Abstract
An examination was made of fluoride content in the mandibular first molars of the permanent teeth of the red fox Vulpes vulpes living in north-west (NW) Poland. The teeth were first dried to a constant weight at 105°C and then ashed. Fluorides were determined potentiometrically, and their concentrations were expressed in dry weight (DW) and ash. The results were used to perform an indirect estimation of fluoride pollution in the examined region of Poland. The collected specimens (n = 35) were classified into one of the three age categories: immature (im, 6–12 months), subadult (subad, from 12 to 20 months) and adult (ad, >20 months). The mean concentrations (geometric mean) of fluoride were similar in the im and subad groups (230 and 296 mg/kg DW and 297 and 385 mg/kg ash, respectively), and significantly smaller than in the ad group (504 and 654 mg/kg, respectively, in DW and ash). Basing on other reports that the ∼400 mg/kg DW concentration of fluoride in bones in the long-lived wild mammals generally reflects the geochemical background, it was found that 57% of the foxes in NW Poland exceeded this value by 9% to 170%. This indirectly reflects a moderate fluoride contamination in the tested region.
Keywords: Fluoride, Red fox Vulpes vulpes, Teeth, Bioindicator, Bioaccumulation
Introduction
The fluoride pollution of anthropogenic origin has been increasing over the last several decades. Its main sources are aluminium, iron and glass mills; plants manufacturing phosphatic fertilizers, cement and bricks; energy plants using coal and petroleum and artificially fluoridated water. Although the adverse effect of fluoride pollution is stronger in aquatic ecosystems than in terrestrial ones (Inorganic Fluorides—PSL1 1993; Camargo 2003; Tataruch and Kierdorf 2004; Ozsvath 2009), the elevated concentrations of fluoride do affect humans and other mammals.
The most important biological materials used for the determination of fluoride in warm-blooded vertebrates are plasma, urine and bone. In bones, including teeth, fluoride binds calcium and replaces the hydroxyl groups in the mineral part of the bone, which is mostly hydroxyapatite (Walton 1988; Den Besten 1994; Harrison 2005). This accumulation reflects the long-term impact of exposure to fluorides (Bezerra de Menezes et al. 2003; Weinstein and Davison 2004; Ozsvath 2009). Although fluoride deficiency is one of the factors contributing to the development of dental caries, the excess levels of this element can lead to dental fluorosis, and in extreme cases, may cause bone fluorosis and carcinogenesis (Vieira et al. 2005; Bassin et al. 2006; Ozsvath 2009; Verkerk 2010).
Many studies have examined the bones and the teeth of wild and domesticated animals for indirect assessment of the degree of environmental contamination with fluorides. They show that the concentrations of fluorides in mammals generally increase with age as a result of bioaccumulation. Bone samples are most often collected from herbivorous ungulates, for example European red deer (Cervus elaphus), white-tailed deer (Odocoileus virginianus), roe deer (Capreolus capreolus), sheep (Ovis aries) and horse (Equus caballus), all considered to be good bioindicators of fluoride pollution (Suttie et al. 1987; Kierdorf et al. 1996; Boulton et al. 1999; Tataruch and Kierdorf 2004; Weinstein and Davison 2004; Choubisa 2008; Macicek and Krook 2008; Telesiński and Śnioszek 2009; Jelenko and Pokorny 2010). Similar studies have been performed in north-west Poland, where a major producer of phosphatic fertilizers is located (“Police” SA). In these studies, fluoride concentrations were determined in the bones and the teeth of deer from the family Cervidae (Dabkowska et al. 1995; Zakrzewska 1996; Gutowska et al. 2004; Zakrzewska et al. 2005). Less frequent ecotoxicological analyses of fluoride were conducted in omnivorous wild mammals (e.g. wild boar Sus scrofa), and were only sometimes performed on carnivores, including canids (Walton 1988; Kierdorf et al. 2000; Weinstein and Davison 2004).
