Abstract
Background
Stroke occurs in 5–10% of children with sickle cell anemia (SCA) and has a high (>50%) risk of recurrence without therapy. Chronic monthly erythrocyte transfusions effectively prevent recurrent stroke, but their long term use is limited by serious side effects, including iron overload. An alternative to transfusion for secondary stroke prevention in SCA is needed, especially one that also improves the management of iron overload.
Methods
Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) is an NHLBI-sponsored Phase III multicenter randomized controlled clinical trial for children with SCA, stroke, and iron overload (NCT00122980). The primary goal of SWiTCH is to compare 30 months of alternative therapy (hydroxyurea and phlebotomy) with standard therapy (transfusions and chelation) for the prevention of secondary stroke and reduction of transfusional iron overload.
Discussion
SWiTCH has several distinctive study features including novel methodological and design components: (1) composite primary endpoint including both stroke recurrence rate and iron burden; (2) non-inferiority design with an ‘acceptable’ increased stroke risk; (3) transfusion goals based on current academic community practices; (4) special oversight for the enrollment and randomization process ; (5) overlap treatment period within the alternative therapy arm; (6) masking of the overall trial Principal Investigator to treatment results; (7) inclusive independent stroke adjudication process for all suspected new neurological events; and (8) periodic therapeutic phlebotomy program to alleviate iron overload.
Conclusion
Investigation of alternative treatments in SWiTCH could lead to changes in the management of cerebrovascular disease for young patients with SCA, stroke, and iron overload.
Keywords: stroke, iron overload, hydroxyurea
INTRODUCTION
Stroke is a devastating clinical complication that occurs in young patients with sickle cell anemia (SCA). Even with prompt recognition and emergency management, the sequelae of stroke are significant, and most affected children have substantial residual motor and cognitive deficits. Because over 50,000 persons have SCA and approximately 2,000 additional affected babies are born annually in the United States, the occurrence and management of stroke in children with SCA has broad implications for national health care and medical resources. Worldwide this issue is even more compelling, due to limited resources and treatment options within developing countries.
Stroke occurs in 5–10% of patients with SCA by adulthood [1,2], and has a very high (>50%) risk of recurrence without therapy [3]. To prevent recurrent (secondary) stroke, children receive chronic monthly erythrocyte transfusions that must be continued indefinitely to be effective [4,5] and have substantial side-effects limiting their long-term use. Although quite safe in the US and Europe, chronic transfusions can be problematic especially in developing countries: transfusions transmit infectious agents [6], often lead to erythrocyte alloantibody or autoantibody formation even with extended phenotype-matched blood [7], and inevitably result in iron overload [8]. Transfusion-acquired iron overload is increasingly associated with morbidity and mortality for teenagers and young adults with SCA and previous stroke [9]. Although current data derive more from studies in thalassemia rather than SCA, transfusional iron overload has been associated with poor growth and development, chronic organ damage including hepatic fibrosis and cirrhosis, diabetes mellitus, cardiac arrhythmias, and early sudden death. Chelation therapy with parenteral deferoxamine (Desferal®) and more recently oral deferasirox (Exjade®) helps prevent iron accumulation but medication non-adherence is common [10]. Among investigators, families, and patients, an alternative to transfusion prophylaxis to reduce the risk of secondary stroke is desired, especially one that also addresses the important issue of transfusion associated iron overload.
Over the past two decades, the laboratory and clinical efficacy of hydroxyurea has been demonstrated for both children and adults with SCA [11–14]. Hydroxyurea helps prevent painful events and acute chest syndrome (ACS), but its utility for cerebrovascular disease and specifically to reduce secondary stroke risk remains unproven. Single-institution pilot data suggest that hydroxyurea has efficacy for reducing the risk of stroke recurrence in children with SCA, with a recurrent stroke rate only slightly higher than the rate with chronic transfusions [15,16]. After transfusions are discontinued, repeated phlebotomy can be used to reduce iron burden. The ability to discontinue transfusions and use hydroxyurea for prevention of secondary stroke, coupled with the removal of excess iron by serial phlebotomy, represents a potentially significant improvement in the current management of young patients with SCA and stroke. Furthermore, if hydroxyurea shows efficacy for the prevention of secondary stroke, it may also warrant investigation for children with SCA at risk for developing primary stroke, such as those with silent infarcts and/or elevated transcranial Doppler (TCD) flow velocities. The SWiTCH trial addresses a clinically important issue; moreover, it is the first NIH-funded prospective randomized clinical trial in SCA to compare hydroxyurea to transfusion therapy. Several distinctive features of the SWiTCH trial design make this an important and potentially pivotal randomized clinical trial for young patients with SCA.
