Abstract
Atoh1 is required for differentiation of sensory hair cells in the vertebrate inner ear. Moreover, misexpression of Atoh1 is sufficient to establish ectopic sensory epithelia, making Atoh1 a good candidate for gene therapy to restore hearing. However, competence to form sensory epithelia appears to be limited to discrete regions of the inner ear. To better understand the developmental factors influencing sensory-competence, we examined the effects of misexpressing atoh1a in zebrafish embryos under various developmental conditions. Activation of a heat shock-inducible transgene, hs:atoh1a, resulted in ectopic expression of early markers of sensory development within 2 hours, and mature hair cells marked by brn3c:GFP began to accumulate 9 hours after heat shock. The ability of atoh1a to induce ectopic sensory epithelia was maximal when activated during placodal or early otic vesicle stages but declined rapidly thereafter. At no stage was atoh1a sufficient to induce sensory development in dorsal or lateral non-sensory regions of the otic vesicle. However, co-misexpression of atoh1a with fgf3, fgf8 or sox2, genes normally acting in the same gene network with atoh1a, stimulated sensory development in all regions of the otic vesicle. Thus, expression of fgf3, fgf8 or sox2 strongly enhances competence to respond to Atoh1.
Keywords: zebrafish, atoh1a/b, sox2, fgf3, fgf8, hair cell, support cell, heat shock
INTRODUCTION
Sensory epithelia of the inner ear, comprising hair cells and support cells, mediate the senses of hearing and balance. One of the most important regulatory factors controlling development of sensory epithelia is the basic helix-loop-helix transcription factor, Atoh1, expression of which is both necessary and sufficient for development of sensory epithelia (Chen et al., 2002; Millimaki et al. 2007; Millimaki et al., 2010; Woods et al., 2004). Atoh1 is best known for its role in differentiation of hair cells. Atoh1 expression is maximal in differentiating hair cells, and conditions that maintain elevated expression promote hair cell differentiation at the expense of support cells (Dabdoub et al., 2008; Jones et al., 2006; Woods et al., 2004; Zheng and Gao, 2000). However, Atoh1 also acts at an earlier stage to establish the prosensory domain from which both hair cells and support cells emerge. Accordingly, Atoh1 is initially expressed in a broad domain containing precursors of both hair cells and support cells (Woods et al., 2004; Yang et al., 2010). Only later does Atoh1 become restricted to differentiating hair cells by a self-limiting process termed lateral inhibition (reviewed by Cotanche and Kaiser, 2010). Disruption of Atoh1 prevents development of both hair cells and support cells, whereas misexpression of Atoh1 can stimulate formation of ectopic sensory epithelia containing both cell types (Millimaki et al., 2007; Woods et al., 2004). Thus, Atoh1 exhibits potent tissue-organizing activity that goes beyond its ability to promote hair cell differentiation.
The basis for Atoh1’s broader organizing activity lies in its ability to activate downstream signaling pathways that diversify cell fates. For example, Atoh1 activates expression of various Notch ligands that facilitate lateral signaling required for support cell specification (Millimaki et al., 2007; Woods et al., 2004). Notch signaling in this context works in part by repressing Atoh1 expression in a subset of precursor cells, resulting in the alternating pattern of hair cells and support cells seen in mature sensory epithelia. Newly formed sensory epithelia also express a number of Fgf genes. It appears that Fgf signaling serves to recruit additional cells into the developing sensory epithelium: Discrete subsets of hair cells and support cells that normally form after the first wave of hair cell production fail to form when Fgf signaling is impaired (Hayashi et al., 2007; Hayashi et al., 2008; Jacques et al., 2007; Millimaki et al., 2007; Pirvola et al., 2002; Puligilla et al., 2007). Notch and Fgf also appear to function upstream to activate Atoh1 expression (Hayashi et al., 2008; Millimaki et al., 2007; Woods et al., 2004), suggesting a complex feedback network that is only partially understood. How these signals influence the effects of Atoh1 misexpression remains to be established.
Despite the organizing effects of Atoh1, competence to respond properly to Atoh1 is not uniform. For example, some regions of the otic vesicle appear to be completely refractory to the effects of Atoh1, failing to produce sensory epithelia even after high-level Atoh1 misexpression (Huang et al., 2009; Kawamoto et al., 2003; Millimaki et al., 2010; Woods et al., 2004; Zheng and Gao, 2000). Even in regions capable of forming ectopic sensory epithelia, ectopic hair cells induced by Atoh1 misexpression often exhibit aberrant morphology or a diminished capacity to survive after differentiation (Izumikawa et al., 2005; Kawamoto et al. 2003; Millimaki et al., 2007). In such cases, it is likely that cells in foreign sites lack essential cofactors needed for normal Atoh1 activity or, alternatively, other regionally expressed factors may interfere with Atoh1. Because Atoh1 is a promising candidate for gene therapy to restore hearing (Izumikawa et al., 2005; Shou et al., 2003), identifying the factors that influence sensory competence remains an important goal of inner ear research.
Here we investigate the effects of atoh1a misexpression in zebrafish by examining temporal and spatial parameters that influence Atoh1 function. We demonstrate that misexpression of atoh1a at various stages of otic development can induce ectopic sensory epithelia composed of both hair cells and support cells. Competence to respond to atoh1a misexpression is already spatially restricted during placodal stages and becomes increasingly restricted after formation of the otic vesicle. Co-misexpressing atoh1a with fgf3, fgf8 or sox2, genes that normally act in the same gene network with atoh1a, promotes sensory development throughout the otic vesicle. These data elucidate a genetic network that is sufficient to enhance competence to respond to Atoh1.
MATERIALS AND METHODS
Strains
The wild-type strain was derived from the AB line (Eugene, OR). The brn3c:gfp line was developed by Xiao et al. (Xiao et al., 2005) and hsp70:dkk1-GFPw32 was developed by Stoick- Cooper et al., (Stoick-Cooper et al., 2007). hsp70:atoh1ax20, hsp70:fgf8x17 and hsp70:sox2x21 lines were previously described (Millimaki et al., 2010). We also generated two new lines, Tg (hsp70:pax2a)x23 and Tg(hsp70:fgf3)x27, using previously described techniques (Millimaki et al., 2010).
Misexpression and gene-knockdown
To assess the effects of gene misexpression or gene knockdown, at least 30 embryos were observed for each time-point. Except where noted in the text, phenotypes were fully penetrant. For most misexpression experiments using heat shock-inducible transgenic lines, embryos were incubated in a water bath at 39°C for 30 minutes at time points described in the results. For experiments involving hsp70:pax2ax23 or both hsp70:pax2ax23 and hsp70:atoh1ax20, embryos were activated at 36°C for 30 minutes. Activation of hsp70:pax2ax23 at higher temperatures causes elevated cell death, whereas activation of both transgenes is highly effective at 36°C (data not shown). Injection of morpholino oligomers to knockdown pax2a, pax2b or pax8 was performed as previously described (Bricaud and Collazo, 2006; Mackereth et al., 2005).
In situ hybridization
In situ hybridization was performed as described previously (Jowett and Yan, 1996; Phillips et al., 2001).
Immunostaining
Antibody staining was performed as described by Riley et al. (Riley et al., 1999). Primary antibodies: anti-Pax2 (Covance diluted at 1:100), anti-GFP (Santa Cruz Biotechnology diluted 1:200) and anti-Caspase 3 (R&D systems diluted 1:100). Secondary antibodies: Alexa 546-conjugated goat anti-rabbit IgG (Molecular Probe diluted 1:200) or HRP-conjugated goat anti-rabbit IgG (Vector laboratories PI-2000 diluted 1:200).
Sections
For cryosectioning of brn3c:gfp, embryos were fixed overnight in MEMFA (0.1 M Mops at pH7.4, 2 mM EGTA, 1 mM MgSO4, 3.7% formaldehyde). Embryos were then washed twice for 5 minutes in 1x PBS followed by two one hour long washes in PBT with 0.5% Triton-X and finally washed twice for 5 minutes in 1x PBS and transferred into a 30% sucrose solution made in PBS. Embryos were embedded in tissue freezing medium (Triangle Biomedical Sciences, H-TFM) and cut into 10 µm sections using a cryostat. Slides were dried overnight, washed in PBS, and then mounted in ProLong Gold (Invitrogen). The same protocol was used for cryosectioning of embryos following wholemount in situ hybridization except that PBT washes were omitted. For double labeling of sox2 and brn3c:gfp, embryos were first stained by wholemount situ hybridization for sox2, then 10 µm cryosections were immunostained for GFP. For resin-sections of sox2 and brn3c:gfp, embryos were stained in wholemount by immunolocalization of GFP followed by in situ hybridization for sox2, then embedded in Immunobed resin (Polysciences No. 17324) and cut into 7 µm sections.