The red fox (Vulpes vulpes), belonging to the family Canidae, is the most numerous and widely hunted carnivorous mammal species in the world. Its natural range covers almost the whole of the northern hemisphere, and in the nineteenth century, the red fox was introduced into Australia (Macdonald and Reynolds 2004). In the 1980s, the concentrations of fluoride were first examined in the bones of many representatives of the red fox population inhabiting the different parts of Great Britain. The results demonstrated that foxes from the vicinity of an aluminium reduction plant in Wales had fluoride concentration several times higher in the bones than foxes from the other regions of Great Britain (Walton 1984). A similar pilot study of fluoride in the teeth of a small group of red foxes, regardless of their age, was first conducted in Poland by Kalisińska and Palczewska (2007). We have found no other data on fluoride concentrations in the bone materials of wild red foxes living in other European countries, Asia, North America and Australia. Little is known about the concentrations of fluorides in wild and domesticated predatory mammals with a chronic exposure to low concentrations of these compounds, including the red fox and dog (Canis familiaris), although a study on the silver fox from breeding farms has shown that chronic ingestion of excessive amounts of fluoride is associated with agalactia, stunted postnatal growth of offspring and high pup mortality (Eckerlin et al. 1988).
It can be assumed that fluorides in the environment accumulate in the teeth of the fox in different quantities, depending on their concentrations and duration of exposure. Some differences may occur related to the age of the animals. The aim of this study was to determine the age-specific concentrations of fluoride in the teeth of red foxes living in north-west Poland and to compare the data with other available reports on the teeth of other mammals, especially those inhabiting the same area. In addition, we aimed to assess the suitability of the red fox as a bioindicator in ecotoxicological studies on fluorides.
Material and methods
Study area
The examined red foxes came from the north-west, NW, of Poland, from the western part of the West Pomeranian Voivodship, WV (districts: Police, Gryfino, Szczecin, Goleniów, Kamień Pomorski and Świnoujście) and from the south-east part of the Pomeranian Voivodship, PV (district Człuchów; Fig. 1). The Człuchów district, located about 200 km E of Szczecin, has no major industrial plants, and the region is considered only slightly contaminated. The city of Szczecin (410,000 inhabitants) is the capital of WV. In the western part of the region, there are large industrial plants (including Chemical Works “Police” SA, Dolna Odra Coal Power Plant, Coal Power Plant Szczecin-Pomorzany), which emit sulphur oxides, nitrogen oxides and water-soluble fluoride compounds. Significant pollution of the fluoride used to be reported primarily in the Police district, where large chemical plants have been operating since 1970 (Dabkowska et al. 1995). Among others, the plants produce phosphoric fertilizers, phosphoric acid and fluorosilicate. In the past several years, they have undergone modernization, and emissions of fluorides to the atmosphere have been minimized. The Chemical Works “Police” SA lies 15 km north of Szczecin at the mouth of the Odra River, which flows to the Szczecin Lagoon. Near Police, on the left bank of the Odra River, there are immense heaps of industrial waste (phosphogypsum), which reach a height of approximately 25 m and cover an area of 280 ha. The mass of the phosphogypsum is estimated at 83 million tonnes (ES WV 2009). The surrounding environment is still being affected by leaks and dust from the not yet fully rehabilitated waste dumps. The prevailing winds in the NW part of Poland blow from the west.
Fig. 1.
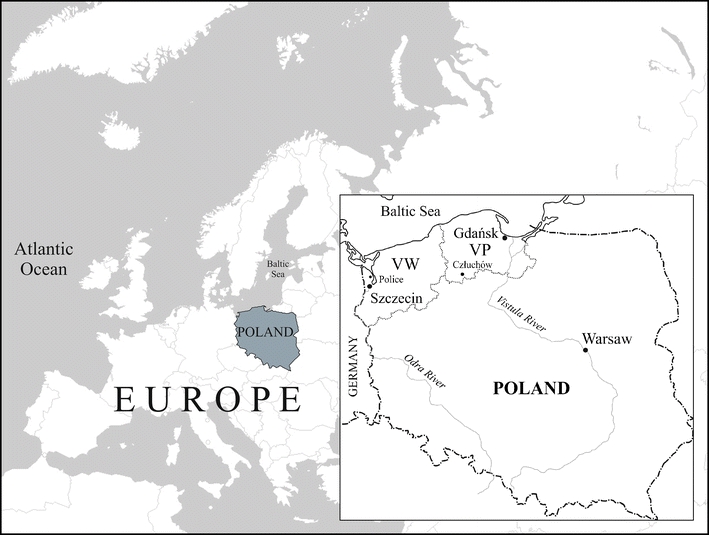
Study area (Szczecin co-ordinates 53.430° N, 14.529° E): VW West Pomeranian Voivodship (województwo zachodniopomorskie), VP Pomeranian Voivodship (województwo pomorskie)
Experimental procedures
The research was carried out on 35 red foxes (V. vulpes, L., 1758) hunted in NW Poland (from West Pomeranian and Pomeranian Voivodships: 26 and 9 individuals, respectively) in the hunting seasons 2004/2005 and 2005/2006. The age of the foxes was determined by the examination of the fusion of cranial sutures, as proposed by Churcher (1960). The foxes were classified into one of the three age categories: immature (im) aged 6–12 months (n = 11), subadult (subad) from 12 to 20 months (n = 10) and adult (ad) older than 20 months (n = 14). Out of the 35 foxes, 40% were over 20 months old and 60% were younger than 20 months (im + subad).
