Abstract
Learning of complex motor skills requires learning of component movements as well as the sequential structure of their order and timing. Using a Serial Interception Sequence Learning (SISL) task, participants learned a sequence of precisely timed interception responses through training with a repeating sequence. Following initial implicit learning of the repeating sequence, functional MRI data were collected during performance of that known sequence and compared with activity evoked during novel sequences of actions, novel timing patterns, or both. Reduced activity was observed during the practiced sequence in a distributed bilateral network including extrastriate occipital, parietal, and premotor cortical regions. These reductions in evoked activity likely reflect improved efficiency in visuospatial processing, spatio-motor integration, motor planning, and motor execution for the trained sequence, which is likely supported by nondeclarative skill learning. In addition, the practiced sequence evoked increased activity in the left ventral striatum and medial prefrontal cortex, while the posterior cingulate was more active during periods of better performance. Many prior studies of perceptual-motor skill learning have found increased activity in motor areas of frontal cortex (e.g., motor and premotor cortex, SMA) and striatal areas (e.g., the putamen). The change in activity observed here (i.e., decreased activity across a cortical network) may reflect skill learning that is predominantly expressed through more accurate performance rather than decreased reaction time.
Keywords: sequence learning, implicit learning, nondeclarative memory, timing, motor control, fMRI
1 Introduction
Skilled motor performance frequently requires executing a sequence of actions with a specific pattern of timing between the individual movements. Studies of the neural basis of learning action sequences have tended to focus on tasks in which participants learn sequences of actions that can be performed increasingly quickly (e.g., Orban et al., 2010; Poldrack et al., 2005; Willingham et al., 2002). However, in many motor expertise domains in the real world, the relative timing among component actions is critical and must be maintained even as the overall speed of behavior increases. For example, the timings between actions are critical for expert performance in athletics and music. Of note, especially in athletic skilled performance, performers are often unable to explicitly describe the sequences they are expressing, suggesting a strong contribution of implicit sequence knowledge.
We have recently used a new perceptual-motor sequence learning task, Serial Interception Sequence Learning (SISL), to examine the integration of action order and timing information in implicit sequence learning (Gobel et al., 2011). The SISL task is similar to the familiar Serial Reaction Time (SRT) task (Nissen and Bullemer, 1987; Robertson, 2007) in using perceptual cues to guide a series of motor responses in which a repeating sequence is covertly embedded. However, instead of simply making a four-alternative forced choice (4-AFC) response as quickly as possible (as in the SRT task), in SISL, the action cues are moving on a display and participants must time their motor response to coincide with the arrival of the cue in a target zone. Our prior research (Sanchez et al., 2010) has found that implicit sequence learning occurs rapidly during SISL practice and with low levels of concomitant explicit sequence knowledge. In Gobel et al. (2011), participants learned a repeating sequence of actions separated by a specific pattern of inter-item timing. Participants exhibited no subsequent transfer to the task with an embedded sequence that had a new timing pattern but the same order, nor any transfer to an embedded sequence with the same timing but random order. This result implies that the representation of the learned sequence obtained from SISL practice is based on tight integration of action order and timing information.
Research that has examined the contribution of inter-item timing to sequence learning using the SRT task has found partial integration of timing information with order information – a correlated timing pattern had a beneficial effect on performance in (and only in) the presence of an ordinal sequence – and observed partial transfer when order was maintained but timing was disrupted (O'Reilly et al., 2008; Shin and Ivry, 2002). These results suggest that while timing information may be secondary to order information, there may be separate representations or learning mechanisms for learning timing and order during SRT practice. This idea is supported by the report of Sakai et al. (2002), which reported separate neural correlates associated with learning of action order (precuneus, right intraparietal sulcus) and timing order (right cerebellum) sequences, albeit under explicit conditions. Sakai et al. (2002) also found that activity in the left intraparietal sulcus increased to either kind of sequence and that several brain regions exhibited increased activity specifically to sequences containing both order and timing information: medial and lateral premotor cortex and dorsolateral prefrontal cortex. This pattern of results suggests that there may be separate learning mechanisms and also regions that integrate information from both sources.
The report of Sakai et al. (2002) did not directly implicate the basal ganglia in sequence learning or integration, although the basal ganglia, particularly the putamen, have been frequently implicated in neuroimaging studies of sequence learning (e.g., Peigneaux et al., 2000). A review of sequence and motor adaptation studies by Doyon et al. (2009) suggested a model in which the basal ganglia and reciprocal circuits connecting to cortical regions support motor sequence learning. In this model, motor adaptation is supported by cerebellar and cortico-cerebellar circuits. While Sakai et al. (2002) reported increased cerebellar activity during learning of a repeated sequence of timing intervals, incorporating timing information into an integrated action sequence might still depend on the basal ganglia. A study of patients with Parkinson's disease (PD) and patients with cerebellar lesions (Shin and Ivry, 2003) found that the PD patients, who have impaired basal ganglia function, were unable to integrate action order and timing information in sequence learning. The patients with damage to the cerebellum showed neither order nor timing sequence learning.
None of the studies to date have looked at the neural correlates of an implicit sequence learning task in which precise timing of responses is intrinsic and essential to performance. After learning a repeating sequence of actions with embedded timing via the SISL task, Gobel et al. (2011) found no transfer to conditions that selectively changed the order or the timing. While this implies a fully integrated representation of order and timing, it is possible that the demand characteristics of the task could mask the expression of order-only or timing-only information (since both are necessary for successful performance). Here, fMRI is used to examine neural changes associated with sequence learning from SISL practice and neural activity associated with transfer to conditions where the action order or timing pattern were independently altered. The current study is also the first to report the changes in neural activity associated with sequence learning in a task where learning is marked by increasingly accurate performance instead of increasingly rapid responding. This element of the SISL task avoids some prior concerns that the reported neural correlates of learning may be influenced by the faster reaction time that is the consequence of improved performance resulting from learning as examined in Orban et al. (2010).
2 Methods
2.1 Participants
Eighteen healthy, right-handed adults (7 male, 11 female) of mean age 24 years (range 19 – 28) were recruited from the Northwestern University community and Chicago area for participation in this study. All participants gave informed consent according to protocol approved by the Northwestern University Institutional Review Board and underwent neuroimaging safety screening. After the experiment, all participants were compensated for their time.