METHODS
Rationale for hydroxyurea in stroke prevention. Hydroxyurea has documented hematological efficacy for both children and adults with SCA. In clinical trials, hydroxyurea led to significant increases in fetal hemoglobin (%HbF), hemoglobin concentration, and mean corpuscular volume (MCV), along with significant decreases in the white blood cell (WBC), neutrophil, reticulocyte, and platelet counts [11,14]. In a placebo-controlled trial, hydroxyurea also had proven clinical efficacy for reducing the frequency of acute symptomatic vaso-occlusion, including painful events and acute chest syndrome [12]. Increasing the HbF level is believed to be the most critical mechanism of action for hydroxyurea in SCA; additional HbF within sickle erythrocytes inhibits intracellular HbS polymerization that leads to in vivo sickling [17]. Moreover, lowering the WBC count may also provide a therapeutic effect of hydroxyurea, as elevated WBC has been associated with an increased risk of developing vaso-occlusive complications including primary stroke [2]. The myelosuppressive effects of hydroxyurea may, therefore, be an additional mechanism by which the drug provides benefit for children with SCA, along with altered cellular adhesion molecules, macrocytosis with reduced viscosity, and possibly local nitric oxide release [18].
In the clinical setting of secondary stroke prevention, hydroxyurea therapy and chronic transfusions have similar beneficial effects on circulating erythrocytes. Both interventions increase the hematocrit, reduce the number of erythrocytes able to undergo sickling, lower the number of adhesive erythrocytes, and improve the rheology and flow characteristics of the circulating erythrocytes. Unlike chronic transfusions, hydroxyurea therapy also lowers the WBC count and changes the rheological characteristics of the endogenous erythrocytes and leukocytes. The clinical efficacy of hydroxyurea is also similar to transfusions in the setting of stroke: Table I lists the published incidences and rates of recurrent stroke in children with SCA, analyzed by therapeutic intervention following the first stroke event. Chronic transfusions significantly lower the recurrent stroke rate to 2–6 events per 100 patient-years. Hydroxyurea prophylaxis also lowers the recurrent stroke rate, but with a slightly higher estimate of 3–7 events per 100 patient-years [15,16].
Table I.
Incidence and rate of secondary (recurrent) stroke in children with SCA, by therapeutic intervention following the first stroke event.
| Author | Year [Reference] | # patients | Recurrence (%) | Secondary stroke rate per 100 patient-years |
|---|---|---|---|---|
| Observation alone | ||||
| Powars et al. | 1980 [19] | 19 | 63 | 13 |
| Moohr et al. | 1982 [20] | 14 | 83 | 54 |
| Russell et al. | 1984 [21] | 10 | 90 | 108 |
| Balkaran et al. | 1992 [22] | 15 | 47 | 25 (estimated) |
| Chronic transfusions | ||||
| Moohr et al. | 1982 [20] | 7 | 0 | 0 |
| Russell et al. | 1984 [21] | 20 | 14 | 2.0 (estimated) |
| Pegelow et al. | 1995 [23] | 61 | 15 | 4.8 |
| Ohene-Frempong et al. | 1998 [2] | 72 | 14 | 6.4 |
| Scothorn et al. | 2002 [24] | 137 | 23 | 2.2 |
| Ware et al. | Unpublished | 44 | 11 | 3.3 |
| Transfusion discontinuation | ||||
| Wilimas et al. | 1980 [4] | 10 | 70 | Unavailable |
| Moohr et al. | 1982 [20] | 7 | 14 | 10 |
| Wang et al. | 1991 [5] | 10 | 50 | 51 (estimated) |
| Rana et al. | 2001 [25] | 9 | 0 | 0 |
| Change from transfusions to hydroxyurea prophylaxis | ||||
| Ware et al. * | 1999 [15] | 15 | 33 | 6.8 |
| Ware et al. † | 2004 [16] | 21 | 10 | 3.1 |
| Ware et al. # | 2004 [16] | 36 | 19 | 5.0 |
subjects who had an abrupt discontinuation of transfusions,
subjects with an overlap period of transfusions and hydroxyurea, and
all subjects who switched from chronic transfusions to hydroxyurea therapy for secondary stroke prophylaxis.
