Abstract
Radionuclide chelators (DOTA, NOTA) functionalized with a monofluorocyclooctyne group were prepared. These materials reacted rapidly and in high yield with a fully deprotected azide-modified peptide via Cu-free click chemistry under mild reaction conditions (aqueous solution, room temperature). The resulting bioconjugates bind with high affinity and specificity to their cell-surface receptor targets in vitro and appear stable to degradation in mouse serum over three hours of incubation at 37 °C.
Keywords: copper free click chemistry, cyclooctyne, peptide synthesis, gallium-68, copper-64, melanocyte stimulating hormone, molecular imaging, PET, radionuclide therapy, PRRT
Radiolabeled peptides are increasingly recognized as effective tools for targeted molecular imaging and therapy of cancer.1–4 A significant challenge in preparing these compounds is selective attachment of chelators for coupling of radionuclides, while maintaining the high affinity binding of the peptide for its in vivo molecular target. A number of protection/deprotection schemes are employed to enable selective installation of the chelator moiety.5–7 Although these methods have proven effective, there is potential for loss of chemical yield with each synthetic step. Thus, simplified methods that enable selective attachment of the chelator, while preserving radiometal coupling, serum stability, and high affinity binding of the peptide for the molecular target can be advantageous.
Click chemistry techniques that employ copper-catalyzed [3+2] cycloaddition between azides and terminal alkynes have received considerable attention recently for use in bioconjugation strategies.8–10 These reactions are characterized by supreme selectivity, high yields, and rapid kinetics, while being amenable to mild aqueous reaction conditions. Although these characteristics have been investigated for the addition of polyamino-macrocyclic chelators to molecular targeting vectors,9, 11 use of the copper-catalyzed reaction for this application is complicated by the need to remove copper that is associated strongly with the chelator moiety prior to radiolabeling.9, 11,12
An alternative to the copper catalyzed cycloaddition reaction is the use of cyclooctyne agents that undergo cycloaddition reactions with azides spontaneously as a consequence of ring strain. In particular, fluoro-substituted cyclooctynes and dibenzocyclooctynes have been shown to participate in [3 + 2] cycloadditions with azides under mild conditions while exhibiting reaction profiles (chemoselectivity, yields, kinetics) comparable with their copper catalyzed counterpart.13–16 Although synthesis of cyclooctyne derivatives suitable for this application has been challenging,17 we recently introduced a versatile monoflouro-substituted cyclooctyne (MFCO, 1) that can be conveniently prepared in only four steps from readily available cyclooctanone in ~40% overall yield (Scheme 1).18 We also demonstrated that 1 could be coupled to a t-butyl protected DOTA (t-DOTA) derivative for selective addition to a resin-bound azide-modified peptide via copper-free click chemistry, followed by deprotection and cleavage by standard methods.19 With success in our initial proof of concept, we embarked to develop an improved bioconjugation strategy that would allow direct attachment of the chelator to bioligands under aqueous conditions without the need for protecting groups. Toward this end, we report here the preparation of fully-deprotected DOTA- and NOTA-MFCO chelators that can be conveniently and selectively attached to fully deprotected azide-modified peptides in aqueous solution at room temperature. To evaluate the potential of the approach for molecular imaging and radionuclide therapy, the DOTA-MFCO and NOTA-MFCO were conjugated to an azide-modified variant of a well-characterized peptide under investigation of molecular imaging and therapy of metastatic melanoma (see below). The chelator modified bioconjugates were efficiently radiolabeled with gallium-68 (68Ga) and copper-64 (64Cu). Competitive binding assays were conducted to evaluate the potential effect of the fused-triazole linkage on the binding affinity and the stability of the chelator-modified peptide conjugates was examined in mouse serum. We further evaluated the effect of metallation on binding affinity by labeling the chelator-modified peptides with stable Ga3+ and comparing the resulting affinity to the unmetallated variant.
Scheme 1.