2.2 Procedure
2.2.1 SISL task
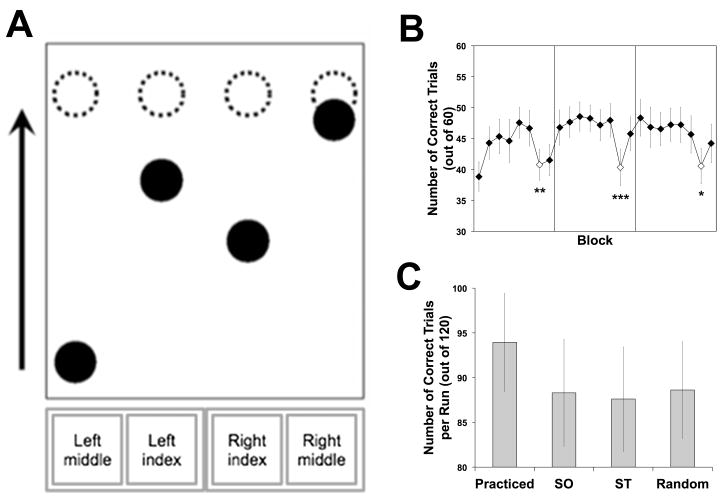
The basic procedure of the Serial Interception Sequence Learning (SISL) task (Figure 1A) is described in Gobel et al. (2011). Participants in the fMRI scanner viewed a screen image projected onto a mirror mounted to the head coil and were given four button boxes to operate with their middle and index fingers of each hand. A target zone was visible as four dashed gold circles (with a diameter of 10% of the screen height) centered on a horizontal 80% from the bottom of the screen. Each button box was assigned to one of the target zones, from left to right. Filled blue circular cues, of the same size as the targets, scrolled up the screen at constant velocity. Cues took 2000 ms from cue appearance at the bottom of the screen to being centered in the appropriate target, after which they continued to scroll for an additional 500 ms to the top of the screen. Participants were simply instructed to press the corresponding button when a cue was centered in its target. A trial, defined as the passage of a cue through its target, was scored as correct if the appropriate button was pressed when the cue was closer to the target zone than any other cue. Only one cue would pass through the target zone at any one time, but three or four other cues were scrolling toward the target zone simultaneously. Incorrect responses, multiple responses, and non-responses were scored as errors. When any button was pressed (correct or incorrect), the corresponding target flashed green briefly as visual feedback. Differential feedback, such as disappearing cues following correct responses, was not provided to minimize any confound of visual input (which would influence neural activity related to visual processing) during periods of better or worse performance.
Figure 1.
The SISL task and behavioral performance during SISL training and scanning runs. Participants saw circular cues scrolling towards a marked target zone (A), with button responses by the middle and index fingers corresponding to the four targets (dashed circles). During training (B), behavioral performance demonstrated implicit learning. Each of the three times that the repeating cue order and timing pattern was switched to a pseudorandom order and the opposite timing pattern, performance (number correct trials out of 60) significantly decreased from the flanking repeating sequence blocks, indicating sequence-specific learning. Pseudorandom blocks are indicated with open diamonds (*p < .05; **p < .01; ***p < .001). During functional imaging (C), participants continued to show sequence-specific learning (Practiced > Random) while failing to demonstrate transfer to the SO or ST conditions. There was a significant order × timing interaction such that when either order or timing was changed from the practiced sequence, performance (number correct trials per run, out of 120) decreased to the level of a pseudorandom sequence, showing a lack of transfer to the altered conditions. The comparison between Practiced and the average of SO and ST (the main contrast for the fMRI analysis, Practiced-New) showed a significant decrease in performance for the transfer conditions.
As in the SRT task, the appearance of the cues followed a repeating sequence of which participants were not informed. Sequence-specific learning is assessed by comparing performance during the covert repeating sequence to conditions where the order of the cues is pseudo-random (or otherwise modified). However, SISL task performance is measured by the proportion of correct interception responses made to the moving cues rather than the reaction time to the onset of the cue. Participants first learned a 12-item second-order conditional (SOC) repeating sequence with an embedded timing pattern over 24 training blocks of 60 trials (block duration = 31.5 s; blocks were presented successively with short participant-terminated breaks after every 8 blocks). Half of the participants trained on practiced sequence B350C700B700D350A350C700A350D700C700D350B350A700 and the other half on D350C700B700D350B350C700A350B700A700D350A350C700, where A, B, C, and D represent target locations from left to right, and responses with the left middle, left index, right index, and right middle fingers, respectively. The subscripted numbers indicate the time in milliseconds between trials. The timing sequence was constrained such that the same interval could not occur more than twice in a row. Since all cues had the same velocity, differences in timing were visible on the screen as differing distances between the cues as they moved vertically up the screen. The sequence was repeated throughout, except for during blocks 7, 15, and 23, during which the cues followed a pseudorandom order and timing sequence. Each of these pseudorandom blocks consisted of five novel, randomly generated 12-item SOC sequences, and the timing pattern was opposite to that of the practiced sequence (assuring minimum overlap). No fMRI data were collected during training, but the standard anatomical T1-weighted structural images were acquired while the participants completed the last 16 blocks of training.
Participants then performed six scanning runs, each of which contained 8 blocks of 60 trials (block duration = 31.5 sec), while T2*-weighted BOLD data were collected. These runs contained four conditions: practiced sequence (Practiced, same order and timing as was practiced during the training runs), same order (SO, same order but opposite timing of the practiced sequence), same timing (ST, same timing as the practiced sequence but pseudorandom order), and pseudorandom (Random, pseudorandom order and opposite timing, as in the pseudorandom blocks of training). The cue orders in the ST and Random blocks were five novel, randomly generated 12-item SOC sequences, in order to prevent learning of a novel sequence, thereby maximizing sensitivity of contrasts with the Practiced condition while maintaining the same statistical structure. To randomize the condition order for the six transfer runs, 12 of the 24 possible orderings of the four conditions were randomly selected and ordered for the first of every two participants (with the other 12 randomly ordered for the subsequent participant). Following these six runs, an additional scan was completed in which a highly predictable sequence (A500B500C500D500) was alternated with the trained sequence.
