Abstract
Context
Insulin-like growth factor (IGF) signaling is essential for achieving optimal body size during fetal development, peak bone mass during puberty, and maximal fecundity in the reproductive period. IGF-II is considered the main fetal IGF, whereas IGF-I is more important postnatally. Pregnancy-associated plasma protein-A (PAPP-A) enhances local IGF signaling through cleavage of inhibitory IGF binding proteins. Conversely, inhibition of PAPP-A results in reduced local IGF action. Thus, PAPP-A knock-out (KO) mice are born as proportional dwarfs due to the dysregulation of IGF-II signaling during early embryogenesis that impacts body size. Relaxation of IgfII imprinting through mutation of a reciprocally imprinted downstream gene, H19, which allowed transcription of IGF-II from the normally silent maternal allele, rescued the dwarf phenotype of PAPP-A KO mice.
Objective
To determine the effect of increased IGF-II expression on postnatal phenotypes of PAPP-A KO mice.
Design
Young adult wild-type (WT), PAPP-A KO, H19 mutant (ΔH19/WT) and ΔH19/PAPP-A KO mice were characterized for skeletal phenotype (peripheral quantitative computed tomography at the midshaft and distal metaphysis of the femur) and reproductive phenotype (time to first litter, time between litters, pups per litter).
Results
Serum IGF-II levels were significantly increased in ΔH19/WT and ΔH19/PAPP-A KO mice compared to WT and PAPP-A KO mice; serum IGF-I levels were not affected by H19 mutation. PAPP-A KO mice had reductions in cortical thickness and in cortical and trabecular area, bone mineral content and bone mineral density compared to WT mice. There were no significant differences between PAPP-A KO and ΔH19/PAPP-A KO mice in any of the bone parameters. PAPP-A KO crossed with (x) PAPP-A KO had a longer time until first litter, normal time between subsequent litters, and significantly reduced number of pups per litter compared to WT × WT. ΔH19/PAPP-A KO × ΔH19/PAPP-A KO had an even longer time to first litter, but also longer time between litters. This phenotype was associated with female ΔH19/PAPP-A KO mice. Furthermore, these ΔH19/PAPP-A KO mouse mothers failed to care for their pups.
Conclusions
An increase in IGF-II expression did not rescue the skeletal and reproductive deficiencies associated with reduced local IGF-I signaling in PAPP-A KO mice. In addition, the data suggest a potential new role for genomic imprinting at the IgfII/H19 locus affecting maternal behavior.
Keywords: Pregnancy-associated plasma protein-A, insulin-like growth factor-II, imprinting, bone mass, reproduction
INTRODUCTION
Insulin-like growth factor (IGF) signaling is essential early in life for achieving optimal body size during fetal development, peak bone mass during puberty, and maximal fecundity in the reproductive period.1–3 On the other hand, increases in IGF in adults are associated with aging and age-related diseases.4 In general, IGF-II appears to be critical during early fetal growth, at least in rodents.5,6 IGF-I appears to be more important postnatally. However, this ‘division of labor’ between IGF-I and IGF-II is not clearcut.
Pregnancy-associated plasma protein-A (PAPP-A), an IGF binding protein protease, enhances IGF activity through cleavage of inhibitory binding proteins in the pericellular environment with the consequent release of IGF ligand for receptor activation.7 Conversely, inhibition of PAPP-A results in reduced local IGF action in vitro and in vivo. PAPP-A knock-out (KO) mice are 60–70% the size of their wild-type littermates at birth and retain the size difference postnatally.8 This proportional dwarfism appears to be linked to the dysregulation of IGF-II signaling during early embryogenesis that impacts body size.9 However, it may be the decrease in local IGF-I bioavailability postnatally that accounts for the negative impact of PAPP-A deletion on the adult skeleton.10 A decrease in local IGF-I bioavailability could also contribute to the compromised reproductive fitness seen in PAPP-A KO mice.11 Nevertheless, a possible role for IGF-II may be overlooked due, in part, to assumptions about IGF-II, and the lack thereof, in adult rodents.
