Abstract
Cannabinoid CB2 receptor has emerged as a very promising target over the last decades. We have synthesized and evaluated a new fluorescent probe designated NMP6 based on 6-methoxyisatin scaffold, which exhibited selectivity and Ki value at hCB2 of 387 nM. We have demonstrated its ability to be an effective probe for visualization of CB2 receptor binding using confocal microscopy and a flow cytometry probe for the analysis of CB2 protein expression. Furthermore, NMP6 was easily obtained in two chemical steps from commercially available building blocks.
The endocannabinoid system consists of the cannabinoid receptors, CB1 and CB2, the endogenous ligands known as endocannabinoids and the proteins involved in the synthesis, transport, and metabolism1. There is also evidence that additional orphan G protein–coupled receptors such as GPR55 are non-CB1 and/or CB2 cannabinoid receptors2. The cannabinoid receptor CB2 is expressed mainly on immune tissues such as the spleen, tonsils, monocytes, and B and T lymphocytes3, 4 and to a lesser degree in the CNS5, 6. Onaivi and colleagues7 reported that CB2 receptors are expressed in the brains of naïve mice and that this expression was enhanced in the presence of chronic mild stress. We and others8–10 have shown that CB2 agonists may be effective for treatment of neuropathic pain without central effects attributable to CB1 activation. CB2 mRNA and/or proteins are increased during different inflammatory conditions11–16. CB2 expression within immune cells, induction of CB2 expression after injury or inflammation, and the discovery that immunomodulation by cannabinoids is absent in CB2 knockout mice 17 are observations that suggest that CB2 plays a key role in immunomodulatory activity and have identified CB2 as an attractive therapeutic target for immunomodulation18, 19. Agonists or antagonists for the CB2 receptors expressed by immune cells inhibit cytokine production, decrease antigen presentation and modulate cell migration20. The therapeutic potential of CB2 modulators in a wide range of diseases and pathological conditions21–25 makes this receptor an attractive target for imaging.
In order to better understand the physiological and pathological involvement of CB2 receptors in immune cells, a fluorescent ligand was designed and synthesized.
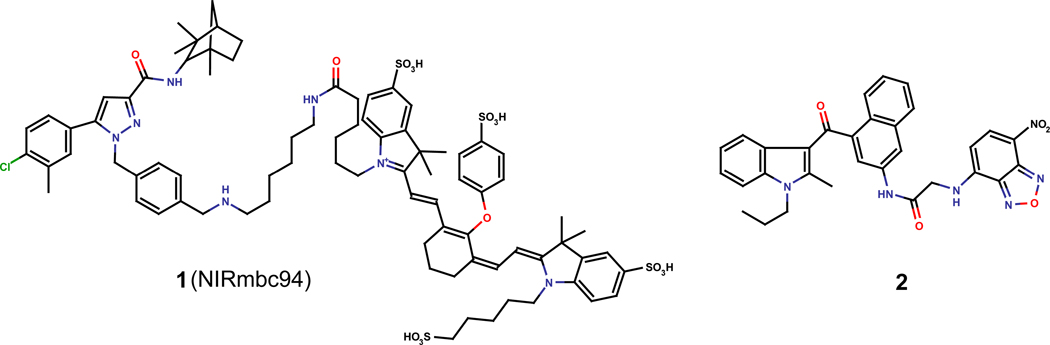
Fluorescent ligands for GPCR have been prepared by conjugation of a well-described ligand with a fluorophore into an area of the structure that would have minimal impact on affinity26. A near-infrared dye, compound 1 (NIRmbc94) resulting from conjugation of the near-infrared dye IRDye 800CW NHS ester with SR144528 (Fig. 1), has been described for CB2 targeted imaging27. However, no binding affinity has been provided. A 7-nitrobenzoxadiazole (NBD) fluorescent conjugate of JWH-015, compound 2 has been described to target CB228. NBD conjugation in this case strongly decreases affinity of JWH-015 at CB2. In order to overcome the disadvantages of conjugation, we sought to incorporate the fluorophore into a scaffold endowed with good affinity for the cannabinoid CB2 receptor.