Following the final run, explicit recognition and recall tests were administered while participants were still in the scanner (though no neuroimaging data were collected). During the recognition test, participants performed the practiced 12-item cue order and four novel 12-item cue orders (all cues were separated by 500 ms for all five sequences) in a random order. After each sequence, the participants were asked to rate their confidence on a scale of 1-100 that the sequence was encountered during the experiment (1 = extremely confident sequence was not encountered; 50 = neutral; 100 = extremely confident sequence was encountered). A recognition score reflecting explicit sequence knowledge was calculated as the rating given for the target minus the average rating given to the four foils (as in Gobel et al., 2011; Sanchez et al., 2010). For the recall test, participants were told that there was a repeating sequence (other than the “highly predictable” sequence) that they encountered during the majority of the experiment, and participants were then asked to reproduce that sequence as accurately as possible by pressing the buttons. Participants were required to continue until 12 responses were made. A recall score was calculated by identifying the longest matching subsequence between the recalled sequence and the training sequence. This score was compared to a baseline of the average longest matching subsequence between the recalled sequence and the foils (as in Gobel et al., 2011; Sanchez et al., 2010).
2.2.2 Neuroimaging
fMRI data were collected using a Siemens TIM TRIO 3.0 T MRI scanner at Northwestern University equipped with a 12-channel head coil. During the second and third runs (training), high-resolution 3D MP-RAGE T1-weighted anatomical scans (voxel size = 1 × 1 × 1 mm; 160 axial slices) were collected. For each fMRI run, whole-brain T2*-weighted EPI (44 axial slices of 3 mm thickness with no gap, positioned to include the cerebellum), were collected every 2.4 s (TR = 2.4 s; TE = 20 ms; flip angle = 80°; 64 × 64 acquisition matrix; FOV 22 × 22 cm; resulting voxel size = 3.44 × 3.44 × 3 mm) for 115 volumes in each run.
2.2.2.1 Preprocessing
Neuroimaging processing and analysis was conducted using AFNI (Cox, 1996). The first four volumes were dropped from each functional run to allow the MR signal to reach equilibrium. The functional data were then motion-corrected over time using a rigid body transformation. Non-brain voxels (average signal < 100 MR units) and erratic voxels (signal change > 30% between successive brain volumes) were clipped from the dataset, followed by spatial smoothing using a 7 mm full-width half-max Gaussian kernel. Structural data were normalized to a standard brain using a 12-parameter transformation, and the participant's functional data were then normalized using the same transformation matrix subsequent to registration to the structural volume. The resulting voxels in the smoothed, realigned, normalized functional images measured 2.5 × 2.5 × 2.5 mm.
2.2.2.2 General Linear Model
The pseudorandom (Random) condition was used as the baseline for neuroimaging data analysis. The hemodynamic response to each of the other three conditions – Practiced, SO and ST – was modeled using a separate block basis function (with block duration set to 31.5 s) provided by AFNI, which convolved an incomplete gamma function with a boxcar function. Relative to block onset, the block basis functions peaked at about 15 s, were at plateau until 31.5 s, and returned to baseline at about 47.3 s. For each participant, a general linear model (GLM) regressed the BOLD response against these basis functions to obtain estimates of evoked neural activity for each condition relative to baseline. Simultaneously, the GLM included a performance-based regressor of activity associated with the number of errors made during each TR, estimated over a period of 2.4 – 12 sec to incorporate hemodynamic delay. Six motion parameters were used as base model regressors of non-interest (to control for movement-related activity).
2.2.2.3 Whole-Brain Group Analysis
For the whole-brain random-effects group analysis, the coefficients were analyzed via one-sample t-tests, yielding a t-value for each voxel in each of the contrasts. A per-voxel t-value threshold of t > 4.5 was used. A Monte Carlo simulation with normally distributed random noise data over the dimensions of the averaged brain determined that at t > 4.5, the minimum cluster volume that would result in an overall alpha level of .05 was 327 mm3.
2.2.2.4 ROI-AL Analyses
The bilateral putamen, caudate, and globus pallidus of the basal ganglia served as a priori regions of interest (ROI) to guide a subsequent cross-subject alignment using the ROI-AL of Yassa and Stark (2009). Using anatomical guidelines, each participant's basal ganglia regions were individually identified and ROI-AL was used to maximize the cross-participant alignment of these regions (instead of aligning across the whole brain as in traditional normalization techniques) and overlap of striatal subregions. Due to the a priori hypotheses and the constrained search volume, a lower t-value threshold of t > 2.5 with cluster volume threshold of V > 582 mm3 (as obtained from a separate Monte Carlo simulation) were used to set the overall alpha level at .05.
3 Results
3.1 Behavioral
During the initial training phase of the SISL task (Figure 1A), participants learned the repeating sequence (Figure 1B). Their performance (number of correct trials) significantly decreased during each pseudorandom block relative to its flanking repeating sequence blocks, with an overall mean difference of 4.7 trials (SE = 1.0), t(17) = 4.65, p < .001 reflecting a drop in performance from 76% correct for the repeating sequence to 68% correct for random trials. This difference was reliable at each training assessment: t(17) = 2.88, p = .01; t(17) = 4.29, p < .001; t(17) = 2.46, p = .02, respectively. The reliable decrease in performance indicates that participants learned the practiced sequence during training. Performance during the six scanning runs was assessed with a 2 (same order or random order) × 2 (same timing or opposite timing) × 6 (run number) repeated-measures ANOVA. There was a significant main effect of changing the response order, F(1,17) = 18.40, p < .001, ηp2 = .52, but there was no main effect of changing inter-response timing, F(1,17) = 2.02, p = .17, ηp2 = .11. Critically, there was a significant order × timing interaction, F(1,17) = 5.58, p = .03, ηp2 = .25, such that participants performed best when neither the order nor the timing was changed (Figure 1C). There was no main effect of run, F(5,85) = 0.58, p = .71, nor did run reliably interact with order, F(5,85) = 0.98, p = .43, with timing, F(5,85) = 0.57, p = .72, or with order × timing, F(5,85) = 0.56, p = .73, suggesting that these effects did not change over the scanning session.
Paired t-tests (collapsed across runs) revealed that performance during the practiced sequence (Practiced) was significantly better than during the Random, t(17) = 3.03, p < .01, and ST (same timing) conditions, t(17) = 3.65, p < .01, and there was a trend towards better performance during the practiced sequence than during the SO (same order) condition, t(17) = 1.94, p = .07 (Figure 1C). There was no difference in performance between the Random, ST, and SO conditions, F(2,34) = 0.27, p = .77. Collapsing across the ST and SO conditions, performance was significantly worse during blocks of the transfer sequences altered on one dimension (M = 88.0 correct trials out of 120, SE = 5.8) than during the blocks of the practiced sequence (M = 94.0, SE = 5.5), t(17) = 2.69, p = .02. During the additional scan at the end, performance during blocks of the highly predicable sequence (M = 200.2 correct trials out of 240, 83% correct, SE = 15.7) did not significantly differ from that during the trained sequence (M = 194.0 correct trials, 81% correct, SE = 12.4), t(17) = 0.82, p = .42.