RESULTS
Effects of hs:atoh1a misexpression in the nascent otic vesicle
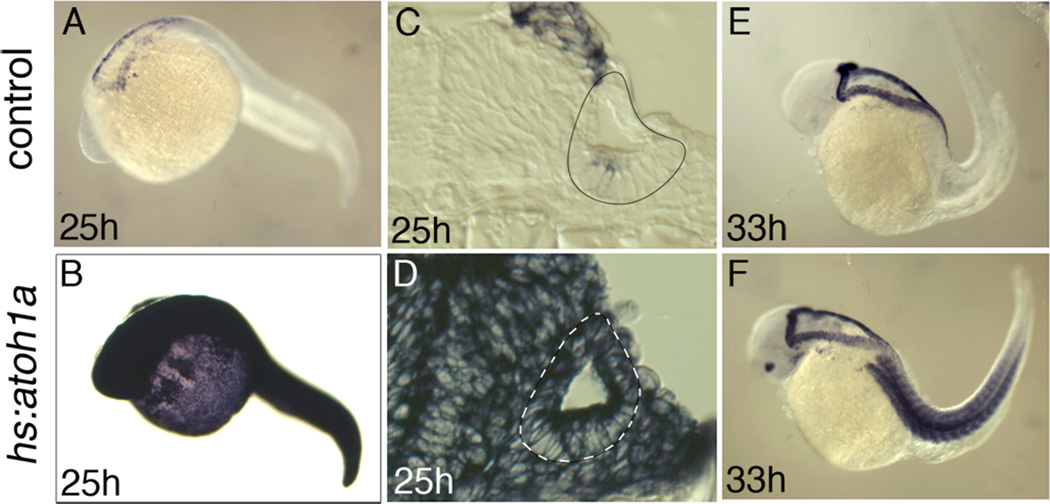
We showed previously that zebrafish atoh1a is necessary and sufficient for hair cell development (Millimaki et al., 2007). To further investigate the effects of atoh1a misexpression and determine the temporal requirements for atoh1a, we utilized a heat shock-inducible transgenic line to misexpress atoh1a (Millimaki et al., 2010). Induction of the hsp70 heat shock promoter typically results in elevated transcript levels of the transgene for 90 minutes, followed by a gradual decay over the next few hours (Hans et al., 2007). However, activation of transgenic hs:atoh1a led to robust expression of atoh1a transcript for at least 6 hours, with moderate upregulation still evident through 9 hours post-activation (Fig. 1E, F). This extended period of upregulation likely occurs through auto-regulatory activation of the endogenous atoh1a locus (Helms et al., 2000; Sun et al., 1998; our unpublished observations). For the purposes of this study it is important to note that the hs:atoh1a transgene is expressed globally, including throughout the otic vesicle (Fig. 1A–D).
Figure 1. atoh1a expression following hs:atoh1a activation at 24 hpf.
Expression of atoh1a at the indicated times in control embryos (A, C, E) and hs:atoh1a transgenic embryos (B, D, F). Images of wholemount specimens (A–B, E–F) are dorsolateral views with anterior to the left and transverse sections (C–D) with dorsal to the top. The otic vesicles are outlined in C–D.
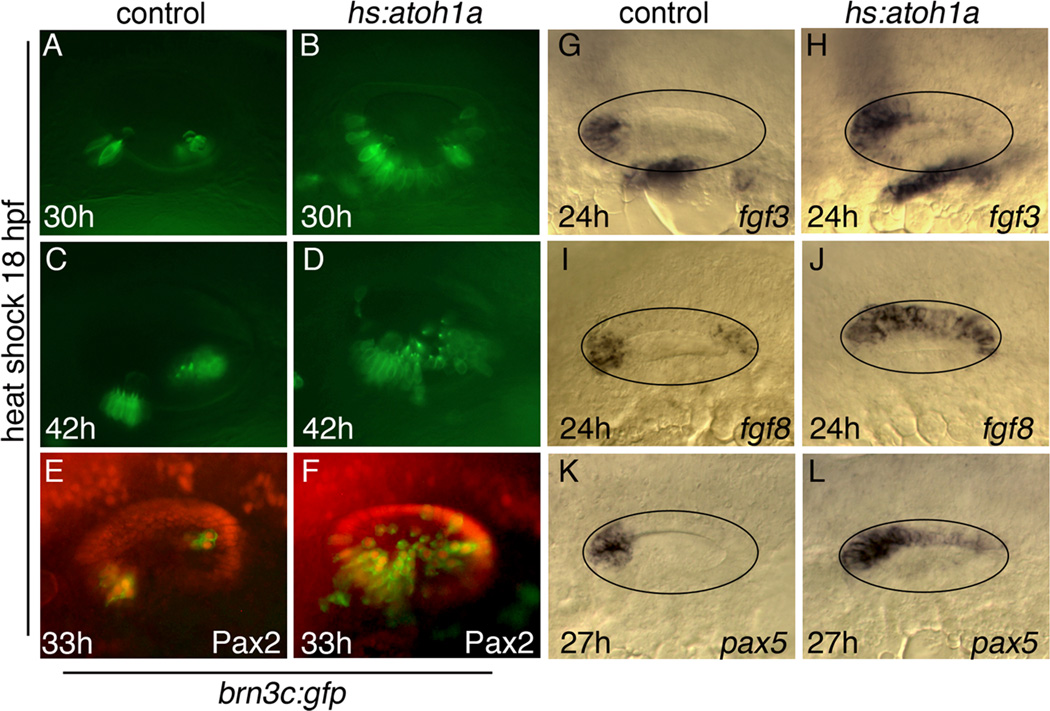
We began our analysis by activating hs:atoh1a at 18 hpf, the time when the otic vesicle first forms. Production of mature hair cells was monitored by following expression of brn3c:gfp (Xiao et al., 2005), which can first be detected in nascent hair cells around 9 hours after activation of hs:atoh1a (Millimaki et al., 2010). Activation of hs:atoh1a at 18 hpf led to production of hair cells throughout the ventromedial quadrant of the ear at 30 hpf (Fig. 2A, B). This region includes the areas normally occupied by the utricular and saccular maculae plus intervening tissue. Ectopic hair cells were stably maintained through at least 42 hpf, and additional hair cells continued to accumulate around the edges of the expanded sensory epithelium (Fig. 2C, D). To further characterize hair cell differentiation under these conditions, we examined Pax2 expression, which normally upregulates during development of all utricular hair cells, as well as the first 2–3 hair cells to form in the saccule (Riley et al., 1999; Kwak et al., 2006). Nearly all cells within the otic vesicle that expressed brn3c:gfp became positive for Pax2 within 15 hours of hs:atoh1a activation (Fig. 2E, F). Additionally, expression of general macular markers fgf3 and fgf8 also expanded following activation of hs:atoh1a, as did the utricular marker pax5 (Fig. 2G–L). Thus, misexpression of atoh1a induced formation of excess and ectopic hair cells in the ventromedial portion of the otic vesicle, with most hair cells expressing markers consistent with an anterior (utricular) fate. In contrast, only a small number of hair cells were seen in the dorsal epithelium and none in the lateral epithelium, indicating that sensory competence is already spatially restricted at the early otic vesicle stage.
Figure 2. Otic vesicle patterning following hs:atoh1a activation at 18 hpf.
(A–F) Expression of brn3c:gfp (green) in the utricle and saccule of control embryos (A, C, E) and in hs:atoh1a transgenic embryos (B, D, F) at the indicated times. (E, F) Co-staining with anti-Pax2 in red. (G–L) Otic expression of fgf3, fgf8, and pax5 in control embryos (G, I, K) and expanded expression in hs:atoh1a transgenic embryos (H, J, L). All images show dorsolateral views with anterior to the left and dorsal up (A–H) or dorsal views with anterior to the left and medial up (I–L).