Figure 1.
Chemical structures of CB2-selective imaging agents.
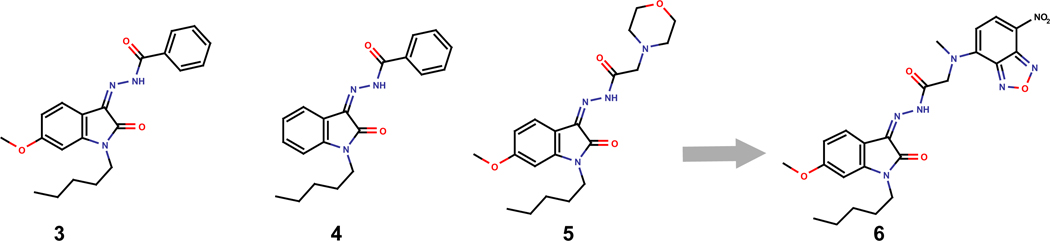
Recently, we reported a series of 6-methoxy-N-alkyl isatin acylhydrazone derivatives that are potent CB2 antagonists29. In this series compound 3 (Fig. 2) exhibited a CB2 functional activity in the nanomolar range (EC50=5.8 nM) and a CB1 activity of 263 nM, using GTPγ[35S] assay. The agonist analog30, compound 4, exhibited a lower CB2 functional activity (EC50=240 nM) and a higher CB1 functional activity (EC50=314 nM). In the antagonist series longer linker between the lipophilic part and the carbonyl are well tolerated as compound 5 did not show any CB1 functional activity and EC50 at CB2 of 13.3 nM. The morpholine moiety of compound 5 fits in the lipophilic pocket formed by residues F2.57, F2.61, F2.64, and F7.35 according to our CB2 model. 4-(N-Hydrazinocarbonylmethyl-N-methylamino)-7-nitro-2,1,3-benzoxadiazole (NBD-CO-Hz) fluorochrome was selected since the fluorescent moiety is connected to a carbonyl by a methylene providing a good flexibility for the benzoxadiazole to fit into the lipophilic pocket.
Figure 2.
Modification of isatin acylhydrazone
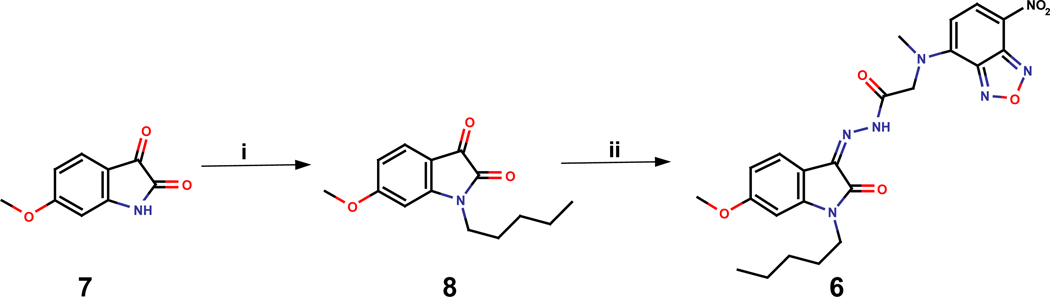
Synthesis is outlined in scheme 129. N-Alkylation of the commercially available 6-methoxyisatin with pentyl bromide using cesium carbonate under microwave irradiation provided 1-pentyl-6-methoxy-isatin in 60% yield. Condensation of the resulting 1-pentyl-6-methoxy-isatin with 4-(N-Hydrazinocarbonylmethyl-N-methylamino)-7-nitro-2,1,3-benzoxadiazole (NBD-CO-Hz) afforded the desired compound NMP6 in 67% yield (scheme 1).
Scheme 1.
Synthesis of compound 6. Reagents and conditions: (i) pentyl bromide, Cs2CO3, DMF, 140°C, µW, 60%; (ii) NBD-CO-Hz, 10 % AcOH, THF/EtOH, r.t. 67%.
