Summary
Menadione (Vitamin K3) has anti-tumoral effects against a wide range of cancer cells. Its potential toxicity to normal cells and narrow therapeutic range limit its use as single agent but in combination with radiation or other anti-neoplastic agents can be of therapeutic use. In this paper, we first evaluated the early (within 3 h) effect of menadione on ongoing DNA replication. In normal rat cerebral cortex mini-units menadione showed an age dependent anti-proliferative effect. In tissue mini-units prepared from newborn rats, menadione inhibited ongoing DNA replication with an IC 50 of approximately 10 μM but 50 μM had no effect on mini-units from prepared adult rat tissue. The effect of short (72 h) and prolonged exposure (1–2 weeks) to menadione alone in the DBTRG.05MG human glioma cells line and in combination with vitamin C was studied. After short period of exposure data show that menadione alone or in combination with vitamin C provided similar concentration-response curves (and IC50 values). Prolonged exposure to these drugs was evaluated by their ability to kill 100% of glioma cells and prevent regrowth when cells are re-incubated in drug-free media. In this long-term assay, menadione:vitamin C at a ratio 1:100 showed higher anti-proliferative activity when compared to each drug alone and allowed to reduce each drug concentration between 2.5 to 5-fold. Similar anti-proliferative effect was demonstrated in 8 patient derived glioblastoma cell cultures. Our data should be able to encourage further advanced studies on animal models to evaluate the potential use of this combination therapy for glioma treatment.
Keywords: Gliomas, Menadione, Vitamin C, Proliferation, DNA replication
Introduction
Menadione (2-methyl-1, 4 naphthoquinone: vitamin K3) inhibits the growth of mammalian tumor cells both in vitro and in vivo, e.g. mouse or rat liver tumors, glioma, melanoma and neuroblastoma, cells [1, 2], human glioma [3], hepatoma cells [4] and urologic tumors [5]. Moreover, a potentiation of radiotherapy by vitamin K has been shown [2] as well as a synergistic effect when given in combination with other anti-tumor agents [6]. Menadione has also effects on MDR1-expressing cancer cells both in culture and animal model systems [7]. It has been suggested that the cytotoxic effect of menadione is related to the generation of reactive oxygen species (ROS) by enzymatic reaction during its metabolism [8]. Menadione can generates ROS by non-enzymatic reaction with protein thiols in plasma [9]. Consistent with this observation, the thiol-depleting agent N-ethylmaleimide (NEM) suppresses menadione-induced ROS generation and cytotoxicity to platelet. Menadione was also found to inhibit topoisomerase II [10], DNA polymerase gamma [11] and binds to tubulin, disrupting the microtubule networks [12]. Menadione, being a small lipid soluble molecule (M.W. 172.2 Da) is a potential drug for the treatment of brain tumors. As pointed out by Pardridge [13], only lipid soluble molecules with a molecular mass less than 400–600 Da are capable of crossing the blood brain barrier (BBB). The anti-proliferative effect of menadione has been investigated in several glioma cell cultures using classical short term (24–72) proliferation assays. In this type of assays, the concentration that inhibits proliferation by 50% (IC50) is the endpoint parameter usually measured. For most drugs, even at concentrations higher than the IC50, the short exposure time does not kill 100% of cells and the IC100 (or lethal concentration 100, LC100) is calculated by interpolation. In most studies, the fate of surviving cells after short term incubation with drugs is not evaluated. For instance, glioma cells can survive prolonged exposure of anti-neoplastic drugs at concentrations much higher than the IC50 [14]. At the clinical level, these surviving cells explain the relapse of tumor when the treatment is discontinued and suggest that drugs that kill 100% of tumoral cells (“pankillers”) will be more effective anti-cancer drugs and may, cure cancer. To circumvent this limitation, we recently postulated that long-proliferation assays and the determination of the minimum concentration and time necessary to kill cancer cells completely and prevent regrowth when the drug is removed from the culture (called Regrowth Concentration Zero, RC0) might help to improve the success of anti-cancer drugs entering clinical trials. In this study, we investigated the anti-proliferative effect of short and prolonged exposure of menadione on glioma cells. We found in the current study that menadione is an effective “pankiller” and its cytotoxic effect can be potentiated when used in combination with vitamin C.