The first permanent molars (Fig. 2) collected from the left mandibular arch were analysed. The air-dry molars (ADMs) were weighed to an accuracy of 0.0001 g. The teeth were dried at 105°C to dry mass (DM105) for 4 days, which allowed the determination of water content (gravimetric method). On macroscopical inspections, none of the analysed teeth showed signs of dental fluorosis. The molars were burned in an oven at 700°C for 8 h. The percentage of dry weight (DW) and ash were determined in ADMs and DM105. From each incinerated tooth, ground in an agate mortar, two samples were taken for analysis, each ca. 100 mg. The sample was dissolved in 5 ml of perchloric acid at a concentration of 1.13 mol/l. We then added 50 ml of 0.2 mol/l solution of sodium citrate, and added double-distilled water to reach 100 ml. Before each determination, pH was regulated to 5.5 using perchloric acid or NaOH. The concentration of fluoride ions (F−) was determined by potentiometry. In the solution with the sample, F− concentration was measured using a fluoride ion-selective electrode (Detektor) and a CX-731 ion meter (Elmetron). The reference electrode for measuring potential difference was a silver–silver chloride electrode with an external coating containing 1 mol/l of KNO3 solution. Statistical analysis used means that were obtained from each pair of the samples. The concentrations of fluorides in the teeth were converted per 1 kg DW of tooth and ash derived from the tooth. These values were expressed in milligrammes per kilogramme.
Fig. 2.
Red fox tooth used for fluoride determination
Statistical analysis involved the determination of the arithmetic mean, standard deviation from that mean and coefficient of variation and the geometric mean with regard to concentrations of fluorides. Taking into account the age categories, the comparisons of pairs were carried out between the percentage of ash content in the ADM and DM105 samples and the concentration of fluorides in the DW and ash. In addition, the significance of difference in fluoride concentrations between red foxes from the more polluted (WV) and low-polluted region (PV) was assessed. These comparisons and correlations were performed using a Student t test. All calculations were performed using the computer program Statistica.
Results
Table 1 lists the mean percentage of dry mass in the ADMs of the examined red foxes and the percentage of ash in the air-dried and dried to constant weight (DM105). All the means in Table 1 are similar and have small coefficients of variation (CV<2.5%). Mean dry mass of a fox molar is 93.6% of ADM, and the proportion of ash in the ADM and DM105 is 72.3% and 77.3%, respectively. The percentage of ash in ADM is significantly correlated (p < 0.001) with the percentage of dry matter content in ADM (r = 0.646) and the percentage of ash in DM105 (r = 0.896). There was no significant relationship between age and the ash content in ADM and DM105.
Table 1.
