Abstract
IL-1 strikingly enhances antigen-driven responses of CD4 and CD8 T cells. It is substantially more effective than LPS and when added to a priming regime of antigen plus LPS, it strikingly enhances cell expansion. The effect is mediated by direct action on CD4 and CD8 T cells; the response occurs when OT-I or OT-II cells are transferred to B6 IL-1R1−/− recipients and only cells that express IL-1 receptors can respond. The major mechanism through which IL-1 enhances responses is by increasing survival of responding cells. IL-1 enhances the proportion of responding CD4 T cells that differentiate into Th17 cells and increases the proportion of responding CD8 cells that express granzyme B. Of a wide range of cytokines tested, only IL-1α and IL-1β mediate this function. The potency of IL-1 as an enhancer of T cell responses suggests that it could act to enhance responses to weak vaccines and that the pathway utilized by IL-1 might be considered in the design of new generations of adjuvants.
Keywords: CD4, CD8, IL-1 receptor, Lipopolysaccharide, IL-1RA
1. Introduction
IL-1 is a highly pleiotropic cytokine, well known for its potent pro-inflammatory effects. Its action as an inducer of adaptive immune responses has not been as clear as many of its effects in orchestrating inflammation. Nonetheless, the history of IL-1 acting to enhance T cell responses is an old one, going back to the pioneering work of Igal Gery, Richard Gershon and Byron Waksman, in which they identified an LPS-induced macrophage product that enhanced the proliferative response of thymocytes to phytohemagglutinin [1]. This product was designated leukocyte activating factor (LAF). With the subsequent discovery of the T cell product, T cell growth factor (TCGF) and the effort to introduce a standardized nomenclature, LAF was designated IL-1 and TCGF, IL-2 [2].
Despite this early attention to the role of IL-1 on T cell responses, the study of IL-1 action on T cells and the consequences of IL-1 stimulation of T cells largely languished. An effort to understand how IL-1 impacted primary immune responses revealed that a single small dose of IL-1 at the time of immunization modestly enhanced T cell responses largely by action on dendritic cells [3].
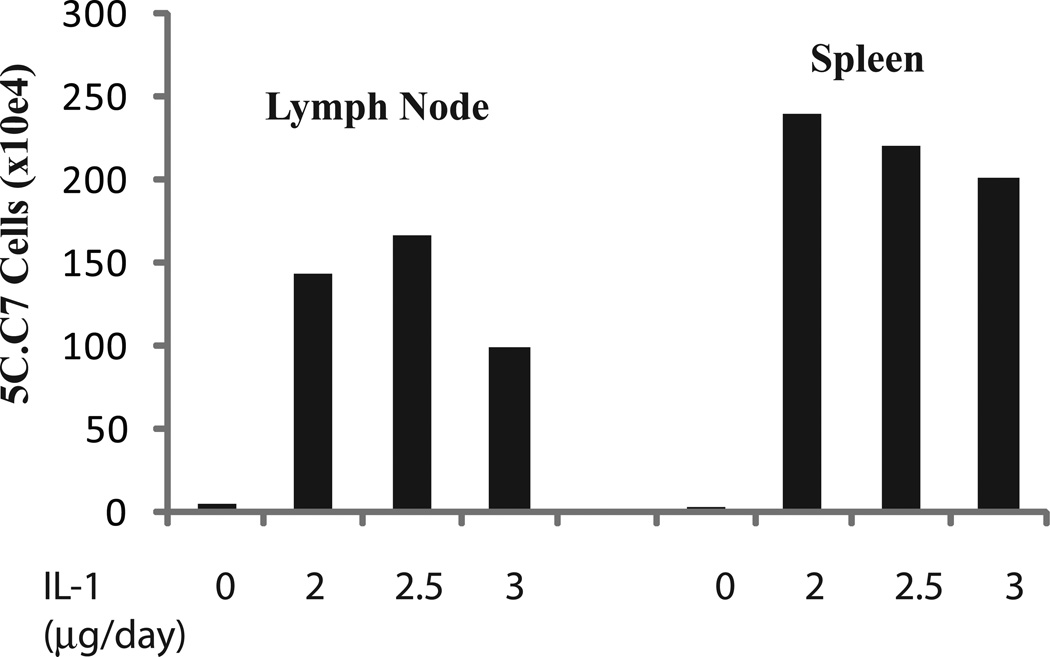
We became interested in the effect of IL-1 as a stimulant of T cell responses in 2005 when we screened a variety of cytokines for their capacity to enhance primary and secondary immune responses in mice. The model we used was to transfer T cell receptor (TCR) transgenic cells to syngeneic recipients and to challenge these animals with the antigen cognate for the TCR expressed by the transgenic T cells [4]. We then compared the expansion of the transferred antigen-specific cells, as measured by the absolute number of transgenic cells at one week after primary immunization or, in a related set of experiments, at a similar time after secondary immunization. Without any added adjuvant, antigen caused little or no expansion of transferred transgenic CD4 T cells. Addition of LPS led to considerable expansion; none of the cytokines we tested performed better than LPS in mediating antigen-driven CD4 T cell expansion with the exception of