Abstract
Background
Present literature and clinical practice provide strong support for the use of aerobic exercise in reducing pain and improving function for individuals with chronic musculoskeletal pain syndromes. However, the molecular basis for the positive actions of exercise remains poorly understood. Recent studies suggest that neurotrophin-3 (NT-3) may act in an analgesic fashion in various pain states.
Objective
The purpose of the present study was to examine the effects of moderate-intensity aerobic exercise on pain-like behavior and NT-3 in an animal model of widespread pain.
Design
This was a repeated-measures, observational cross-sectional study.
Methods
Forty female mice were injected with either normal (pH 7.2; n=20) or acidic (pH 4.0; n=20) saline in the gastrocnemius muscle to induce widespread hyperalgesia and exercised for 3 weeks. Cutaneous (von Frey monofilament) and muscular (forceps compression) mechanical sensitivity were assessed. Neurotrophin-3 was quantified in 2 hind-limb skeletal muscles for both messenger RNA (mRNA) and protein levels after exercise training. Data were analyzed with 2-factor analysis of variance for repeated measures (group × time).
Results
Moderate-intensity aerobic exercise reduced cutaneous and deep tissue hyperalgesia induced by acidic saline and stimulated NT-3 synthesis in skeletal muscle. The increase in NT-3 was more pronounced at the protein level compared with mRNA expression. In addition, the increase in NT-3 protein was significant in the gastrocnemius muscle but not in the soleus muscle, suggesting that exercise can preferentially target NT-3 synthesis in specific muscle types.
Limitations
Results are limited to animal models and cannot be generalized to chronic pain syndromes in humans.
Conclusions
This is the first study demonstrating the effect of exercise on deep tissue mechanical hyperalgesia in a rodent model of pain and providing a possible molecular basis for exercise training in reducing muscular pain.
Chronic widespread pain is complex and poorly understood and affects about 12% of the adult population in developed countries.1–3 Many laboratory animal models of pain have been produced to mimic human painful conditions. The acid model used in the present study is a noninflammatory muscle pain model and is considered to mirror some aspects of human fibromyalgia and other related syndromes that display referred hypersensitivity to mechanical stimuli. Acidic saline produces mechanical hypersensitivity of cutaneous,4–9 visceral,10 and muscular tissue11,12 that lasts up to 3 to 4 weeks. It is suggested that the influx of acid stimulates the acid-sensing ion channel-3 (ASIC3) receptors expressed by muscle afferent sensory fibers, resulting in increased nociceptive input to the spinal cord. Convergent input in the spinal cord from both muscle and paw sensory axons and receptive field plasticity of wide dynamic range (WDR) spinal neurons are believed to cause and maintain widespread secondary hyperalgesia (widespread hypersensitivity).4,6 No muscle tissue damage or gross motor/sensory loss is associated with this model of pain,4 thus allowing the investigation of exercise effects on widespread pain-like behavior.
Management of chronic pain syndromes poses challenges for health care practitioners, and pharmacological interventions offer limited efficacy.13,14 Exercise training has been long suggested to reduce pain and improve functional outcomes.13,15–20 Surprisingly, the current literature is mainly limited to human studies where the molecular basis for exercise training cannot be easily determined. Relatively few animal studies have addressed the effects and mechanisms of exercise on sensory modulation of chronic pain. These studies demonstrate that exercise training is capable of reducing or reversing hypersensitivity associated with chronic pain in various animal models.21,22 However, they are limited to examining cutaneous sensation and have not investigated the effects of exercise on deep tissue pain, which is a major clinical complaint of many people with chronic pain syndromes. To date, no animal studies have evaluated the effect of exercise on muscular hypersensitivity.
Pain states are influenced by members of the nerve-growth factor (NGF) family of neurotrophins, which include brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4). These neurotrophins support sensory neurons during development and continue to modify their function, particularly related to nociception in adulthood.23 The concept of exercise-induced analgesia via modulation of neurotrophins has only recently emerged. Studies by Gómez-Pinilla and colleagues24–27 have shown that various exercise regimens (voluntary or forced) increase neurotrophins at central and peripheral sites in healthy and injured animals. However, these studies were largely limited to biochemical approaches, and studies correlating behavioral improvements, neurotrophin production, and exercise are lacking.
In recent years, evidence has emerged concerning the role of NT-3 as a pain modulator for thermal,28,29 mechanical,9 and inflammatory30 hyperalgesia. Previous studies from our laboratory revealed that increased levels of NT-3 (either genetically overexpressed or delivered intramuscularly) abolished mechanical hypersensitivity that developed in response to intramuscular acid injections.9 If exercise increases NT-3 synthesis and NT-3 reduces cutaneous and thermal hyperalgesia, the next logical step is to test whether exercise-induced analgesia can be achieved in a muscular pain model. Thus, the goals of the present study were: (1) to examine the effect of exercise training on widespread hypersensitivity (cutaneous and muscular) in a mouse model of chronic muscle pain and (2) to test whether exercise induces an increase in muscle-derived NT-3 synthesis.
Method
Animals
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center and adhered to the university's animal care guidelines. Forty CF-1 female mice* (weight=25 g) were used to examine the effects of moderately intense exercise on primary (muscular) and secondary (cutaneous) hyperalgesia and NT-3 synthesis. Because women develop widespread pain syndromes at a greater rate than age-matched men,31–33 hyperalgesia was induced in female mice. The mice were exposed to a 12-hour light/dark cycle and had access to food and water ad libitum. The mice received two 20 μL injections of either acidic saline (mean [±SD] pH=4.0±0.1) or normal saline (mean [±SD] pH=7.4±0.1) 2 days apart into the right gastrocnemius muscle to induce chronic widespread hyperalgesia or pain-like behavior.4,6–8 The acid-pain model injections were made with a 1-mL syringe† in 10-μL increments, as previously reported.9,12
Experimental Design
Initially, the mice were randomly assigned to either the acidic saline injection (experimental) group or the normal saline injection (placebo) group. Five days after inducing hyperalgesia with acidic saline injections into the right limb, the animals were further assigned to either exercise or no-exercise groups, as follows: experimental with exercise group (n=10), experimental without exercise group (n=10), placebo with exercise group (n=10), and placebo without exercise group (n=10). The assignment to exercise or no-exercise groups was stratified within each group (acidic versus normal saline) based on the animals’ postinjection mechanical hypersensitivity, as they displayed some variability in their cutaneous paw sensitivity both before and after injection. Thus, all groups contained a range of mechanical hyperalgesia, from mild to high levels, to ensure that the groups were not biased in relation to their mechanical sensitivity.