Materials and methods
Reagents and enzymes
Dimethylsulfoxide (DMSO), menadione and vitamin C were purchased from Sigma (Sweden). Dulbecco modified Eagle’s medium with glutamine and 4500 mg/l D-glucose (DMEM) and (Iscove's modified Dulbecco medium) IMDM were purchased from GIBCO/Life-technologies (Sweden). [methyl-3H]-thymidine (86.0 Ci/mmol) was purchased from Amersham (U.K.). All other reagents were of analytical grade or the highest grade available.
Cell lines
Stock cultures of human DBTRG-05MG glioma cell line were obtained from the European Collection of Cell Culture (ECACC). Cells were routinely cultured as previously described [14]. Patients-derived glioblastoma cells hGCL1- hGCL8 were kindly provided by Dr Peter Siesjö (Lund University). The usage of patient glioblastoma multiforme tumor samples has been accepted by the Local Ethical Board of the University of Lund.
Preparation of drugs
Menadione was prepared as stock solutions (100 mM) in DMSO and stored at −20°C. Vitamin C was diluted in distilated sterile water and stored at −20°C as 500 mM stock solution. The final dilutions were done in culture media, keeping the DMSO concentration below 1% (v/v).
Experimental procedures
Determination of ongoing DNA replication
Generation of tissue mini-units, determination of [methyl-3H]-thymidine incorporation into DNA and ongoing DNA synthesis rate, protein quantitation and determination of effect of menadione were performed as previously reported [15, 16]. Briefly, mini-units of normal rat cerebral tissue were generated immediately after killing the animals. These mini-units were then incubated in microwell plates (Nunc, Denmark) with DMEM containing 2 μCi/ml [methyl-3H]-thymidine and drugs at the appropriate concentration or their corresponding vehicle (DMSO or H2O). Each experimental point was determined by at least quadruplicate. The DNA synthesis rate was calculated as cpm/mg of protein/min. The effect of drugs was determined as change (%) of DNA synthesis rate compared to the corresponding control or percentage of control DNA synthesis rate. Animal experiments were approved by the Animal Ethical Committee at Huddinge University Hospital, Sweden.
Short term proliferation assay: Cell were plated in 96 well microplates (~5,000 cell/well) and allowed to adhere overnight. Drugs at the appropriate concentration were added and incubated for 72 h. Cell viability was measured by the cell counting kit (CCK Kit) (Sigma, Sweden) following manufacturer’s instructions.
Long term proliferation assay: For prolonged effect of drugs on cell cultures, cells were plated in 96 well microplates (~5,000 cell/well) and allowed to grow for 3–4 days. Drugs were added and maintained for 2 weeks (media and drugs were changed twice a week). After 2 weeks, cells were incubated in drug-free media (changed twice a week) for 2–4 weeks. Regrowth was evaluated using a routine inverted microscope.