The percentage content of dry mass in the air dried tooth, ADM and of ash in ADM as well as in a tooth dried to constant mass at 105°C (DM105)
| Age group (n) | Parameter | Percent content of dry mass in a tooth (ADM) | Percent ash content | |
|---|---|---|---|---|
| ADM teeth | DM105 teeth | |||
| Immature, im (n = 11) | AM ± SD | 93.6 ± 0.8 | 72.7 ± 1.4 | 77.7 ± 0.9 |
| CV | 0.90 | 1.97 | 1.15 | |
| Range | 92.0–94.7 | 70.1–74.5 | 76.2–79.0 | |
| Subadult, subad (n = 10) | AM ± SD | 93.4 ± 0.9 | 71.9 ± 1.6 | 77.0 ± 1.4 |
| CV | 0.92 | 2.26 | 1.80 | |
| Range | 91.9–94.2 | 69.4–74.6 | 74.96–79.24 | |
| Adult, ad (n = 14) | AM ± SD | 93.8 ± 0.6 | 72.3 ± 1.0 | 77.1 ± 1.0 |
| CV | 0.67 | 1.34 | 1.31 | |
| Range | 92.3–94.6 | 71.0–74.5 | 75.9–78.8 | |
| im + subad + ad (n = 35) | AM ± SD | 93.6 ± 0.8 | 72.3 ± 1.3 | 77.3 ± 1.1 |
| CV | 0.82 | 1.84 | 1.44 | |
| Range | 91.9–94.7 | 69.4–74.6 | 75.0–79.2 | |
GM geometric mean, AM arithmetic mean, SD standard deviation, CV coefficient of variation in %, range minimum–maximum
The concentration of F− in the DW of teeth of red foxes generally ranged from 12.9 to 795.5 mg/kg and in ash, from 16.9 to 1,040.1 mg/kg. The largest concentration was recorded in a molar of a 5–6-month fox from Mielin Island (∼1,096 and 1,387 mg F−/kg, respectively, in DW and ash). That island (lying in the northern part of the Odra estuary called the Szczecin Lagoon) is home to a large colony of great cormorant (Phalacrocorax carbo). Both great cormorant chicks, which fall from nests, and the fish dropped by the adult birds were the major diet of foxes in the time of rearing young (Kalisińska et al. 2009). That specific diet of the young fox was a likely cause of such a large accumulation of F− in its tooth. As the concentration of F− in the tooth of the Mielin Island fox was very different from the other immature foxes, this case was excluded from further statistical analysis. Table 2 shows the levels of F− in the teeth of foxes belonging to different age groups and for all individuals taken together. The mean concentration of F− in DW was 77.2% of the concentration of F− in ash, ranging across the age groups from 77.0% to 77.6%. The correlation coefficient between the concentration of F− in the DW and ash, which was determined for all the individuals included in the statistical analysis (n = 34), had a very high value (r = 0.999; p < 0.0001).
Table 2.
Concentration of fluorides (mg/kg) in the first molar of red foxes from the respective age groups in dry weight (DW) and ash
| Age category (n) | Parameter | Concentration of fluorides | |
|---|---|---|---|
| DW | Ash | ||
| Immaturea, im (n = 10) | GM | 230.4 | 297.0 |
| AM ± SD | 351.6 ± 202.0 | 451.5 ± 259.6 | |
| CV | 57.5 | 57.5 | |
| Range | 12.9–614.3 | 16.9–789.9 | |
| Subadult, subad (n = 10) | GM | 296.4 | 384.9 |
| AM ± SD | 364.4 ± 224.0 | 470.7 ± 287.7 | |
| CV | 61.5 | 60.7 | |
| Range | 112.6–726.3 | 112.6–726.3 | |
| im + subad (n = 20) | GM | 261.3 | 338.1 |
| AM ± SD | 357.8 ± 207.7 | 461.1 ± 265.9 | |
| CV | 58.1 | 57.7 | |
| Range | 12.9–726.3 | 16.9–926.5 | |
| Adult, ad (n = 14) | GM | 503.8 | 653.7 |
| AM ± SD | 532.2 ± 169.1 | 692.1 ± 224.0 | |
| CV | 31.8 | 32.4 | |
| Range | 258.3–795.5 | 332.0–1,040.1 | |
| im + subad + ada (n = 34) | GM | 342.4 | 443.5 |
| AM ± SD | 429.6 ± 209.1 | 556.2 ± 271.6 | |
| CV | 48.7 | 48.8 | |
| Range | 12.9–795.5 | 16.90–1,040.1 | |
aOne im specimen from the Mielin Island has been excluded due to its abnormally high F− concentration (1,096 mg F−/kg in DW and 1,387 mg F−/kg in ash)
GM geometric mean, AM arithmetic mean, SD standard deviation, CV coefficient of variation in %, range minimum–maximum
The highest and the lowest values of the mean concentrations of F− in the molars (both in DW and ash) were found in ad and im groups, respectively (Table 2). Comparing the im and subad individuals, we found that in im foxes the concentrations of F− in DW and ash were slightly smaller (by 7.5% and 29.6%, respectively), but these differences were not confirmed statistically (DW and ash, p > 0.80). The greatest concentrations of F− were found in the ad group, with statistically significant differences in F− concentrations in DW and ash found between this group and two other groups (ad vs im—DW t = 2.38, p = 0.03 and ash t = 2.43, p = 0.02; ad vs subad—DW t = 2.10, p = 0.05 and ash t = 2.13, p = 0.04). The im and subad groups combined (im + subad) differed from the ad foxes even more distinctly (ad vs im + subad—DW t = 2.59, p < 0.02 and ash t = 2.65, p < 0.02). There was a significant correlation (p < 0.03) between the age of a fox and the concentration of F− in all the investigated molars (DW r = 0.381; ash r = 0.390).