Exercise Training
Two 6-lane, motorized treadmills‡ were used for exercise training. The exercise training was conducted 5 days per week for 3 weeks. The desired speed and exercise duration were gradually increased over the 3-week period, as follows: 13 m/min for 30 minutes during the first week, 14 m/min for 40 minutes during the second week, and 15 to 16 m/min for 45 minutes during the third week. The exercise protocol included 2 to 3 minutes of warm-up and 3 minutes of cool-down. Although treadmill running in rodents is considered forced activity,34,35 we observed that most animals ran for the majority of the exercise period without any encouragement. The animals did not appear overly fatigued, which is an important consideration, as exhaustive exercise training may alter the levels of lactic acid36,37 and cause fatigue that may increase pain sensitivity.11 All mice were acclimated and trained for 3 days on the treadmill at 13 m/min before being assigned to an experimental or placebo group. After exercise training, all mice were tested for cutaneous and muscular mechanical sensitivity and then killed to quantify NT-3 levels in the soleus and gastrocnemius muscles. The investigator (N.S.) carrying out the behavioral and biochemical assessments was blinded to group assignments.
Behavioral Testing
Animals were acclimated for 3 days to the behavioral testing paradigms (2 times per day for von Frey monofilament and 3 times per day for muscle squeeze testing) at each time point of the behavior testing. For acclimation, the animals were brought to the testing laboratory and placed under the testing environment without actually conducting the test. For cutaneous testing, the animals were placed under a plastic chamber for 20 minutes for each acclimation period. For the muscle squeeze test, they were placed in a customized holder and remained in the holder for 5 minutes for each acclimation period. All exercise sessions were conducted between 4 and 6 pm toward the end of the animals’ sleep cycle. Behavioral testing was always conducted between 7 and 10 am to avoid potential confounding factors such as stress-induced antinociception or acute effects of exercise training. Behavior testing was conducted at baseline (before injection), following the second acidic saline injection (1 day after injection for von Frey monofilament and 3 days after injection for muscle squeeze) and every week thereafter, alternating with cutaneous and deep tissue tests to avoid excessive animal handling and overstimulation.
Mechanical testing of cutaneous sensitivity.
Mechanical hyperalgesia to an innocuous stimulus was assessed using von Frey monofilaments. Clear plastic chambers (3 × 8 × 12 cm) were placed in an inverted position on a wire-mesh tabletop. The mice were placed under the plastic chambers and allowed to acclimate for 20 minutes prior to each test. A single von Frey monofilament§ of 1.0g bending force was applied to the plantar surface of each foot. A positive response to the von Frey stimulus was defined as a retraction of paw with or without licking. Three trials, each consisting of 5 applications (each 30 seconds apart) of the von Frey monofilament to the plantar surface of the ipsilateral and the contralateral hind paws, were conducted. The percent response for each hind limb was obtained by determining the number of withdrawals out of 5 monofilament applications. For statistical analysis, percent responses were averaged to obtain group means.
Mechanical testing of muscle sensitivity.
The mice were placed in a customized holder, and deep tissue mechanical sensitivity to a noxious stimulus was tested with a forceps compression device similar to the apparatus described by Yu et al.38 A modified version of the device was built internally at the University of Kansas Medical Center Neuromuscular Research Laboratory to accommodate the small size of the mice. The device is described elsewhere.12 In brief, the device consisted of a forceps, a pressure sensor (LCKD subminiature compression load cells∥) attached to the inner tip flat surface of the forceps, a signal amplifier, and a laptop computer. A manual force was applied to each gastrocnemius muscle from a marked area on the forceps by an examiner who was blinded to group assignment. The signal from the load cells was amplified, digitized, and stored in a laptop computer. The recorded signal from the pressure sensor was analyzed with a custom-written Matlab computer program (Matlab 6.5).# The loading period and peak threshold level were recorded for each hind limb. The loading period was defined as the total time from the beginning of the loading to the peak withdrawal force. The peak threshold level was defined as the peak force and demonstrated by withdrawal response or vocalization upon compression. Three consecutive squeezes on the right and left sides were conducted on each mouse. A total of 3 trials were performed. The mean of 9 force peaks over 3 trials was calculated for each hind limb. Only force peaks with a loading time period of less than 1.0 second were used for the final calculation.
Biochemical Assays
Levels of NT-3 messenger RNA (mRNA) and protein in skeletal muscles were measured using standard measures of quantitative, real-time polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA), respectively. After the completion of 3 weeks of exercise training, mice from all groups were killed 24 hours after the last exercise session. The gastrocnemius and soleus muscles were removed separately and frozen at −80°C. The soleus muscle often is chosen in rat studies because of its high level of recruitment during treadmill training.24,25,27 In our previous study using NT-3 to reduce acid-induced mechanical sensitivity, we chose the gastrocnemius muscle.9 We included both muscles in the present study. Five animals from each group were randomly chosen for biochemical analysis. The tissue samples from the remaining 5 animals were fixed for future analysis and were not included in this study. Two to 3 independent NT-3 real-time PCR and protein assays per animal were conducted.
NT-3 mRNA.