IL-1α and IL-1β (Fig. 1). Among the cytokines we have tested thus far are: TNFα, IL-2, IL-4, IL-7, IL-9, IL-15, IL-18, and IL-33. IL-1α and IL-1β used with antigen, both substantially outperformed LPS, often by factors of 5- to 10-fold. In most other experiments, we compared immunization with antigen plus LPS to antigen plus LPS plus IL-1β. That model also showed that the presence of IL-1β enhanced antigen plus LPS-driven responses, resulting in a 5- to 10-fold greater response to antigen, LPS and IL-1 than that observed with antigen plus LPS.
Fig. 1.
IL-1 enhances responses of specific cells to immunization with antigen plus LPS. 5C.C7 cells, derived from TCR transgenic B10.A mice, were transferred to syngeneic B10.A recipients. The mice were immunized with cytochrome C plus LPS and on days 2 through 6 received PBS or IL-1 (2.0, 2.5, or 3.0 µg per day). Animals were sacrificed on day 7 and numbers of 5C.C7 cells in spleen and lymph nodes determined.
The route of administration of IL-1β varied. In early experiments, we administered the cytokine continuously, using miniosmotic pumps that would deliver the cytokine over a 7-day period. Generally, 5 to 10 µg of IL-1β was placed in the miniosmotic pump. However, we have had comparable success in administering the IL-1β subcutaneously together with antigen and LPS. While many of our experiments have utilized daily injections for five days, with 1 to 2 µg per dose, we have had success with as few as two injections, on day 1 and day3 of the immune response. We have also had success administering IL-1 and antigen intratracheally and measuring the response in the mediastinal lymph node.
2. What cell is the target of IL-1
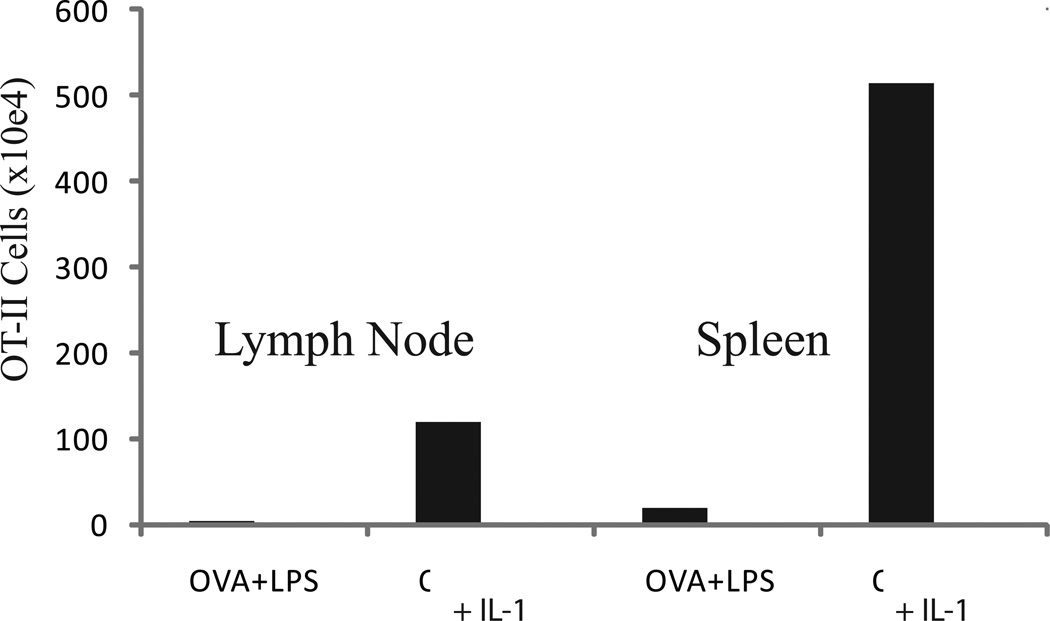
We initially considered the possibility that dendritic cell activation was the mechanism through which IL-1 mediated its enhancing action on CD4 T cell expansion. However, in a direct test it became clear that IL-1β acting on antigen-specific CD4 T cells led to a very considerable expansion of the responding cells [4]. The critical experiments utilized the transfer of T cells from C57BL/6 mice transgenic for a TCR specific for an ovalbumin peptide. These mice, designated OT-II, were bred onto a Rag2−/− background so that all the CD4 T cells from the donor were ovalbumin-specific; no second receptors were expressed by the transgenic T cells, and virtually all the transgenic T cells would be naive. OT-II cells from Rga2−/− donors were then transferred into wild-type C57BL/6 mice or IL-1R1-deficient C57BL/6 recipients. In the latter setting, the only IL-1-receptor-expressing cells would be the transferred OT-II cells and, if those cells showed an enhanced expansion when IL-1 was added to the OVA plus LPS immunization regime, it would indicate that IL-1 acted on the T cells. Indeed, IL-1 enhanced the expansion of the OT-II cells in the IL-1R1-deficient recipients (Fig. 2). The degree of IL-1-mediated enhancement was actually greater than the enhancement observed when OT-II cells were transferred to wild-type recipients. This greater enhancement can probably be ascribed to the higher serum levels of IL-1 achieved when IL-1 is infused or injected into IL-1R1-deficient mice since the only cells that can “consume” the IL-1 are the transferred T cells. We directly measured serum IL-1β concentration in the IL-1R1-deficient recipients and observed that it was several fold higher than in the wild-type recipients immunized with OVA, LPS plus IL-1.