Justification of an increased acceptable stroke risk. Early in protocol development, twenty SWiTCH clinical investigators reached a consensus on two important points that influenced the trial design. First, interest in hydroxyurea as an alternative for secondary stroke prevention was strongly linked to the possibility of improving management of iron overload. This consensus point led to a unique composite endpoint combining stroke recurrence rate and iron overload. Second, hydroxyurea did not need to have equal efficacy for secondary stroke prevention as transfusions, if the management of iron overload could be improved. Pilot data suggested that hydroxyurea has a similar, but not necessarily equivalent, efficacy for preventing recurrent stroke in SCA [15,16]. Accordingly, SWiTCH was not designed as an equivalence trial between treatment arms, but as a non-inferiority trial that allows a small but increased number of recurrent stroke events using hydroxyurea, with the prospect of also reducing iron overload. The acceptance among the investigators of a modestly increased stroke risk is a key feature of the non-inferiority design in SWiTCH.
Management of iron overload. The use of chelation for transfusional iron overload is problematic in clinical practice, due to cost and non-adherence. For SWiTCH subjects who randomize to continued transfusions, daily chelation is available as oral deferasirox provided free of charge for study subjects by Novartis Pharmaceuticals, Inc. For subjects who randomize to hydroxyurea, iron overload is managed by serial phlebotomy, thereby avoiding the issues of chelation cost and non-adherence.
Study Design. SWiTCH is a Phase III randomized clinical trial comparing alternative therapy to standard therapy for both the prevention of secondary stroke AND the reduction of iron overload in pediatric patients with SCA and previous stroke, who have been on chronic transfusions for at least 18 months. Subjects from 26 sickle cell centers across the United States were enrolled to achieve at least 65 randomized subjects per treatment arm. Each peripheral clinical site is led by a pediatric hematologist (Clinical Investigator) with experience in the management of stroke in SCA and the use of hydroxyurea in this young patient population. SWiTCH uses hydroxyurea under an IND (69,909) for use in children with SCA and secondary stroke prophylaxis.
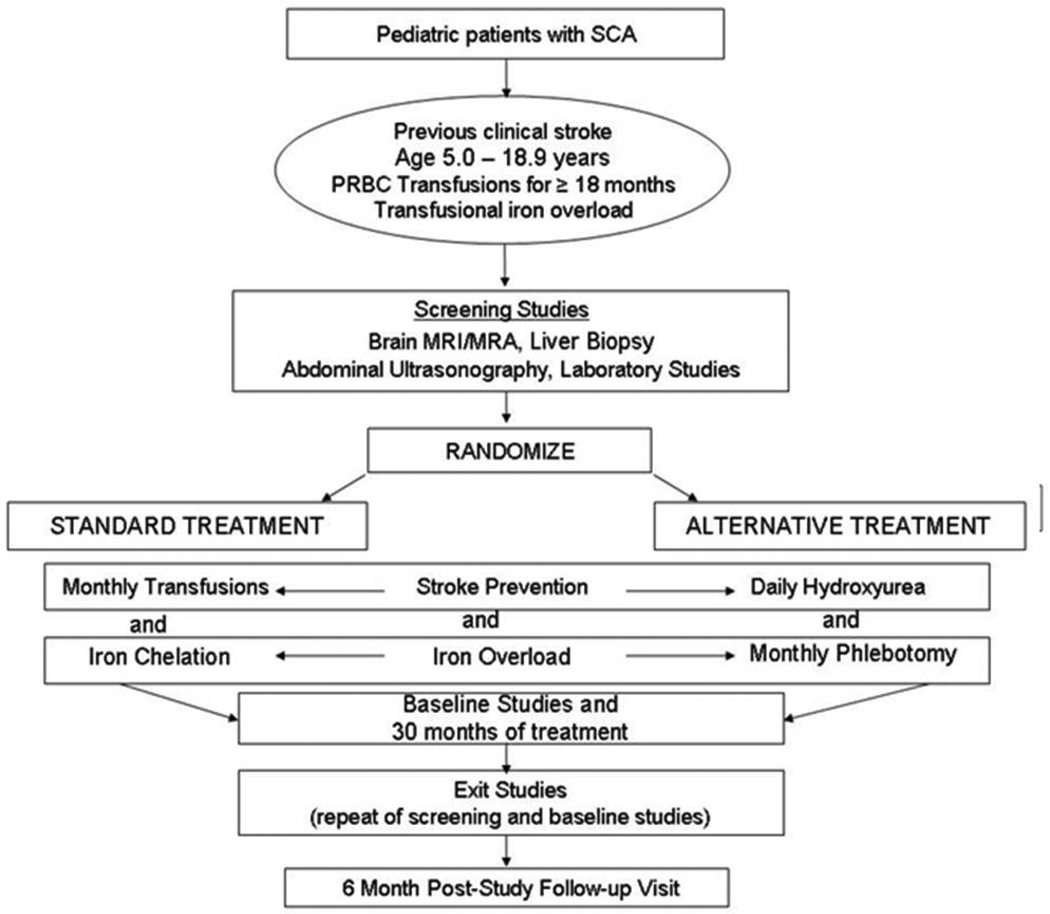
Figure 1 provides an overview of the SWiTCH trial. After enrollment, subjects underwent screening tests including non-contrast brain MRI/MRA, liver biopsy to measure liver iron content and histology, formal neurological examination by a pediatric neurologist, abdominal ultrasonography, neurocognitive testing including quality of life evaluation, and laboratory studies before randomization to either Standard Treatment (transfusions/chelation) or Alternative Treatment (hydroxyurea/phlebotomy). Subjects then receive study treatment for 30 months, after which all evaluations are repeated. At each clinic visit, adherence with all study treatments is emphasized and medication compliance is monitored through pharmacy logs and direct questioning. A single safety follow-up clinic visit is captured 6 months after study treatment is completed. During the treatment phase all possible stroke events are adjudicated within approximately 1 month of symptoms; positive decisions mark a primary endpoint and lead to cessation of study treatment for that subject. The SWiTCH inclusion and exclusion criteria are listed in Supplemental Table I.