Effects of hs:atoh1a misexpression at later stages
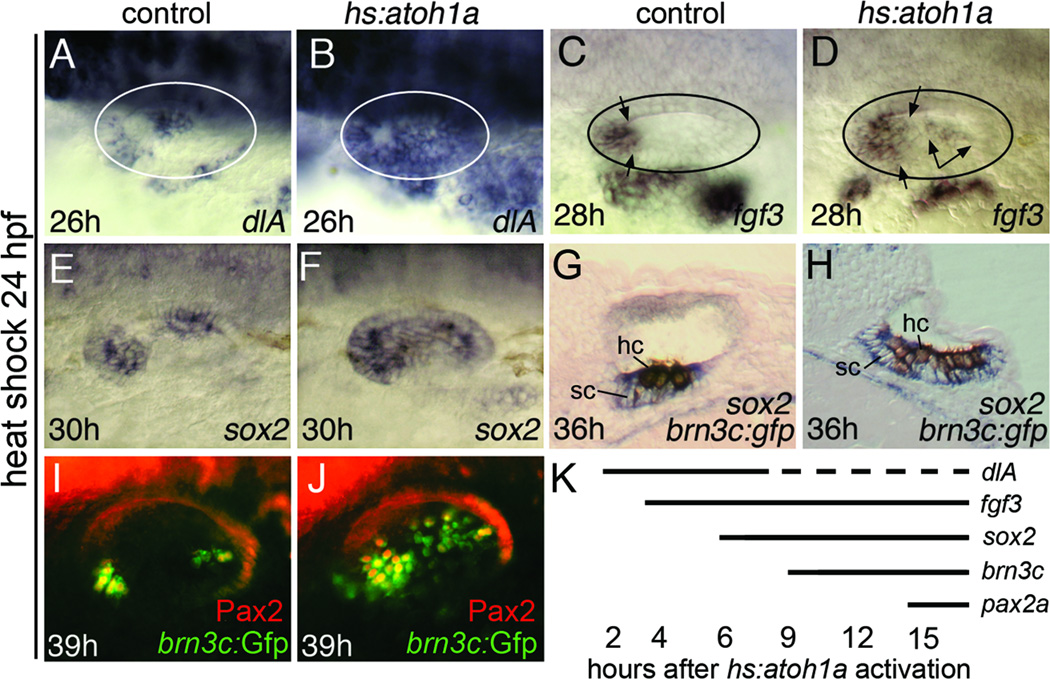
We next characterized the effects of activating hs:atoh1a at 24 hpf, by which time the first mature hair cells have formed and maculae have started to expand (Haddon and Lewis, 1996; Riley et al., 1999). We showed previously that activation of hs:atoh1a at this time leads to production of hair cells throughout the ventromedial wall in a manner comparable to activating hs:atoh1a at 18 hpf (Millimaki et al., 2010). We extended that work by examining early markers of macular development. Some of the earliest targets of atoh1a/b in the zebrafish otic placode and vesicle are Notch pathway genes deltaA and deltaD (Millimaki et al., 2007). Accordingly, activation of hs:atoh1a at 24 hpf led to a rapid expansion of the macular domains of deltaA to cover the entire ventromedial wall of the otic vesicle by 26 hpf (Fig. 3A, B). This was followed by expansion of fgf3 into the medial wall at 28 hpf, including medial expansion of utricular expression and upregulation in the saccular macula (Fig. 3C, D). Expression of sox2, which normally follows atoh1a/b and initially marks both hair cells and support cells, showed intense expression throughout the ventromedial wall of the otic vesicle by 30 hpf, 6 hours after heat shock (Fig. 3E, F; Millimaki et al., 2010). Because sox2 is also induced by Fgf and Notch (Millimaki et al., 2010), it is possible that Atoh1a induced sox2 indirectly through activation of Fgf and Notch pathways. Ectopic hair cells marked with brn3c:gfp were first observed by 33 hpf, 9 hours after activation of hs:atoh1a (Millimaki et al., 2010, and data not shown). Transverse sections of embryos differentially stained for brn3c:gfp and sox2 confirmed that atoh1a misexpression expanded production of both hair cells and support cells (Fig. 3G, H). Many hair cells in the anterior half of the ear, and a few randomly scattered hair cells in the posterior, became Pax2-positive by 39 hpf (Fig. 3I, J; Millimaki et al., 2010). The timeframe of responses of various macular genes to hs:atoh1a activation is summarized in Fig. 3K.
Figure 3. Otic vesicle patterning following hs:atoh1a activation at 24 hpf.
(A–F) Expression at the indicated times of dlA, fgf3 and sox2 in control embryos (A, C, E) and hs:atoh1a transgenic embryos (B, D, F). To assist in interpretation of images, otic vesicles are outlined in A–D and the spatial limits of fgf3 expression are marked by arrows (C, D). (G, H) Transverse sections showing expression of sox2 (blue) and anti-GFP (brown) at 36 hpf in a control embryo (G) and a hs:atoh1a transgenic embryo (H). Positions of hair cells (hc) and support cells (sc) are indicated. (I, J) Expression of brn3c:gfp (green) and Pax2 (red) in otic hair cells at 39 hpf in a control embryo (I) and a hs:atoh1a transgenic embryo (J). (K) Summary of the onset of expanded or ectopic expression of various otic markers following activation of hs:atoh1a at 24 hpf. Most markers were stably expressed, except for dlA. Expression of dlA was lost in a subset of cells after several hours, presumably reflecting the process of lateral inhibition. Images of wholemount specimens (A–F, I, J) are dorsolateral views with anterior to the left and dorsal to the top.
Although many genes showed similar responses to hs:atoh1a activation at 18 hpf compared to 24 hpf, there were several notable exceptions. For example, activation of hs:atoh1a at 24 hpf or later did not expand the domains of fgf8 and pax5 expression as it did with earlier hs:atoh1a activation (data not shown). Similarly, upregulation of pax2a was limited mostly to anterior hair cells following hs:atoh1a activation at 24 hpf, whereas virtually all hair cells expressed pax2a following hs:atoh1a activation at 18 hpf (compare Figs. 2F and 3J). These data suggest that atoh1a misexpression at 24 hpf does not expand anterior otic fates as it does at earlier stages. The reason for this change is not clear.
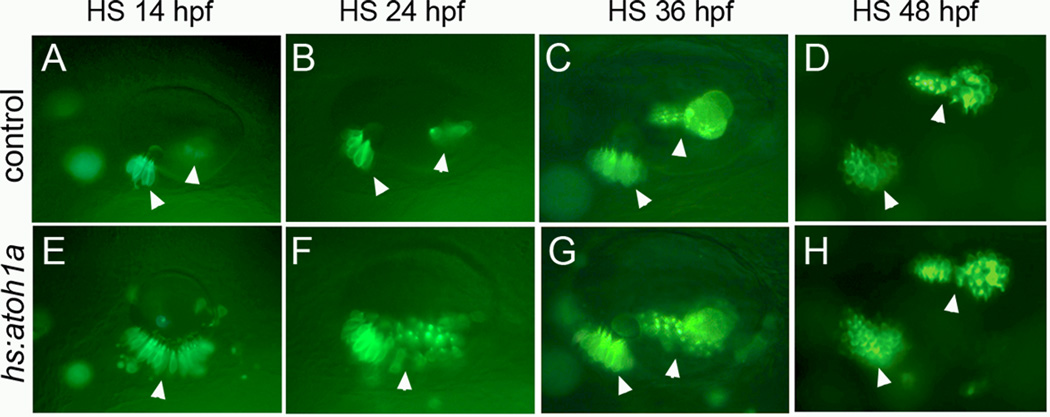
The effects of atoh1a misexpression diminished after 24 hpf. For example, compared to the broad medial expansion of hair cells following activation of hs:atoh1a at 24 hpf (Fig. 4B, F), activation of hs:atoh1a at 36 hpf resulted in production of two discrete but enlarged maculae, with an intervening region devoid of hair cells (Fig. 4C, G). Activation of hs:atoh1a at 48 hpf caused only a slight increase in hair cell production within the endogenous maculae and cristae but did not promote sensory development in ectopic locations (Fig. 4D, H). These data indicate that competence to respond to hs:atoh1a becomes increasingly restricted at later developmental stages.
Figure 4. Spatial restriction of competence to respond to hs:atoh1a at different stages.
