky MI. Cytochrome bd from Azotobacter vinelandii: evidence for high-affinity oxygen binding. Biochemistry. 2007;46:11177–11184. doi: 10.1021/bi700862u. [DOI] [PubMed] [Google Scholar]
- 242.Azarkina N, Borisov V, Konstantinov AA. Spontaneous spectral changes of the reduced cytochrome bd. FEBS Lett. 1997;416:171–174. doi: 10.1016/s0014-5793(97)01196-4. [DOI] [PubMed] [Google Scholar]
- 243.Jiang FS, Zuberi TM, Cornelius JB, Clarkson RB, Gennis RB, Belford RL. Nitrogen and proton ENDOR of cytochrome d, hemin, and metmyoglobin in frozen solutions. J Am Chem Soc. 1993;115:10293–10299. [Google Scholar]
- 244.Hirota S, Mogi T, Anraku Y, Gennis RB, Kitagawa T. Resonance Raman study on axial ligands of heme irons in cytochrome bd-type ubiquinol oxidase from Escherichia coli. Biospectroscopy. 1995;1:305–311. [Google Scholar]
- 245.Hori H, Tsubaki M, Mogi T, Anraku Y. EPR study of NO complex of bd-type ubiquinol oxidase from Escherichia coli. J Biol Chem. 1996;271:9254–9258. doi: 10.1074/jbc.271.16.9254. [DOI] [PubMed] [Google Scholar]
- 246.Mogi T. Probing the heme d-binding site in cytochrome bd quinol oxidase by site-directed mutagenesis. J Biochem. 2009;145:763–770. doi: 10.1093/jb/mvp033. [DOI] [PubMed] [Google Scholar]
- 247.Pudek MR, Bragg PD. Redox potentials of the cytochromes in the respiratory chain of aerobically grown Escherichia coli. Arch Biochem Biophys. 1976;174:546–552. doi: 10.1016/0003-9861(76)90382-9. [DOI] [PubMed] [Google Scholar]
- 248.Lorence RM, Miller MJ, Borochov A, Faiman-Weinberg R, Gennis RB. Effects of pH and detergent on the kinetic and electrochemical properties of the purified cytochrome d terminal oxidase complex of Escherichia coli. Biochim Biophys Acta. 1984;790:148–153. doi: 10.1016/0167-4838(84)90218-8. [DOI] [PubMed] [Google Scholar]
- 249.Junemann S, Wrigglesworth JM, Rich PR. Effects of decyl-aurachin D and reversed electron transfer in cytochrome bd. Biochemistry. 1997;36:9323–9331. doi: 10.1021/bi970055m. [DOI] [PubMed] [Google Scholar]
- 250.Trutko SM, Akimenko VK, Suzina NE, Anisimova LA, Shlyapnikov MG, Baskunov BP, Duda VI, Boronin AM. Involvement of the respiratory chain of gram-negative bacteria in the reduction of tellurite. Arch Microbiol. 2000;173:178–186. doi: 10.1007/s002039900123. [DOI] [PubMed] [Google Scholar]
- 251.Trutko SM, Suzina NE, Duda VI, Akimenko VK, Boronin AM. Participation of the bacterial respiratory chain in reduction of potassium tellurite. Dokl Akad Nauk (in Russian) 1998;358:836–838. [PubMed] [Google Scholar]
- 252.Matsumoto Y, Muneyuki E, Fujita D, Sakamoto K, Miyoshi H, Yoshida M, Mogi T. Kinetic mechanism of quinol oxidation by cytochrome bd studied with ubiquinone-2 analogs. J Biochem (Tokyo) 2006;139:779–788. doi: 10.1093/jb/mvj087. [DOI] [PubMed] [Google Scholar]
- 253.Zhang J, Hellwig P, Osborne JP, Gennis RB. Arginine 391 in subunit I of the cytochrome bd quinol oxidase from Escherichia coli stabilizes the reduced form of the hemes and is essential for quinol oxidase activity. J Biol Chem. 2004;279:53980–53987. doi: 10.1074/jbc.M408626200. [DOI] [PubMed] [Google Scholar]
- 254.Oden KL, Gennis RB. Isolation and characterization of a new class of cytochrome d terminal oxidase mutants of Escherichia coli. J Bacteriol. 1991;173:6174–6183. doi: 10.1128/jb.173.19.6174-6183.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Borisov VB. Interaction of bd-type quinol oxidase from Escherichia coli and carbon monoxide: Heme d binds CO with high affinity. Biochemistry (Moscow) 2008;73:14–22. doi: 10.1134/s0006297908010021. (translated from Biokhimiya (in Russian) (2008), 73, 18–28) [DOI] [PubMed] [Google Scholar]
- 256.Junemann S, Wrigglesworth JM. Stoichiometry of CO binding to the cytochrome bd complex of Azotobacter vinelandii. Biochem Soc Trans. 1993;21:345S. doi: 10.1042/bst021345s. [DOI] [PubMed] [Google Scholar]
- 257.Junemann S, Wrigglesworth JM. Cytochrome bd oxidase from Azotobacter vinelandii. Purification and quantitation of ligand binding to the oxygen reduction site. J Biol Chem. 1995;270:16213–16220. doi: 10.1074/jbc.270.27.16213. [DOI] [PubMed] [Google Scholar]