Total RNA was isolated using Trizol reagent** as per the manufacturer's protocol. Frozen tissue samples of the right gastroctrocnemius muscle and the right soleus muscle were homogenized separately in 1 mL of Trizol reagent and precipitated with isopropanol. RNA pellets were washed with 75% ethanol and resuspended in deionized diethylpyrocarbonate water. The RNA concentration was determined using a BioRad spectrophotometer.†† Samples were tested for the quality of RNA using an electrophoretic separation technique (Agilent 2100 bioanalyzer‡‡ tracer with Eukaryote total RNA nano assay) prior to real-time PCR analysis.39 Following the assessment of RNA quality, 0.653 μg of RNA was reverse transcribed to complementary DNA (cDNA) using the iScript cDNA synthesis kit.§§ The thermal cycling conditions for reverse transcription were programmed such that each sample cycled at 25°C for 5 minutes, at 42°C for 30 minutes, and at 85°C for 5 minutes for cDNA synthesis.
Real-time PCR amplification of NT-3 cDNA was performed using 0.2 μg of total cDNA (from 0.653 μg reverse-transcribed cDNA) and SYBR green master mix (BioRad iCycler§§). The thermal cycling conditions for real-time PCR were set at 95°C and 60°C for 40 cycles (MyiQ real-time PCR detection system§§). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a reference gene.24,26,27,40 The primer sequences used for real-time PCR were as follows:
NT-3 forward: 5′AACGGACACAGAGCTACTACG-3′
NT-3 reverse: 5′CCATTAGGTATAAGGGAGGGGG-3′
GAPDH forward: 5′AGGTCGGTGTGAACGGATTTG-3′
GAPDH reverse: 5′TGTAGACCATGTAGTTGAGGTCA-3′
Samples were run in triplicate, and control reactions were run with each amplification series. Control reactions were derived from the gastrocnemius muscle from a mouse that genetically overexpressed NT-3 levels in skeletal muscles. Threshold cycle (CT) values were averaged, and relative changes in RNA levels were determined by subtracting sample CT values from the reference gene CT values (ΔCT). The ΔCT values were averaged to obtain group means. A higher average CT value suggests that a larger number of cycles were needed to obtain a defined threshold level, indicating less mRNA in the sample.
NT-3 protein analysis.
The left gastrocnemius and left soleus muscles were used to assess NT-3 protein levels. Frozen sections of samples were separately homogenized in buffer consisting of 20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 1% NP40, 1 mM PMSF, 10% glycerol, 10 μg/mL aprotinin, 1 μg/mL leupeptin, 0.5 mM sodium vanadate, and 4% Triton X-100 using electrical homogenizers. The homogenates were centrifuged, and supernatants were collected. The total protein concentration was measured using the Bradford method (Bio-Rad protein reagent). The NT-3 protein was quantified using an ELISA kit (NT-3 Emax immunoassay system kit∥∥).26,27 Equal amounts of protein extracts from each group were used to quantify NT-3 protein levels (10 μg for the soleus muscle and 5 μg for the gastrocnemius muscle per well) and were analyzed in duplicate using the manufacturer's instructions.
Data Analysis
All data were analyzed using SPSS 15.0 for Windows.## Hypersensitivity following acidic saline injections and the effect of exercise on behavioral measures initially were analyzed using repeated-measures analysis of variance (ANOVA) on the ipsilateral and contralateral sides separately. In addition, a one-way ANOVA was conducted to examine the group differences related to cutaneous and muscle hyperalgesia at different time points when interactions (time × hypersensitivity or time × exercise) were significant. The Fisher least significant difference post hoc analysis of behavioral measures was used to conduct pair-wise comparisons when groups were significantly different using ANOVA. Comparison between experimental and placebo groups (acidic versus normal saline) was used to indicate hypersensitive status of the mice. Comparison between both experimental groups (with and without exercise) was used to indicate the effect of exercise training. The NT-3 mRNA measurements were analyzed with the Pair-Wise Fixed Reallocation Randomization Test (Pfaffl method) to determine pair-wise comparisons between animals from different groups.41 The Pair-Wise Fixed Reallocation Randomization Test is a nonparametric test similar to the Mann-Whitney U test and was used for mRNA analysis because our data were derived from ratios (NT-3 − GAPDH). In the Pair-Wise Fixed Reallocation Randomization Test, various data points are randomly selected from samples and repeatedly analyzed (random sampling) for effect size (expression of ratio is based on mean values of reference and target genes within control and intervention groups) with mathematical analysis. The NT-3 protein levels were analyzed with one-way ANOVA for group differences. Values were considered significant at an alpha level of <.05.
Role of the Funding Source
This study was funded by National Institutes of Health/National Institute of Neurological Disorders and Stroke grant R01NS43314 and a Foundation for Physical Therapy Research PODS II scholarship.
Results
Behavioral Assessments
Changes in scores of cutaneous and muscle hyperalgesia were calculated by subtracting the mean value of each time point from the preinjection (baseline) value and are presented in Figures 1 and 2. The actual mean scores and 95% confidence intervals are presented in Tables 1 and 2.
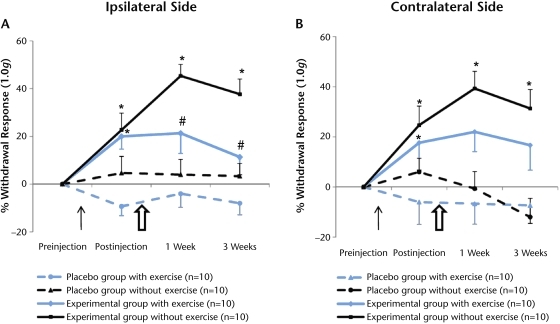
Figure 1.