Some red foxes came from the less contaminated area (PV), and some from an area with a long-term exposure to fluorides (WV). As we demonstrated a significant correlation between the age of the animals and the content of F− in their teeth, the comparison of foxes from PV and WV was carried out for specimens from the same age group with a similar number of individuals: in red foxes under the age of 20 months (VP, n = 7 and WV, n = 13). In VP, the concentration of F− in DW and ash was, respectively, 392 and 303 mg/kg, and in WV foxes, 312 and 241 mg/kg. There were no statistically significant differences in this respect (p > 0.40).
Discussion
In ecotoxicological studies, F− concentration is usually determined in bone—skeletal elements and teeth. Analyses usually involve the examination of the whole teeth or separately, enamel, dentin, cementum, as well as crowns and roots. Dentin, in contrast to enamel, is formed throughout the life of the tooth (Gutiérrez-Salazar and Reyes-Gasga 2003; Vieira et al. 2004; Richter et al. 2010). Among hard tissues, dentin constitutes the greatest part of the tooth, as it fills the interior of both crown and root. It contains only fluoride that has been incorporated through systemic ingestion. Mature enamel is an avascular tissue, and enamel fluoride content is therefore related to mammals’ F− intake at the time the tooth was forming (Suttie et al. 1987; Den Besten 1994). In humans, and laboratory, domestic and wild mammals, the intake of excessive amounts of F− results in dental fluorosis connected with frequent fractures of teeth, irregularly worn teeth and brown to black staining of enamel (Li et al. 1996; Zemek et al. 2006; Choubisa 2008; Macicek and Krook 2008; Saiani et al. 2009; Richter et al. 2010).
In wild and laboratory mammals as well as people not exposed to elevated F− levels, fluoride concentrations in teeth did not exceed 400 mg F−/kg; in enamel, they are distinctly lower than in dentin and bone (Suttie et al. 1987; Li et al. 1996; Vieira et al. 2004; 2005). Dentin structure and physical properties are similar to bone, but dentin is more mineralized (Qin et al. 2001). In the bones of medium-sized wild mammals, which live in non-contaminated areas with fluoride, for example in red foxes from Great Britain, GB (Aberdeen) and white-tailed deer from USA (South Carolina), the concentrations of F− are ∼280 mg/kg (Table 3).
Table 3.