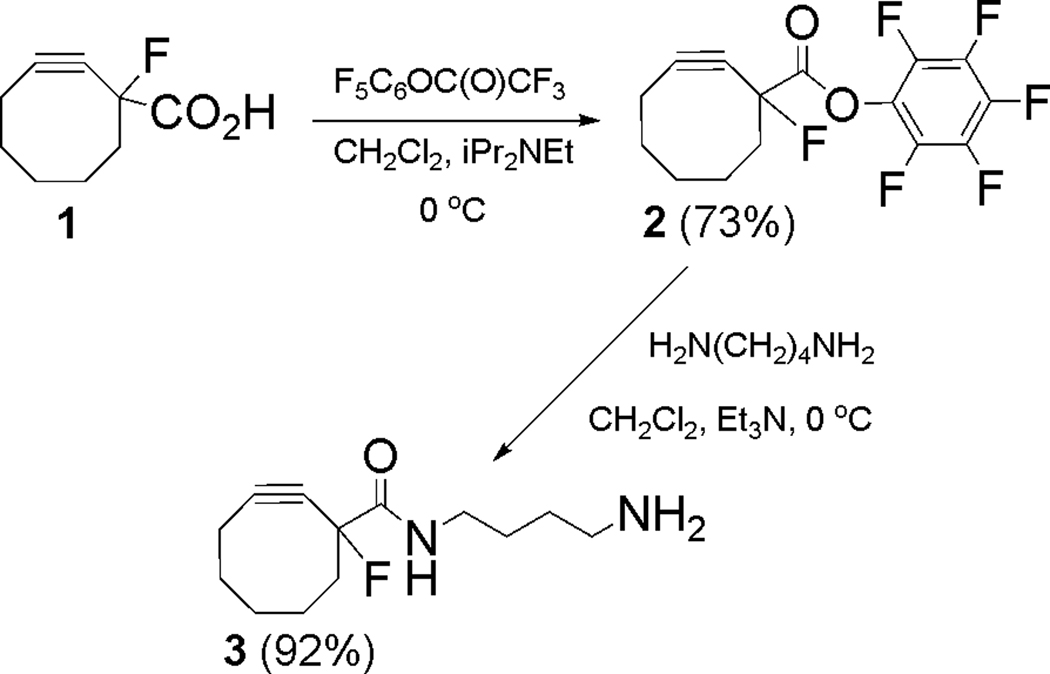
Synthesis of MFCO-amine (3)
The synthesis of DOTA- and NOTA-MFCO†† began with conversion of the acid (1) to the corresponding pentafluorophenyl ester 2 (Scheme 1). This material was previously prepared and used without isolation in the synthesis of a biotin-MFCO conjugate.17 In the present case, however, it was found that 2 could be easily isolated/purified and was stable for months at −20 °C. With the purified MFCO-PFP ester in hand, amine-MFCO (3) was readily obtained upon treatment of 2 with an excess of 1,4-diaminobutane in CH2Cl2. A simple aqueous workup of the reaction mixture afforded the desired MFCO-amine (3) as a pale yellow gummy solid in 92% yield. Material obtained in this manner proved suitable for use in subsequent coupling reactions without further purification.