Compound 6 was screened at 10 µM in competitive binding experiments against CB1, CB2, 5-HT2A and 5-HT2C receptors. Compound 6 did not show any significant inhibition of radioligand binding (<40 %) at CB1, 5-HT2A ([3H]Ketanserin) and 5-HT2C ([3H]Mesulergine). In contrast, Compound 6 exhibited a Ki value at hCB2 of 387 nM (hCB2 recombinant expressed in CHO-K1 cells). HU-210 was used as a reference compound and exhibited a Ki value of 2.66 nM and [3H]-CP55940 was used as a radioligand for CB1 and CB2 experiments.
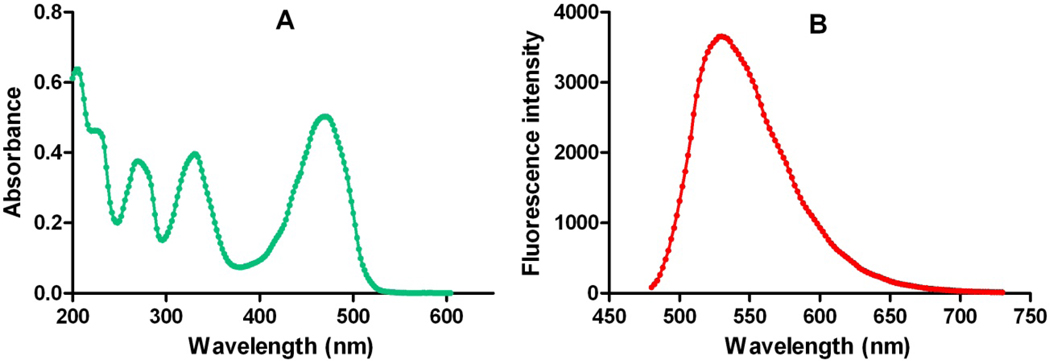
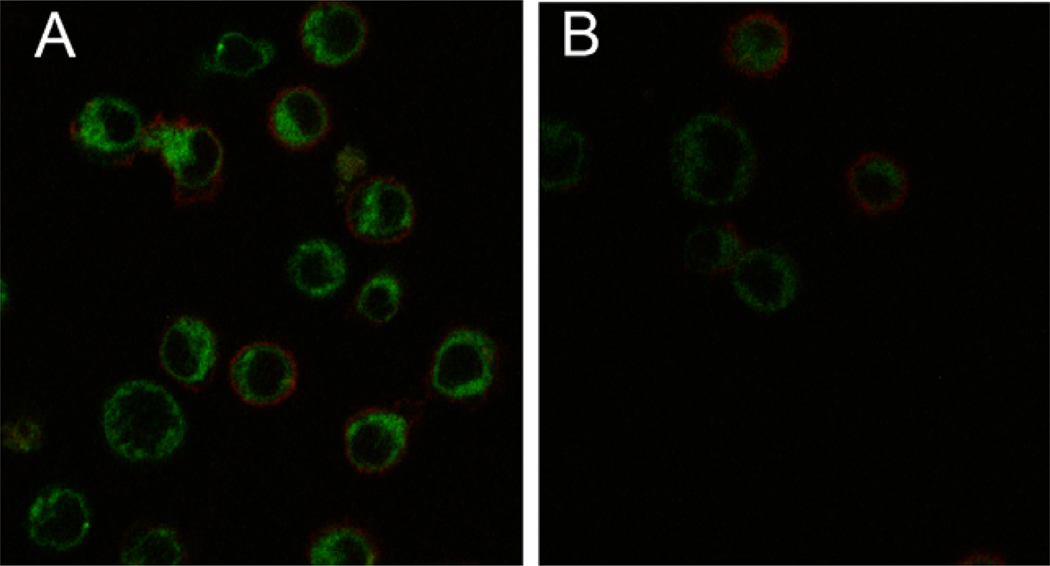
Absorption and emission spectra of the compound after the background correction were collected in acetonitrile (Fig. 3). The emission spectrum was collected by exciting the sample at 470nm. We next demonstrated that the fluorescent CB2 ligand NMP6 can be used as probe for visualization of CB2 receptor using confocal microscopy. To test whether NMP6 reliably binds to the CB2 receptor in non transfected cells, T cells purified from peripheral lymph nodes of BALB/C mice were stained with PE-conjugated anti-CD4 antibody and different concentrations of NMP6 and then visualized by confocal microscopy. As shown in Figure 4a, 5 µM NMP6 stained the CD4+ T cells. Little or no staining was observed using 1.0 µM and 0.1 µM concentrations of NMP6 (data not shown). To test specificity of NMP6, a competition study with the selective CB2 agonist GW842166X31 was done (Fig. 4B). The receptor binding was blocked by preincubating the cells with GW842166X.