Fluoride concentration (mg/kg) in bone materials of selected mammals, with regard to their age and origin
| Age | Material | Localization | Source | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tooth | Bone (mandible) | |||||||||
| Enamel | Whole teeth | Molar | ||||||||
| Crown | Root | |||||||||
| DW | Ash | DW | DW | DW | Ash | DW | ||||
| White-tailed deer, Odocoileus virginianus | ||||||||||
| All age groups | Before 1979 | 149 ± 73 | USA, South Carolina, Mount Hollya | Suttie et al. (1987) | ||||||
| After 1980 | 457 ± 333 | |||||||||
| 1.5 years | Before 1979 | 182 ± 69 | ||||||||
| After 1983 | 586 ± 215 | |||||||||
| ≥2.5 years | Before 1979 | 286 ± 100 | ||||||||
| After 1983 | 1,275 ± 624 | |||||||||
| Wild boar, Sus scrofa | ||||||||||
| <24 months | UP: 33.6 ± 26.7 | UP: 304.7 ± 91 | W Germany | Kierdorf et al. (2000) | ||||||
| P: 165.2 ± 38.3 | P: 490.8 ± 135.1 | Germany, Saxony | ||||||||
| P: 382.1 ± 125.0 | P: 754.3 ± 149.6 | Czech Rep., Bohemia | ||||||||
| Red deer, Cervus elaphus | ||||||||||
| 1–2 years | P: 159.0 | P: 231.8 ± 30.8 (alveolus) | NW Poland | Zakrzewska et al. (2005) | ||||||
| UP: 114.0 | UP: 150.2 ± 36.0 (alveolus) | |||||||||
| 6–8 years | P: 470.1 | P: 637.8 ± 121.8 (alveolus) | ||||||||
| UP: 211.1 | UP: 341.0 ± 91.4 (alveolus) | |||||||||
| 1–2 years | P: 147.5 ± 19.4 | |||||||||
| UP: 98.1 ± 15.2 | ||||||||||
| 6–8 years | P 342.7 ± 296.1 | |||||||||
| UP: 179.2 ± 54.5 | ||||||||||
| 1–2 years | P: 212.9 ± 14.1 | |||||||||
| UP: 144.1 ± 5.8 | ||||||||||
| 6–8 years | P: 493.3 ± 190.9 | |||||||||
| UP: 264.0 ± 119.8 | ||||||||||
| Red fox, Vulpes vulpes | ||||||||||
| 28.8 months | 551 | GB, Wales | Walton (1984) | |||||||
| 16.6 months | 476 | GB, Anglesey (excluding Holyheada) | ||||||||
| Unknown | UP: 283 | GB, Aberdeen | ||||||||
| Unknown | 1,650 | GB, Anglesey, Holyheada | ||||||||
| <20 months | 357.8 ± 207.7 | NW Poland | This work | |||||||
| >20 months | 532.2 ± 169.1 | |||||||||
DW dry weight, P polluted area, UP unpolluted area, GB Great Britain
aThe areas with aluminium reduction plant; in Mount Holly the plant started working in 1980
In the case of people and other long-lived mammals, including ungulates and carnivores, the concentration of F− clearly increases with their age (Walton 1984; Suttie et al. 1987; Dabkowska et al. 1995; Machoy et al. 1995; Zakrzewska et al. 2005; Weinstein and Davison 2004; Jelenko and Pokorny 2010; Richter et al. 2010). Also in the red fox, there is a clear relationship between age and the fluoride concentration in bone tissues. Walton (1984) and these authors demonstrated the statistically significant correlation coefficient (r) concerning the studied mandibles of red foxes from GB (r = 0.59, p < 0.001, n = 180) and the teeth of the population from NW Poland (r = 0.38, p < 0.03, n = 34). This trend implies the need of taking into account the age in the intra- and interspecies comparisons of F−. Comparing the concentrations of F− in foxes of similar age from GB (∼17 and >24 months, respectively, from Anglesey and Wales) and NW Poland (<20 and >20 months), one can see a significant similarity between older foxes, with approximately 530–550 mg F−/kg DW (Table 3). In younger foxes inhabiting Poland, F− concentration was more than 30% lower compared to the Anglesey population.