After scanning, participants' explicit sequence knowledge was assessed. The average recognition score was reliably greater than zero (M = 24.3, SE = 4.7), t(17) = 5.23, p < .001, indicating that participants obtained some explicit knowledge of the repeating sequence. However, the recognition score was not correlated with the implicit sequence learning score obtained during training, r = .20, p = .42. In addition, those with the lowest explicit recognition scores (by median split) still demonstrated reliable implicit learning scores, with an overall mean difference of 3.8 trials (SE = 0.7), t(8) = 5.16, p < .001. In their attempt to recall the repeating sequence, the sequences produced did not reliably match the learned sequence (M = 4.6 consecutive matches, SE = 0.5) better than they matched the recognition foils (M = 4.0, SE = 0.1), t(17) = 1.16, p = .26.
3.2 Neuroimaging
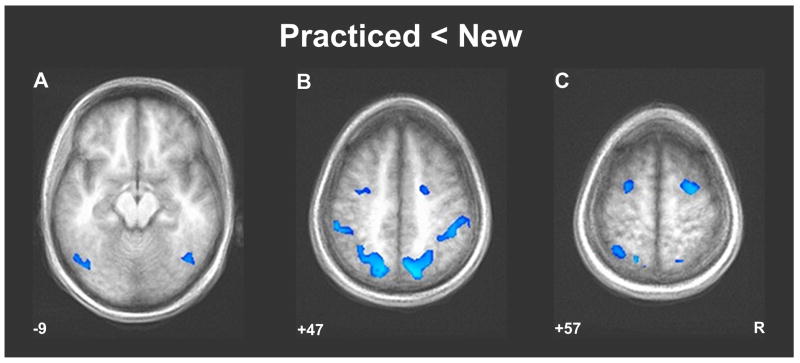
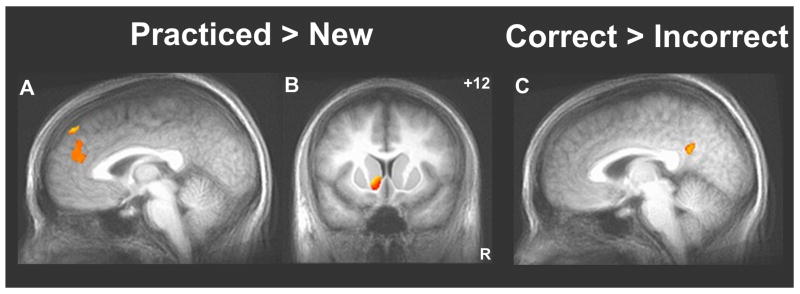
No reliable differences in evoked activity were observed between the SO, ST, and Random conditions (neither in the whole-brain nor the ROI-AL analyses), providing no evidence here for separate neural representations of action order and response timing. To identify the neural correlates of sequence learning in the SISL task, both of the transfer conditions (with performance at the level of the Random baseline) were combined (as ‘New’ sequences) and activity was contrasted with the repeating sequence (Practiced) condition. During the practiced sequence, significant decreases in activity were observed across a bilateral cortical network consisting of premotor, parietal (superior parietal/precuneus medially and inferior parietal laterally), and extrastriate occipital cortices (Figure 2; Table 1). Accompanying this deactivated network for the practiced sequence (relative to the transfer conditions) was significantly increased activity in medial prefrontal cortical regions (Figure 3A). Regardless of the sequence condition, activity in the posterior cingulate was inversely correlated with number of errors (Figure 3C); higher activity was observed during periods of time when participants are making more correct responses (fewer errors) in general.
Figure 2.
Decreased activity across a distributed bilateral cortical network during performance of the practiced sequence relative to the transfer conditions. The Practiced-New contrast shows that there was a decrease in neural activity in the extrastriate occipital (A), parietal (B), and premotor (C) cortices while participants were performing the practiced sequence relative to the SO and ST conditions (“New”). Significantly deactivated clusters were those consisting of deactivated voxels (t > 4.5) that passed a minimum cluster volume threshold of V > 327 mm3.
Table 1.
Volume-thresholded clusters (t > 4.5, V > 327 mm3) of regions that differentially activated to the practiced sequence versus transfer sequences (New). Coordinate order is LPI: left, posterior, and inferior are negative
| Contrast | Talairach coordinates (x, y, z) of CMass | Cluster size (mm3) |
|---|---|---|
| Brain region | ||
| Practiced < New | ||
| Rt Precuneus / SPL (BA 7) | (+20, -70, +41) | 6984 |
| Rt Inferior Parietal Lobule | (+45, -35, +43) | 5516 |
| Lt Precuneus / SPL (BA 7) | (-23, -64, +48) | 5391 |
| Lt Inferior Parietal Lobule | (-50, -35, +40) | 2719 |
| Rt Middle Frontal Gyrus (premotor / BA 6) | (+25, -4, +54) | 1828 |
| Lt Middle Occipital Gyrus | (-38, -79, +12) | 1703 |
| Lt Middle Frontal Gyrus (premotor / BA 6) | (-27, -5, +53) | 1375 |
| Rt Inferior Occipitotemporal (V5 / hMT+) | (+41, -61, -5) | 828 |
| Lt Inferior Occipitotemporal (V5 / hMT+) | (-45, -64, -10) | 484 |
| Practiced > New | ||
| Medial Frontal Gyrus / Anterior Cingulate | (-2, +44, +24) | 1813 |
| Medial Superior Frontal Gyrus | (+2, +47, +43) | 422 |
Figure 3.
Increased activity in targets of the mesolimbic dopamine pathway while performing the practiced sequence relative to the transfer conditions and in the posterior cingulate during periods of better performance. The Practiced-New contrast showed greater activity in the medial prefrontal cortex (A; whole-brain contrast, t > 4.5 and V > 327 mm3) and in the left ventral striatum (B; constrained search volume following ROI-AL of the striatum, t > 2.5 and V > 582 mm3; within the mask, left ventral striatum cluster volume is 731 mm3 and center of mass is at -8.4, +13.1, -1.5 mm). Activity in the posterior cingulate cortex (C) was higher during periods of more successful performance of the SISL task, i.e., was negatively correlated with number of errors (whole-brain contrast, t > 4.5 and V > 327 mm3; posterior cingulate cluster volume is 1234 mm3 and center of mass is at +0.6, -49.7, +28.8 mm).