Figure 3.
Absorption (A) and fluorescence emission (B) spectra of NMP6 in CH3CN with excitation λ= 470 nm.
Figure 4.
Visualization of CB2 receptor binding by CD4+ T cells using NBD-labeled CB2 ligand by confocal microscopy. A: Mouse T cells were stained with PE-conjugated anti-CD4 antibody (red) and the NMP6 at 5 µM, on ice for 30 minutes (green) and then visualized by confocal microscopy, (x40). B: The receptor binding was blocked by preincubating the cells (on ice for 5 min) with the CB2 agonist GW 842166X at 100 µM, (Cayman Chemical).
Figure 5.
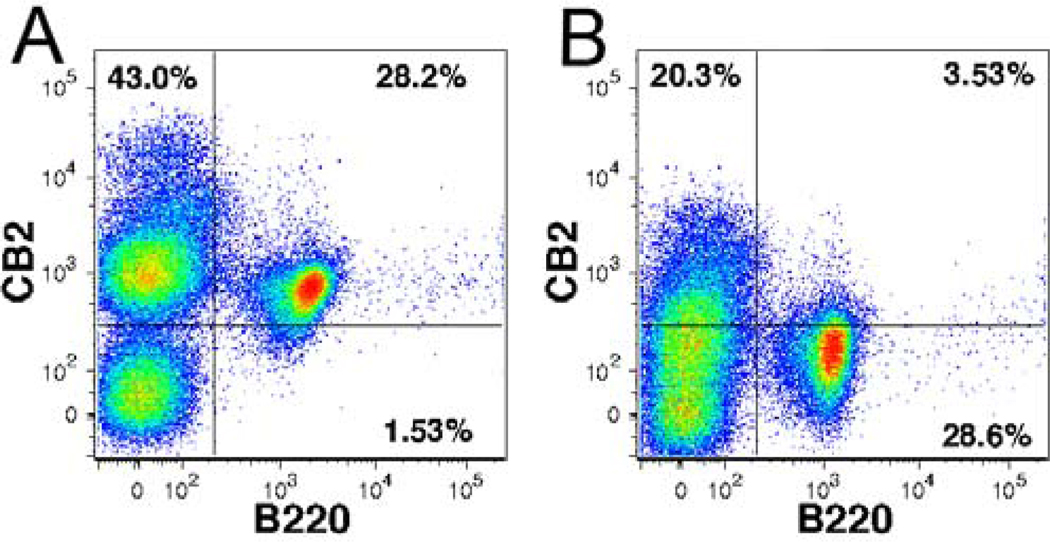
Visualization of CB2 receptor binding by B220 B cells using NBD-labeled ligand by flow cytometry. A: Mouse lung mononuclear B cells were stained with PE-conjugated anti-B220 antibody and NMP6 (5 µM, on ice for 30 minutes) and then visualized by flow cytometry using FACSAria II (BD Biosciences). B: The receptor binding was blocked by preincubating the cells (on ice for 5 min) with with the CB2 agonist GW842166X at 100 µM.