Effect of moderately intense exercise on cutaneous hyperalgesia. Acidic saline injections significantly increased cutaneous withdrawal responses on the ipsilateral and contralateral sides. (A) Moderately intense exercise significantly decreased withdrawal responses at 1-week and 3-week time points on the ipsilateral side (P<.05). (B) A similar effect of exercise was noted on the contralateral side, but it was not significant (P>.05) at any time points. The thin black arrow indicates the injection of acidic or normal saline. The larger, open arrow indicates the initiation of exercise training. Data are represented as change in mean score from before injection to each time point; error bars indicate standard error of the mean. * denotes status of hypersensitivity and P<.05 for difference between experimental and placebo groups (acidic versus normal saline) at the postinjection time points. # denotes effects of exercise training and P<.05 for difference between exercise and no-exercise groups at the postexercise time points.
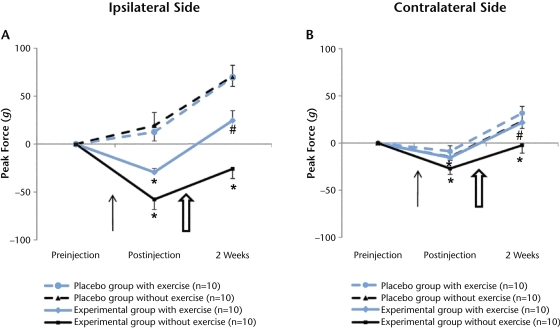
Figure 2.
The effect of moderately intense exercise on muscle hyperalgesia. Acidic saline injections significantly decreased muscle withdrawal thresholds on the ipsilateral and contralateral sides. Moderately intense exercise training significantly increased the muscle withdrawal thresholds of both hind limbs (A and B, P<.05). The thin black arrow indicates the injection of acidic or normal saline. The larger, open arrow indicates the initiation of exercise training. Data are represented as change in mean score from before injection to each time point; error bars indicate standard error of the mean. * denotes P<.05 for difference between experimental and placebo status (acidic versus normal saline) at the postinjection time point. # denotes P<.05 for difference between exercise and no exercise at the postexercise time point.
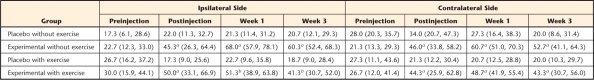
Table 1.
Mean Scores and 95% Confidence Intervals for the Cutaneous Behavioral Test
a P<.05 for experimental group mice that developed cutaneous hyperalgesia following acidic saline injections.
b P<.05 for exercise group mice compared with their no-exercise counterparts.
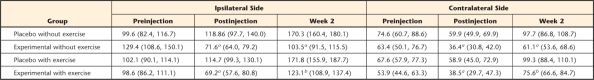
Table 2.
Mean Scores and 95% Confidence Intervals for the Muscle Behavioral Test
a P<.05 for experimental group mice that developed muscle hyperalgesia following acidic saline injections.
b P<.05 for exercise group mice compared with their no-exercise counterparts.
Cutaneous mechanical hypersensitivity.
The withdrawal responses remained relatively unaffected on the ipsilateral and contralateral sides in both placebo groups of mice that received normal saline injections (Fig. 1), suggesting that neither normal saline injection nor exercise influenced mechanical sensitivity. In contrast, the percentage of withdrawal increased in both experimental groups of mice that received acidic saline injections on the ipsilateral and contralateral sides, consistent with mechanical secondary hyperalgesia (Tab. 1, Fig. 1). The increase in withdrawal response was significant for the ipsilateral and contralateral sides (P<.05) from before injection to 1 day after acidic saline injection. The withdrawal response remained increased on both sides in the experimental group of mice that did not exercise. In comparison, our post hoc analysis showed that the experimental group of mice that exercised showed a significant decrease in withdrawal response on the ipsilateral side at 1 week and 3 weeks of exercise training (Fig. 1A; P<.05). The effect of exercise training on the contralateral side was not significant at any time points (Fig. 1B; P>.05). Overall, these results indicate that intramuscular acidic saline injection induced secondary hyperalgesia bilaterally in the hind paws of the mice. Furthermore, moderate-intensity exercise training significantly reduced the cutaneous hypersensitivity on the ipsilateral side.
Muscular mechanical hypersensitivity.
Following acidic saline injections, the mice displayed bilateral muscle hyperalgesia that lasted up to 2 weeks, and exercise decreased muscle hyperalgesia (Tab. 2, Fig. 2). The muscle withdrawal threshold increased on the ipsilateral and the contralateral sides 2 weeks following normal saline injections in both placebo groups of mice. These results suggest that the saline-injected mice became acclimated to the muscle compression test and were able to tolerate greater compression over time.
In contrast, muscle withdrawal thresholds decreased after acidic saline injections in both experimental groups of mice. In the experimental group that did not exercise, ipsilateral muscle withdrawal thresholds decreased from preinjection to postinjection levels and remained decreased 2 weeks later. The muscle withdrawal threshold on the contralateral side also decreased from preinjection to postinjection levels and returned to baseline values 2 weeks later. In the experimental group that exercised, ipsilateral muscle withdrawal thresholds also decreased from preinjection to postinjection testing, but then increased after 2 weeks of exercise training (Tab. 2, Fig. 2A; P<.05). Muscle withdrawal thresholds on the contralateral side also decreased from preinjection to postinjection testing but then increased beyond baseline after 2 weeks of exercise training (Fig. 2B; P<.05). These results indicate that acidic saline significantly induced bilateral muscular hyperalgesia in the hind limbs of the mice and that exercise training significantly reduced the muscular hypersensitivity on the ipsilateral and contralateral sides. Thus, exercise training increased muscle withdrawal thresholds in an animal model of chronic pain.
Biochemical Assessments
NT-3 mRNA.