We previously reported that relaxation of IgfII imprinting through mutation of a reciprocally imprinted downstream gene, H19, which allowed transcription of IGF-II from the normally silent maternal allele, could rescue the dwarf phenotype and the delayed skeletal ossification of PAPP-A KO mice seen at birth.9 For the present study, we used these novel mouse models to address questions about the role of IGF-I versus IGF-II in early adulthood. We hypothesized that the increase in IGF-II expression would not affect postnatal phenotypes associated with reduced IGF-I signaling. To test this hypothesis, wild-type, PAPP-A KO, H19 mutant (ΔH19), and ΔH19/PAPP-A KO mice were characterized for skeletal and reproductive phenotypes. The latter was of particular interest because PAPP-A KO and ΔH19/PAPP-A KO mice live 30–40% longer than their wild-type littermates.12–14 Whether extension of lifespan has a fecundity cost is an open question.
MATERIALS and METHODS
Mice
Characterization of the strains of mice used in this study, which carry targeted mutations in the Pappa and H19 loci, and genotyping have been detailed in previous publications.8,9 Mice receiving the H19 mutation from the maternal allele (H19m−/+) have approximately two-fold increased levels of IGF-II in the embryo due to expression from both the maternal and paternal alleles, with consequent fetal overgrowth.15 In contrast, paternal transmission of the mutation has no phenotypic consequences for the progeny. The Pappa gene is not imprinted and both males and females with homozygous deletion (PAPP-A−/−) are 60–70% of the body weight of wild-type and heterozygous litter-mates.8 Accordingly, paternally-transmitted female H19 heterozygous mice were first crossed with PAPP-A−/− males. Of these offspring, females inheriting the H19 mutant allele and heterozygous for Pappa deletion were then mated to male PAPP-A−/− mice to generate embryos belonging to four genotypes designated as wild-type (WT; H19+/+PAPP-A+/+), H19 mutant (ΔH19/WT; H19m−/+PAPP-A+/+), PAPP-A knock-out (KO; H19+/+PAPP-A−/−), and ΔH19/PAPP-A KO (H19m−/+PAPP-A−/−), the latter being the ‘rescue’ of the dwarf phenotype. Genotypes were confirmed for all mice with tail DNA collected at the end of each experiment. All experiments in this study were approved by the Institutional Animal Care and Use Committee of Mayo Clinic.
Mouse IGF assays
Serum levels of mouse IGF-II were measured by enzyme-linked immunosorbent assay (ELISA). Serum samples were extracted with acid/ethanol reagent.16 Briefly, 100 μL acid/ethanol reagent (12.5% 2N HCl, 87.5% ethanol) was added to 20 μL serum sample. The mixture was incubated at room temperature for 30 min followed by microcentrifugation at 10,000g for 10 min. Fifty microliter supernatant was neutralized with 200 μL neutralization buffer (100 mM sodium phosphate, pH 7.8, 40 mM NaCl, 0.01% Thimerosal, 0.1% Tween 20, 0.25% BSA, 500 ng/ml mouse IGF-I).17
For the ELISA, 96-well microtiter plates were coated with purified monoclonal rat anti-mouse IGF-II antibody (R&D, Minneapolis, MN) at 0.5 μg/well in 200 μL of phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4), incubated overnight on a shaker, then washed 3 times with wash buffer (PBS, 0.05% Tween-20) and 3 times with SuperBlock T20(PBS) blocking buffer (Thermo Scientific, Rockford, IL). Standards were prepared by diluting recombinant mouse IGF-II (R&D, Minneapolis, MN) in assay buffer in concentrations ranging from 0 to 50 ng/mL. Standards, controls or samples (100 μL/well) and 50 ng (100 μL) biotinylated goat anti-mouse IGF-II antibody (R&D, Minneapolis, MN) were added to the appropriate wells and incubated at room temperature for 3 hr on a shaker. Wells were then washed 3 times with wash buffer, followed by the addition of streptavidin-HRP conjugate (200 μL/well) and incubated for 30 min at room temperature. After 4 washes with wash buffer, 200 μL/well of o-phenylenediamine dihydrochloride solution (1 mg/mL in hydrogen peroxide substrate) was added to each well and incubated for an additional 10–20 min. The reaction was stopped by the addition of 50 μL 2N H2SO4 and absorbance was determined on a plate spectrophotometer (Molecular Designs, Sunnyvale, CA) at 490 nm. The intra and inter-assay CVs are <10% and recovery of added mouse IGF-II is >90%. This assay has a sensitivity of 0.1 ng/ml and there is no cross-reactivity with mouse IGF-I or human IGF-I.