Results
Age dependent inhibition of DNA synthesis by menadione in normal rat cerebral cortex
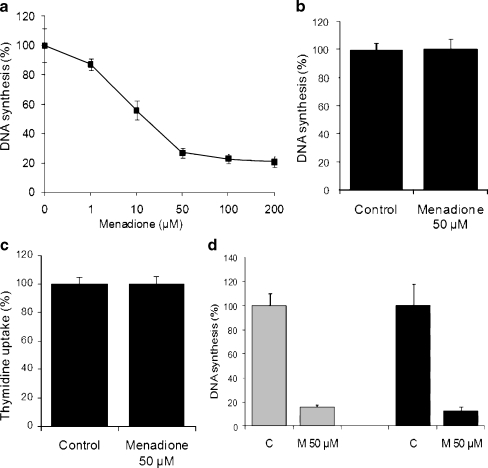
The results obtained from tissue mini-units prepared from 5 days old rats showed a dose-dependent inhibition of the DNA synthesis in menadione treated mini-units (IC50 ≅ 10 μM) (Fig. 1a). This inhibitory effect was of short time onset as well as time-dependent since the inhibitory effect of 50 μM menadione at 30 and 60 min were 34,66 ± 2,90% and 84,36 ± 2,25% respectively (data not shown). When tissue mini-units were prepared from adult rats (>60 days old), menadione (50 μM) failed to decrease the rate of DNA synthesis (Fig. 1b). When uptake experiments were performed (see “Materials and methods”), menadione (50 μM) did not inhibit the cellular uptake of [methyl-3H]-thymidine prior to incorporation into DNA (Fig. 1c). Further, the reversibility of menadione effect on the incorporation [methyl-3H]-thymidine into DNA showed that, a) 60 min of pre-incubation with menadione gave a strong (84,36 ± 2,25%) inhibition of DNA synthesis (Fig. 1d, grey columns), and b) removal of menadione from the medium failed to recover the capacity of the tissue to incorporate the radioactive precursor into DNA (Fig. 1d, black columns). In all cases, DMSO was used as control in concentrations below 1% (v/v) since it has been shown that DMSO at concentrations up to 1% (v/v) for 3 h has no effect on DNA synthesis rate.
Fig. 1.
Age-dependent inhibitory effect of menadione on normal rat cerebral cortex. a Concentration-dependent effect of menadione on DNA synthesis in tissue mini-units prepared from 5 days old rat cerebral cortex. b Effect of menadione (50 μM) on DNA synthesis in tissue mini-units prepared from >60 days postnatal rat cerebral cortex. Control mini-units (expressed as: 100% DNA synthesis) were incubated with equivalent concentrations of vehicle (0.2% DMSO). c Effect of menadione on [methyl-3H]-thymidine uptake. The tissue mini-units prepared from the cerebral cortex from 5 days postnatal age rats were incubated with 50 μM menadione or equivalent concentration of DMSO. After 60 min [methyl-3H]-thymidine (final concentration 4 μCi/ml) was added to each well, incubated for 15 min and the amount of radioactivity incorporated into the cell was determined as described in Materials and Methods. d Reversibility of menadione-mediated DNA synthesis inhibition. Mini-units were incubated with DMSO alone (C) or menadione 50 μM (M) for 60 min with DMEM containing 4 μCi/ml [methyl-3H]-thymidine (grey columns) or without [methyl-3H]-thymidine (black columns). After incubation, the samples were processed for measurement of [methyl-3H]-thymidine incorporation into DNA (grey columns) or washed, incubated with DMEM containing 4 μCi/ml [methyl-3H]-thymidine for 90 min and then processed for measurement of [methyl-3H]-thymidine incorporation into DNA (black columns). All data are the results (mean±SEM) of 2-3 independent experiments performed by quadruplicate
Effect of menadione on DBTRG.05MG human glioma cells
The relative lack of inhibition by menadione on ongoing DNA synthesis in adult normal rat cerebral cortex suggested that the drug might be useful as anticancer drugs for brain tumors in adults because of potential lower toxicity to normal cells. In an adult animal model of glioma, we previously showed that the ongoing DNA synthesis rate was relatively high in tumor tissue compared to normal brain tissue [17]. Unfortunately, the tissue mini-unit system does not allow the study of prolonged drug incubation time. For this reason we next evaluated the anti-proliferative effect of menadione on the human DBTRG.05MG glioma cells using a standard short term (72 h) proliferation assay. Since menadione effect might be serum dependent we performed concentration- responses experiments in serum-free as well as serum-containing media. Menadione alone inhibits cell proliferation with similar efficiency in both media (data not shown). Vitamin C alone in the range 0.1-5 mM slightly inhibited cell proliferation and the combination of both drugs in the ratio 1:100 (e.g. menadione 1 μM + Vitamin C 0.1 M) showed modest increase in potency compared to menadione alone (Fig. 2).
Fig. 2.