In addition, NMP6 was used for visualization of CB2 receptor binding in B lymphocytes using flow cytometric analysis (Fig. 5A). Mouse lung mononuclear were prepared by colagenase digestion of lung tissue32 and cells were stained with PE-conjugated anti-B220 antibody and 5 µM of NMP6 and then visualized by flow cytometry. The receptor binding was blocked by preincubating the cells with the selective CB2 agonist GW842166X (Fig. 5B). The ability of GW842166X to displace NMP6 clearly demonstrated selectivity for CB2.
In the course of our structure-activity relationship studies of CB2 selective antagonists, we discovered that introduction of bulky lipophilic moiety connected by an acylhydrazone to 6-methoxy-N-alkyl isatin scaffold provided CB2 selective antagonists. Based on this series we designed a fluorescent ligand obtained by condensation of NBD-CO-Hz with 6-methoxy-N-pentyl isatin. The good selectivity and affinity at CB2 of the target probe designated NMP6, sugest that attachment of a fluorochrome to the isatin scaffold is a good strategy to design CB2 fluorescent ligands. In previous attempt to synthesize CB2 fluorescent ligand, the fluorochrome was conjugated to previously described CB2 agonists resulting in a loss of affinity. The ability of GW842166X to displace NMP6 showed that NMP6 can be used for receptor visualization but also to rank-order the relative affinities of potential CB2 ligands. Furthermore, NMP6 will provide a valuable tool for investigating CB2 receptor expression in immune cells.
In conclusion, we have synthesized and evaluated a new 6-methoxyisatin based fluorescent probe, which exhibited selectivity and good affinity for CB2 receptor. We have demonstrated its ability to be an effective probe for visualization of CB2 receptor binding using confocal microscopy and a flow cytometry probe for the analysis of CB2 protein expression. NMP6 could be used for the assessment of relative binding of compounds that interact with CB2 receptor. Furthermore, NMP6 was easily obtained in two chemical steps from commercially available building blocks.
Acknowledgements
The work was supported by National Institutes of Health (NIH) grant P30-NS055022 and COBRE grant (P20RR017670). We acknowledge the Fluorescence Cytometry and Molecular Biology Facility Core, the Molecular Histology and Fluorescence Imaging Core, BioSpectroscopy Core and the CSFN COBRE grant P20 RR-15583. We thank Beverly Parker and Dr. Ayesha Sharmin for experimental assistance. Radioligand binding assays were performed by the National Institute of Mental Health’s Psychoactive Drug Screening Program Contract # HHSN-271-2008-00025-C (NIMH PDSP). NIMH PDSP is directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Basavarajappa BS. Curr. Neuropharmacol. 2007;5:81. doi: 10.2174/157015907780866910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. Br. J. of Pharmacol. 2007;152:1092. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munro S, Thomas KL, Abu-Shaar M. Nature. 1993;365:61. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 4.Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Proc. Natl. Acad. Sci. U S A. 1995;92:3376. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Science. 2005;310:329. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 6.Onaivi ES, Ishiguro H, Sejal P, Meozzi PA, Myers L, Tagliaferro P, Hope B, Leonard CM, Uhl GR, Brusco A, Gardner E. Methods mol. med. 2006;123:291. doi: 10.1385/1-59259-999-0:291. [DOI] [PubMed] [Google Scholar]