Mouse IGF-I levels were measured using an in-house ELISA assay as described previously.18
Skeletal phenotype
Volumetric bone mineral density (BMD) was measured by peripheral quantitative computed tomography (pQCT) at the midshaft and distal metaphysis of the femur using a Stratec XCT Research SA Plus scanner (Norland Medical Systems, Fort Atkinson, WI) as described previously.10
Reproductive phenotype
Pair breedings of mice were started at 8 weeks of age. Time to first litter, time between litters, and number of pups per litter were monitored for 4 months. Pups remained in the cage to foster normal maternal instincts. Breeding cages were checked every day including weekends and holidays. Number of pups borne (alive + dead) and date of birth were recorded. Surviving pups were kept in the cage with the mother and checked daily with minimal disturbance. Final number of pups surviving was recorded on day 19.
Statistical Analyses
Except where indicated, data are expressed as mean ± SEM. ANOVA followed by post-hoc t-test was used for comparisons of multiple groups. A non-parametric Fisher’s exact test was used when data were not normally distributed. P value < 0.05 was considered statistically significant.
RESULTS
Serum IGF levels
Mouse IGF-II levels were measured in serum from 4-week-old WT, PAPP-A KO, ΔH19/WT, and ΔH19/PAPP-A KO mice. IGF-II was detectable in adult mouse sera, and levels were significantly increased in ΔH19/WT and ΔH19/PAPP-A KO mice compared to WT and PAPP-A KO mice (Fig. 1). Although lower in PAPP-A KO compared to WT, serum IGF-I levels were not affected by H19 mutation.
Figure 1.
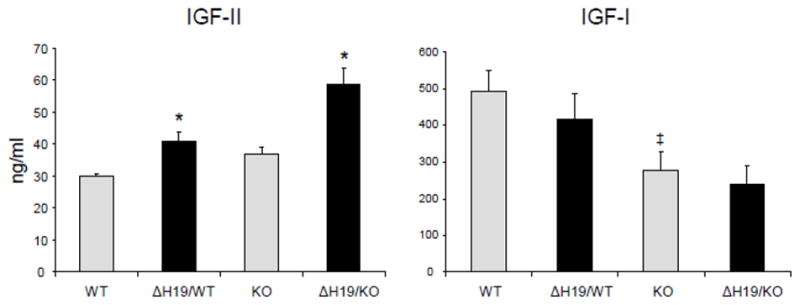
Serum levels of IGFs in 4-week-old mice.
Results are mean ± SEM, N = 9–19.
*Significant effect of ΔH19, P < 0.05.
‡Significant difference between wild-type (WT) and PAPP-A KO
Skeletal phenotype
Femoral pQCT data are presented in Table 1 (male mice) and Table 2 (female mice). Male PAPP-A KO mice had significantly reduced cortical thickness, area, and bone mineral content (BMC) at the midshaft of the femur (primarily cortical bone), and significantly reduced area, BMC, and bone mineral density (BMD) at the distal metaphysis of the femur (primarily trabecular bone) compared to WT mice. Male ΔH19/PAPP-A KO mice also had significant reductions in cortical thickness, area, BMC and BMD in the midshaft, and reduced area and BMC in the distal femur compared to WT. However, BMD in the distal femur was not different from WT. Similar findings were seen in the female PAPP-A KO mice (Table 2). Thus, cortical thickness, area and BMC were significantly reduced in the midshaft, and area, BMC, and BMD were significantly reduced in the distal femur of female PAPP-A KO compared to WT mice. Female ΔH19/PAPP-A KO mice had similar reductions in cortical thickness, area and BMC in the midshaft, and reduced BMD in the distal femur. However, reductions in midshaft BMD, and distal area and BMC did not reach statistical significance. There were no significant differences between PAPP-A KO and ΔH19/PAPP-A KO mice in any of the skeletal parameters. Therefore, the increase in IGF-II did not ‘rescue’ the skeletal phenotype of the PAPP-A KO mouse. Interestingly, the ΔH19/WT mice had a mixed skeletal phenotype. There were significant reductions in midshaft cortical thickness, BMC and BMD and a significant increase in distal area in male ΔH19/WT compared to WT mice. In females, the only significant difference was a reduction in BMD in the distal femur.
Table 1.