Expression of brn3c:gfp in control embryos (A–D) and hs:atoh1a transgenic embryos (E–H). Embryos were heat shocked at the times indicated across the top and photographed 12–13 hours later. Arrowheads mark positions of endogenous and expanded sensory epithelia. Images show dorsolateral views with anterior to the left and dorsal to the top.
Effects of hs:atoh1a misexpression at placodal stages
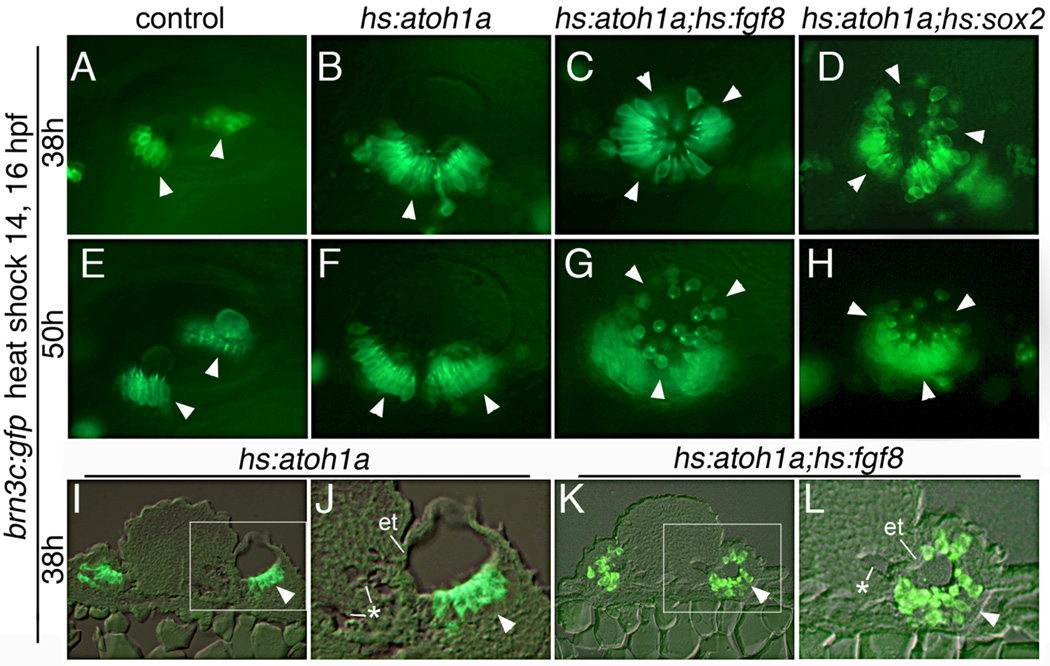
In zebrafish, a broad prosensory domain is established in the preplacode by 10.5 hpf and the first hair cells are specified by 14 hpf, just as the otic placode becomes morphologically visible (Millimaki et al., 2007). We reasoned that competence to respond to Atoh1a may be more widespread at these early stages. Misexpression at different times showed that activating hs:atoh1a at 14 hpf had the greatest effect on sensory development (Fig. 4A, E). In contrast, activation of hs:atoh1a at 12 hpf resulted in a more modest expansion of sensory epithelia; and heat shock initiated at 10 hpf had little or no effect on macular development (data not shown). The likely reason for the weak response to transgene activation at 10 hpf or 12 hpf is that the endogenous atoh1b locus normally shows widespread expression in the otic placode at these times (Millimaki et al., 2007), such that a brief pulse of transgene activity is superfluous. We therefore focused on transgene activation at the most sensitive stage to assess the spatial limits of sensory competence. Although heat shock at 14 hpf caused a dramatic expansion of sensory epithelium, the sensory epithelium was generally limited to the ventral epithelium of the otic vesicle (Fig. 4A, E). In rare cases, a small number of ectopic hair cells were observed in more dorsal positions (Fig. 4E), though none were detected in the lateral wall. The same results were obtained when embryos were subjected to serial heat shocks at 14 hpf and 16 hpf to prolong expression of hs:atoh1a (Fig. 5A, B, and data not shown). Transverse sections revealed few if any cells on the dorsal, medial, or lateral walls of the otic vesicle (Fig. 5I, J). Thus, the zone of sensory competence is already spatially restricted at the earliest stages when embryos are maximally responsive to hs:atoh1a.
Figure 5. Co-misexpression of atoh1a with fgf8 or sox2.
(A–L) Expression of brn3c:gfp after serial heat shock at 14 and 16 hpf in a control (A, E), hs:atoh1a (B, F, I, J), hs:atoh1a;hs:fgf8 (C, G, K, L) and hs:atoh1a;hs:sox2 (D, H) embryos. Embryos were fixed and processed at the indicated times. Arrowheads mark positions of endogenous and ectopic sensory epithelia. Images in I–L show transverse sections with dorsal to the top. The boxed areas in I and K are enlarged in J and L, respectively. The hindbrain shows sporadic formation of microvesicles (asterisks), suggesting tissue disruption, and the adjacent wall of the otic vesicle shows marked epithelial thinning (et). All other images show dorsolateral views, with anterior to the left and dorsal to the top.
Enhancement of sensory competence by misexpression of Fgf8 or Sox2
Fgf is one of the factors required to activate atoh1a/b in the developing otic placode and vesicle (Millimaki et al., 2007). We speculated that Fgf might influence sensory competence by activating additional factors that work in concert with Atoh1. To test this, we examined the effects of co-misexpression of hs:atoh1a and hs:fgf8. A single heat shock at 14 hpf yielded a large sensory epithelium in the ventral floor, as well as a few scattered hair cells in the lateral wall (not shown). Prolonging misexpression by serial co-activation of hs:atoh1a and hs:fgf8 at 14 hpf and 16 hpf led to a much more dramatic expansion of hair cells throughout the otic vesicle, including the dorsal and lateral walls (Fig. 5C). Similar results were obtained by serial co-activation of hs:atoh1a and hs:fgf3 (Fig. S1 F). Transverse sections of hs:atoh1a;hs:fgf8 embryos confirmed the presence of a contiguous sensory epithelium covering the entire vesicle, with the exception of a small region in the medial wall (Fig. 5K, L). Absence of hair cells in this region correlated with notable thinning of the epithelium and the presence of multiple microvesicles in adjacent hindbrain tissue, suggesting some degree of tissue disruption. Nevertheless, these data show that early co-misexpression of hs:atoh1a and either hs:fgf8 or hs:fgf3 can dramatically expand sensory competence into virtually all regions of the otic vesicle. Moreover, regions of ectopic sensory development exhibited a thickened pseudostratified morphology typical of normal sensory epithelia. In contrast, activation of hs:fgf8 or hs:fgf3 alone was not sufficient to induce ectopic sensory epithelia, though the saccular macula was broken into 2 discrete domains in these backgrounds (Fig. S1 B, C).
We next examined the ability of sox2 to enhance hair cell production following activation of hs:atoh1a. sox2 is normally induced by Fgf and Notch and is co-expressed with atoh1a/b in developing sensory epithelia (Millimaki et al., 2010). Similar to co-misexpression of atoh1a and fgf8, serial activation of hs:atoh1a and hs:sox2 at 14 hpf and 16 hpf produced hair cells located throughout the otic vesicle (Fig. 5D). Serial activation of hs:sox2 alone had little effect on hair cell production (Fig. S1 A). Hair cells produced after misexpression of atoh1a with either fgf8 or sox2 were still present at 50 hpf, indicating these cells are relatively stable. Although hair cells in the lateral wall appeared more widely separated at later stages (Fig. 5G, H), this appears to result from expansion of intervening tissue rather than death of hair cells based on monitoring GFP patterns over time. Anti-Caspase 3 staining confirmed that double-transgenic embryos did not exhibit an elevated number of apoptotic cells (Fig. S2).
We also examined the ability of pax genes to influence sensory competence. Expression of pax8 and pax2a are also regulated by Fgf during otic development and are known to affect development and survival of hair cells (Kwak et al., 2006; Millimaki et al., 2007; Riley et al., 1999). However, serial co-activation of hs:atoh1a with either hs:pax2a or hs:pax8 did not alter the production of hair cells compared to activation of hs:atoh1a alone (Fig. S1 D, E, I, and data not shown). Likewise, disruption of either pax8 or pax2a and pax2b did not diminish the ability of hs:atoh1a to induce ectopic hair cells following heat shock activation at 14 hpf or 24 hpf (Fig. S1 G, H, and data not shown).