Figure 1.
Overview of the Phase III SWiTCH randomized clinical trial (NCT00122980).
Main study objective and primary endpoint. The main objective for the SWiTCH trial is to compare alternative therapy (hydroxyurea treatment with repeated phlebotomy) to standard therapy (monthly erythrocyte transfusions with daily iron chelation) for the prevention of secondary stroke and reduction of transfusional iron overload in pediatric subjects with SCA and previous stroke. In order for the alternative treatment regimen to be declared better than the standard treatment regimen, the hydroxyurea-phlebotomy group must have both a similar rate of recurrent stroke and a statistically significant decrease in liver iron levels.
The primary endpoint for the SWiTCH trial has two distinct components, related both to the important issue of secondary stroke prevention and the management of transfusional iron overload. This composite primary endpoint for SWiTCH is therefore occurrence of a secondary stroke during the 30-month treatment period and quantitative liver iron level change-from-baseline, as measured by liver biopsy at baseline and at 30 months or exit from the study.
Secondary study objectives and endpoints. The secondary objectives of this study include the comparison of alternative therapy to standard therapy for effects on quality of life, the frequency of non-stroke neurological events and other sickle cell-related events, and growth and development. Specific secondary endpoints of the SWiTCH trial reflect both efficacy and safety, and include the following: non-stroke neurological events (isolated radiological findings on TCD, brain MRI, or MRA); non-neurological sickle cell events (vaso-occlusive pain, ACS, splenic sequestration, priapism, hospitalization, death); Quality of Life Assessment (Child Health Questionnaire [CHQ-50] and PedsQL); functional evaluation (Barthel Index); neurocognitive decline (Woodcock Johnson Tests of Cognitive Abilities – III (WJ-C), Woodcock Johnson Tests of Achievement – III (WJ-III), Conners’ Continuous Performance Test – II (CPT-II); growth and development (height, weight, Tanner staging); transfusion-related complications (erythrocyte autoantibody and alloantibody formation, infections, transfusion reactions); chelation-related complications (gastrointestinal symptoms, renal effects, allergic reactions, ophthalmological toxicity, ototoxicity, non-compliance); hydroxyurea-related complications (excessive myelosuppression, non-compliance); phlebotomy-related complications (anemia, hypotension, syncope); liver biopsy-related complications (bleeding, pain); and adverse events and serious adverse events.
Statistical hypotheses and analyses. The primary statistical analyses of efficacy and safety will be performed on the Intent to Treat Population, which consists of all subjects who were randomized to a study treatment and for whom outcome data are available. Secondary safety and efficacy analyses will also be performed, using the Per Protocol Population, which consists of all subjects who meet the study’s eligibility criteria and who have no major protocol violations, as determined by a treatment-masked data review committee after database lock and prior to construction of final analysis datasets. The following statistical plan, which also includes two interim analyses, has been approved by the NHLBI-appointed Data and Safety Monitoring Board.