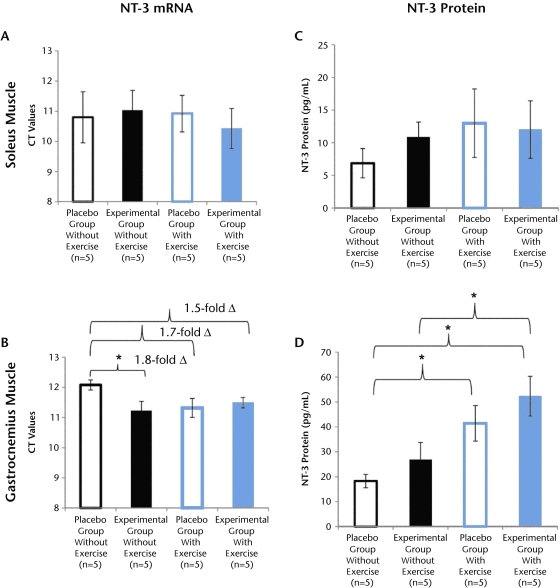
The NT3 mRNA data are presented in CT values, where a larger number indicates less mRNA in the tissue samples (Fig. 3). Our results show that exercise training had no effect on NT-3 mRNA levels in the soleus muscle (Fig. 3A; P>.05), but a trend for increased levels of mRNA (1.7-fold increase; P=.06) was noted in the gastrocnemius muscle when comparing both placebo groups of mice (Fig. 3B). In addition, no significant differences in NT-3 mRNA levels were observed between both experimental groups in either the soleus or gastrocnemius muscle. Interestingly, acidic saline injection increased NT-3 mRNA in the gastrocnemius muscle when NT-3 levels were compared between the 2 groups of mice that did not exercise (Fig. 3B; P<.05), suggesting that acidic saline itself may affect NT-3 mRNA levels.
Figure 3.
Effect of exercise on neurotrophin-3 (NT-3) messenger RNA (mRNA) and protein synthesis. Moderately intense exercise training did not change NT-3 mRNA expression in the soleus or gastrocnemius muscle (A and B, P>.05). In contrast, the effect of exercise training did increase NT-3 protein levels (C and D). The increased NT-3 protein levels were not significant in the soleus muscle (C, P>.05) but were significant in the gastrocnemius muscle (D, P<.05). The NT-3 mRNA data (A and B) are represented in threshold cycle (CT) value, where lower values indicate a higher amount of mRNA in the sample. In addition, comparisons between groups (B) are indicated by fold change (2ΔCT-ΔCT). Data represented as mean scores; error bars indicate standard error of the mean. Group comparisons with P<.05 are denoted with asterisk (*), indicating that both exercise groups of mice were different from the placebo group of mice that did not exercise (D).
NT-3 protein.
In contrast to mRNA, a significant increase in protein levels was noted following 3 weeks of moderate-intensity exercise training in the mice. In the soleus muscle, a trend for increased levels of protein was noted in both exercise groups, but it was not significant (Fig. 3C; P>.05). However, the protein level of the gastrocnemius muscle was significantly upregulated. Both exercise groups had significantly greater amounts of NT-3 protein compared with the placebo group that did not exercise (Fig 3D; P<.05). These results indicate that exercise training can increase NT-3 abundance, as reported previously.25–27 Additionally, it appears that exercise can differentially increase NT-3 protein levels in muscles with different properties of fiber types.
Discussion
Because most animal studies related to exercise and chronic pain have evaluated cutaneous sensation in neuropathic pain models, there is a significant gap in our knowledge about how exercise alters deep tissue hypersensitivity in a chronic muscular pain model. Here, we analyzed the effect of moderate-intensity exercise training on widespread cutaneous and muscle hyperalgesia induced by acidic saline injection. We further examined a possible molecular correlate (NT-3 synthesis) that could explain the changes in skeletal muscles. The findings of our study suggest that exercise training attenuates acidic saline-induced cutaneous and muscle hypersensitivity in both limbs. In addition, our findings suggest that moderately intense treadmill running can increase muscle-derived NT-3 protein levels in mice and that this effect is selective to the gastrocnemius muscle. Overall, these results show, for the first time, that exercise decreases chronic muscle hyperalgesia in rodents, supporting the growing literature indicating the role of NT-3 in pain modulation and providing a potential molecular correlate for exercise-induced analgesia in chronic muscular pain.
Aerobic Exercise Decreases Mechanical Hyperalgesia Associated With Intramuscular Acidic Saline Injection
Clinical studies using various types of exercise interventions for people with chronic pain syndromes are increasing.13,15–19,42 These human experiments provide insight into important treatment avenues, but it is clear that additional animal studies are needed to extend our knowledge about exercise-induced analgesia and its mechanisms. A limited number of animal studies have attempted to address this issue. Bement and Sluka21 recently reported that low-intensity treadmill training (3.05 m/min for 30 minutes) for 5 days reversed acid-induced cutaneous mechanical allodynia via an opioid-based mechanism in rats. However, their study examined an immediate effect of exercise, as seen in most animal studies.43 Another study22 demonstrated a decrease in pain responses following 9 days of swimming exercise, yet it was limited to assessing cutaneous sensation. The primary goal of the present study was to examine the long-term effect of exercise training on muscle hypersensitivity, which is the predominant complaint in people with various chronic pain syndromes.44 Our results are consistent with those of previous studies21,22,25 and suggest that moderate-intensity exercise can be effective in reducing widespread hyperalgesia that is chronic in nature, potentially by modulating NT-3 levels.