To further examine possible differences in activity associated with the SO and ST conditions (and separate representations of order and timing), an exploratory functional ROI analysis was conducted with ROIs defined by the parietal, premotor, and occipital clusters from the Practiced-New contrast. During the ST condition, activity was numerically higher than the Random baseline across the ROIs, although the effect was only reliable for the left precuneus and left inferior parietal ROIs, t(17) = 2.37, p = .030; t(17) = 2.14, p = .047; respectively. Activity during the SO condition did not appear to differ from the Random baseline for any of the ROIs. Activity was reliably lower in the Practiced condition than the Random baseline across regions, but this reflects the fact that the ROIs were selected on this basis. During the additional scanning run at the end, no reliable activity differences were observed between the highly predicable sequence and the trained sequence, which did not significantly differ in performance level.
Since activity changes in the basal ganglia are very commonly reported in neuroimaging studies of sequence learning, a ROI-AL analysis was used to assess activity changes with maximum sensitivity in the striatum. No reliable differences in activity between the practiced sequence (Practiced) and New sequences (SO, ST) were observed in dorsal parts of the basal ganglia (caudate, putamen) after ROI alignment (nor in the whole-brain analysis). However, a region in the left ventral striatum (Figure 3B), near the nucleus accumbens, was found in which increased activity was associated with performing the repeating sequence.
4 Discussion
Participants learned the repeating sequence during training with the SISL task, as reflected in a significant decrease in their performance (increased error rate) when the cues did not follow the practiced order and timing. The sequence-specific learning demonstrated here replicates our previous findings with the novel SISL task, a paradigm that requires temporal accuracy in sequential responses (Gobel et al., 2011). Participants did demonstrate some concomitant explicit knowledge of the sequence on the recognition task as a group. While we cannot be sure that explicit knowledge did not contribute to performance in some way, it was not correlated with the performance benefit, and even those with low recognition scores showed a reliable sequence-specific improvement. Furthermore, if explicit knowledge was driving performance, one might have expected it to benefit performance in the SO condition (where the cue order was maintained, but timing changed), but transfer to this condition was not observed. These behavioral observations combined with the neural correlates of learning strongly suggest that sequence-specific improvement was a product of implicit learning of the sequence. The lack of even partial transfer to conditions where the order was maintained and the timing was altered (SO) or the timing pattern was maintained and the order was changed (ST) suggests that implicit sequence-specific learning here depends on an integrated representation of order and timing.
The primary consequence of perceptual-motor sequence learning on brain activity was reliably less evoked cortical activity in a widespread cortical network during performance of the learned sequence relative to untrained sequences. Areas exhibiting less activity for the practiced sequence included bilateral premotor cortex (BA 6), which is involved with movement selection and initiation (e.g., Weinrich and Wise, 1982), and has previously been reported to show increased activity during learning of a practiced sequence during the SRT task (Grafton et al., 1995; Hazeltine et al., 1997; but was also found to be deactivated in Aizenstein et al., 2004). Lower levels of evoked activity were also observed in the precuneus and anterior inferior parietal lobule, regions that are likely part of the dorsal visual stream involved in spatio-motor integration (Goodale and Milner, 1992) and that are associated with motor planning (Hanakawa et al., 2008) and motor sequencing (Jubault et al., 2007). The occipito-temporal deactivation, particularly in the right hemisphere, is close to areas reported as hMT+ in Maruyama et al. (2009). Area hMT+ is the purported human analogue of primate area MT, the motion-processing area of extrastriate cortex, which has been implicated in other interception tasks (e.g., Bosco et al., 2008).
The network of regions exhibiting reduced activity for the trained sequence were brain areas likely involved in performing the SISL task. These differences likely reflect increased fluidity of neural processing (i.e., facilitation) while performing the practiced sequence relative to novel sequences. As in other studies of implicit learning, the critical regions for learning may be the same as regions involved in performance (e.g., Reber et al., 2003, 2005), reflecting a learning process that improves efficiency in performance networks specifically for the trained information. However, it may also be the case that the differential activity in this network reflects a consequence of learning that occurred in a brain region outside of this network. Furthermore, a region selectively involved solely in the learning process might be missed here because participants had some initial practice with the repeating sequence prior to functional imaging, or possibly because learning may be occurring for both the repeating and novel sequences. Since implicit learning is automatic (and outside awareness), conditions that do not engage sequence learning processes may be difficult to construct without also introducing perceptual or motor confounds.
The ubiquity of learning across conditions may explain the null result obtained when contrasting the practiced sequence with the predictable sequence. The predictable sequence is rapidly learned (reaching performance levels that did not differ from the trained sequence), but is still far from being executed flawlessly during scanning (performed at 83% correct), which reflects the overall difficulty level of the SISL task (due to the need for precise timing of responses). Both sequences were learned to the point that performance was facilitated to a similar degree, yet it is likely that learning was also ongoing during this final scan. Therefore, simultaneous learning processes and improvements in performance to similar degrees for both the predicable sequence and the trained sequence (along with the small number of brain volumes acquired during the predictable sequence) could lead to the lack of a reliable difference in brain activity observed here.
Regions associated specifically with learning might be expected to exhibit greater activity for the practiced sequence than the untrained sequences. We observed two such candidate regions. During performance of the practiced sequence, the ventral striatum and medial PFC were more active, which may reflect reward-related processing (Schultz et al., 1992) or the detection of salient predictable events (Matsumoto and Hikosaka, 2009) that underlies sequence learning, though these activations might also reflect the rewarding nature of more successful performance or “flow” as discussed below. In addition, there was greater activity in the posterior cingulate during periods of better performance, which may reflect more successful anticipatory control of spatial attention (Small et al., 2003) preceding correct responses. It is also possible that the posterior cingulate and medial prefrontal activations may reflect activity in the default mode network (DMN; Raichle et al., 2001). These areas, particularly the posterior cingulate, have been proposed as important hubs of convergence in the DMN (Buckner et al., 2008). During the presumably less challenging and less attention-demanding periods of the practiced sequence and/or better performance, cognitive resources could be more free for the internally-directed mental processing that is presumed to underlie the DMN. However, other core hubs of the DMN either showed deactivations (precuneus and inferior parietal lobule) or no reliable activity differences (medial temporal lobe) associated with the practiced sequence.