The primary alternative hypothesis to be tested in this study is as follows: The probability that a subject in the alternative therapy treatment group will experience a secondary stroke during the 30-month treatment period is less than or equal to the corresponding probability for the standard treatment group plus 0.20 AND there will be a statistically significant decrease in liver iron levels in the Alternative Treatment group compared to the Standard Treatment.
The component (stroke and iron) hypotheses and the overall composite hypotheses to be tested in this study can be expressed precisely as follows:
Let:
πH denote the probability that a subject in the alternative treatment group will experience a secondary stroke during the 30-month observation period, and
πT denote the probability that a subject in the standard treatment group will experience a secondary stroke during the 30-month observation period.
The null and alternative hypotheses for the STROKE component of the primary endpoint are
The STROKE hypothesis will be tested using the following Z test statistic:
where pT and pH are the Kaplan-Meier estimates of πT and πH, respectively, and the variance estimates v(pT) and v(pH) are computed using Greenwood’s estimator:
Let:
μHCB denote the normal distribution mean for the alternative treatment group change-from-baseline of log-transformed values, i.e., MEAN[log(study-exit Fe) – log(baseline Fe)], and
μTCB denote the normal distribution mean for the standard treatment group change-from-baseline of log-transformed values, i.e., MEAN[log(study-exit Fe) – log(baseline Fe)].
The null and alternative hypotheses for the LIVER iron component of the primary endpoint are
The IRON null hypothesis will be tested using a one-tail t-test with unequal variances, adjusted for baseline iron concentration and age. Exit iron (Fe) is assessed by liver biopsy iron concentration.
The component hypotheses will be combined into the following OVERALL composite set of hypotheses:
The STROKE and IRON hypotheses will each be tested separately at the alpha = 0.05 level using standard methods. The OVERALL null hypothesis will be rejected in favor of the OVERALL alternative hypothesis only if both the STROKE and IRON null hypotheses are rejected.
DISCUSSION
The impetus for the design of the multicenter SWiTCH trial is the current management dilemma for children with SCA and stroke. Indefinite chronic transfusion therapy has benefits for reducing the risk of recurrent stroke, but also has numerous complications including inevitable transfusion-associated iron overload that is arguably the most difficult problem to address. Treatment with daily chelation is problematic and expensive, and non-adherence is very common. Transfusion therapy to prevent recurrent stroke in SCA without proper management of iron overload is inadequate and should not be considered acceptable.
Since the initial reports of success over 30 years ago [27,28], therapeutic intervention to reduce the risk of secondary stroke in children with SCA has consisted of chronic erythrocyte transfusions, designed to increase the circulating hemoglobin concentration and reduce %HbS. Monthly transfusions, typically provided with a goal of maintaining HbS <30% are effective in preventing stroke recurrence [20,21,23,24]. Transfusions reduce the incidence of secondary stroke to 10–15% and reduce the recurrence rate to 2–6 events per 100 patient-years, representing about 90% reduction in the event rate compared to no intervention (Table I). The published risk of recurrent stroke on hydroxyurea prophylaxis is not equivalent, but only slightly higher at 3–7 events per 100 patient-years [16]. The allowed acceptable stroke risk using hydroxyurea, as agreed upon by the study investigators, helps form the trial’s non-inferiority margin.
Within SWiTCH, there are several distinctive study features including novel elements in the design and treatment plan deserving specific mention and discussion. First is the composite primary endpoint that includes both stroke recurrence rate and iron burden. The dual objectives of secondary stroke prevention and improved management of iron overload are inextricably linked in this clinical setting; this realization leads naturally to the unique composite (“AND”) primary endpoint proposed for the SWiTCH trial. SWiTCH is not simply a head-to-head trial of hydroxyurea versus transfusions for prevention of secondary stroke; only the accompanying opportunity to improve the management of iron overload makes the study worth doing. Consequently, the SWiTCH trial primary endpoint contains two equal components: stroke recurrence and iron overload, both of which must be successful for overall trial success.
Another important feature of the study is the non-inferiority design with an acceptable increased stroke risk. A non-inferiority margin that represents an acceptable stroke risk, given a significantly reduced iron burden, within the Alternative Therapy arm was determined by repeated discussions and consensus expert opinion among the investigators before the trial was started. All investigators agreed that the increased stroke risk with hydroxyurea treatment must be < 0.2 (20%). This inferiority margin is included in the statistical analysis as the upper confidence limit.