Patterning associated with global sensory development
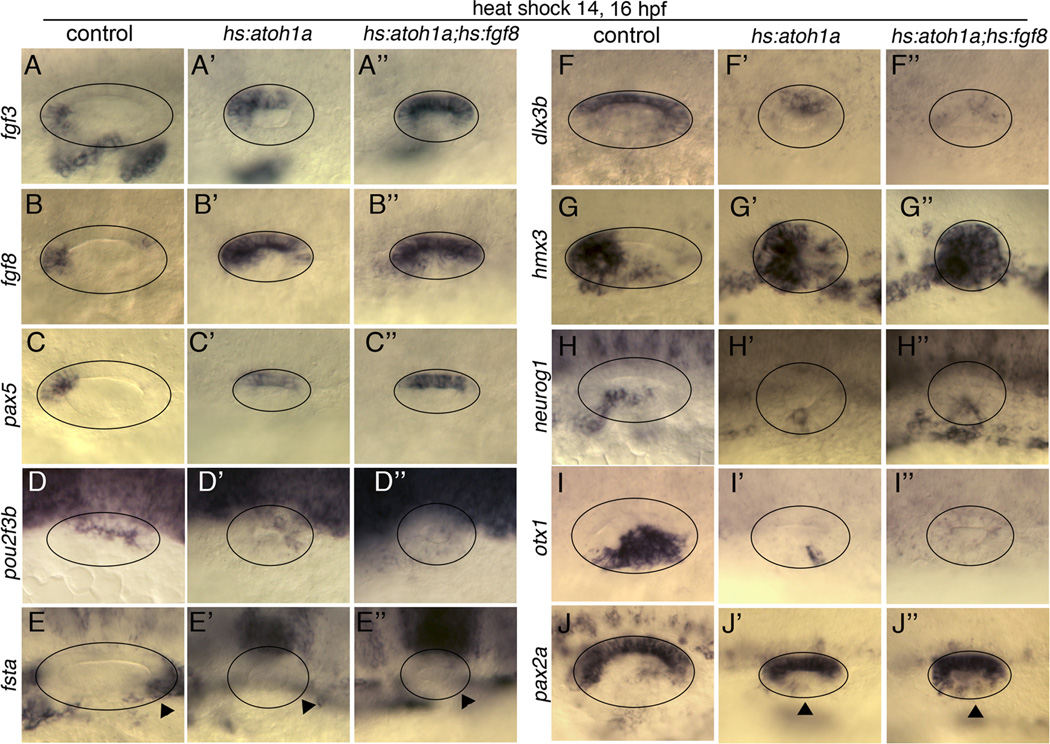
The nearly global expansion of sensory development following co-misexpression of hs:atoh1a with either hs:fgf8 or hs:sox2 suggested dramatic changes in axial patterning within the otic vesicle. To test this, we examined expression of numerous spatial markers after serial activation of hs:atoh1a alone or in combination with hs:fgf8. Several anterior markers, fgf8, fgf3 and pax5 were all expanded posteriorly following activation of hs:atoh1a alone and were more strongly expressed following co-misexpression of hs:atoh1a and hs:fgf8 (Fig. 6A–C”). Consistent with anteriorization of the otic vesicle, the posterior marker pou2f3b (previously zp23) was reduced by activation of hs:atoh1a and nearly absent following activation of hs:atoh1a and hs:fgf8, while the posterior marker fsta was completely absent after misexpression of atoh1a or atoh1a and fgf8 (Fig. 6D–E”). Expression of the dorsal marker dlx3b was reduced in hs:atoh1a and nearly absent after co-activation of hs:atoh1a and hs:fgf8 (Fig. 6F–F”). An anterior/ventral marker hmx3 (previously nkx5.1) was somewhat expanded by activating hs:atoh1a alone and was expressed nearly globally in hs:atoh1a; hs:fgf8 double transgenic embryos (Fig. 6G–G”). Expression of the neuronal specifier neurog1 was restricted to a small antero-lateral patch following activation of hs:atoh1a (Fig. 6H, H’). This is consistent with data from mouse showing Neurog1 and Atoh1 antagonize one another (Raft et al., 2007). In hs:atoh1a; hs:fgf8 embryos the domain of neurog1 was similarly reduced but shifted to a slightly more posterior position (Fig. 6H”). The lateral/posterior marker otx1 was severely diminished in the otic vesicles of hs:atoh1a embryos and completely eliminated in double transgenic animals (Fig. 6I-I”). Consistent with loss of lateral markers we observed expansion of the medial marker pax2a into more lateral regions in hs:atoh1a and more strongly so in hs:atoh1a; hs:fgf8 double transgenic embryos (Fig. 6J-J”). Nevertheless, expansion of medial fate was incomplete, since pax2a expression was not as strong laterally as medially (Fig. 6J”) and pax5 did not show appreciable lateral expansion (Fig. 6C”). In contrast to the above results, serial activation of hs:fgf8 alone led to ectopic expression of some anterior markers in posterior domains but otherwise did not strongly affect axial patterning in the otic vesicle (Fig. S3). Taken together, these data indicate that atoh1a misexpression can expand anterior/ventral/medial identity within the otic vesicle but only to a certain extent on its own. Co-activation of hs:fgf8 and hs:atoh1a enhances this activity.
Figure 6. Axial patterning following co-activation of hs:atoh1a and hs:fgf8.
Expression of various otic markers in control embryos (A–J), hs:atoh1a transgenic embryos (A’–J’) and hs:atoh1a;hs:fgf8 double transgenic embryos (A”–J”). Embryos were serially heat shocked at 14 and 16 hpf and fixed for processing at 26 hpf. Images show dorsal views (A–C”) or dorsolateral views (D–J”), with anterior to the left. Circles outline the otic vesicle. Arrowheads in E-E” mark expected location of fsta in the posterior otic vesicle. Arrowheads in J-J” indicate expanded domains of pax2a in the lateral wall of the otic vesicle.
Expansion of sensory competence at later stages
Because the effects of atoh1a misexpression become severely limited at later stages of development, we asked whether co-misexpression of fgf8 or sox2 can enhance sensory competence after 24 hpf. In an initial series of experiments, we observed that delivering two heat shocks separated by either a 2- or 3-hour rest interval was optimal for increasing hair cell production, whereas two heat shocks separated by a 4-hour rest interval gave results that were indistinguishable from a single heat shock. Delivering a third heat shock offered no advantage relative to two heat shocks. For all experiments below, embryos were subjected to two heat shocks separated by a 3-hour rest interval, and sensory development was examined 24 hours after the final heat shock.