Femoral pQCT data from 6-month-old male mice
| WT (26) | PAPP-A KO (17) | Δ H19/KO (18) | ΔH19/WT (20) | |
|---|---|---|---|---|
| Midshaft | ||||
| Cort th | 0.309 ± 0.006 | 0.264 ± 0.009* | 0.250 ± 0.008* | 0.257 ± 0.008* |
| Area | 1.56 ± 0.04 | 1.22 ± 0.07* | 1.32 ± 0.07* | 1.49 ± 0.09 |
| BMC | 1.77 ± 0.05 | 1.27 ± 0.07* | 1.30 ± 0.06* | 1.46 ± 0.08* |
| BMD | 1131 ± 9 | 1046 ± 17 | 985 ± 32* | 990 ± 29* |
| Distal metaphysis | ||||
| Area | 4.60 ± 0.10 | 3.46 ± 0.11* | 3.65 ± 0.22* | 5.1 ± 22* |
| BMC | 2.40 ± 0.07 | 1.70 ± 0.07* | 1.90 ± 0.07* | 2.60 ± 0.10 |
| BMD | 527 ± 13 | 490 ± 9* | 532 ± 34 | 516 ± 21 |
Cort th: Cortical thickness (mm).
Area: (mm2).
BMC: Bone mineral content (per 1 mm slice).
BMD: Bone mineral density (mg/cm3).
Results are mean ± SEM, with numbers of mice per group in parentheses.
Significantly different from WT, P < 0.05.
Table 2.
Femoral pQCT data from 6-month-old female mice
| WT (23) | PAPP-A KO (17) | ΔH19/KO (15) | ΔH19/WT (29) | |
|---|---|---|---|---|
| Midshaft | ||||
| Cort th | 0.288 ± 0.009 | 0.246 ± 0.009* | 0.235 ± 0.006* | 0.284 ± 0.005 |
| Area | 1.49 ± 0.05 | 1.01 ± 0.04* | 1.12 ± 0.03* | 1.44 ± 0.03 |
| BMC | 1.56 ± 0.06 | 1.03 ± 0.05* | 1.10 ± 0.04* | 1.56 ± 0.04 |
| BMD | 1047 ± 23 | 1027 ± 19 | 989 ± 25 | 1091 ± 15 |
| Distal metaphysis | ||||
| Area | 4.30 ± 0.13 | 3.38 ± 0.11* | 3.63 ± 0.09 | 4.60 ± 0.10 |
| BMC | 2.21 ± 0.08 | 1.55 ± 0.08* | 1.62 ± 0.07 | 2.10 ± 0.05 |
| BMD | 519 ± 9 | 455 ± 11* | 444 ± 14* | 462 ± 6* |
Cort th: Cortical thickness (mm).
Area: (mm2).
BMC: Bone mineral content (per 1 mm slice).
BMD: Bone mineral density (mg/cm3).
Results are mean ± SEM, with numbers of mice per group in parentheses.
Significantly different from WT, P < 0.05.
Reproductive phenotype
General reproductive fitness was assessed by successful pregnancies and litter size (Table 3). WT crossed with WT mice on average had 21.8 days from mating to first litter, 20.7 days between litters, and 8.0 pups per litter. PAPP-A KO crossed with PAPP-A KO mice had a longer time until first pregnancy (25.7 days), but normal time between litters after the first one (20.8 day) and significantly reduced number of pups/litter (5.8). Interestingly, ΔH19/PAPP-A KO crossed with ΔH19/PAPP-A KO mice had an even longer time to first pregnancy (28.0 days) but also longer times between litters (25.2 days). Litter size was somewhat reduced (6.7 pups/litter), but was not significantly different from Control. ΔH19/WT crossed with ΔH19/WT mice, which evaluated the effect of elevated IGF-II per se, showed moderately delayed time to first litter (24.2 days), normal times between litters and normal litter sizes. In order to determine whether the compromised reproductive phenotype of the ΔH19 was predominantly in the male or female, we performed additional crosses. If male ΔH19/PAPP-A KO mice were bred with female WT mice, then the reproductive phenotype was similar to WT crosses. If female ΔH19/PAPP-A KO mice were bred to male WT mice, then fecundity was compromised although litter size was normal. Interestingly, the percentage of pups surviving in the perinatal period was dramatically affected in female ΔH19/PAPP-A KO mice crossed with a male ΔH19/PAPP-A KO or with a male WT mouse. In these pairings, few pups survived (median value 0%) compared to normally 50–90% survival. Pup death was 4 times more likely to occur between birth and day 19 than at birth when the female had the H19 mutation. These mothers did not appear to react appropriately to the pups and failed to care for them.