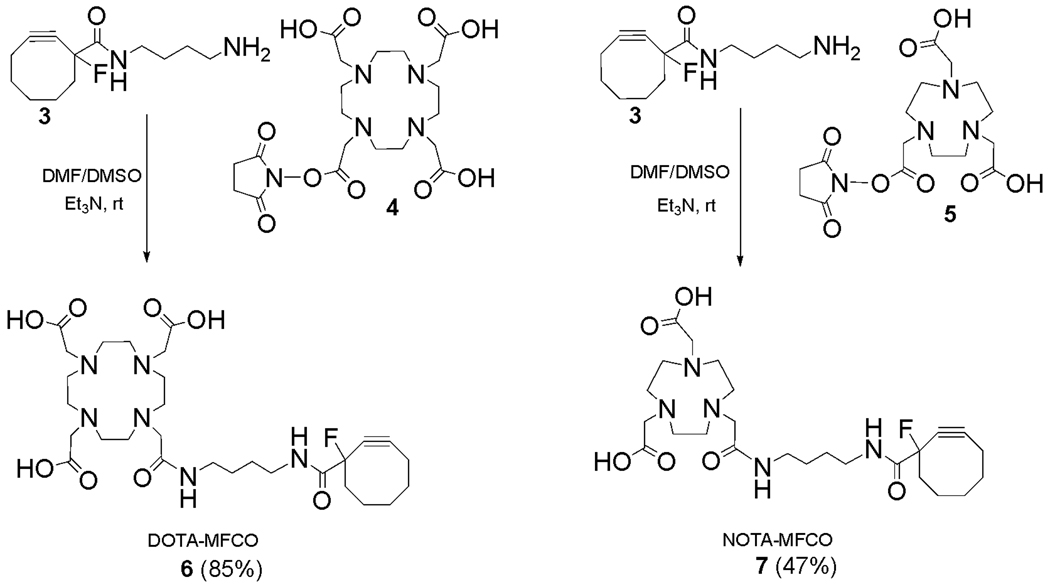
The preparation of MFCO-functionalized DOTA and NOTA derivatives (Scheme 2) proceeded smoothly by reaction of 3 with commercially available DOTA and NOTA N-hydroxysuccinimide esters (NHS esters) 4 and 5. These couplings were performed in DMF/DMSO at room temperature, and each reaction proceeded to completion within 2 h as determined by HPLC monitoring. The desired products 6 and 7 were isolated and purified by HPLC in good chemical yield (85% and 47%, respectively) and their identities were confirmed by mass spectrometry.
Scheme 2.
Synthesis of DOTA-MFCO (6) and NOTA-MFCO (7).
With 6 and 7 in hand, selective attachment of these new chelators to an azide-modified peptide via Cu-free click chemistry was next explored. The peptide chosen is a variant of α-melanocortin-stimulating hormone (α-MSH) in which two residues have been replaced with unnatural amino acids (Nle4,D-Phe7) to form NDP-α-MSH, which exhibits enhanced affinity for melanocortin receptors relative to the natural ligand. Since binding assays involving NDP-α-MSH are well established,20–27 this ligand offers a means to test the effect of DOTA(NOTA)-MFCO conjugation via fused triazole linkages on binding affinity to its cell-surface receptor target (i.e., melanocortin receptor subtype 1; MC1R).
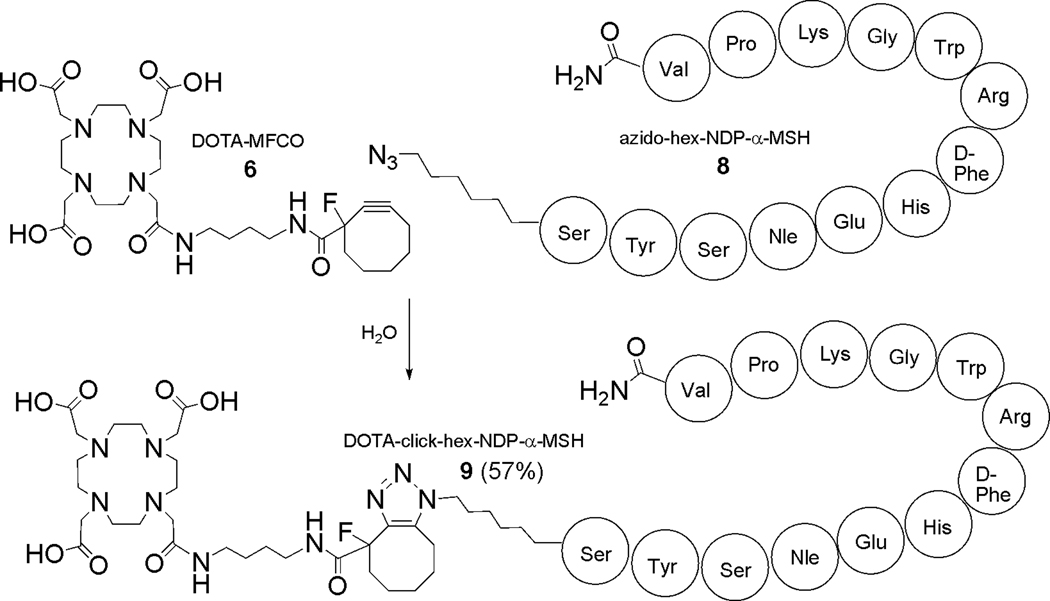
An azide-modified derivative of NDP-α-MSH (8) was prepared by standard solid phase peptide synthesis. Incorporation of the azide function was accomplished by coupling 6-azido hexanoic acid to the N-terminus of the fully side-chain protected peptide on resin, followed by deprotection and cleavage using routine procedures and purification/characterization by RP-HPLC and LC-MS.