- 258.Muntyan MS, Bloch DA, Drachev LA, Skulachev VP. Kinetics of CO binding to putative Na+-motive oxidases of the o-type from Bacillus FTU and of the d-type from Escherichia coli. FEBS Lett. 1993;327:347–350. doi: 10.1016/0014-5793(93)81018-u. [DOI] [PubMed] [Google Scholar]
- 259.Junemann S, Rich PR, Wrigglesworth JM. CO flash photolysis of cytochrome bd from Azotobacter vinelandii. Biochem Soc Trans. 1995;23:157S. doi: 10.1042/bst023157s. [DOI] [PubMed] [Google Scholar]
- 260.Hubbard JAM, Hughes MN, Poole RK. Nitrite, but not silver, ions induce spectral changes in Escherichia coli cytochrome d. FEBS Lett. 1983;164:241–243. doi: 10.1016/0014-5793(83)80293-2. [DOI] [PubMed] [Google Scholar]
- 261.Hubbard JAM, Hughes MN, Poole RK. In: Poole RK, Dow CS, editors. Academic Press; London: 1985. pp. 231–236. [Google Scholar]
- 262.Bonner FT, Hughes MN, Poole RK, Scott RI. Kinetics of the reactions of trioxodinitrate and nitrite ions with cytochrome d in Escherichia coli. Biochim Biophys Acta. 1991;1056:133–138. doi: 10.1016/s0005-2728(05)80279-8. [DOI] [PubMed] [Google Scholar]
- 263.Kauffman HF, van Gelder BF, DerVartanian DV. Effect of ligands on cytochrome d from Azotobacter vinelandii. J Bioenerg Biomembr. 1980;12:265–276. doi: 10.1007/BF00744688. [DOI] [PubMed] [Google Scholar]
- 264.Junemann S, Wrigglesworth JM. Binding of NO to the oxygen reaction site of cytochrome bd from Azotobacter vinelandii. Biochem Soc Trans. 1996;24:38S. doi: 10.1042/bst024038s. [DOI] [PubMed] [Google Scholar]
- 265.Sarti P, Giuffre A, Forte E, Mastronicola D, Barone MC, Brunori M. Nitric oxide and cytochrome c oxidase: mechanisms of inhibition and NO degradation. Biochem Biophys Res Commun. 2000;274:183–187. doi: 10.1006/bbrc.2000.3117. [DOI] [PubMed] [Google Scholar]
- 266.Lemon DD, Calhoun MW, Gennis RB, Woodruff WH. The gateway to the active site of heme-copper oxidases. Biochemistry. 1993;32:11953–11956. doi: 10.1021/bi00096a002. [DOI] [PubMed] [Google Scholar]
- 267.Kauffman HF, Van Gelder BF. The respiratory chain of Azotobacter vinelandii. II. The effect of cyanide on cytochrome d. Biochim Biophys Acta. 1973;314:276–283. doi: 10.1016/0005-2728(73)90112-6. [DOI] [PubMed] [Google Scholar]
- 268.Kauffman HF, Van Gelder BF. The respiratory chain of Azotobacter vinelandii. III. The effect of cyanide in the presence of substrates. Biochim Biophys Acta. 1974;333:218–227. doi: 10.1016/0005-2728(74)90006-1. [DOI] [PubMed] [Google Scholar]
- 269.Pudek MR, Bragg PD. Inhibition by cyanide of the respiratory chain oxidases of Escherichia coli. Arch Biochem Biophys. 1974;164:682–693. doi: 10.1016/0003-9861(74)90081-2. [DOI] [PubMed] [Google Scholar]
- 270.Pudek MR, Bragg PD. Reaction of cyanide with cytochrome d in respiratory particles from exponential phase Escherichia coli. FEBS Lett. 1975;50:111–113. doi: 10.1016/0014-5793(75)80468-6. [DOI] [PubMed] [Google Scholar]
- 271.Poole RK. In: Bacterial Energy Transduction. Anthony C, editor. Academic Press; London: 1988. pp. 231–291. [Google Scholar]
- 272.Krasnoselskaya I, Arutjunjan AM, Smirnova I, Gennis R, Konstantinov AA. Cyanide-reactive sites in cytochrome bd complex from E. coli. FEBS Lett. 1993;327:279–283. doi: 10.1016/0014-5793(93)81004-j. [DOI] [PubMed] [Google Scholar]
- 273.Keyhani E, Minai-Tehrani D. The binding of cyanide to cytochrome d in intact cells, spheroplasts, membrane fragments and solubilized enzyme from Salmonella typhimurium. Biochim Biophys Acta. 2001;1506 doi: 10.1016/s0005-2728(01)00176-1. [DOI] [PubMed] [Google Scholar]
- 274.Kauffman HF, DerVartanian DV, van Gelder BF, Wampler J. EPR studies on cytochrome components in phosphorylating particles of Azotobacter vinelandii. J Bioenerg. 1975;7:215–222. [Google Scholar]
- 275.Sun J, Osborne JP, Kahlow MA, Kaysser TM, Gennis RB, Loehr TM. Resonance Raman studies of Escherichia coli cytochrome bd oxidase. Selective enhancement of the three heme chromophores of the “as-isolated” enzyme and characterization of the cyanide adduct. Biochemistry. 1995;34:12144–12151. doi: 10.1021/bi00038a007. [DOI] [PubMed] [Google Scholar]