The transfusion goals were selected based on current academic community practices. Although the time-honored target of chronic transfusions is to maintain the HbS level ≤30%, this goal is hard to achieve in actual clinical practice and is sometimes relaxed to ≤50% after several years of transfusions. Because monthly transfusions serve as the Standard Treatment Arm, the investigators elected to provide transfusions as currently used in their academic practice. A pre-trial survey of the SWiTCH investigators indicated that the average HbS was about 34% for their patients receiving secondary stroke prophylaxis, with substantial excursions above 40% [26]. Some local discretion is allowed, therefore, with regard to the transfusion type (simple, partial exchange, or erythrocytapheresis), interval (4±1 week), and volume. The pre-transfusion target goal remains at 30% HbS, but protocol deviations occur only when the HbS level exceeds 45%.
Special oversight is provided for the enrollment eligibility and randomization process. To avoid the possibility that a subject could enroll in SWiTCH without being truly eligible and potentially begin screening studies that impart risk to the subject (e.g., liver biopsy), the study used an electronic gating system to confirm entry criteria. For each potential subject, clinical site personnel accessed the Enrollment Eligibility function (EEF) of the screening, enrollment, and randomization system (RhoRAND™); they responded to the enrollment criteria questions based on the subject’s medical history. Because these were yes/no responses, no prior informed consent was required. Upon qualifying a subject via the EEF, the system assigned a SWiTCH Subject ID number, and site staff obtained consent. Afterward, site staff completed the RhoRAND Enrollment Verification Function (EVF) by entering the actual medical history values (i.e., protected health information and specific medical information including laboratory results); these values were evaluated by computer algorithms to further confirm that subjects qualified for the study. This two-tiered strategy allows us to demonstrate the effectiveness of subjecting inclusion/exclusion data to algorithmic confirmation. Once a subject was qualified for enrollment, screening assessments were completed and entered into the electronic case report form. These data again received algorithmic checks to confirm that subjects’ newly acquired screening data qualified them for randomization. Additionally, the screening data were reviewed by the study’s Medical Monitor and the overall trial Principal Investigator (PI) to confirm continued suitability for randomization. Only after subjects were approved for randomization, could they actually be randomized on the study.
For safety purposes, an overlap treatment period is provided within the Alternative Treatment Arm. The subjects who randomize to alternative treatment do not abruptly discontinue transfusions, since this potentially increases their stroke risk to an unsafe level. Instead, these subjects have an overlap period of approximately 6–9 months, during which time the hydroxyurea dose is be escalated to the maximum tolerated dose (MTD), which is defined by specific hematological toxicity criteria within the protocol. Only after a subject reaches a stable hydroxyurea MTD are transfusions discontinued.
Another key feature of the SWiTCH trial design is the masking of the Principal Investigator to treatment results. By the nature of the study treatments used to prevent recurrent stroke (blood transfusions versus hydroxyurea), it is impractical to make SWiTCH a blinded (masked) study. However, at the request of the NHLBI-appointed DSMB, the overall trial PI (REW) is masked to all study treatment data including primary endpoint determinations. This request is to help prevent any possible bias or influence on study conduct or results by the PI. In addition, all investigators at the peripheral clinical sites are masked to study treatment results outside of their own clinical center.
The inclusive independent stroke adjudication process for all suspected new neurological events is a novel feature of the study. Stroke recurrence is a primary study endpoint but also is a critical safety endpoint for the SWiTCH trial. Accordingly, it was necessary to develop an inclusive process by which all potential stroke events were recognized and systematically adjudicated using a standardized protocol and masked consultants. Subjects who develop any acute neurological change are promptly evaluated for possible stroke. In addition, site personnel are provided with a written script to use at each interval clinic visit, to ensure that subjects and families are asked each month about any signs and symptoms of stroke. After a new neurological event is suspected, the stroke adjudication process begins. The clinical history and neurological exam are reviewed by 3 independent neurologists without knowledge of the imaging findings. Simultaneously, the radiological evaluation is reviewed by 3 independent masked neuroradiologists without knowledge of the clinical history or neurological examination. Only after their independent consensus opinions are formed are these two opinions reconciled into a final stroke adjudication decision; a diagnosis of stroke requires new neurological findings with corresponding radiological changes.