DOTA-click-hex-NDP-α-MSH (9) was synthesized by reacting 100 nmol of azido-hex-NDP-α-MSH (8) with a 10-fold excess of DOTA-MFCO (6) in 0.5 mL of H2O at rt (Scheme 3). The corresponding NOTA analogue was prepared similarly by treating 8 with a 20-fold excess of 7. Reaction progress was monitored by observing the disappearance of starting material using RP-HPLC (214 nm). The Cu-free click reaction between DOTA-MFCO (6) and azido-hex-NDP-α-MSH (8) formed the desired product (9) in 2 h while complete formation of NOTA-click-hex-NDP-α-MSH (10, not shown in Scheme 3) required 5.5 h. The differences in reaction kinetics under these conditions are unclear, but may be related to differences in solubility of the NOTA-MFCO and DOTA-MFCO bifunctional chelators. Further optimization of the reaction parameters are the subject of ongoing research. No evidence of side reactions was observed by monitoring the 214 nm absorbance of the HPLC trace during the reaction period. The peptide conjugates were purified by HPLC, lyophilized, converted to the acetate form, and stored at −20°C or −80 °C (see Supporting Information). HPLC purification provided excellent isolated chemical yields and purity for these reactions for both DOTA-click-hex-NDP-α-MSH (9, 57.1%; ESI-MS: observed 2370.4 amu; theoretical: 2370.7 amu); and NOTA-click-hex-NDP-α-MSH (10, 70.3%; ESI-MS: observed 2269.4 amu; theoretical: 2269.58 amu). While the formation of 1,4 and 1,5 triazole regioisomers was expected, the species were not distinguishable by radio- or UV-HPLC methods applied here (see Figure 1; and Supporting Information Figure 4). Although radioHPLC traces for 64Cu suggested the presence of the expected regioisomers, efforts to separate these species further by modification of HPLC parameters proved unsuccessful (data not shown). On the other hand, mass spectral analysis of the resulting bioconjugates indicated that the fluorine substituent is labile over a period of months despite storage at −20 °C and −80 °C (see Supporting Information Figure 4). However, subsequent radiolabeling and in vitro competitive binding assays and stability analysis in serum indicate that loss of fluorine (as HF) neither degrades the binding affinity of the peptides for their cognate receptor target, nor promotes degradation of the chelator-peptide bioconjugate.
Scheme 3.
Synthesis of DOTA-click-hex-NDP-α-MSH (9).