- 7.Onaivi ES, Ishiguro H, Gong J-P, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu Q-R, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Ann. N.Y. Acad. Sci. 2006;1074:514. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- 8.Naguib M, Diaz P, Xu JJ, Astruc-Diaz F, Craig S, Vivas-Mejia P, Brown DL. Br. J. Pharmacol. 2008;155:1104. doi: 10.1038/bjp.2008.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao BB, Hsieh GC, Frost JM, Fan Y, Garrison TR, Daza AV, Grayson GK, Zhu CZ, Pai M, Chandran P, Salyers AK, Wensink EJ, Honore P, Sullivan JP, Dart MJ, Meyer MD. Br. J. Pharmacol. 2008;153:390. doi: 10.1038/sj.bjp.0707568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiteside GT, Lee GP, Valenzano KJ. Curr. Med. Chem. 2007;14:917. doi: 10.2174/092986707780363023. [DOI] [PubMed] [Google Scholar]
- 11.Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Neurosci. 2005;135:235. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. Eur. J. Neurosci. 2006;23:1530. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. Eur. J. Neurosci. 2003;17:2750. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 14.Walczak JS, Pichette V, Leblond F, Desbiens K, Beaulieu P. Neuroscience. 2005;132:1093. doi: 10.1016/j.neuroscience.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Merriam FV, Wang Z-y, Guerios SD, Bjorling DE. Neurosci. Lett. 2008;445:130. doi: 10.1016/j.neulet.2008.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalski CW, Laukert T, Sauliunaite D, Pacher P, Bergmann F, Agarwal N, Su Y, Giese T, Giese NA, Bátkai S, Friess H, Kuner R. Gastroenterology. 2007;132:1968. doi: 10.1053/j.gastro.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M, Zimmer A. Eur. J. Pharmacol. 2000;396:141. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 18.Lunn CA, Reich EP, Fine JS, Lavey B, Kozlowski JA, Hipkin RW, Lundell DJ, Bober L. Br. J. Pharmacol. 2008;153:226. doi: 10.1038/sj.bjp.0707480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muccioli GG. Chemistry & Biodiversity. 2007;4:1805. doi: 10.1002/cbdv.200790153. [DOI] [PubMed] [Google Scholar]
- 20.Miller AM, Stella N. Br. J. Pharmacol. 2008;153:299. doi: 10.1038/sj.bjp.0707523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Ruiz J, Pazos MR, Garcia-Arencibia M, Sagredo O, Ramos JA. Mol. Cell. Endocrinol. 2008;286:S91. doi: 10.1016/j.mce.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Arevalo-Martin A, Garcia-Ovejero D, Gomez O, Rubio-Araiz A, Navarro-Galve B, Guaza C, Molina-Holgado E, Molina-Holgado F. Br. J. Pharmacol. 2008;153:216. doi: 10.1038/sj.bjp.0707466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centonze D, Rossi S, Finazzi-Agro A, Bernardi G, Maccarrone M. Int. Rev. Neurobiol. 2007;82:171. doi: 10.1016/S0074-7742(07)82009-5. [DOI] [PubMed] [Google Scholar]
- 24.Maccarrone M, Battista N, Centonze D. Prog. Neurobiol. 2007;81:349. doi: 10.1016/j.pneurobio.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Pacher P, Batkai S, Kunos G. Pharmacol. Rev. 2006;58:389. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leopoldo M, Lacivita E, Berardi F, Perrone R. Drug Discovery Today. 2009;14:706. doi: 10.1016/j.drudis.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Bai M, Sexton M, Stella N, Bornhop DJ. Bioconjugate Chem. 2008;19:988. doi: 10.1021/bc700419e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yates AS, Doughty SW, Kendall DA, Kellam B. Bioorg. Med. Chem. Lett. 2005;15:3758. doi: 10.1016/j.bmcl.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 29.Diaz P, Phatak SS, Xu J, Astruc-Diaz F, Cavasotto CN, Naguib M. J. Med. Chem. 2009;52:433. doi: 10.1021/jm801353p. [DOI] [PubMed] [Google Scholar]
- 30.Diaz P, Xu J, Astruc-Diaz F, Pan H-M, Brown DL, Naguib M. J. Med. Chem. 2008;51:4932. doi: 10.1021/jm8002203. [DOI] [PubMed] [Google Scholar]
- 31.Giblin GMP, O'Shaughnessy CT, Naylor A, Mitchell WL, Eatherton AJ, Slingsby BP, Rawlings DA, Goldsmith P, Brown AJ, Haslam CP, Clayton NM, Wilson AW, Chessell IP, Wittington AR, Green R. J. Med. Chem. 2007;50:2597. doi: 10.1021/jm061195+. [DOI] [PubMed] [Google Scholar]
- 32.Girtsman T, Jaffar Z, Ferrini M, Shaw P, Roberts K. J. Leukocyte Biol. 2010;88:537. doi: 10.1189/jlb.0110044. [DOI] [PMC free article] [PubMed] [Google Scholar]