Finally, a periodic therapeutic phlebotomy program is offered to alleviate iron overload. The Alternative Treatment Arm includes serial phlebotomy procedures (10 mL/kg/procedure) to remove excess iron. Although phlebotomy seems paradoxical for anemic patients, it is well tolerated in many situations of iron overload including SCA [15,16]. Because some centers did not use this therapeutic option before joining the SWiTCH trial, standard operating procedures and training were provided so local nursing staff could perform this monthly procedure on subjects randomized to the Alternative Treatment Arm.
Additional challenges for the SWiTCH trial include the following: the likelihood of some hydroxyurea non-adherence as subjects change from a monthly administered transfusion stroke prophylaxis to a daily oral medication; standardizing liver biopsy procedures across multiple clinical sites, allowing for local discretion for the service performing the biopsy and the pre-procedural preparation; and the exclusion criterion of siblings that impacts several families with multiply affected children.
Taken together, the SWiTCH trial offers a novel and creative approach to the management of children with SCA who are on chronic transfusion therapy. Almost all children with SCA and stroke receive erythrocyte transfusions indefinitely, and at some clinical sites more subjects have died from complications of iron overload than from the recurrent stroke. Young patients with SCA need an alternative therapy both to prevent secondary stroke and to reduce their iron burden. Pilot data suggest that hydroxyurea may effectively prevent secondary stroke, while serial phlebotomy can lead to complete resolution of iron overload. The significance of the uniquely designed SWiTCH trial for children with SCA is substantial: study results could lead to important changes in the approach to the prevention of recurrent cerebrovascular disease in these patients. A similar investigation about the potential use of hydroxyurea for children on chronic transfusions to reduce primary stroke risk is warranted, especially the growing cohort of children with silent infarcts or abnormal intracranial TCD flow velocities.
Supplementary Material
Acknowledgments
Funding Sources: NHLBI U01 HL078787 (REW) and U01 HL078987 (RWH); deferasirox study treatment was provided by Novartis Inc.
Footnotes
No authors have conflicts to declare.
CONFLICT OF INTEREST
No author has a conflict of interest to report.
REFERENCES
- 1.Balkaran B, Char G, Morris JS, et al. Stroke in a cohort of patients with homozygous sickle cell disease. J Pediatr. 1992;120:360. doi: 10.1016/s0022-3476(05)80897-2. [DOI] [PubMed] [Google Scholar]
- 2.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood. 1998;91:288. [PubMed] [Google Scholar]
- 3.Powars D, Wilson B, Imbus C, Pegelow C, Allen J. The natural history of stroke in sickle cell disease. Am J Med. 1978;65:461. doi: 10.1016/0002-9343(78)90772-6. [DOI] [PubMed] [Google Scholar]
- 4.Wilimas J, Goff JR, Anderson JR, Jr, Langston JW, Thompson E. Efficacy of transfusion therapy for one to two years in patients with sickle cell disease and cerebrovascular accidents. J Pediatr. 1980;96:205. doi: 10.1016/s0022-3476(80)80803-1. [DOI] [PubMed] [Google Scholar]
- 5.Wang WC, Kovnar EH, Tonkin ILe, et al. High risk of recurrent stroke after discontinuance of five to twelve years of transfusion therapy in patients with sickle cell disease. J Pediatr. 1991;118:377. doi: 10.1016/s0022-3476(05)82150-x. [DOI] [PubMed] [Google Scholar]
- 6.Dodd RY. Current risk of transfusion transmitted infections. Curr Opin Hematol. 2007;14:671. doi: 10.1097/MOH.0b013e3282e38e8a. [DOI] [PubMed] [Google Scholar]
- 7.Castellino SM, Combs MR, Zimmerman SA, et al. Erythrocyte autoantibodies in pediatric patients receiving transfusion therapy: Frequency, characteristics, and significance. Br J Haematol. 1999;104:189. doi: 10.1046/j.1365-2141.1999.01127.x. [DOI] [PubMed] [Google Scholar]
- 8.Harmatz P, Butensky E, Quirolo K, et al. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood. 2000;96:76. [PubMed] [Google Scholar]