Fig. 2.
IL-1 enhances antigen-driven expansion of CD4 T cells transferred to IL-1R1−/− mice. OT-II cells, derived from TCR transgenic B6 mice, were transferred to syngeneic IL-1R1−/− recipients. The mice were immunized subcutaneously with ovalbumin (OVA) plus LPS, with our without IL-1 (administered on days 2–6). Animals were sacrificed on days 7 and the number of OT-II cells in lymph node and spleen measured. Results represent means of four individual animals.
3. How does IL-1 enhance expansion of antigen-specific CD4 T cells
Although we still lack a molecular mechanism underlying the enhanced expansion of naive CD4 T cells when primed in the presence of IL-1, we have examined the relative proliferative rates of 5C.C7 cells primed in vivo with antigen plus LPS with or without IL-1. In these experiments, the transferred cells were first labeled with CFSE so that we could measure the division history of the responding cells as well as the absolute number of transgenic cells. The striking result was that by days 3 and 4 after immunization, the presence of IL-1 in the immunization regime resulted in a 5- to 7-fold greater degree of expansion of the transferred TCR transgenic cells but the mean number of cell divisions had increased by less than 1. Therefore, enhanced cell division could account for only a relatively small proportion of the antigen-driven expansion observed when IL-1 is added to the priming regime. Thus, we conclude that the principal IL-1-mediated expansion mechanism is enhanced cell survival, which is not unanticipated in view of the capacity of the IL-1R1-Myd88 pathway to activate NF-κB and the well known role of NF-κB in enhancing cell survival [5]. What is enigmatic, as we discuss below, is that many of the cells that undergo expansion would be expected to express very limited numbers of IL-1R1 chains and thus limited numbers of functional IL-1 receptors [6]. Would the engagement of such small numbers of receptors be sufficient to activate the NF-κB pathway?
4. Do responding cells need to express IL-1R1
While the transfer experiment established that IL-1 targeting of antigen-specific CD4 T cells can account for the enhanced response, it does not rule out the possibility that the T cells responding to IL-1 produce a product that causes other antigen-specific cells to respond more vigorously even if those cells did not receive an IL-1-generated signal. To examine this point, we prepared CD4 T cells from wild-type OT-II mice and IL-1R1-deficient OT-II mice, expressing distinct CD45 markers. When transferred together to IL-1R1-deficient B6 recipients, only the wild-type OT-II cells showed a greater degree of expansion when IL-1β was included in the immunization regime. The IL-1R1-deficient OT-II cells showed the same degree of antigen-driven expansion in the presence or absence of IL-1, indicating that the response of the wild-type cells did not allow the IL-1R1-deficient cells to respond more vigorously.