Table 3.
Reproductive phenotype
| Male × Female | Days to 1st litter | Days between litters | Pups per litter | Perinatal Survival (%) |
|---|---|---|---|---|
| WT × WT | 21.8 ± 0.2 | 20.7 ± 0.4 | 8.0 ± 0.6 | 89 |
| KO × KO | 25.7 ± 1.8‡ | 20.8 ± 0.3 | 5.8 ± 0.5* | 67 |
| ΔH19/KO × ΔH19/KO | 28.0 ± 2.0* | 25.2 ± 2.1* | 6.7 ± 0.7 | 0* |
| ΔH19/WT × ΔH19/WT | 24.4 ± 2.3 | 21.0 ± 0.8 | 8.1 ± 0.6 | 57 |
| ΔH19/KO × WT | 22.0 ± 1.5 | 22.1 ± 1.1 | 7.3 ± 0.5 | 86 |
| WT × ΔH19/KO | 24.0 ± 1.0‡ | 24.5 ± 1.8* | 7.8 ± 0.5 | 0* |
Data for days to 1st litter, days between litters, and pups per litter are mean ± SEM of N = 5, 10–19, and 14–24, respectively. Perinatal survival data, which were not normally distributed, are presented as median values.
Significantly different from WT × WT, P < 0.05.
Different from WT × WT, P = 0.06.
DISCUSSION
In this study, increases in IGF-II expression that rescued the fetal growth defect did not rescue the compromised skeletal and reproductive phenotypes of PAPP-A KO mice. These data support the hypothesis that increases in IGF-II expression do not affect postnatal phenotypes associated with reduced local IGF-I signaling.
Whereas IGF-II has a clearly established role in embryonic development, its role during postnatal life is unclear. IGF-II expression is known to decrease markedly after birth in rodents, and a physiological function in the adult has largely been dismissed. However, recent data from Qiu et al.19 challenged the assumption that IGF-II is absent in adult rodents by demonstrating the persistence of circulating levels of IGF-II in adult rats. In addition, IGF-II knock-out mice show resistance to the development of atherosclerosis, implicating a possible pathophysiological role for IGF-II in the vasculature of adult mice.20 Therefore, it was important to evaluate IGF-II and postnatal phenotypes in this study. In a newly validated mouse IGF-II assay, serum levels of IGF-II were significantly elevated 40–60% in ΔH19 mutant mice. This moderate increase fits with disruption of IgfII imprinting and consequent transcription of IgfII from both maternal and paternal alleles; normally the maternal allele is silent.15 However, the increase in IGF-II did not rescue the bone phenotype of PAPP-A KO mice. There were no significant differences between PAPP-A KO and ΔH19/PAPP-A KO mice in any of the parameters measured in midshaft and distal metaphysis of the femurs. Thus, in spite of the increase in IGF-II, ΔH19/PAPP-A KO mice retained the skeletal insufficiencies in cortical and trabecular mass and density seen in male and female PAPP-A KO mice. Similarly, Moerth et al.21 showed that even higher, 6- to 11-fold, elevated levels of IGF-II in the postnatal period did not rescue the skeletal growth defects of IGF-I KO mice. Using IGF-I and IGF-II KO mice, Mohan et al.22 also concluded that IGF-I, but not IGF-II, is critical for the increase in BMD that occurs during puberty in mice. In a conditional knock-out mouse model, local IGF-I was found to be essential for optimal peak BMD, even in the face of normal circulating IGF-I levels.23 Thus, our data are in agreement with others that IGF-I, but not IGF-II, is important for optimal skeletal growth postnatally. Nonetheless, IGF-II expression may be relevant in the injury response in rodents.20 Our unpublished data indicate that IGF-II, but not IGF-I expression, is increased during fracture repair in mice.