Moderately Intense Exercise Training Can Induce NT-3 Synthesis
In adulthood, NT-3 is primarily synthesized in skeletal muscles, within muscle spindles and smooth muscles surrounding the arterial supply (unpublished observations and Wright et al45). The exact mechanism by which exercise induces NT-3 synthesis is unknown. However, it is possible that the ability of exercise to increase levels of NT-3 may be associated with potentially increased muscle spindle activity or increased in muscle perfusion from exercise training. Some studies24,25,27 have shown that exercise training can induce NT-3 synthesis. Various types of exercise training, such as treadmill training,24 general physical activity of swimming or hind-paw standing,25 and voluntary wheel running,27 have increased peripheral or spinal NT-3 synthesis. In a spinal cord injury model, 28 days of wheel running was required to stimulate NT-3 synthesis.25 Our results are consistent with those of these studies and demonstrate that moderately intense exercise training was sufficient to abolish muscle-originated hyperalgesia. Activity-dependent changes in muscle may have a strong effect on NT-3 levels via activation of proprioceptive and muscle afferents that could result in exercise-induced analgesia. Muscle activity provides signals to innervating neurons and can alter the synthesis of neurotrophic factors.24–26,46,47
One surprising finding from this study was that we observed significant exercise-induced increases in NT-3 only in the gastrocnemius muscle. We did observe a trend in increased NT-3 protein in the soleus muscle, consistent with previous rat studies.24–27 The functions of the soleus and gastrocnemius muscles are different, and these muscles are composed of very different fiber types. The soleus muscle consists of both type I and type II fibers, whereas the gastrocnemius muscle has predominantly type II muscle fibers.48,49 It is not known whether NT-3 is preferentially expressed more in slow-twitch fibers or in fast-twitch fiber types under normal conditions, but our studies suggest that this possibility could account for the differences in NT-3 expression between gastrocnemius and soleus muscles in rodents. Neurotrophin-3 has been shown to favorably improve reinnervation of fast-twitch muscles (gastrocnemius and extensor digitorum longus muscles) over slow-twitch muscles (soleus muscle),50 and myogenic and transgenic mice that overexpress NT-3 and develop excessive muscle spindles do so only in the gastrocnemius muscle and not in the soleus muscle.45 Collectively, these data suggest that NT-3 is differentially synthesized in select muscle fiber types, and our results suggest that aerobic exercise training may be associated with this differential increase in NT-3 levels.
Antinociceptive Effects of NT-3 on Chronic Pain
Neurotrophin-3 is an antinociceptive molecule and a biomarker for testing activation of large beta and proprioceptive fibers. Once, NT-3 is synthesized in peripheral tissues, it preferentially binds to its receptors located on about 75% of muscle afferents30 and is retrogradely transported to cell bodies of the dorsal root ganglia.30,51–53 Given its anatomical location and ability to be altered under physiological stress (ie, exercise), NT-3 is positioned to influence afferent input associated with chronic pain, in terms of both peripheral afferent activity and modulation of spinal neurons. In addition, the effects of NT-3 are not restricted to large-diameter afferent fibers, as C-fibers and Aδ-fibers also are known to respond to NT-3.9,29 Therefore, NT-3 is capable of modulating different sensory fiber types after certain injuries.29,54 Neurotrophin-3 also has been shown to reduce the expression of tyrosine kinase A receptors for nerve growth factor and substance P in dorsal root ganglion neurons, both of which are involved in inflammatory pain.28 The antagonistic role of NT-3 on secondary hyperalgesia has been tested in many pain models. Intrathecal administration of NT-3 prevents thermal hyperalgesia and suppresses the injury-induced overexpression of these receptors in the dorsal root ganglia and the spinal cord of rats receiving a sciatic nerve injury.29 A single systemic dose of NT-3 into hyperalgesic rats results in mechanical but not thermal hypoalgesia 24 hours following its administration.30 Either overexpression of NT-3 or intramuscular injection of NT-3 decreases mechanical hyperalgesia from acid injection.9 Collectively, these findings strengthen the role of NT-3 as an antinociceptive neurotrophin in treating muscle pain and provide further support for exercise training as a therapeutic intervention for the field of pain medicine.
Limitations
The duration of the exercise training and lack of frequent behavioral testing may be a limitation of this study and should be considered in future studies. To comprehend the full effect of exercise training on chronic pain, future studies should consider extending exercise training for longer periods and conducting frequent behavioral measures. This study demonstrated a possible correlation between NT-3 synthesis and decrease in muscle and cutaneous hyperalgesia from exercise training in a muscle pain model. Future studies could be conducted by blocking the NT-3 receptor tyrosine kinase C to establish a mechanism of exercise-induced analgesia via NT-3 synthesis.
Demonstration of actions of exercise on neurotrophin levels has been restricted to animal models and it may be difficult to relate the results of the study to clinical syndromes of chronic pain in humans. However, the study provides a possible physiological correlate for exercise-induced relief of muscle pain reported in clinical studies. It will be interesting to find out whether these observations hold true in human studies. Blood samples and muscle biopsies following exercise training might provide some insight into possible exercise effects on select neurotrophins in human experiments.
Clinical Significance
The mechanisms of exercise-induced analgesia in patients with chronic pain are poorly understood. Two possible mechanisms have been reported. The most widely accepted explanation involves modulation of central opioid receptors.17,21,55,56 Alternatively, activation of muscle and proprioceptive afferents may affect descending inhibitory pathways and central sensitization.39,57 The theory of activation of proprioceptive input to override pain has gained interest in physical therapy rehabilitation. Our results provide a possible correlation between activity-related NT-3 synthesis (presumably through activation of muscle afferent fibers from exercise training) and an exercise-induced decrease in hyeralgesia. The correlation of pain modulation and NT-3 changes through exercise training can provide support for exercise prescriptions.
It is important to determine the intensity of exercise training required to activate muscle afferents and synthesize neurotrophins. Although more studies are needed to examine this issue further, our results suggest that moderate-intensity exercise training is sufficient to induce NT-3 synthesis. Human studies provide similar evidence for exercise training for people with fibromyalgia. Long-term aerobic training at a moderate level (50%–60% of maximum heart rate) with gradual progression from pool walking to land jogging for 30 minutes, 3 to 5 times per week, decreases symptom severity and improves physical function and aspects of self-care in individuals with fibromyalgia.15,16,18
Conclusion
We have demonstrated that moderate-intensity exercise training did not cure but significantly reduced cutaneous and deep tissue mechanical hypersensitivity induced by acidic saline injection. This finding is consistent with the findings of human studies, as exercise does not reverse the painful condition but rather decreases pain and improves function. The data also demonstrate an increase in activity-dependent NT-3 levels in selected peripheral tissues. Based on emerging views about the analgesic properties of NT-3, it is plausible to suggest that the decrease in mechanical hypersensitivity following exercise may be due, in part, to elevated levels of NT-3 protein. However, the mechanism by which NT-3 modulates mechanoreceptors is still unknown and remains to be investigated.