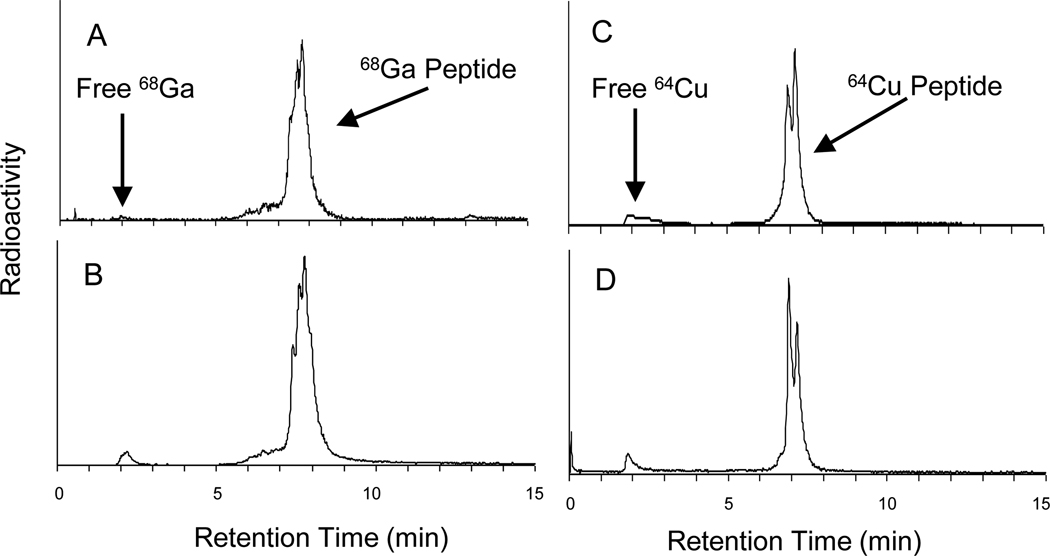
Figure 1.
Radiochemical purity of 64Cu and 68Ga labeled DOTA- and NOTA-click-hex-NDP-a-MSH (9, 10): (A) [68Ga]DOTA-peptide; (B) [68Ga] NOTA-peptide; (C) [64Cu] DOTA-peptide; (D) [64Cu] NOTA-peptide. Radiochemical purity in excess of 98% is achieved in all cases.
The ability to incorporate radionuclides into the purified bioconjugates was next determined through radiolabeling with positron emitters 64Cu and 68Ga. Radiolabeling reactions were carried out by methods described previously.18, 28, 29 Briefly, 64Cu (Washington University in Saint Louis USA) was incubated for 45 min at 70 °C with 5 and 10 nmol of peptide in 50 µL acetate buffer (pH 6.0). For 68Ga, radiolabeling was carried out through the use of a 68Ga/68Ge generator system IGG100 (Eckert Ziegler, GmbH, Berlin, Germany) with a total 68Ge activity of approximately 900 MBq at the time of the experiments presented here. 68Ga was eluted in 10 mL 0.1 M HCl, purified by cation exchange and incubated with 5 to 10 nmoles of chelator modified peptide at 100 °C for 12 minutes. Preparation of 68Ga labeled variants required the insertion of a final purification step using a disposable C-18 cartridge post-radiolabeling to achieve suitable radiochemical purity (Figure 1A & B). In each case radioHPLC analysis demonstrated excellent radiochemical purity (>98%) and specific activity (68Ga, 48 MBq nmole−1 and 64Cu, 35 MBq nmole−1). It is anticipated that the specific activity of the 64Cu variant could be improved as no final purification step was required to obtain >98% radiochemical purity at specific activity of 35 MBq nmole−1 (Figure 1C & D).
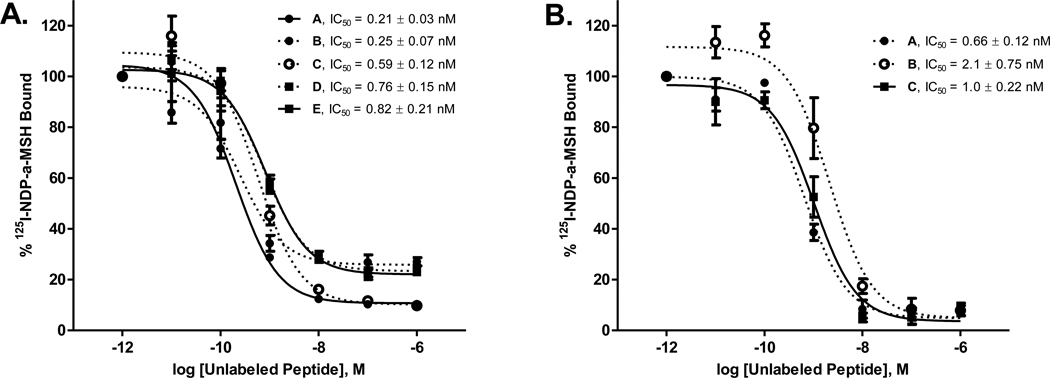
Finally, the biological activities of DOTA and NOTA-click-hex-NDP-á-MSH peptide conjugates 9 and 10 were evaluated through in vitro cell-binding assays. For these experiments, the effect of the fused triazole-MFCO linkage on receptor binding was determined by comparison to binding affinities exhibited by α-MSH peptide derivatives lacking this motif. The effect of metallation on receptor binding affinity was examined using stable Ga3+ labeled DOTA-click-hex-NDP-α-MSH. Specifically, the binding of 9 (metallated and unmetallated) and 10 to B16-F10 murine melanoma cells expressing MC1R was compared to binding affinities obtained for NDP-α-MSH, azido-hex-NDP-α-MSH (8), and a DOTA-NDP-α-MSH derivative prepared by standard N-amide bond conjugation. By using stable Ga3+ for these experiments, we were able to make this comparison using the same iodine-125 (125I) labeled native α-MSH competitor and melanoma cells, thus enabling a fair comparison of observed binding affinities of the unmetallated species conducted by us and according to methods described previously.30 Briefly, cell suspensions were