Anti-proliferative effect of short term incubation with menadione (M), vitamin C (VC) and menadione:vitamin C (M+VC). DBTRG-05MG glioma cells were incubated with the indicated concentration of drugs for 72 h. Cell proliferation was measured by the CCK kit. Results are representative of two independent experiments performed by quadruplicates
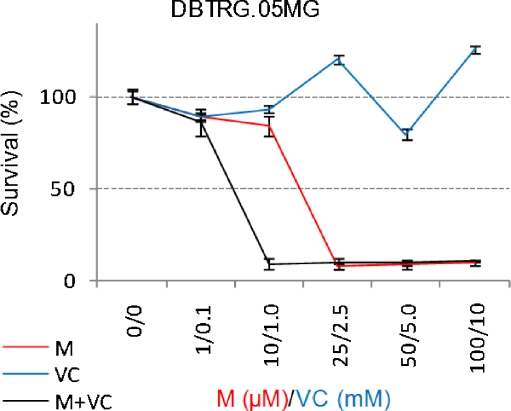
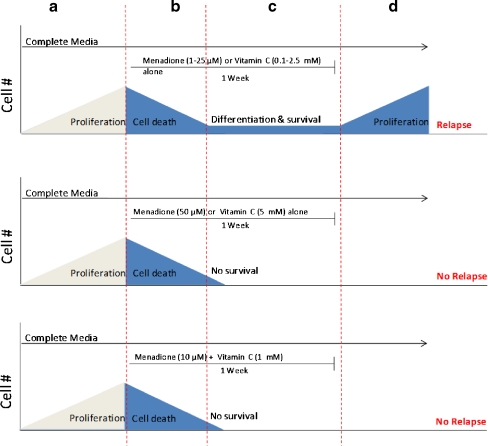
In order to evaluate the effect of long term incubation of these drugs, DBTRG.05MG cells were incubated with different concentrations of each drug alone or in combination for 2 weeks as described in Materials and Methods and the RC0 parameter was determined. Menadione (1–25 μM) alone or vitamin C alone (0.1–2.5 mM) failed to kill 100% of glioma cells: a small fraction of cells survived up to 1 week exposure and were able to resume growth when cells were cultured in drug-free media (Fig. 3 Top). To prevent regrowth of DBTRG.05MG cells, menadione at 50 μM for one week (Fig. 3 Middle) or 25 μM for 2 weeks (data not shown) were required. Vitamin C alone failed to prevent regrowth unless a very high concentration (> 5 mM) for 1 week was used (Fig. 3 Top and Middle). When used in combination at relatively low doses (M:VC at 10 μM:1 mM) for one week M:VC was able to prevent regrowth (Fig. 3 Bottom). Thus, the M:VC combination is a “pankiller” being able to prevent regrowth of glioma cells when the drug is removed from the media.
Fig. 3.
Menadione:vitamin C combination inhibit cell proliferation and prevent regrowth of human glioma cells at doses lower than individual drugs. Anti-proliferative effect of long -term incubation with menadione alone (M), vitamin C (VC) alone or Menadione:vitamin C at a 1:100 ratio. Top: Exponentially growing cells were incubated in complete media for 2–3 days (a), exposed to 1–25 μM menadione or 0.1–2.5 mM vitamin C for 1 week (b-c). During this period the media and the drugs were changed twice a week. A clear decrease in the cell density was observed by microscopic examination indicating extensive cell death (b) followed by a stable low cell density (c). When the drug was removed, the surviving cells resumed proliferation and formed a monolayer indicated as regrowth (d). Middle: same as top but cells were incubated with menadione 50 μM or vitamin C 5 mM for 1 week. Bottom: same as top but cells were incubated with menadione 10 μM + vitamin C 1 mM for 1 week
Menadione:vitamin C (10 μM:1 mM) shows similar antiproliferative activity in a panel of 8 patient derived cell cultures
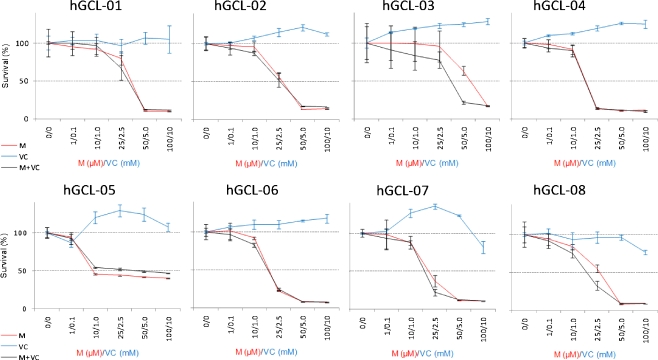
In order to evaluate the possibility that menadione:vitamin C might show differential potency for other glioma cells, we investigated the effect of selected drug treatment in a panel of 8 different patient derived cell cultures (hGCL1-8, Table 1). Short term assays (72 h) showed modest or no difference between menadione alone and menadione+vitamin C. In long-term proliferation assays, despite the fact that some glioma cells were slightly resistant to menadione alone (e.g. few live cells were present after 2 weeks of menadione treatment with 25–50 μM) the M:VC combination for 1 week (10 μM:1 mM) was able to prevent the regrowth of all cell cultures when the drug was removed from the culture (Table 1).