5. Are CD4 T cell products important in IL-1-mediated expansion
The dual transfer experiment does not exclude the possibility that a product of the responding cells may be important in or even essential for the enhanced expansion of the responder cells. It only establishes that such a factor or factors cannot enhance responses unless the cells have also received an IL-1-induced signal. An obvious candidate to act in this manner would be IL-6 since IL-6 can enhance responses of naive cells in vitro [7] and IL-1 often induces IL-6 production and makes cells more sensitive to IL-6 [8]. We directly tested whether IL-6 was essential for IL-1-mediated expansion of OT-II cells. We transferred IL-6-deficient TCR transgenic CD4 T cells into IL-6-deficient recipient cells. The cells showed an enhanced response to antigen plus LPS and IL-1, compared to antigen plus LPS alone, indicating that IL-6 was not required for the mediating the IL-1 effect. In a similar type of experiment, we also showed that CD-28 was not required for the IL-1 effect; CD-28-deficient OT-II cells showed an enhanced response to IL-1, LPS and antigen.
Although CD4 T cell secreted products cannot mediate IL-1-mediated enhanced expansion of antigen-specific CD4 T cells expansion independent of IL-1 action on the responding cells, there is a striking effect of administering IL-1 during a T cell response. Spleen and lymph nodes expand in size in IL-1R1−/− recipients of wild-type OT-II cells that are immunized with antigen, LPS and IL-1. This CD4 T cell dependent expansion is not cell selective. It affects CD4, CD8 and B cells equivalently.
6. IL-1 administration promotes Th17 CD4 T cell responses
Analysis of the cytokine producing potential of T cells primed in the presence of IL-1 yields several quite interesting points. There is a striking increase in the frequency of cells capable of producing IL-17 upon in vitro stimulation with antigen or with PMA and ionomycin. This is an exciting but perhaps anticipated result. IL-1 is known to play a role in Th17 priming in vitro [9, 10], particularly in human cells [11], and Th17 cells express large amounts of mRNA for IL-1 receptors whereas Th1 cells have almost undetectable levels of IL-1R1 mRNA [6]. Th2 cells, while expressing much less IL-1R1 mRNA than Th17 cells, do express substantially more that do Th1 cells.
Thus, it is perhaps not surprising that the frequency of IL-17-producing cells is increased by priming with antigen, LPS and IL-1, compared to priming with antigen and LPS. There is a modest increase in the frequency of IL-4-producing cells but this increase translates into quite a substantial increment in serum concentrations of IgE and IgG1, implying that the increase in Th2 cells is real. This increase is in keeping with the intermediate amounts of IL-1R1 mRNA expressed by Th2 cells.
7. What accounts for the enhanced expansion of Th1 cells in response to the presence of IL-1 in the priming regime
What is surprising is that, while Il-1 results in only a modest increase in the proportion of antigen-specific CD4 T cells that produce IFNγ, there is certainly no fall in the proportion of IFNγ-producing cells. Since the total number of antigen-specific cells increases 5- to 10-fold, the total number of Th1 cells increases strikingly in immunization protocols that include IL-1. The experiment cited above that shows that only cells expressing IL-1R1 undergo enhanced expansion as a result of priming in the presence of IL-1 implies that IL-1 must act directly on the responding cells. Thus, do the precursors of Th1 cells express more IL-1 receptors than the mature cells and does this account for their enhanced expansion or are the very limited numbers of IL-1 receptors on Th1 cells sufficient to drive their expansion? If the latter, are the molecular mechanisms through which IL-17-producing cells and IFNγ-producing cells undergo enhanced expansion in total number when IL-1 is added to the priming regime the same or different? This needs careful analysis if we are to understand the mechanisms through which IL-1 mediates its striking in vivo expansion effects.
8. CD8 T cells undergo enhanced expansion in response to antigen, LPS and IL-1
In a model similar to that used to assess the capacity of IL-1β to increase the expansion of OT-II or 5C.C7 cells, we undertook an analysis of the role of IL-1 in enhancing the expansion of CD8 TCR transgenic cells that are specific for an OVA peptide (OT-I cells), in response to immunization with antigen plus LPS. We observed that splenic OT-I cells showed a massive expansion when IL-1 was added to the priming regime and a larger proportion of these cells express granzyme B suggesting that they might be more effective effector cells. Preliminary experiments indicate that the expansion of CD8 T cells could occur in a model in which the CD8 T cells expressed IL-1R1 but the recipient C57BL/6 mice lacked IL-1R1, indicating that IL-1 acted directly on the responding CD8 cells.