Although fertile, PAPP-A KO mice produced smaller litter sizes compared to WT mice. This is presumably because of reduced ovulation. Ovaries of PAPP-A KO mice have an increased number of primordial and atretic follicles while the number of healthy growing antral follicles is decreased.11 Importantly for this study, the increase in IGF-II in the ΔH19/PAPP-A KO mice did not rescue the mild sub-fertility seen in PAPP-A KO mice. Indeed, it seemed to worsen it. Thus, there was an even longer time to first litter, as well as increased time between litters in ΔH19/PAPP-A KO mice. Furthermore, perinatal mortality of newborns of ΔH19/PAPP-A KO breedings or female ΔH19/PAPP-A KO mice bred to male WT mice was significantly higher than that of newborns of WT crosses or PAPP-A KO crosses (median survival values of 0 compared to 50–90%). Pups of mouse mothers with the H19 deletion were frequently observed to be abandoned outside the nestlet with no milk in their stomach. The reason for the high mortality of offspring from these crosses is not certain, but it may be that increases in IGF-II interfere with maternal behavior; the maternal IgfII allele is normally silent. On the other hand, the H19 gene encodes an untranslated RNA and its loss may have as yet unidentified consequences. Although not specifically investigated for the IgfII/H19 locus, genomic imprinting has been shown to influence the quality of care that a mouse mother gives to her offspring.24–26 Interestingly, this linkage between IgfII and H19 and their imprinted features are conserved in humans.27 Thus, the disruption of maternal imprinting of IgfII appears to have unexpected negative consequences on sexual maturity and maternal behavior.
Reduced IGF-I signaling likely contributes to the finding that PAPP-A KO mice have lifespan extension of 30–40%, since ΔH19/PAPP-A KO mice retained the enhanced lifespan.14 These data in conjunction with the findings of the current study appear to support the idea that longevity is a secondary consequence of reproductive immaturity and/or decline in reproductive function.28–30 The disposable soma theory proposes a trade-off between resources available for reproduction on one hand and for maintenance and life extension on the other.31 However, studies in the long-lived Snell dwarf mice suggest that, at least for males, longevity does not depend on prepubertal immaturity.32 Also, male PAPP-A KO and ΔH19/PAPP-A KO mice did not show a compromised reproductive phenotype in this study even though these mice have an extended lifespan.12–14 Thus, much remains to be learned about stages in development and aging at which mice are susceptible to improvement long-term health by manipulation of PAPP-A and IGF signaling.
Acknowledgments
This work was supported by grant AG028141 from The National Institutes of Health and a Senior Scholar Award from the Ellison Medical Foundation (CAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.D’ercole AJ. Insulin-like growth factors and their receptors in growth. Endocrinol Metab Clinics N Am. 1996;25:573–590. doi: 10.1016/s0889-8529(05)70341-8. [DOI] [PubMed] [Google Scholar]
- 2.Yakar S, Rosen CJ. From mouse to man: redefining the role of insulin-like growth factor-I in the acquisition of bone mass. Exp Biol Med. 2003;228:245–252. doi: 10.1177/153537020322800302. [DOI] [PubMed] [Google Scholar]
- 3.Sonntag WE, Carter CS, Ikeno Y, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- 4.Bartke A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell. 2008;7:285–290. doi: 10.1111/j.1474-9726.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- 5.Rotwein P. Structure, evolution, expression and regulation of insulin-like growth factors I and II. Growth Factors. 1991;5:3–18. doi: 10.3109/08977199109000267. [DOI] [PubMed] [Google Scholar]
- 6.Chao W, D’Amore PA. IGF2: epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev. 2008;19:111–120. doi: 10.1016/j.cytogfr.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res. 2007;17:10–18. doi: 10.1016/j.ghir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Conover CA, Bale LK, Overgaard MT, et al. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131:1187–1194. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- 9.Bale LK, Conover CA. Disruption of insulin-like growth factor-II imprinting during embryonic development rescues the dwarf phenotype of mice null for pregnancy-associated plasma protein-A. J Endocrinol. 2005;186:325–331. doi: 10.1677/joe.1.06259. [DOI] [PubMed] [Google Scholar]