Table 1.
Regrowth of glioma cells after prolonged treatment with Menadione (M), vitamin C (VC) or Menadione + Vitamin C (M:VC). Exponentially growing cells were incubated in complete media for 2–3 days, exposed to the indicated concentration of menadione , vitamin C or menadione:Vitamin C for 1 week and evaluated for regrowth after incubation in drug-free media for 2–4 weeks. (+) and (−) indicates ability to resume growth when the drug was removed from the culture media
| hGCL1 | hGCL2 | hGCL3 | hGCL4 | hGCL5 | hGCL6 | hGCL7 | hGCL8 | ||
|---|---|---|---|---|---|---|---|---|---|
| M (μM) | 10 | + | + | + | + | + | + | + | + |
| 25 | +a | − | +a | − | − | +a | − | − | |
| VC (mM) | 0.1 | + | + | + | + | + | + | + | + |
| 2.5 | + | + | + | + | + | + | + | + | |
| M:VC (μM:mM) | 10:1 | − | − | − | − | − | − | − | − |
| M:VC (μM:mM) | 25:2.5 | − | − | − | − | − | − | − | − |
aFew cells were observed
Discussion
In the present study, we first showed an age-dependent inhibitory effect of menadione on normal rat cerebral cortex (Fig. 1) suggesting that menadione might preferentially kill fast proliferating cells. Since glioma mostly occurs in the adult population where menadione toxicity to normal tissue might be low, menadione can be exploited to kill fast proliferating tumor cells with relatively low toxicity to slow proliferating normal cells. Indeed, while 50 μM menadione showed no effect on ongoing DNA replication in adult normal rat cerebral cortex (Fig. 1), the IC50 for the human DBTRG.05MG glioma line and patient-derived glioma cells was in the 10–25 μM range (Figs. 2 and 4). In our study, the data obtained from the tissue mini-unit system and the cell lines cannot be compared because they are different experimental systems and different exposure times were used. However, several groups had reported that low menadione concentration (10–50 μM) are relatively well tolerated by normal glial cells [18, 19]. The tissue mini-unit system preserves the metabolic and proliferative properties of the tissue in situ and thus, suggests that menadione might be also less toxic for other neural cell types on adult tissue compared to young brain tissue. The early effect of menadione on ongoing DNA replication was irreversible (Fig. 1d), suggesting that the drug does not need to be continuously present to exert its anti-proliferative effect. Before clinical trials, toxicity to other normal neural cell types (neurons and neural stem cells) should be evaluated. Unfortunately, the tissue mini-unit system does not allow the evaluation of prolonged drug exposure and our assay did not measure viability of non-proliferating cells (e.g. neurons). On the other hand, even if available, studies on cell lines representing each neural cell type will not solve this problem since they are no representative of intact tissue due to loss of cell-cell interactions. Future studies on animal models are needed to address these important issues about toxicity. Regardless of this issue, strategies to potentiate the anti-proliferative effect of low menadione concentrations might help to selectively kill tumor cells. In this context, the combination of menadione+vitamin C has been explored in several cancer cell lines with encouraging results since this combination seems to selectively kill cancer cells by a novel mechanism of cell death called autoschizis [20, 21]. The cell injury of the menadione+vitamin C has been associated with oxidative stress that affect the cytoeskeletal architecture [22]. Additional DNA damage might be caused by reactivation of DNAse activity that has been found to be negative in malignant tumors of the central nervous system [22, 23]. The anti-proliferative effect of menadione has been studied in several glioma cell lines and showed IC50 values ranging between 13.5 μM to ~25 μM [1, 24] . We recently suggested that drug concentrations much higher than the IC50 might be required to eliminate 100% of tumor cells and that the IC50 is not the best parameter to evaluate anti-cancer drugs because it does not select for useful clinical drugs [14].