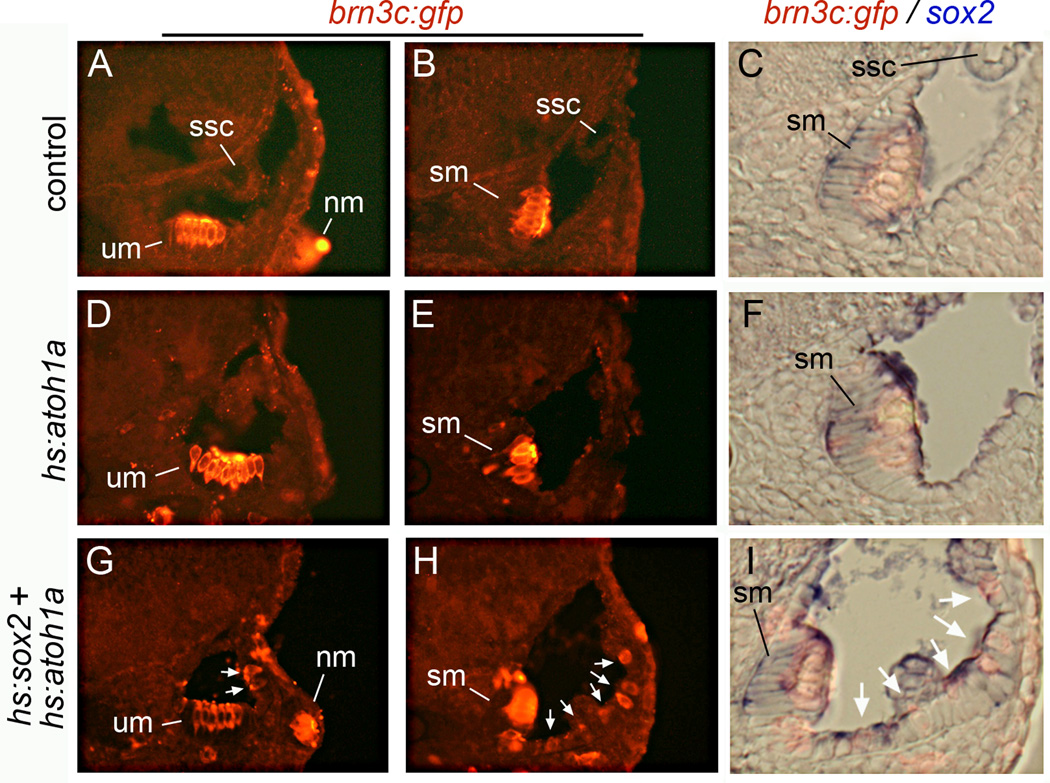
Serial activation of hs:atoh1a at 24 hpf or 45 hpf yielded greater production of excess and ectopic hair cells than a single heat shock. However, production of ectopic hair cells was still mostly seen in the medial and ventral portions of the otic vesicle (Fig. 7D, E). Formation of hair cells in the lateral wall was rare, with an average of about 2 ± 2 lateral-wall hair cells per otic vesicle (n=13) (Fig. S4). Semicircular canals failed to form normally under these conditions, suggesting perturbation of non-sensory development. Serial co-activation of hs:atoh1a and hs:fgf8 after 24 hpf did not appreciably expand the domain of sensory development compared to serial activation of hs:atoh1a alone (not shown). In contrast, serial co-activation of hs:atoh1a and hs:sox2 beginning at 24 hpf or 45 hpf led to a marked increase in hair cell production, including in the lateral wall of the otic vesicle (Fig. 7G, H). On average 9 ± 3 hair cells were observed in the lateral wall per otic vesicle (n=12) (Fig. S4). Ectopic hair cells usually formed as widely scattered single cells or small clusters. The epithelium surrounding ectopic hair cells was notably thickened and exhibited a pseudostratified morphology (compare Fig. 7C, F, I). Additionally, small patches of sox2 expression were usually detected near ectopic hair cells, suggesting the presence of support cells (Fig. 7I). Excess and ectopic hair cells were not observed following activation of hs:sox2 alone, nor in control embryos (Fig. S4 and Millimaki et al., 2010). Thus, co-misexpression of sox2 plus atoh1a can significantly enhance sensory competence at later stages of development.
Figure 7. Sox2 expands sensory competence at later stages.
Transverse sections showing otic expression of brn3c:gfp (red) and sox2 (blue) in a control embryo (A–C), a hs:atoh1a transgenic embryo (D–F) and a hs:atoh1a;hs:sox2 double transgenic embryo (G–I). Embryos were serially heat shocked at 45 and 48 hpf and fixed at 72 hpf for staining and sectioning. Shown are sections passing through the anterior end (A, D, G) or the posterior end (B, C, E, F, H, I) of the otic vesicle. Positions of the utricular macula (um), saccular macula (sm), semicircular canals (ssc) and lateral line neuromasts (nm) are indicated. White arrows (G–I) mark ectopic hair cells. Specimens in (C, F, I) are enlargements of images (B, E, H) and are shown in brightfield with fluorescence to clarify the spatial relationship between hair cells and sox2 expression.
Finally, we examined the effects of co-misexpressing atoh1a with either pax2a or the Wnt-inhibitor dkk1. Despite the involvement of pax2a in sensory development (Kwak et al., 2006; Riley et al., 1999), co-activation of hs:atoh1a and hs:pax2a after 24 hpf did not lead to production of ectopic hair cells, similar to results obtained at earlier stages (Fig. S1 E and data not shown). Wnt signaling is thought to induce non-sensory fates in the otic vesicle (Lecaudey et al., 2007; Riccomagno et al., 2005), raising the possibility that blocking Wnt via dkk1 misexpression might enhance sensory competence. However, the effects of co-activating hs:atoh1a and hs:dkk1 were indistinguishable from activating hs:atoh1a alone (data not shown). Thus, not all genes associated with medial sensory development or lateral non-sensory development can affect sensory competence under the conditions used here.
DISCUSSION
We have characterized the effects of Atoh1 misexpression and identified important cofactors that potentiate its ability to promote sensory development in the zebrafish inner ear. Misexpressing atoh1a greatly expands the spatial domain of sensory development, typically resulting in formation of a single large macula covering the ventral/medial region of the otic vesicle. Responsiveness to atoh1a misexpression is maximal during placodal through early otic vesicle stages and diminishes soon thereafter, presumably reflecting progressive differentiation of non-sensory fates in the developing inner ear. Even during placodal stages, cells in the lateral portion of the otic placode are refractory to the effects of Atoh1a. By co-misexpressing the upstream regulator fgf8, which normally predominantly affects ventral/medial (sensory and neural) fates (Alsina et al., 2004; Hatch et al., 2007; Kwak et al., 2002; Kwak et al., 2006; Vásquez-Echeverría et al., 2008), the entire otic epithelium is rendered competent to respond appropriately to atoh1a. Likewise, co-misexpressing sox2, which is normally induced in parallel with atoh1a/b in response to Fgf and Notch (Millimaki et al., 2010), globally expands sensory development during placodal stages. At later stages sox2 can still potentiate the ability of atoh1a to promote ectopic sensory development, whereas fgf8 loses this ability. These findings refine our understanding of the genetic network that influences Atoh1 function and sensory competence.
Profile of gene expression in expanded and ectopic sensory epithelia
A highly conserved feature of Atonal gene regulation in insects and vertebrates is a robust auto-amplification loop that acts during the initial stages of proneural/prosensory development (Helms et al., 2000; Sun et al., 1998; our unpublished observations). This is followed by a non-autonomous negative feedback loop in which upregulation of Delta mediates lateral inhibition/lateral specification (Millimaki et al, 2007; Woods et al., 2004). Accordingly, we find that a relatively brief pulse of transgenic atoh1a expression is sufficient to activate prolonged expression of endogenous atoh1a/b genes within 1 hour, and delta genes are activated within 2 hours (Figs. 1, 3B and data not shown). Thus efficient induction of both feedback loops accounts for why transient expression of hs:atoh1a causes a dramatic increase in both hair cells and support cells. An expanded domain of sox2 expression is seen within 6 hours and brn3c:gfp expression is detected in new hair cells within 9–10 hours (Fig. 3), a timeframe similar to the course of normal sensory development. A notable difference in ectopic sensory development, however, is that upregulation of pax2a in hair cells is not observed until 15 hours after hs:atoh1a activation. Normally pax2a expression precedes or coincides with hair cell differentiation, as both processes are initially coordinately regulated by localized Fgf signaling from the hindbrain. In contrast, the first exposure to local Fgf signaling in ectopic sensory patches comes 4–6 hours after activation of hs:atoh1a as expanded macular domains of fgf3 and fgf8 begin to form. Hence the delay in expression of pax2a could reflect the distinctive timing of Fgf signaling in ectopic sensory epithelia.
Mechanisms that promote sensory competence
Our findings implicate Fgf and Sox2 as important mediators of sensory competence in zebrafish. Neither factor alone is sufficient to promote ectopic sensory development, but they synergize with Atoh1a to promote global sensory development. How Fgf and Sox2 function in this context is not clear. Fgf signaling influences axial fates in the otic placode and vesicle (Alsina et al., 2004; Hatch et al., 2007; Kwak et al., 2002; Kwak et al., 2006; Vásquez-Echeverría et al., 2008), raising the possibility that transgenic Fgf8 expands a regional identity compatible with sensory development. Indeed, analysis of regional markers in the otic vesicle following co-misexpression of atoh1a and fgf8 indicates there is a near global expansion of ventral/medial/anterior identity, which is normally associated with the utricular macula. Similar but less pronounced changes in axial markers are seen following misexpression of atoh1a alone, including expansion of the domains of fgf3 and fgf8 expression. Nevertheless, expansion of endogenous fgf3/8 expression by hs:atoh1a is not sufficient to promote sensory development in dorsal/lateral regions of the otic vesicle. It is possible that transgenic Fgf8 boosts the overall level of Fgf signaling above the threshold required for more complete axial respecification. Transgenic Sox2 also strongly promotes sensory competence, although its role in axial specification in the inner ear is unknown. A distinct alternative model is that Fgf8 and Sox2 promote sensory competence by inducing a state of increased pluripotency. Both factors can promote formation of stem cells or multi-potent progenitors associated with early stages of tissue development (Graham et al., 2003; Nyeng et al., 2011; Tucker et al., 2010; reviewed by Lanner and Rossant, 2010). Thus, elevating Fgf8 or Sox2 could reverse early stages of differentiation of non-sensory cell types, thereby making cells more susceptible to Atoh1 activity. Whether Fgf and Sox2 are required before Atoh1 to enhance sensory competence, or instead act simultaneously with Atoh1 to form an optimal combinatorial code, remains to be established.