- 9.Ballas SK. Iron overload is a determinant of morbidity and mortality in adult patients with sickle cell disease. Sem Hematol. 2001;38:30. doi: 10.1016/s0037-1963(01)90058-7. [DOI] [PubMed] [Google Scholar]
- 10.Treadwell MJ, Law AW, Sung J, et al. Barriers to adherence of deferoxamine usage in sickle cell disease. Pediatr Blood Cancer. 2005;44:500. doi: 10.1002/pbc.20290. [DOI] [PubMed] [Google Scholar]
- 11.Charache S, Dover GJ, Moore RD, et al. Hydroxyurea: Effects on hemoglobin F production in patients with sickle cell disease. Blood. 1992;79:2555–2565. [PubMed] [Google Scholar]
- 12.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on frequency of painful crisis in sickle cell anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 13.Kinney TR, Helms RW, O’Branski EE, et al. Safety of hydroxyurea in children with sickle cell anemia: Results of the HUG-KIDS study, a Phase I/II trial. Blood. 1999;94:1550–1554. [PubMed] [Google Scholar]
- 14.Zimmerman SA, Schultz WH, Davis JS, Pickens CV, Mortier NA, Howard TA, Ware RE. Sustained long-term hematological efficacy of hydroxyurea therapy at maximum tolerated dose for children with sickle cell disease. Blood. 2004;103:2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 15.Ware RE, Zimmerman SA, Schultz WH. Hydroxyurea as an alternative to blood transfusions for the prevention of recurrent stroke in children with sickle cell disease. Blood. 1999;94:3022. [PubMed] [Google Scholar]
- 16.Ware RE, Zimmerman SA, Sylvestre PB, et al. Prevention of secondary stroke and resolution of transfusional iron overload in children with sickle cell anemia using hydroxyurea and phlebotomy. J Pediatr. 2004;145:346. doi: 10.1016/j.jpeds.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 17.Platt OS. Hydroxyurea for the treatment of sickle cell anemia. New Engl J Med. 2008;358:1362. doi: 10.1056/NEJMct0708272. [DOI] [PubMed] [Google Scholar]
- 18.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powars D, Imbus C. Cerebral vascular accidents in sickle cell anemia. Texas Reports Biol Med. 1980;40:293. [PubMed] [Google Scholar]
- 20.Moohr JW, Wilson H, Pang EJ-M. Strokes and their management in sickle cell disease. In: Fried W, editor. Comparative clinical aspects of sickle cell disease. Elsevier North Holland: Amsterdam; 1982. p. 101. [Google Scholar]
- 21.Russell MO, Goldberg HI, Hodson A, et al. Effect of transfusion therapy on arteriographic abnormalities and on recurrence of stroke in sickle cell disease. Blood. 1984;63:162. [PubMed] [Google Scholar]
- 22.Balkaran B, Char G, Morris JS, et al. Stroke in a cohort of patients with homozygous sickle cell disease. J Pediatr. 1992;120:360. doi: 10.1016/s0022-3476(05)80897-2. [DOI] [PubMed] [Google Scholar]
- 23.Pegelow CH, Adams RJ, McKie V, et al. Risk of recurrent stroke in patients with sickle cell disease treated with erythrocyte transfusions. J Pediatr. 1995;126:896. doi: 10.1016/s0022-3476(95)70204-0. [DOI] [PubMed] [Google Scholar]
- 24.Scothorn DJ, Price C, Schwartz D, et al. Risk of recurrent stroke in children with sickle cell disease receiving blood transfusion therapy for at least five years after initial stroke. J Pediatr. 2002;140:348. doi: 10.1067/mpd.2002.122498. [DOI] [PubMed] [Google Scholar]
- 25.Rana S, Houston PE, Surana N, et al. Discontinuation of long-term transfusion therapy in patients with sickle cell disease and stroke. J Pediatr. 1997;131:757. doi: 10.1016/s0022-3476(97)70108-2. [DOI] [PubMed] [Google Scholar]
- 26.Aygun B, McMurray MA, Schultz WH, et al. Chronic transfusion practice for children with sickle cell anaemia and stroke. Br J Haematol. 2009;145:524. doi: 10.1111/j.1365-2141.2009.07630.x. [DOI] [PubMed] [Google Scholar]
- 27.Russell MO, Goldberg HU, Reis L, et al. Transfusion therapy for cerebrovascular abnormalities in sickle cell disease. J Pediatr. 1976;88:382. doi: 10.1016/s0022-3476(76)80251-x. [DOI] [PubMed] [Google Scholar]
- 28.Sarnaik S, Soorya D, Kim J, et al. Periodic transfusion for sickle cell anemia and CNS infarction. Am J Dis Child. 1979;133:1254. doi: 10.1001/archpedi.1979.02130120046009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.