Fig. 4.
Menadione alone and menadione:vitamin C showed similar anti-proliferative potency in a panel of 8 different patients derived glioma cells. Anti-proliferative effect of short term incubation with menadione alone (M), vitamin C (VC) alone or menadione:vitamin C at a 1:100 ratio. hGCL1-8 cells were incubated with the indicated concentration of drugs for 72 h. Cell proliferation was measured by the CCK kit
In our study, the IC50 for the DBTRG.05MG human cell line and other patient derived cells hGCL1-8 were around 10–25 μM but at least one week exposure to 50 μM menadione alone was necessary to prevent regrowth of surviving cells. Increasing menadione concentration will also increase toxicity to normal cells. To circumvent this problem, combination with other drugs might selectively kill cancer cells without increasing toxicity to normal cells. In the present study, the addition of vitamin C at a 1:100 ratio showed that it is possible to reduce the concentration of menadione needed to kill 100% of glioma cells from 25–50 μM to 10 μM. The combination also reduced the vitamin C concentration needed to kill 100% of glioma cells from 5 to 1 mM. Thus, this combination exhibited anti-tumor activity (cytotoxicity) at concentrations that were 2.5- to 5-fold lower than for the individual drugs. As suggested by Matzno et al. [25], the use of lower menadione concentration is advantageous because it makes easier to control the useful therapeutic range below toxic values due to the steepness of the concentration-response curve (Figs. 2 and 4). In agreement with the literature , anti-tumor activity at concentrations that were 4- to 61-fold lower than for the individual vitamins was found in other types of cancer [26]. However, results from our present data and those reported should be carefully evaluated considering methods and experimental designs. For instance, our values between 2 to 5-fold lower were obtained measuring regrowth concentration zero (RC0) as endpoint parameter while the reported values of 4- to 61 –fold values were obtained by determination of CD50 values by the MTT assay following 1 h treatment [27]. When 5 days continuous exposure was used, a lower synergistic effect (between 6 to 7 fold lower) was obtained [27]. It is possible that the RC0 may be similar for most cancer cells and only 2.5 to 5-fold reduction can be obtained for each drug. Due to the heterogeneous nature of gliomas, the evaluation of several cell lines is important since some cell lines might show different sensitivity to a particular drug as it has been reported for temozolomide [28]. In conclusion, the menadione:vitamin C combination at 10 μM:1 mM may be more selective against glioma cells and less toxic for normal brain tissue and should be a strong argument to encourage animal studies in order to evaluate the anti-proliferative effect on glioma models in addition to the overall systemic toxicity.
Acknowledgements
This study was supported by grants from the Swedish Research Council and the Karolinska Institute. We thank Dr Peter Siesjö for providing the patient derived cells (hGCL1-8).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Marina F. Vita and Nivedita Nagachar contributed equally to this work.