Studies in mouse and chick suggest that a somewhat different mechanism operates in amniotes, though there is likely to be some conservation of function as well. Misexpression of Atoh1 in rodents induces formation of ectopic sensory epithelia but only in regions close to endogenous sensory epithelia, indicating that competence to respond to Atoh1 is spatially restricted in mammals, too. Mammalian and avian sensory epithelia are normally specified by Jag1-Notch signaling (Brooker et al., 2006; Daudet et al., 2007; Kiernan et al., 2001; Kiernan et al., 2006). Notch plays a dual role in sensory development in birds and mammals, with an initial prosensory phase followed by a robust inhibitory phase associated with lateral inhibition/lateral specification (Brooker et al., 2006; Daudet and Lewis, 2004; Daudet et al., 2007). In mouse early misexpression of NICD, the intracellular domain of Notch, leads to global expression of prosensory markers Jag1 and Sox2 throughout the otic epithelium (Hartman et al., 2010; Pan et al., 2010). Under these conditions otic development arrests and mature hair cells and support cells are not observed. However, localized Cre-mediated expression of NICD at later stages results in formation of scattered ectopic sensory epithelia, even in non-sensory regions far from endogenous sensory epithelia. Thus prosensory Notch activity in mammals can reprogram virtually any otic cell to adopt a sensory fate. In chick, misexpression of NICD or Jag1 can induce formation of ectopic sensory epithelia, but not within the dorsal half of the otic vesicle (Daudet and Lewis, 2004; Neves et al., 2011). However, misexpression of the Notch target gene Sox2 can yield scattered sensory epithelia in virtually any part of the otic vesicle in chick (Neves et al., 2011). In zebrafish, activation of NICD strongly upregulates sox2 expression throughout the medial wall, but this is not sufficient to activate hair cell formation, nor does NICD activate sox2 expression in lateral cells (Millimaki et al., 2010). Despite these species-differences, Sox2 appears to be an important effector of sensory-competence in all vertebrates: Sox2 is essential for sensory development in mammals (Kiernan et al., 2005), it is sufficient to activate sporadic sensory development in chick (Neves et al., 2011), and it is sufficient to render all otic cells competent to respond to Atoh1 in zebrafish (this work). Whether Fgf signaling can also promote Sox2 expression or ectopic sensory development in mammals and birds has not been reported.
Implications for regeneration
In non-mammalian vertebrates, hair cell regeneration is efficiently mediated by support cells, which can transdifferentiate directly into hair cells or undergo asymmetric cell division to yield new hair cell-support cell pairs (Millimaki et al., 2010; Schuck and Smith, 2009; reviewed by Brignull et al., 2009; Cotanche and Kaiser, 2010). However, regeneration fails to occur in the adult mammalian cochlea because support cells lose the ability to divide or transdifferentiate during neonatal development. This transition correlates with a significant decline in Sox2 expression during cochlear maturation (Smeti et al., 2010). Because Sox2 is essential for hair cell regeneration in zebrafish (Millimaki et al., 2010), it seems likely that the decline in Sox2 levels in the mammalian cochlea contributes to loss of regenerative capacity. Interestingly, forced expression of Atoh1 in rodents can stimulate transdifferentiation of support cells and thereby foster some regeneration, though recovery of hair cells is inefficient and morphology is often abnormal (Izumikawa et al., 2005; Kawamoto et al., 2003; Shou et al., 2003; Zheng and Gao., 2000). Whether Sox2 can augment Atoh1-mediated regeneration in mammals remains an open question. In apparent contradiction, one study in mouse showed that co-misexpression of Sox2 and Atoh1 induced many fewer ectopic hair cells than did Atoh1 alone (Dabdoub et al., 2008). However that study utilized vectors designed to promote strong constitutive expression, conditions that clearly override normal feedback mechanisms. Based on our studies, we speculate that transient co-misexpression would allow endogenous Atoh1 and Sox2 promoters to respond freely to natural regulatory mechanisms and potentiate sensory development and hair cell regeneration (e.g. see Woods et al. 2004).
Highlights.
Misexpression of Atoh1a in zebrafish expands development of sensory epithelia in the otic vesicle, but the response is spatially limited and declines with age.
Early co-misexpression of Atoh1a with Fgf3, Fgf8 or Sox2 promotes global expansion of sensory development throughout the otic vesicle.
Sox2 misexpression potentiates sensory development even at later stages when Atoh1a-responsiveness normally declines.
We conclude that Sox2 and Fgf3/8 act as competence factors that facilitate Atoh1a’s ability to promote sensory development.
Supplementary Material
Expression of brn3c:gfp after serial heat shock at 14 and 16 hpf in the indicated transgenic (A–F, H, I) and non-transgenic (G) backgrounds. Embryos in G and H were also injected at the one-cell stage with 5 ng each of pax2a and pax2b morpholino (pax2a/b MO). Embryos were fixed and processed at 38 hpf. All images are dorsolateral views with anterior to the left and dorsal to the top.
Expression of brn3c:gfp (green) and Caspase 3 (red) at 44 hpf following serial heat shock at 14 and 16 hpf in a control, hs:fgf8, hs:atoh1a and hs:atoh1a;hs:fgf8 embryo. Means and standard deviations of the number of Caspase-positive cells is indicated for each background. Sample sizes and p-values from t-tests (relative to controls) were as follows: controls, n=16. hs:atoh1a, n=17, p=0.01. hs:fgf8, n=13, p=0.07. hs:atoh1a;hs:fgf8, n=20, p=0.94. Thus, only hs:atoh1a embryos showed a statistically significant, albeit small, increase in cell death. All images are dorsolateral views with anterior to the left and dorsal to the top.
Expression of various otic markers in control (A–H) and hs:fgf8 transgenic embryos (A’–H’). Embryos were serially heat shocked at 14 and 16 hpf and fixed for processing at 26 hpf. Arrowheads mark ectopic sites of expression in (A’, B’, H’). Images show dorsal views (A–B’) or dorsolateral views (C–H’), with anterior to the left. Circles outline the otic vesicle.