- 10.Tanner SJ, Hefferan TE, Rosen CJ, Conover CA. Impact of pregnancy-associated plasma protein-A deletion on the adult murine skeleton. J Bone Miner Res. 208;23:655–662. doi: 10.1359/JBMR.071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyegaard M, Overgaard MT, Su YQ, et al. Lack of functional pregnancy-associated plasma protein-A (PAPPA) compromises mouse ovarian steroidogenesis and female fertility. Biol Reprod. 2010;82:1129–1138. doi: 10.1095/biolreprod.109.079517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conover CA, Bale LK. Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell. 2007;6:727–729. doi: 10.1111/j.1474-9726.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- 13.Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RJ. Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. J Gerontol Biol Sci Med Sci. 2010;65:590–599. doi: 10.1093/gerona/glq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conover CA, Bale LK, Grell JA, Mader JR, Mason MA. Longevity is not influenced by prenatal programming of body size. Aging Cell. 2010;9:647–649. doi: 10.1111/j.1474-9726.2010.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leighton PA, Ingram RS, Eggenschwiler J, Efstratiadis A, Tilghman SM. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature. 1995;375:34–39. doi: 10.1038/375034a0. [DOI] [PubMed] [Google Scholar]
- 16.Daughaday WH, Mariz IK, Blethen SL. Inhibition of access of bound somatomedin to membrane receptor and immunobinding sites: a comparison of radioreceptor and radioimmunoassay of somatomedin in native and acid-ethanol-extracted serum. J Clin Endocrinol Metab. 1980;51:781–788. doi: 10.1210/jcem-51-4-781. [DOI] [PubMed] [Google Scholar]
- 17.Blum WF, Ranke MB, Bierich JR. A specific radio immunoassay for insulin-like growth factor II: interference of IGF binding proteins can be blocked by excess of IGF-I. Acta Endocrinol. 1988;118:374–380. doi: 10.1530/acta.0.1180374. [DOI] [PubMed] [Google Scholar]
- 18.Hwang DL, Lee PD, Cohen P. quantitative ontogeny of murine insulin-like growth factor (IGF)-I, IGF-binding protein-3 and the IGF-related acid-labile subunit. Growth Horm IGF Res. 2008;18:65–74. doi: 10.1016/j.ghir.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu Q, Jiang JY, Bell M, Tsang BK, Gruslin A. Activation of endoproteolytic processing of insulin-like growth factor-II in fetal, early postnatal, and pregnant rats and persistence of circulating levels in postnatal life. Endocrinology. 2007;148:4803–4811. doi: 10.1210/en.2007-0535. [DOI] [PubMed] [Google Scholar]
- 20.Zaina S, Pettersson L, Ahren B, et al. Insulin-like growth factor II plays a central role in atherosclerosis in a mouse model. J Biol Chem. 2002;277:4504–4511. doi: 10.1074/jbc.M108061200. [DOI] [PubMed] [Google Scholar]
- 21.Moerth C, Schneider MR, Renner-Mueller I, et al. Postnatally elevated levels of insulin-like growth factor (IGF)II fail to rescue the dwarfism of IGF-I-deficient mice except kidney weight. endocrinology. 2007;148:441–451. doi: 10.1210/en.2006-0385. [DOI] [PubMed] [Google Scholar]
- 22.Mohan S, Richman C, Guo R, et al. Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and –independent mechanisms. Endocrinology. 2003;144:929–936. doi: 10.1210/en.2002-220948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1α2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology. 2007;148:5706–5715. doi: 10.1210/en.2007-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkins JF, Haig D. Inbreeding, maternal care and genomic imprinting. J Theor Biol. 2003;221:559–564. doi: 10.1006/jtbi.2003.3206. [DOI] [PubMed] [Google Scholar]
- 25.Hager R, Johnstone RA. The genetic basis of family conflict resolution in mice. Nature. 2003;421:533–535. doi: 10.1038/nature01239. [DOI] [PubMed] [Google Scholar]
- 26.Moore AJ. Behavioral genetics: all in the family. Nature. 2004;429:517–518. doi: 10.1038/429517a. [DOI] [PubMed] [Google Scholar]
- 27.Zemel S, Bartolomei MS, Tilghman SM. Physical linkage of two mammalian imprinted genes, H19 and insulin-like growth factor 2. Nat Genet. 1992;2:61–65. doi: 10.1038/ng0992-61. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y-F, Wu C-Y, Kao C-H, Tsai T-F. Longevity and lifespan control in mammals: lessons from the mouse. Ageing Res Rev. 2010;9S:S28–S35. doi: 10.1016/j.arr.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Westendorp RGJ, Kirkwood TBL. Human longevity at the cost of reproductive success. Nature. 1998;396:743–746. doi: 10.1038/25519. [DOI] [PubMed] [Google Scholar]
- 31.Kirkwood TBL. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- 32.Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated Snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol Biol Sci. 2004;59A:1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]