References
- 1.Prasad KN, Edwards-Prasad J, Sakamoto A. Vitamin K3 (menadione) inhibits the growth of mammalian tumor cells in culture. Life Sci. 1981;29:1387–1392. doi: 10.1016/0024-3205(81)90683-4. [DOI] [PubMed] [Google Scholar]
- 2.Taper HS, Keyeux A, Roberfroid M. Potentiation of radiotherapy by nontoxic pretreatment with combined vitamins C and K3 in mice bearing solid transplantable tumor. Anticancer Res. 1996;16:499–504. [PubMed] [Google Scholar]
- 3.Sun LK, Yoshii Y, Miyagi K. Cytotoxic effect through Fas/APO-1 expression due to Vitamin K in human glioma cells. J Neurooncol. 2000;47:31–38. doi: 10.1023/A:1006443422488. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa Y, Carr BJ, Wang M, Kar S, Finn F, Dowd P, Zheng ZB, Kerns J, Naganathan S. Growth inhibition of hepatoma cells induced by vitamin K and its analogs. J Biol Chem. 1995;270:28304–28310. doi: 10.1074/jbc.270.47.28304. [DOI] [PubMed] [Google Scholar]
- 5.Venugopal M, Jamison MJ, Gilloteaux J, Koch JA, Summers M, Giammar D, Sowick C, Summers JL. Synergistic antitumor activity of vitamins C and K3 on human urologic tumor cell lines. Life Sci. 1996;59:1389–1400. doi: 10.1016/0024-3205(96)00466-3. [DOI] [PubMed] [Google Scholar]
- 6.Liao WC, Wu FY, Wu WC. Binary/ternary combined effects of vitamin K3 with other antitumor agents in nasopharyngeal carcinoma CG1 cells. Int J Oncol. 2000;17:323–328. [PubMed] [Google Scholar]
- 7.Chen AY, Liu LF. Mechanisms of resistance to topoisomerase inhibitors. Cancer Treat Res. 1994;73:263–281. doi: 10.1007/978-1-4615-2632-2_13. [DOI] [PubMed] [Google Scholar]
- 8.Okayasu H, Ishihara M, Satoh K, Sakagami H. Cytotoxic activity of vitamins K1, K2 and K3 against human oral tumor cell lines. Anticancer Res. 2001;21:2387–2392. [PubMed] [Google Scholar]
- 9.Chung SH, Chung SM, Lee JY, Kim SR, Park KS, Chung JH. The biological significance of non-enzymatic reaction of menadione with plasma thiols: enhancement of menadione-induced cytotoxicity to platelets by the presence of blood plasma. FEBS Lett. 1999;449:235–240. doi: 10.1016/S0014-5793(99)00452-4. [DOI] [PubMed] [Google Scholar]
- 10.Chen AY, Yu C, Lee WH, Peng LF, Liu LF. Menadione (vitamin K3) induces topoisomerase II-mediated DNA cleavage. Proc Am Assoc Cancer Res. 1992;33:2588. [Google Scholar]
- 11.Sasaki R, Suzuki Y, Yonezawa Y, Ota Y, Okamoto Y, Demizu Y, Huang P, Yoshida H, Sugimura K, Mizushina Y. DNA polymerase gamma inhibition by vitamin K3 induces mitochondria-mediated cytotoxicity in human cancer cells. Cancer Sci. 2008;99:1040–1048. doi: 10.1111/j.1349-7006.2008.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acharya BR, Choudhury D, Das A, Chakrabarti G. Vitamin K3 disrupts the microtubule networks by binding to tubulin: a novel mechanism of its antiproliferative activity. Biochemistry. 2009;48:6963–6974. doi: 10.1021/bi900152k. [DOI] [PubMed] [Google Scholar]
- 13.Pardridge WM. CNS drug design based on principles of blood-brain barrier transport. J Neurochem. 1998;70:1781–1792. doi: 10.1046/j.1471-4159.1998.70051781.x. [DOI] [PubMed] [Google Scholar]
- 14.Avramidis D, Cruz M, Sidén Å, Tasat DR, Yakisich JS. Regrowth concentration zero (RC0) as complementary endpoint parameter to evaluate compound candidates during preclinical drug development for cancer treatment. J Canc Sci Ther. 2009;1:19–24. doi: 10.4172/1948-5956.1000003. [DOI] [Google Scholar]