Footnotes
Dr Sharma and Dr Wright provided concept/idea/research design, writing, project management, and fund procurement. Dr Sharma and Ms Ryals provided data collection. Dr Sharma and Dr Gajewski provided data analysis. Dr Wright provided facilities/equipment. Ms Ryals provided clerical support. The authors thank Megan Johnson and Karra Muller for helpful comments on the manuscript and Jennifer Koch, Elizabeth Phelps, and Melissa Sindt for helping with exercise training.
A poster presentation of this work was given at the Combined Sections Meeting of the American Physical Therapy Association; February 9–12, 2009; Las Vegas, Nevada.
This study was funded by National Institutes of Health/National Institute of Neurological Disorders and Stroke grant R01NS43314 and a Foundation for Physical Therapy Research PODS II scholarship.
Charles River Laboratories International Inc, 251 Ballardville St, Wilmington, MA 01887.
Becton Dickinson, 1 Becton Dr, Franklin Lakes, NJ 07417.
Columbus Instruments, 950 N Hague Ave, Columbus, OH 43204.
Stoelting Co, Ste A, 620 Wheat Ln, Wood Dale, IL 60191.
Omega Engineering Inc, PO Box 4047, Stamford, CT 06907-0047.
The MathWorks Inc, PO Box 845428, Boston, MA 02284-5428.
Sigma-Aldrich Corporate Offices, 3050 Spruce St, St Louis, MO 63103.
Biocompare, 395 Oyster Point Blvd, #405, South San Francisco, CA 94080.
Quantum Analytics Inc, 363 Vintage Park Dr, Foster City, CA 94404.
Bio-Rad Laboratories, 1000 Alfred Nobel Dr, Hercules, CA 94547.
Promega Corp, 2800 Woods Hollow Rd, Madison, WI 53711-5399.
SPSS Inc, 233 Wacker Dr, Chicago, IL 60606.
References
- 1. Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol. 2003;17:547–561 [DOI] [PubMed] [Google Scholar]
- 2. Neumann L, Buskila D. Epidemiology of fibromyalgia. Curr Pain Headache Rep. 2003;7:362–368 [DOI] [PubMed] [Google Scholar]
- 3. Rohrbeck J, Jordan K, Croft P. The frequency and characteristics of chronic widespread pain in general practice: a case-control study. Br J Gen Pract. 2007;57:109–115 [PMC free article] [PubMed] [Google Scholar]
- 4. Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46 [DOI] [PubMed] [Google Scholar]
- 5. Sluka KA, Rohlwing JJ, Bussey RA, et al. Chronic muscle pain induced by repeated acid Injection is reversed by spinally administered mu- and delta-, but not kappa-, opioid receptor agonists. J Pharmacol Exp Ther. 2002;302:1146–1150 [DOI] [PubMed] [Google Scholar]
- 6. Sluka KA, Price MP, Breese NM, et al. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239 [DOI] [PubMed] [Google Scholar]
- 7. Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98:69–78 [DOI] [PubMed] [Google Scholar]
- 8. Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci. 2003;23:5437–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gandhi R, Ryals JM, Wright DE. Neurotrophin-3 reverses chronic mechanical hyperalgesia induced by intramuscular acid injection. J Neurosci. 2004;24:9405–9413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miranda A, Peles S, Rudolph C, et al. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089 [DOI] [PubMed] [Google Scholar]
- 11. Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle fatigue increases the probability of developing hyperalgesia in mice. J Pain. 2007;8:692–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma NK, Ryals JM, Liu H, et al. Acidic saline-induced primary and secondary mechanical hyperalgesia in mice. J Pain. 2009;10:1231–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA. 2004;292:2388–2395 [DOI] [PubMed] [Google Scholar]
- 14. Wang LX, Wang ZJ. Animal and cellular models of chronic pain. Adv Drug Deliv Rev. 2003;55:949–965 [DOI] [PubMed] [Google Scholar]
- 15. Meiworm L, Jakob E, Walker UA, et al. Patients with fibromyalgia benefit from aerobic endurance exercise. Clin Rheumatol. 2000;19:253–257 [DOI] [PubMed] [Google Scholar]
- 16. Gowans SE, deHueck A. Effectiveness of exercise in management of fibromyalgia. Curr Opin Rheumatol. 2004;16:138–142 [DOI] [PubMed] [Google Scholar]
- 17. Hoffman MD, Shepanski MA, Ruble SB, et al. Intensity and duration threshold for aerobic exercise-induced analgesia to pressure pain. Arch Phys Med Rehabil. 2004;85:1183–1187 [DOI] [PubMed] [Google Scholar]
- 18. Whiteside A, Hansen S, Chaudhuri A. Exercise lowers pain threshold in chronic fatigue syndrome. Pain. 2004;109:497–499 [DOI] [PubMed] [Google Scholar]
- 19. Redondo JR, Justo CM, Moraleda FV, et al. Long-term efficacy of therapy in patients with fibromyalgia: a physical exercise-based program and a cognitive-behavioral approach. Arthritis Rheum. 2004;51:184–192 [DOI] [PubMed] [Google Scholar]
- 20. Mannerkorpi K. Exercise in fibromyalgia. Curr Opin Rheumatol. 2005;17:190–194 [DOI] [PubMed] [Google Scholar]
- 21. Bement MK, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil. 2005;86:1736–1740 [DOI] [PubMed] [Google Scholar]
- 22. Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain. 2007;8:989–997 [DOI] [PubMed] [Google Scholar]
- 23. Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538 [DOI] [PubMed] [Google Scholar]
- 24. Gómez-Pinilla F, Ying Z, Opazo P, et al. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084 [DOI] [PubMed] [Google Scholar]