- 276.Poole RK, Williams HD. Formation of the 680-nm-absorbing form of the cytochrome bd oxidase complex of Escherichia coli by reaction of hydrogen peroxide with the ferric form. FEBS Lett. 1988;231:243–246. doi: 10.1016/0014-5793(88)80740-3. [DOI] [PubMed] [Google Scholar]
- 277.Borisov V, Gennis R, Konstantinov AA. Peroxide complex of cytochrome bd: Kinetics of generation and stability. Biochem Mol Biol Int. 1995;37:975–982. [PubMed] [Google Scholar]
- 278.Junemann S, Butterworth PJ, Wrigglesworth JM. A suggested mechanism for the catalytic cycle of cytochrome bd terminal oxidase based on kinetic analysis. Biochemistry. 1995;34:14861–14867. doi: 10.1021/bi00045a029. [DOI] [PubMed] [Google Scholar]
- 279.Junemann S, Wrigglesworth JM. Antimycin inhibition of the cytochrome bd complex from Azotobacter vinelandii indicates the presence of a branched electron transfer pathway for the oxidation of ubiquinol. FEBS Lett. 1994;345:198–202. doi: 10.1016/0014-5793(94)00372-6. [DOI] [PubMed] [Google Scholar]
- 280.Koland JG, Miller MJ, Gennis RB. Reconstitution of the membrane-bound, ubiquinone-dependent pyruvate oxidase respiratory chain of Escherichia coli with the cytochrome d terminal oxidase. Biochemistry. 1984;23:445–453. doi: 10.1021/bi00298a008. [DOI] [PubMed] [Google Scholar]
- 281.Wikstrom M, Bogachev A, Finel M, Morgan JE, Puustinen A, Raitio M, Verkhovskaya M, Verkhovsky MI. Mechanism of proton translocation by the respiratory oxidases. The histidine cycle. Biochim Biophys Acta. 1994;1187:106–111. doi: 10.1016/0005-2728(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 282.Gibson Q, Greenwood C. Reactions of cytochrome oxidase with oxygen and carbon monoxide. Biochem J. 1963;86:541–555. doi: 10.1042/bj0860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Borisov VB, Forte E, Sarti P, Giuffre A. Catalytic intermediates of cytochrome bd terminal oxidase at steady-state: Ferryl and oxy-ferrous species dominate. Biochim Biophys Acta. 2011;1807:503–509. doi: 10.1016/j.bbabio.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 284.Mason MG, Nicholls P, Cooper CE. The steady-state mechanism of cytochrome c oxidase: redox interactions between metal centres. Biochem J. 2009;422:237–246. doi: 10.1042/BJ20082220. [DOI] [PubMed] [Google Scholar]
- 285.Meunier B, Madgwick SA, Reil E, Oettmeier W, Rich PR. New inhibitors of the quinol oxidation sites of bacterial cytochromes bo and bd. Biochemistry. 1995;34:1076–1083. doi: 10.1021/bi00003a044. [DOI] [PubMed] [Google Scholar]
- 286.Junemann S, Wrigglesworth JM. Inhibitors of electron transport in the cytochrome bd complex of Azotobacter vinelandii. Biochem Soc Trans. 1994;22:287S. doi: 10.1042/bst022287s. [DOI] [PubMed] [Google Scholar]
- 287.Jones CW, Redfearn ER. The cytochrome system of Azotobacter vinelandii. Biochim Biophys Acta. 1967;143:340–353. doi: 10.1016/0005-2728(67)90088-6. [DOI] [PubMed] [Google Scholar]
- 288.Konishi K, Ouchi M, Kita K, Horikoshi I. Purification and properties of a cytochrome b560-d complex, a terminal oxidase of the aerobic respiratory chain of Photobacterium phosphoreum. J Biochem. 1986;99:1227–1236. doi: 10.1093/oxfordjournals.jbchem.a135586. [DOI] [PubMed] [Google Scholar]
- 289.Kavanagh EP, Callis JB, Edwards SE, Poole RK, Hill S. Redox poise and oxygenation of cytochrome bd in the diazotroph Azotobacter vinelandii assessed in vivo using diode-array reflectance spectrophotometry. Microbiology. 1998;144(Pt 8):2271–80. doi: 10.1099/00221287-144-8-2271. [DOI] [PubMed] [Google Scholar]
- 290.Yang K, Borisov VB, Konstantinov AA, Gennis RB. The fully oxidized form of the cytochrome bd quinol oxidase from E. coli does not participate in the catalytic cycle: direct evidence from rapid kinetics studies. FEBS Lett. 2008;582:3705–3709. doi: 10.1016/j.febslet.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]