- 15.Yakisich JS, Sidén Å, Idoyaga Vargas V, Eneroth P, Cruz M. Fast and sensitive method for simultaneous measurement of cell proliferation rate and drug sensitivity in rat cerebral cortex. Exp Neurol. 1998;151:194–202. doi: 10.1006/exnr.1998.6814. [DOI] [PubMed] [Google Scholar]
- 16.Yakisich JS, Sidén Å, Idoyaga Vargas V, Eneroth P, Cruz M. Early effects of protein kinase modulator on DNA synthesis in rat cerebral cortex. Exp Neurol. 1999;159:164–176. doi: 10.1006/exnr.1999.7121. [DOI] [PubMed] [Google Scholar]
- 17.Yakisich JS, Vita MF, Siden A, Tasat DR, Cruz M. Strong inhibition of replicative DNA synthesis in the developing rat cerebral cortex and glioma cells by roscovitine. Invest New Drugs. 2009;28:299–305. doi: 10.1007/s10637-009-9254-4. [DOI] [PubMed] [Google Scholar]
- 18.Abe K, Saito H. Menadione toxicity in cultured rat cortical astrocytes. Jpn J Pharmacol. 1996;72:299–306. doi: 10.1254/jjp.72.299. [DOI] [PubMed] [Google Scholar]
- 19.Öztopcu-Vatan P, Kabadere S. The effects of menadione on rat glial cell proliferation. J Neurol Sci (Turkish) 2007;24:25–28. [Google Scholar]
- 20.Gilloteaux J, Jamison JM, Lorimer HE, Jarjoura D, Taper HS, Calderon PB, Neal DR, Summers JL. Autoschizis: a new form of cell death for human ovarian carcinoma cells following ascorbate:menadione treatment. Nuclear and DNA degradation. Tissue Cell. 2004;36:197–209. doi: 10.1016/j.tice.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Gilloteaux J, Jamison JM, Neal DR, Summers JL. Cell death by autoschizis in TRAMP prostate carcinoma cells as a result of treatment by ascorbate: menadione combination. Ultrastruct Pathol. 2005;29:221–235. doi: 10.1080/01913120590951239. [DOI] [PubMed] [Google Scholar]
- 22.Gilloteaux J, Jamison JM, Neal DR, Loukas M, Doberzstyn T, Summers JL. Cell damage and death by autoschizis in human bladder (RT4) carcinoma cells resulting from treatment with ascorbate and menadione. Ultrastruct Pathol. 2010;34:140–160. doi: 10.3109/01913121003662304. [DOI] [PubMed] [Google Scholar]
- 23.Taper HS, Brucher JM, Fort L. Activity of alkaline and acid nucleases in tumors of the human central nervous system. Histochemical study. Cancer. 1971;28:482–490. doi: 10.1002/1097-0142(197108)28:2<482::AID-CNCR2820280230>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Oztopçu P, Kabadere S, Mercangoz A, Uyar R. Comparison of vitamins K1, K2 and K3 effects on growth of rat glioma and human glioblastoma multiforme cells in vitro. Acta Neurol Belg. 2004;104:106–110. [PubMed] [Google Scholar]
- 25.Matzno S, Yamaguchi Y, Akiyoshi T, Nakabayashi T, Matsuyama K. An attempt to evaluate the effect of vitamin K3 using as an enhancer of anticancer agents. Biol Pharm Bull. 2008;31:1270–1273. doi: 10.1248/bpb.31.1270. [DOI] [PubMed] [Google Scholar]
- 26.Gilloteaux J, Jamison JM, Arnold D, Neal DR, Summers JL. Morphology and DNA degeneration during autoschizic cell death in bladder carcinoma T24 cells induced by ascorbate and menadione treatment. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:58–83. doi: 10.1002/ar.a.20276. [DOI] [PubMed] [Google Scholar]
- 27.Jamison JM, Gilloteaux J, Nassiri MR, Venugopal M, Neal DR, Summers JL. Cell cycle arrest and autoschizis in a human bladder carcinoma cell line following Vitamin C and Vitamin K3 treatment. Biochem Pharmacol. 2004;67:337–351. doi: 10.1016/j.bcp.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Sankar A, Thomas DG, Darling JL. Sensitivity of short-term cultures derived from human malignant glioma to the anti-cancer drug temozolomide. Anticancer Drugs. 1999;10:179–185. doi: 10.1097/00001813-199902000-00006. [DOI] [PubMed] [Google Scholar]