Immunization with IL-1 enhanced the cytotoxic activity of the responding cells, as assessed with an in vivo cytotoxicity assay. Immunization of C57BL/6 mice with ovalbumin plus LPS with or without IL-1 induced a striking ovalbumin-specific cytotoxic activity if IL-1 was present during priming but only minimal cytotoxic activity when IL-1 was not added. Thus priming in the presence of IL-1 causes a striking enhancement in expansion of CD8 cells, an increase in the frequency of the cells that have granzyme B and enhanced in vivo cytotoxicity.
9. Can the IL-1 pathway be employed to enhance the efficacy of weak vaccines
The very striking capacity of IL-1 to enhance expansion and differentiation of CD4 and CD8 T cells to efficient effectors suggests that if used with weak vaccines, there might be sufficient enhancement in the magnitude and quality of the immune response to allow these vaccines to be efficacious. An obvious concern would be the very severe toxicities that IL-1 would induce at the relatively high concentrations that are used in our experiments. However, if IL-1 acts only on the antigen-stimulated CD4 T cells, much of the toxicity is lost. That was clearly observed in models in which OT-II cells were transferred into IL-1R1−/− recipients. Challenge of these mice with OVA, LPS and IL-1 induced a very large degree of expansion of antigen-specific CD4 T cells but limited overt toxicity. Probably, because of the very large number of transgenic cells used, splenomegaly and lymphadenopathy were noted, but if the number of responding T cells were limited, as would be the case in physiologic immunization, we suspect that these side-effects would be markedly diminished. The challenge is to develop a strategy that would allow IL-1 or some surrogate to be used in such a way that only T cells (or indeed only the responding T cells) were targeted. An alternative would be to find pharmacologic manner to reproduce the IL-1 effect and to target that effect to the CD4 or CD8 cell populations. Whether either approach will be feasible will await direct future work.
Highlights.
IL-l is a potent enhancer of CD4 and CD8 T cell responses to antigen. It leads to greater antigen-driven responses than LPS and, when paired with LPS can cause expansion that is 5-10-fold greater than LPS alone
The IL-l effect in enhancing CD4 T cell expansion is mediated by direct action on the antigen-specific T cells.
IL-l strikingly enhances the differentiation of the responding CD4 T cell population to Th17 cells, suggesting it might enhance responses that were protective against extracellular bacteria and fungi.
Acknowledgments
This work was supported by the NIAID Division of Intramural Research through Project 1-Z01-AI000926-09-LI. The care and handling of the animals used in our studies were in accordance with the guidelines of the National Institutes of Health Animal Care and Use Committee. We thank Shirley Starnes for excellent editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gery I, Gershon RK, Waksman BH. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J. Exp. Med. 1972;136:128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arden L, et al. Revised nomenclature for antigen-nonspecific T cell prolioferation and helper factors. J. Immunol. 1979;123:2928–2929. [PubMed] [Google Scholar]
- 3.Khoruts A, Osness RE, Jenkins MK. IL-2 acts on antigen presenting cells to enhance the in vivo proliferation of antigen-stimulated naïve CD4 T cells via a CD28-dependent mechanism that does not involve increased expression of CD28 ligands. Eur. J. Immunol. 2004;34:10085–10090. doi: 10.1002/eji.200324170. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. USA. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Ann. Rev. Immuol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 6.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. USA. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rochman I, Paul WE, Ben-Sasson SZ. IL-6 increases primed cell expansion and survivial. J. Immunol. 2005;174:4761–4767. doi: 10.4049/jimmunol.174.8.4761. [DOI] [PubMed] [Google Scholar]
- 8.Helle M, Boeije L, Aarden LA. IL-6 is an intermediate in IL-1-induced thymocyte proliferation. J. Immunol. 1989;142:43335–43338. [PubMed] [Google Scholar]
- 9.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulen MF, Kang Z, Bulek K, Youzhong W, Kim TW, Chen Y, Altuntas CZ, Sass Bak-Jensen K, McGeachy MJ, Do JS, Xiao H, Delgoffe GM, Min B, Powell JD, Tuohy VK, Cua DJ, Li X. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32:54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Evnon EE, Flavell RA, Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]