incubated with a constant quantity of 125I labeled NDP-α-MSH ([125I]-[Nle4,D-Phe7]-α-MSH) and increasing concentrations of peptide analog ranging from 10−6 to 10−11 M. Following incubation, cells were pelleted by centrifugation, the media was aspirated, pellets were transferred to 12×75 mm glass tubes, and the radioactivity of the cell pellet was determined by gamma counter. Each experiment was performed in quadruplicate and binding curves were plotted; IC50 values and their associated standard errors were calculated with GraphPad Prism 5 curve-fitting software (GraphPad Prism version 5.01 for Windows, GraphPad Software, San Diego, CA). The observed IC50 values for the DOTA- and NOTA-click-hex-NDP-α-MSH (9 and 10) calculated in this way were less than 1 nM (0.82 nM and 0.76 nM, respectively) (Figure 2). No significant difference in binding affinity was observed for Ga3+ metallated NOTA-click-hex-NDP-α-MSH (1.0 ± 0.22 nM) and the unmetallated species (0.82 ± 0.21 nM). Comparison of affinity values for Ga3+ metallated DOTA-amide-NDP (0.66 ± 0.12 nM) and unmetallated conjugate (0.59 ± 0.12 nM) also yielded no significant difference associated with metallation of the chelator modified peptide. The metallated-species affinity values are slightly elevated in comparison to unmetallated conjugates and overall are slightly higher than standard N-terminal amide bond formation, although the difference is not statistically significant. Furthermore, 9 and 10 display IC50 concentrations comparable to those of azido-hex-NDP-α-MSH (8) and NDP-α-MSH.21, 24, 27, 31–33 On the other hand, the binding affinity of the Ga3+ metallated DOTA-click-hex-NDP-α-MSH conjugate (2.1 ± 0.75 nM) appeared somewhat higher than the unmetallated counterpart (0.76 ± 0.15), although the uncertainty obtained for the metallated conjugate is rather large in comparison to other experimental results suggesting the potential for an artifact that requires further investigation. In general, these findings suggest that the presence of fused triazole-MFCO linkages does not significantly alter the binding affinity or selectivity of the molecular targeting vector in vitro. It is anticipated that this quality will be preserved for conjugation of the MFCO-family of chelators to other molecular targeting vectors as well.
Figure 2.
Competitive inhibition of [125I]-NDP-α-MSH binding to B16-F10 mouse melanocytes by α-MSH peptides. (A) Peptides A through E (n=4) in the figure legend correspond to the following: (A) NDP- α-MSH; (B) azido-hex-NDP-α-MSH (8); (C) DOTA-amide-NDP-α-MSH; (D) DOTA-click-hex-NDP-α-MSH (9); and (E) NOTA-click-hex-NDP-α-MSH (10). (B) Peptides A through C in the figure legend correspond to the following: A = [Ga]DOTA-NDP-α-MSH, B = [Ga]DOTA-Click-NDP-α-MSH, and C = [Ga]NOTA-Click-NDP-α-MSH.