Expression of brn3c:gfp at 72 hpf following serial heat shock at 45 and 48 hpf in a control, hs:sox2, hs:atoh1a and hs:atoh1a;hs:sox2 embryo. White arrowheads indicate ectopic hair cells in the lateral wall. Hair cells are also evident in the anterior crista (ac), lateral crista (lc), posterior crista (pc), and neuromasts of the lateral line (nm). Images show lateral views with anterior to the left and dorsal up.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alsina B, Abelló G, Ulloa E, Henrique D, Pujades C, Giraldez F. FGF signaling is required for determination of otic neuroblasts in the chick embryo. Dev. Biol. 2004;267:119–134. doi: 10.1016/j.ydbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Bricaud O, Collazo A. The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear. J. Neuroscience. 2006;26:10438–10451. doi: 10.1523/JNEUROSCI.1025-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignull HR, Raible DW, Stone JS. Feathers and fins: Non-mammalian models for hair cell regeneration. Brain Res. 2009;1277:12–23. doi: 10.1016/j.brainres.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Kaiser CL. Hair cell fate decisions in cochlear development and regeneration. Hearing Res. 2010;266:18–25. doi: 10.1016/j.heares.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KSE, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc. Natl. Acad. Sci. 2008;105:18397–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2004;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J. Comp Neurol. 1996;365:113–128. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hans S, Christison J, Liu D, Westerfield M. Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev. Biol. 2007;7:5. doi: 10.1186/1471-213X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc. Natl. Acad. Sci. 2010;107:15792–15797. doi: 10.1073/pnas.1002827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EP, Noyes CA, Wang X, Wright TJ, Mansour SL. Fgf3 is required for dorsal patterning and morphogenesis of the inner ear epithelium. Development. 2007;134:3615–3625. doi: 10.1242/dev.006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Cunningham D, Bermingham-McDonogh O. Loss of FGFR3 leads to excess hair cell development in the mouse organ of Corti. Dev. Dyn. 2007;235:525–533. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ray CA, Bermingham-McDonogh O. Fgf20 is required for sensory epithelial specification in the developing cochlea. J. Neurosci. 2008;28:5991–5998. doi: 10.1523/JNEUROSCI.1690-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Huang Y, Chi F, Han Z, Yang J, Gao W, Li Y. New ectopic vestibular hair cell-like cells induced by Math1 gene transfer in postnatal rats. Brain Res. 2009;1276:31–38. doi: 10.1016/j.brainres.2009.04.036. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 2005;3:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M, Kelley MW. Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development. 2007;134:3021–3029. doi: 10.1242/dev.02874. [DOI] [PubMed] [Google Scholar]
- Jones JM, Montcouquiol M, Dabdoub A, Woods C, Kelley MW. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during development of the organ of Corti. J. Neurosci. 2006;26:550–558. doi: 10.1523/JNEUROSCI.3859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowett T, Yan YL. Double fluorescent in situ hybridization to zebrafish embryos. Trends Genet. 1996;12:387–389. doi: 10.1016/s0168-9525(96)90091-8. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J. Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Ahituv N, Fuchs H, Balling R, Avraham KB, Steel KP, de Angelis MH. The Notch ligand Jagged1 is required for inner ear sensory development. Proc. Natl. Acad. Sci. 2001;98:3873–3878. doi: 10.1073/pnas.071496998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KKH, Tang ASP, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KSE. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genetics. 2006;2(1):e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak S-J, Phillips B, T. Heck R, Riley BB. An expanded domain of fgf3 expression in the hindbrain of zebrafish valentino mutants results in mis-patterning of the otic vesicle. Development. 2002;129:5279–5287. doi: 10.1242/dev.129.22.5279. [DOI] [PubMed] [Google Scholar]
- Kwak S-J, Vemaraju S, Moorman SJ, Zeddies D, Popper AN, Riley BB. Zebrafish pax5 regulates development of the utricular macula and vestibular function. Dev. Dyn. 2006;235:3026–3038. doi: 10.1002/dvdy.20961. [DOI] [PubMed] [Google Scholar]
- Lecaudey V, Ulloa E, Anselme I, Stedman A, Schneider-Maunoury S, Pujades C. Role of the hindbrain in patterning the otic vesicle: A study of the zebrafish vhnf1 mutant. Dev. Biol. 2007;303:134–143. doi: 10.1016/j.ydbio.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Lanner F, Rossant J. The role of FGF/Erk signaling in pluripotent stem cells. Development. 2010;137:3351–3360. doi: 10.1242/dev.050146. [DOI] [PubMed] [Google Scholar]
- Mackereth MD, Kwak J-J, Fritz A, Riley BB. Zebrafish pax8 is required for otic placode induction and plays a redundant role with Pax2 genes in the maintenance of the otic placode. Development. 2005;132:371–382. doi: 10.1242/dev.01587. [DOI] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Dhason MS, Riley BB. Zebrafish atoh1 genes: Classic proneural activity in the inner ear and regulation by Fgf and Notch. Development. 2007;134:295–305. doi: 10.1242/dev.02734. [DOI] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Riley BB. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev. Bio. 2010;338:262–269. doi: 10.1016/j.ydbio.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J, Parada C, Chamizo M, Giráldez F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development. 2011;138:735–744. doi: 10.1242/dev.060657. [DOI] [PubMed] [Google Scholar]
- Nyeng P, Bjerke MA, Norgaard GA, Qu X, Kobberup S, Jensen J. Fibroblast growth factor 10 represses cell differentiation during establishment of the intestinal progenitor niche. Dev. Biol. 2011;349:20–34. doi: 10.1016/j.ydbio.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc. Natl. Acad. Sci. 2010;107:15798–15803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BT, Bolding K, Riley BB. Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev. Biol. 2001;235:351–365. doi: 10.1006/dbio.2001.0297. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of Fibroblast Growth Factor Receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev. Dyn. 2007;236:1905–1917. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quinones H, Jayaseba CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–4415. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes & Dev. 2005;19:1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BB, Chiang M-Y, Farmer L, Heck R. The deltaA gene of zebrafish mediates lateral inhibition of hair cells in the inner ear and is regulated by pax2.1. Development. 1999;126:5669–5678. doi: 10.1242/dev.126.24.5669. [DOI] [PubMed] [Google Scholar]
- Schuck JB, Smith ME. Cell proliferation follows acoustically-induced hair bundle loss in the zebrafish saccule. Hearing Res. 2009;253:67–76. doi: 10.1016/j.heares.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J, Zheng JL, Gao W-Q. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol. Cell. Neurosci. 2003;23:169–179. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Smeti I, Savary E, Capelle V, Hugnot JP, Uziel A, Zine A. Expression of candidate markers for stem/progenitor cells in the inner ears of developing and adult GFPA and nestin promoter-GFP transgenic mice. Gene Expression Patterns. 2010;11:22–32. doi: 10.1016/j.gep.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jan LY, Jan YN. Transcriptional regulation of atonal during development of the Drosophila peripheral nervous system. Development. 1998;125:3731–3740. doi: 10.1242/dev.125.18.3731. [DOI] [PubMed] [Google Scholar]
- Tucker ES, Lehtinen MK, Maynard T, Xirsinger M, Dulac C, Rawson N, Penvy L, LaMantia A-S. Proliferative and trancscriptional identity of distinct classes of neural precursors in the mammalian olfactory epithelium. Development. 2010;137:2471–2481. doi: 10.1242/dev.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez-Echeverría C, Domininguez-Frutos E, Charnay P, Schimmang T, Pujades C. Analysis of mouse kreisler mutants reveals new roles of hindbrain-derived signals in the establishment of the otic neurogenic domain. Dev. Biol. 2008;322:167–178. doi: 10.1016/j.ydbio.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat. Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Xiao T, Roeser T, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 2005;132:2955–2967. doi: 10.1242/dev.01861. [DOI] [PubMed] [Google Scholar]
- Yang H, Xie X, Deng M, Chen X, Gan L. Generation and characterization of Atoh1-Cre knock-in mouse line. Genesis. 2010;48:407–413. doi: 10.1002/dvg.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of brn3c:gfp after serial heat shock at 14 and 16 hpf in the indicated transgenic (A–F, H, I) and non-transgenic (G) backgrounds. Embryos in G and H were also injected at the one-cell stage with 5 ng each of pax2a and pax2b morpholino (pax2a/b MO). Embryos were fixed and processed at 38 hpf. All images are dorsolateral views with anterior to the left and dorsal to the top.
Expression of brn3c:gfp (green) and Caspase 3 (red) at 44 hpf following serial heat shock at 14 and 16 hpf in a control, hs:fgf8, hs:atoh1a and hs:atoh1a;hs:fgf8 embryo. Means and standard deviations of the number of Caspase-positive cells is indicated for each background. Sample sizes and p-values from t-tests (relative to controls) were as follows: controls, n=16. hs:atoh1a, n=17, p=0.01. hs:fgf8, n=13, p=0.07. hs:atoh1a;hs:fgf8, n=20, p=0.94. Thus, only hs:atoh1a embryos showed a statistically significant, albeit small, increase in cell death. All images are dorsolateral views with anterior to the left and dorsal to the top.
Expression of various otic markers in control (A–H) and hs:fgf8 transgenic embryos (A’–H’). Embryos were serially heat shocked at 14 and 16 hpf and fixed for processing at 26 hpf. Arrowheads mark ectopic sites of expression in (A’, B’, H’). Images show dorsal views (A–B’) or dorsolateral views (C–H’), with anterior to the left. Circles outline the otic vesicle.
Expression of brn3c:gfp at 72 hpf following serial heat shock at 45 and 48 hpf in a control, hs:sox2, hs:atoh1a and hs:atoh1a;hs:sox2 embryo. White arrowheads indicate ectopic hair cells in the lateral wall. Hair cells are also evident in the anterior crista (ac), lateral crista (lc), posterior crista (pc), and neuromasts of the lateral line (nm). Images show lateral views with anterior to the left and dorsal up.