Most previous studies of perceptual-motor sequence learning using the SRT task have reported higher activity for known sequences in the basal ganglia (Albouy et al., 2008; Doyon et al., 1996; Grafton et al., 1995; Hazeltine et al., 1997; Peigneux et al., 2000; Rauch et al., 1995; Rauch et al., 1997; Schendan et al., 2003; Seidler et al., 2005; Wächter et al., 2009; Willingham et al., 2002), including both those focusing primarily on increases throughout acquisition (Grafton et al., 1995; Hazeltine et al., 1997; caudate in Schendan et al., 2003; Seidler et al., 2005) and sequence-specific activations observed for a sequence that has already been learned to some degree, relative to random sequences (Albouy et al., 2008; Doyon et al., 1996; Peigneux et al., 2000; Rauch et al., 1995; Rauch et al., 1997; Schednan et al., 2003; Wächter et al., 2009; Willingham et al., 2002). In addition, many have also found higher activity in the motor or premotor cortex (Grafton et al., 1995; Hazeltine et al., 1997; Rauch et al., 1995; Rauch et al., 1997; Seidler et al., 2005) and in parietal cortex (Doyon et al., 1996; Grafton et al., 1995; Hazeltine et al., 1997; Rauch et al., 1997; Seidler et al., 2005; Willingham et al., 2002). As a result, Doyon and Benali (2005) proposed a model of motor sequence learning that predicts increased activity in the basal ganglia supporting production of well-learned motor sequences. Our results stand in contrast with the findings above, as the primary neural correlates of performing a known sequence in the SISL task were lower levels of evoked cortical activity relative to unknown sequences. However, our results are consistent with several reports of deactivations in these regions associated with a learned sequence (Aizenstein et al., 2004; Berns et al., 1997; Fletcher et al., 2005; Poldrack et al., 2005). The inconsistency in these findings may be partly due to the difficulty of separating the neural correlates associated with the consequence of learning from those involved in the representation of sequence knowledge. In the SRT task, learning is expressed through increasingly rapid reaction times. Faster reaction times, in general, are known to be associated with greater activity in the motor cortex and putamen (Dai et al., 2001; Orban et al., 2010; Turner et al., 2003). Nonspecific performance effects associated with a learned sequence could therefore complicate the interpretation of learning-related changes in activity assessed with the SRT task.
In the SISL task, sequence knowledge is expressed as increasingly accurate timed responses to intercept moving cues in sequence, a more challenging task than a simple 4-AFC button press. The sequence-specific cortical deactivations reflect less effort required to perform the SISL task (i.e., facilitation or fluidity of processing) during a learned sequence, which is specific to both the order and timing pattern of the sequenced actions. This facilitation implies a higher level of neural efficiency following practice with a sequence. Improved neural efficiency is also seen in expert or highly trained marksmen and golfers, who show refined cortical dynamics (e.g., improved EEG alpha power) during the preparatory aiming period, interpreted as deactivation of cortical areas nonessential to the task (Hatfield et al., 2004; Kerick et al., 2004). Our observation of less activity in brain regions likely to be essential to the SISL task may reflect sequence-specific improved neural efficiency leading to increasing ease and fluidity in performance rather than a state of extreme concentration present in the aiming period of expert marksmen and golfers. Nonetheless, the neural efficiency hypothesis is also supported by findings with more automatized tasks that do not require high concentration. For example, during a single-leg balancing task, the alpha power decrease (i.e., increased cortical activity) observed was smaller in magnitude (i.e., less of an increase in cortical activity) for elite fencing and karate athletes (Del Percio et al., 2009), and training with a visuomotor adaptation task has been shown to result in increased alpha power (Gentili et al., 2011), reflecting decreased cortical activity. Therefore, a number of neural efficiency studies as well as our fMRI results with the SISL task reflect brain deactivation and increased fluidity accompanying well-practiced skills. The sense of increasing ease and fluidity as learning proceeds invokes the idea of “flow” that occurs in highly trained skilled performance (Csikszentmihalyi, 1990; Ericsson and Ward, 2007). The interface to the SISL task is derived from a number of currently popular video games, likely reflecting a conscious element of game design aimed at creating a positive emotional state during successful performance.
The idea of “flow” in skilled performance, associated with a positive emotional state, is consistent with the idea that activations in the ventral striatum and medial PFC may reflect reward-related processing. Both of these regions, which exhibit greater activity during performance of the known sequence, are targets of the mesolimbic dopamine pathway from the ventral tegmental area (VTA). The ventral striatum has been frequently found to exhibit higher activity during performance of a known sequence (Doyon et al., 1996; Grafton et al., 1995; Hazeltine et al., 1997; Peigneaux et al., 2000; Rauch et al., 1995; Schendan et al., 2003; Seidler et al., 2005; Wachter et al., 2009). The ventral striatum may activate to predictable environmental events related to expectation of reward (Schultz et al., 1992) – for example, the rewarding nature of a successful response – so these findings might reflect dopamine-related reward processing that supports building the associations between actions into the representation of the sequence during learning. Alternately, dopaminergic neurons may have a role in making outcome predictions regardless of reward. In addition to dopaminergic neurons whose firing patterns were associated with predictability of reward, Matsumoto and Hikosaka (2009) found a large number of macaque nigrostriatal dopaminergic neurons that increased in activity to stimuli predicting positive or negative events. Firing rates increased with predictability of outcome, suggesting increased activity should be observed as learning occurs. This suggests that the dopaminergic system may also be broadly involved in learning sequential associations, regardless of valence/reward.
If the sequence-specific activation in the ventral striatum reflects a modulatory change in dopamine, a key question remains about the neural substrate of the representation of sequence learning. Impairments in sequence learning observed in patients with Parkinson's disease (Siegert et al., 2006) suggest that dopamine dysfunction affects sequence learning and that corticostriatal circuits normally participate in sequential learning. Corticostriatal circuits connect the basal ganglia with traditional motor areas (such as the motor cortex, premotor cortex, and SMA) most directly associated with motor control as well as most other cortical regions (Middleton and Strick, 2000). Previous studies that have found cortical deactivations associated with implicit learning have interpreted these changes as experience-related improvements in processing efficiency. This phenomenon has been observed in repetition priming (Reber et al., 2005; Schacter et al., 2007) as well as visual category learning (Aizenstein et al., 2004; Reber et al., 2003). The sequence-specific decrease in activity found here suggests that the neural representation of sequence learning is improved processing efficiency in inferior and posterior parietal cortex, precuneus, occipito-temporal cortex, and premotor cortex that depends on dopamine-gated plasticity in corticostriatal circuits.