In summary, two novel chelator moieties (DOTA-MFCO 6 and NOTA-MFCO 7) were successfully synthesized in high yield and used for copper-free click conjugation to an azide-modified peptide. Specifically, these MFCO-modified chelators were selectively attached to an azide-modified fully-deprotected analogue of NDP-α-MSH under aqueous conditions at room temperature within 2 h (DOTA-MFCO) and 5.5 h (NOTA-MFCO). The new NOTA and DOTA peptide variants were each radiolabeled with 64Cu and 68Ga in excellent radiochemical purity and specific activity. In vitro binding assays suggest that the fused-triazole conjugation strategy provides a stable coupling and does not significantly alter the binding affinity of the peptide for its cognate MC1R receptor. Metallation with Ga3+ did not significantly alter the binding affinity of the NOTA-click-hex-NDP-α-MSH or DOTA- click-hex-NDP-α-MSH for their molecular target. The “click” bioconjugates were stable in mouse serum when incubated at 37 °C for three hours (a relevant time frame for peptide-based molecular targeting), with evidence of a stability advantage over an amide-linked DOTA-NDP-α-MSH variant that is the subject of ongoing research (see Supplemental Figures 5–6, Supporting Information). Thus, the DOTA-MFCO (6) and NOTA-MFCO (7) entities represent a new class of bifunctional chelator that enables selective attachment to fully-deprotected azide-modified peptides under aqueous conditions at room temperature with excellent chemical yields.
Supplementary Material
Acknowledgements
The authors acknowledge support of the University of Iowa Holden Comprehensive Cancer Center, the American Cancer Society (IRG-77004-31), and the Roy J. Carver Charitable Trust (RJCCT 01-224 and 09-3279).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information
†Electronic Supplementary Information (ESI) available: Detailed synthesis of 2, 3, 6–10; RP-HPLC purification and ESI-MS of chelators and peptides; cell culturing and binding assay details; and detailed radiolabeling procedures. See DOI: 10.1039/b000000x/
DOTA = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid; NOTA = 1,4,7-triazacyclononane-1,4,7-triacetic acid.
References
- 1.Bushnell DL, Jr, O'Dorisio TM, O'Dorisio MS, Menda Y, Hicks RJ, Van Cutsem E, Baulieu JL, Borson-Chazot F, Anthony L, Benson AB, Oberg K, Grossman AB, Connolly M, Bouterfa H, Li Y, Kacena KA, LaFrance N, Pauwels SA. J Clin Oncol. 2010;28(10):1652. doi: 10.1200/JCO.2009.22.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menda Y, O'Dorisio MS, Kao S, Khanna G, Michael S, Connolly M, Babich J, O'Dorisio T, Bushnell D, Madsen M. J Nucl Med. 2010;51(10):1524. doi: 10.2967/jnumed.110.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miao Y, Quinn TP. Crit Rev Oncol Hematol. 2008;67(3):213. doi: 10.1016/j.critrevonc.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, Papathanasiou ND, Pepe G, Oyen W, De Cristoforo C, Chiti A. Eur J Nucl Med Mol Imaging. 2010;37(10):2004. doi: 10.1007/s00259-010-1512-3. [DOI] [PubMed] [Google Scholar]
- 5.De Leon-Rodriguez LM, Kovacs Z. Bioconjug Chem. 2008;19(2):391. doi: 10.1021/bc700328s. [DOI] [PubMed] [Google Scholar]
- 6.Brechbiel MW. Qrtly J Nucl Med and Mol Imaging. 2008;52(2):166. [PMC free article] [PubMed] [Google Scholar]
- 7.Dijkgraaf I, Liu S, Kruijtzer JA, Soede AC, Oyen WJ, Liskamp RM, Corstens FH, Boerman OC. Nucl Med Biol. 2007;34(1):29. doi: 10.1016/j.nucmedbio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 8.El-Sagheer AH, Brown T. Chem Soc Rev. 2010;39(4):1388. doi: 10.1039/b901971p. [DOI] [PubMed] [Google Scholar]