- 25. Hutchinson KJ, Gómez-Pinilla, Crowe MJ, et al. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127(pt 6):1403–1414 [DOI] [PubMed] [Google Scholar]
- 26. Ying Z, Roy RR, Edgerton VR, Gómez-Pinilla Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–419 [DOI] [PubMed] [Google Scholar]
- 27. Ying Z, Roy RR, Edgerton VR, Gómez-Pinilla Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003;987:93–99 [DOI] [PubMed] [Google Scholar]
- 28. Gratto KA, Verge VM. Neurotrophin-3 down-regulates trkA mRNA, NGF high-affinity binding sites, and associated phenotype in adult DRG neurons. Eur J Neurosci. 2003;18:1535–1548 [DOI] [PubMed] [Google Scholar]
- 29. Wilson-Gerwing TD, Dmyterko MV, Zochodne DW, et al. Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons. J Neurosci. 2005;25:758–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watanabe M, Endo Y, Kimoto K, et al. Inhibition of adjuvant-induced inflammatory hyperalgesia in rats by local injection of neurotrophin-3. Neurosci Lett. 2000;282:61–64 [DOI] [PubMed] [Google Scholar]
- 31. Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol. 2003;17:547–561 [DOI] [PubMed] [Google Scholar]
- 32. Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128–134 [DOI] [PubMed] [Google Scholar]
- 33. Yunus MB. Gender differences in fibromyalgia and other related syndromes. J Gend Specif Med. 2002;5:42–47 [PubMed] [Google Scholar]
- 34. Aguiar AS, Jr, Tuon T, Pinho CA, et al. Intense exercise induces mitochondrial dysfunction in mice brain. Neurochem Res. 2008;33:51–58 [DOI] [PubMed] [Google Scholar]
- 35. Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019:84–96 [DOI] [PubMed] [Google Scholar]
- 36. Sastre J, Asensi M, Gasco E, et al. Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am J Physiol. 1992;263(5 pt 2):R992–995 [DOI] [PubMed] [Google Scholar]
- 37. Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol. 2005;98:1258–1263 [DOI] [PubMed] [Google Scholar]
- 38. Yu YC, Koo ST, Kim CH, et al. Two variables that can be used as pain indices in experimental animal models of arthritis. J Neurosci Methods. 2002;115:107–113 [DOI] [PubMed] [Google Scholar]
- 39. Schroeder A, Mueller O, Stocker S, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mahoney DJ, Carey K, Fu MH, et al. Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise. Physiol Genomics. 2004;18:226–231 [DOI] [PubMed] [Google Scholar]
- 41. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Droste C. Transient hypoalgesia under physical exercise: relation to silent ischaemia and implications for cardiac rehabilitation. Ann Acad Med Singapore. 1992;21:23–33 [PubMed] [Google Scholar]
- 43. Koltyn KF. Analgesia following exercise: a review. Sports Med. 2000;29:85–98 [DOI] [PubMed] [Google Scholar]
- 44. Borg-Stein J. Management of peripheral pain generators in fibromyalgia. Rheum Dis Clin North Am. 2002;28:305–317 [DOI] [PubMed] [Google Scholar]
- 45. Wright DE, Zhou L, Kucera J, Snider WD. Introduction of a neurotrophin-3 transgene into muscle selectively rescues proprioceptive neurons in mice lacking endogenous neurotrophin-3. Neuron. 1997;19:503–517 [DOI] [PubMed] [Google Scholar]
- 46. Molteni R, Zheng JQ, Ying Z, et al. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci USA. 2004;101:8473–8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xie K, Wang T, Olafsson P, et al. Activity-dependent expression of NT-3 in muscle cells in culture: implications in the development of neuromuscular junctions. J Neurosci. 1997;17:2947–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221:177–190 [DOI] [PubMed] [Google Scholar]
- 49. Ashmore CR, Doerr L. Comparative aspects of muscle fiber types in different species. Exp Neurol. 1971;31:408–418 [DOI] [PubMed] [Google Scholar]
- 50. Simon M, Terenghi G, Green CJ, Coulton GR. Differential effects of NT-3 on reinnervation of the fast extensor digitorum longus (EDL) and the slow soleus muscle of rat. Eur J Neurosci. 2000;12:863–871 [DOI] [PubMed] [Google Scholar]
- 51. Wright DE, Williams JM, McDonald JT, et al. Muscle-derived neurotrophin-3 reduces injury-induced proprioceptive degeneration in neonatal mice. J Neurobiol. 2002;50:198–208 [DOI] [PubMed] [Google Scholar]
- 52. Malcangio M, Garrett NE, Cruwys S, Tomlinson DR. Nerve growth factor- and neurotrophin-3-induced changes in nociceptive threshold and the release of substance P from the rat isolated spinal cord. J Neurosci. 1997;17:8459–8467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Munson JB, Johnson RD, Mendell LM. Neurotrophin-3 and maintenance of muscle afferent function. Prog Brain Res. 1999;123:157–163 [PubMed] [Google Scholar]
- 54. Saragovi HU, Gehring K. Development of pharmacological agents for targeting neurotrophins and their receptors. Trends Pharmacol Sci. 2000;21:93–98 [DOI] [PubMed] [Google Scholar]
- 55. Goldfarb AH, Jamurtas AZ. Beta-endorphin response to exercise: an update. Sports Med. 1997;24:8–16 [DOI] [PubMed] [Google Scholar]
- 56. Schwarz L, Kindermann W. Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med. 1992;13:25–36 [DOI] [PubMed] [Google Scholar]
- 57. Farrell PA, Gustafson AB, Morgan WP, Pert CB. Enkephalins, catecholamines, and psychological mood alterations: effects of prolonged exercise. Med Sci Sports Exerc. 1987;19:347–353 [PubMed] [Google Scholar]