The post-hoc functional ROI analysis revealed that activity in the left precuneus and left inferior parietal cortex was reliably higher in the ST condition than in the Random baseline. Although speculative, these differences may suggest that interference is occurring when a novel action sequence is executed with a learned rhythm; the motor system could be preparing the incorrect actions at specific times, resulting in decreased processing efficiency. Perhaps this explains why timing has been found to be subordinate to order (O'Reilly et al., 2008; Shin and Ivry, 2002), since knowing the timing but not the action order does not facilitate preparation of the appropriate movements.
5 Conclusions
The new SISL paradigm provides a model for studying the acquisition of increasingly fluid and accurate sequential motor performance and sequence-specific learning. The representation of the known sequence integrates both action order and timing information such that transfer is essentially non-existent to sequences with only order or timing maintained. The neural correlate of this sequence-specific learning is a pattern of deactivation indicating improved processing efficiency across cortical regions involved in motor planning, spatio-motor integration, visuospatial processing, and visual motion processing. Higher activity during fluid performance of a known sequence was observed in the ventral striatum and medial prefrontal cortex, reflecting the likely involvement of dopamine-gated plasticity in corticostriatal circuits necessary for sequence learning. The changes observed here and the mechanisms supporting these changes are likely to contribute significantly to the neural basis of expert motor skill learning, particularly improvements based on repeated practice of specific motor sequences that are precisely timed.
Acknowledgments
This research was supported by the National Institutes of Health training grant T32 NS047987 awarded to Eric W. Gobel from the National Institute of Neurological Disease and Stroke and was partially supported by a Research Grant from the University Research Grants Committee at Northwestern University. The authors would like to acknowledge Daniel J. Sanchez for his comments on an earlier draft of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Eric W. Gobel, Email: egobel@u.northwestern.edu.
Todd B. Parrish, Email: toddp@northwestern.edu.
References
- Aizentstein HJ, MacDonald AW, Stenger VA, Nebes RD, Larson JK, Ursu S, Carter CS. Complimentary category learning systems identified using event-related functional MRI. J Cogn Neurosci. 2000;12:977–987. doi: 10.1162/08989290051137512. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Stenger VA, Cochran J, Clark K, Johnson M, Nebes RD, Carter CS. Regional brain activation during concurrent implicit and explicit sequence learning. Cereb Cortex. 2004;14:199–208. doi: 10.1093/cercor/bhg119. [DOI] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang-Vu T, Darsaud A, Ruby P, Luppi PH, Degueldre C, Peigneux P, Luxen A, Maquet P. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron. 2008;58:261–272. doi: 10.1016/j.neuron.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Berns GS, Cohen JD, Mintun MA. Brain regions responsive to novelty in the absence of awareness. Science. 1997;276:1272–1275. doi: 10.1126/science.276.5316.1272. [DOI] [PubMed] [Google Scholar]
- Bosco G, Carrozzo M, Lacquaniti F. Contributions of the human temporoparietal junction and MT/V5+ to the timing of interception revealed by transcranial magnetic stimulation. J Neurosci. 2008;28:12071–12084. doi: 10.1523/JNEUROSCI.2869-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Cerminara NL, Apps R, Marple-Horvat DE. An internal model of a moving visual target in the lateral cerebellum. J Physiol. 2009;587:429–442. doi: 10.1113/jphysiol.2008.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M. Flow: The Psychology of Optimal Experience. Harper and Row; New York: 1990. [Google Scholar]
- Dai TH, Liu JZ, Sahgal V, Brown RW, Yue GH. Relationship between muscle output and functional MRI-measured brain activation. Exp Brain Res. 2001;140:290–300. doi: 10.1007/s002210100815. [DOI] [PubMed] [Google Scholar]
- Del Percio C, Babiloni C, Marzano N, Iacoboni M, Infarinato F, Vecchio F, Lizio R, Aschieri P, Fiore A, Toràn G, Gallamini M, Baratto M, Eusebi F. “Neural efficiency” of athletes' brain for upright standing: A high-resolution EEG study. Brain Res Bull. 2009;79:193–200. doi: 10.1016/j.brainresbull.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehéricv S, Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC. Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomorgraphy. Eur J Neurosci. 1996;8:637–648. doi: 10.1111/j.1460-9568.1996.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Ward P. Capturing the naturally occurring superior performance of experts in the laboratory: Toward a science of expert and exceptional performance. Curr Dir Psychol Sci. 2007;16:346–350. [Google Scholar]
- Fletcher PC, Zafiris O, Frith CD, Honey RAE, Corlett PR, Zilles K, Fink GR. On the benefits of not trying: Brain activity and connectivity reflecting the interactions of explicit and implicit sequence learning. Cereb Cortex. 2005;15:1002–1015. doi: 10.1093/cercor/bhh201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili RJ, Bradberry TJ, Oh H, Hatfield BD, Contreras Vidal JL. Cerebral cortical dynamics during visuomotor transformation: Adaptation to a cognitive-motor executive challenge. Psychophysiology. 2011;48:813–824. doi: 10.1111/j.1469-8986.2010.01143.x. [DOI] [PubMed] [Google Scholar]
- Gobel EW, Sanchez DJ, Reber PJ. Integration of temporal and ordinal information during serial interception sequence learning. J Exp Psychol Learn Mem Cogn. 2011 doi: 10.1037/a0022959. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. J Cogn Neurosci. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Dimyan MA, Hallett M. Motor planning, imagery, and execution in the distributed motor network: A time-course study with functional MRI. Cereb Cortex. 2008;18:2775–2788. doi: 10.1093/cercor/bhn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield BD, Haufler AJ, Hung TM, Spalding TW. Electroencephalographic studies of skilled psychomotor performance. J Clin Neurophysiol. 2004;21:144–156. doi: 10.1097/00004691-200405000-00003. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry RB. Attention and stimulus characteristics determine the locus of motor sequence learning: A PET study. Brain. 1997;120:123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Hore J, Timmann D, Watts S. Disorders in timing and force of finger opening in overarm throws made by cerebellar subjects. Ann N Y Acad Sci. 2002;978:1–15. doi: 10.1111/j.1749-6632.2002.tb07551.x. [DOI] [PubMed] [Google Scholar]