- 9.Lebedev AY, Holland JP, Lewis JS. Chem Comm. 2010;46(10):1706. doi: 10.1039/b924784j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moses JE, Moorhouse AD. Chem Soc Rev. 2007;36(8):1249. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 11.Knor S, Modlinger A, Poethko T, Schottelius M, Wester HJ, Kessler H. Chemistry. 2007;13(21):6082. doi: 10.1002/chem.200700231. [DOI] [PubMed] [Google Scholar]
- 12.Nwe K, Brechbiel MW. Cancer Biother Radiopharm. 2009;24(3):289. doi: 10.1089/cbr.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. PNAS. 2007;104(43):16793. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. JACS. 2008;130(34):11486. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sletten EM, Bertozzi CR. Org Lett. 2008;10(14):3097. doi: 10.1021/ol801141k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ning X, Guo J, Wolfert MA, Boons GJ. Angew Chem Int Ed Engl. 2008;47(12):2253. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jewett JC, Bertozzi CR. Chem Soc Rev. 2010;39(4):1272. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz MK, Parameswarappa SG, Pigge FC. Org Lett. 2010;12(10):2398. doi: 10.1021/ol100774p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin ME, Parameswarappa SG, O'Dorisio MS, Pigge FC, Schultz MK. Bioorg Med Chem Lett. 2010;20(16):4805. doi: 10.1016/j.bmcl.2010.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao Y, Gallazzi F, Guo H, Quinn TP. Bioconjug Chem. 2008;19(2):539. doi: 10.1021/bc700317w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao Y, Whitener D, Feng W, Owen NK, Chen J, Quinn TP. Bioconjug Chem. 2003;14(6):1177. doi: 10.1021/bc034069i. [DOI] [PubMed] [Google Scholar]
- 22.Raposinho PD, Correia JD, Oliveira MC, Santos I. Biopolymers. 2010;94(6):820. doi: 10.1002/bip.21490. [DOI] [PubMed] [Google Scholar]
- 23.Ren G, Liu S, Liu H, Miao Z, Cheng Z. Bioconjug Chem. 2010 doi: 10.1021/bc100391a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei L, Butcher C, Miao Y, Gallazzi F, Quinn TP, Welch MJ, Lewis JS. J Nucl Med. 2007;48(1):64. [PubMed] [Google Scholar]
- 25.Wei L, Miao Y, Gallazzi F, Quinn TP, Welch MJ, Vavere AL, Lewis JS. Nucl Med Biol. 2007;34(8):945. doi: 10.1016/j.nucmedbio.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Guo H, Gallazzi F, Berwick M, Padilla RS, Miao Y. Bioconjug Chem. 2009;20(8):1634. doi: 10.1021/bc9001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Guo H, Padilla RS, Berwick M, Miao Y. Bioorg Med Chem. 2010;18(18):6695. doi: 10.1016/j.bmc.2010.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson CJ, Wadas TJ, Wong EH, Weisman GR. Qrtly J Nucl Med and Mol Imaging. 2008;52(2):185. [PMC free article] [PubMed] [Google Scholar]
- 29.Rockey WM, Huang L, Kloepping KC, Baumhover NJ, Giangrande PH, Schultz MK. Bioorg & Med Chem. 2011 doi: 10.1016/j.bmc.2011.05.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eberle AN, Verin VJ, Solca F, Siegrist W, Kuenlin C, Bagutti C, Stutz S, Girard J. J Recept Res. 1991;11(1–4):311. doi: 10.3109/10799899109066410. [DOI] [PubMed] [Google Scholar]
- 31.Miao Y, Hoffman TJ, Quinn TP. Nucl Med Biol. 2005;32(5):485. doi: 10.1016/j.nucmedbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Miao Y, Quinn TP. Front Biosci. 2007;12:4514. doi: 10.2741/2406. [DOI] [PubMed] [Google Scholar]
- 33.Quinn T, Zhang X, Miao Y. G Ital Dermatol Venereol. 2010;145(2):245. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.