- Jubault T, Ody C, Koechlin E. Serial organization of human behavior in the inferior parietal cortex. J Neurosci. 2007;27:11028–11036. doi: 10.1523/JNEUROSCI.1986-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerick SE, Douglass LW, Hatfield BD. Cerebral cortical adaptations associated with visuomotor practice. Med Sci Sports Exerc. 2004;36:118–129. doi: 10.1249/01.MSS.0000106176.31784.D4. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Palomo DD, Ioannides AA. Stimulus-contrast-induced biases in activation order reveal interaction between V1/V2 and human MT+ Hum Brain Mapp. 2009;30:147–162. doi: 10.1002/hbm.20495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant H, Georgopoulos AP. Neurophysiology of perceptual and motor aspects of interception. J Neurophysiol. 2006;95:1–13. doi: 10.1152/jn.00422.2005. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cogn Psychol. 1987;19:1–32. [Google Scholar]
- Nissen MJ, Knopman DS, Schacter DL. Neurochemical dissociation of memory systems. Neurology. 1987;37:789–794. doi: 10.1212/wnl.37.5.789. [DOI] [PubMed] [Google Scholar]
- Orban P, Peigneux P, Lungu O, Albouy G, Breton E, Laberenne F, Benali H, Maquet P, Doyon J. The multifaceted nature of the relationship between performance and brain activity in motor sequence learning. NeuroImage. 2010;49:694–702. doi: 10.1016/j.neuroimage.2009.08.055. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, McCarthy KJ, Capizzi M, Nobre AC. Acquisition of the temporal and ordinal structure of movement sequences in incidental learning. J Neurophysiol. 2008;99:2731–2735. doi: 10.1152/jn.01141.2007. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Maquet P, Meulemans T, Destrebecqz A, Laureys S, Degueldre C, Delfiore G, Aerts J, Luxen A, Frank G, Cleeremans A. Striatum forever, despite sequence learning variability: A random effect analysis of PET data. Hum Brain Mapp. 2000;10:179–194. doi: 10.1002/1097-0193(200008)10:4<179::AID-HBM30>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ. Neural correlated of motor skill automaticity. J Neurosci. 2005;25:5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Brown HD, Curran T, Alpert NM, Kendrick A, Fischman AJ, Kosslyn SM. A PET investigation of implicit and explicit sequence learning. Hum Brain Mapp. 1995;3:271–286. [Google Scholar]
- Rauch SL, Whalen PJ, Savage CR, Curran T, Kendrick A, Brown HD, Bush G, Breiter HC, Rosen BR. Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Hum Brain Mapp. 1997;5:124–132. [PubMed] [Google Scholar]
- Reber PJ, Gitelman DR, Parrish TB, Mesulam MM. Dissociating explicit and implicit category knowledge with fMRI. J Cogn Neurosci. 2003;15:574–583. doi: 10.1162/089892903321662958. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Gitelman DR, Parrish TB, Mesulam MM. Priming effects in the fusiform gyrus: Changes in neural activity beyond the second presentation. Cereb Cortex. 2005;15:787–795. doi: 10.1093/cercor/bhh179. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Parallel brain systems for learning with and without awareness. Learn Mem. 1994;1:217–229. [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Encapsulation of implicit and explicit memory in sequence learning. J Cogn Neurosci. 1998;10:248–263. doi: 10.1162/089892998562681. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Stark CEL, Squire LR. Cortical areas supporting category learning identified using functional MRI. Proc Natl Acad Sci U S A. 1998;95:747–750. doi: 10.1073/pnas.95.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM. The serial reaction time task: implicit motor skill learning? J Neurosci. 2007;27:10073–10075. doi: 10.1523/JNEUROSCI.2747-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Ramnani N, Passingham RE. Learning of sequences of finger movements and timing: Frontal lobe and action-oriented representation. J Neurophysiol. 2002;88:2035–2046. doi: 10.1152/jn.2002.88.4.2035. [DOI] [PubMed] [Google Scholar]
- Sanchez DJ, Gobel EW, Reber PJ. Performing the unexplainable: Implicit task performance reveals individually reliable sequence learning without explicit knowledge. Psychon Bull Rev. 2010;17:790–796. doi: 10.3758/PBR.17.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Curr Opin Neurobiol. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Purushotham A, Kim SG, Ugurbill K, Willingham D, Ashe J. Neural correlates of encoding and expression in implicit sequence learning. Exp Brain Res. 2005;165:114–124. doi: 10.1007/s00221-005-2284-z. [DOI] [PubMed] [Google Scholar]
- Siegert RJ, Taylor KD, Weatherall M, Abernethy DA. Is implicit sequence learning impaired in Parkinson's disease? A meta-analysis. Neuropsychology. 2006;20:490–495. doi: 10.1037/0894-4105.20.4.490. [DOI] [PubMed] [Google Scholar]
- Shin JC, Ivry RB. Concurrent learning of temporal and spatial sequences. J Exp Psychol Learn Mem Cogn. 2002;28:445–457. doi: 10.1037//0278-7393.28.3.445. [DOI] [PubMed] [Google Scholar]
- Shin JC, Ivry RB. Spatial and temporal sequence learning in patients with Parkinson's disease or cerebellar lesions. J Cogn Neurosci. 2003;15:1232–1243. doi: 10.1162/089892903322598175. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. NeuroImage. 2003;18:633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Timmann D, Watts S, Hore J. Failure of cerebellar patients to time finger opening precisely causes ball high-low inaccuracy in overarm throws. J Neurophysiol. 1999;82:103–114. doi: 10.1152/jn.1999.82.1.103. [DOI] [PubMed] [Google Scholar]
- Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST. Motor subcircuits mediating the control of movement extent and speed. J Neurophysiol. 2003;90:3958–3966. doi: 10.1152/jn.00323.2003. [DOI] [PubMed] [Google Scholar]
- Wächter T, Lungu OV, Liu T, Willingham DT, Ashe J. Differential effect of reward and punishment on procedural learning. J Neurosci. 2009;29:436–443. doi: 10.1523/JNEUROSCI.4132-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich M, Wise SP. The premotor cortex of the monkey. J Neurosci. 1982;2:1329–1345. doi: 10.1523/JNEUROSCI.02-09-01329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham DB, Greeley T, Bardone AM. Dissociation in a serial response time task using a recognition measure: comment on Perruchet and Amorim (1992) J Exp Psychol Learn Mem Cogn. 1993;19:1424–1430. [Google Scholar]
- Williingham DB, Salidis J, Gabrieli JDE. Direct comparison of neural systems mediating conscious and unconscious skill learning. J Neurophysiol. 2002;88:1451–1460. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. A quantitative evaluation of cross-participant alignment techniques for MRI studies of the medial temporal lobe. NeuroImage. 2009;44:319–327. doi: 10.1016/j.neuroimage.2008.09.016. [DOI] [PubMed] [Google Scholar]