In the 1990s, F− concentration was examined in the mandible and the enamel of wild boar in Central Europe (age <24 months, n = 35). The specimens came from one unpolluted area (W Germany) and two areas heavily contaminated by fluorides—Saxony in Germany and N Bohemia in the Czech Republic (Table 3). In the bone of wild boar from the most polluted sites (N Bohemia), the mean F− level was about 750 mg/kg DW, and in unpolluted ones, it was about 300 mg F−/kg DW (Kierdorf et al. 2000). In comparison with the bones of wild boar in W Germany, the teeth of the red fox in this study (im + subad) from NW Poland had a 20% higher F− concentration (Table 3). It can therefore be assumed that NW Poland is more exposed to F− than W Germany. However, data on F− in the mandibles of red deer in the same NW region of Poland are not as clear. The samples (from different places of the mandible, crown and root of the first permanent molar as well as the whole first incisor) were taken from red deer in 1996–1997, i.e. already during the modernization of chemical plants in Police (Zakrzewska et al. 2005). The red deer were partially from an area more exposed to the effects of fluorides (P, polluted; W, part of the West Pomeranian Voivodship, covering Szczecin and surrounding area, including the Police district), and partially from areas with a much smaller industrial pollution (UP, unpolluted; the E part of the Voivodship). In the P area, F− concentration in the bone of red deer aged 1–2 years ranged from 169 to 240 mg/kg (mean value ∼204 mg F−/kg). The mean level fluoride concentration was 40–50% lower than the values reported for animals of similar age which inhabited the areas not contaminated by F−, e.g. wild boar in W Germany and white-tailed deer in South Carolina, USA (Table 3). Based on this comparison, the P area near Szczecin was not much contaminated with flouride and UP area was even less polluted. In the case of red deer from the P area, the concentration of F− in the tooth specimens from deer aged 1–2 years and im + subad foxes were, respectively, 160 and 360 mg F−/kg DW, i.e. 120% higher in the fox (Table 3). It indicates that the predatory red fox accumulated F− more intensely than the herbivorous red deer. This observation can be explained by the biology of these species and the most frequent preys of red fox—Micromammalia, mainly small herbivorous (Rodentia) and insectivorous (Insectivora) species. In Europe, red fox consumes mainly rodents from genus Microtus and less frequently insectivores—moles and shrews (Walton 1984; Gołdyn et al. 2003; Sidorovich et al. 2006). The fox and its main prey, living near the sources of F− emissions in Holyhead (Anglesey, GB), had concentrations of 1,650 and from 1,000 to 11,000 mg F−/kg DW of bone, respectively. The bones of red foxes evidently did not accumulate the amounts of F− in excess of that found in their prey (Walton 1988). One needs to take into account the fact that the fluoride-contaminated bones in the gastrointestinal tract of the red fox and other carnivores are not digested and mostly are excreted (Walton 1984; Weinstein and Davison 2004). Therefore, it can be assumed that the source of F− for the red fox are mostly the soft tissues of rodents, with a very small concentration of F− (Inkielewicz et al. 2003). A small amount of F− also enters the fox body with ingested plants, drinking water and inhaled air. When increased quantities of F− can be found in the environment of a fox, especially in the diet, its bone F− concentrations are considerable, as demonstrated by the work of Walton (1984, 1988) and our own findings, including the red fox from Mielin Island. It appears that F− concentrations accumulated in the bone materials of the fox enable a much better indirect assessment of pollution, including the human environment, as compared to deer and wild boar, usually living away from people and their dwellings. Unfortunately, data in this field are scarce (Walton 1984; Kalisińska and Palczewska 2007).
Using cautious estimations, 300–400 and 500 mg F−/kg DW, respectively, in the teeth and bones of long-lived mammals, may be regarded as reflecting the geochemical background. An additional argument in this regard is the concentration of fluorides in the mandible of canids from unpolluted areas: red fox from GB (Aberdeen) and coyote (Canis latrans) from North America. Their bones had mean F− concentrations 280–290 mg/kg DW (Kay et al. 1975; Walton 1984). Among foxes from NW Poland, concentrations of F− in the teeth <400, 400–500 and >500 mg/kg DW were found in 15, 5 and 15 specimens, with the latter two groups combined representing more than 57% of the examined population. In this part of the population, the geochemical level of F− was exceeded by 9% to 170%. It can therefore be concluded the examined area was moderately exposed to fluorides.
The red fox fulfils the conditions required for good bioindicators (Ellenberg 1991). It has a large geographical range, but not too large home range; its local populations are stable; it has a permanent position in the food chain; it is relatively easy to determine its age and it is also game, so it is not difficult to obtain. For these reasons, it is sometimes used in studies on different types of environmental pollution (Corsolini et al. 1999; Dip et al. 2001; Kalisińska et al. 2009). Currently, pollution with anthropogenic substances, including F−, is so widespread that there is a reasonable need to determine concentrations in common mammals living in the vicinity of man, including the red fox. Results of such research could be used in broader, comparative ecotoxicological studies of any region, country and continent.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bassin EB, Wypij D, Davis RB, Mittelman MA. Age-specific fluoride exposure in drinking water and osteosarcoma (United States) Cancer Causes Control. 2006;17:421–428. doi: 10.1007/s10552-005-0500-6. [DOI] [PubMed] [Google Scholar]
- Bezerra de Menezes LM, Volpato MC, Rosalen PL, Cury JA. Bone as a biomarker of acute fluoride toxicity. Forensic Sci Inter. 2003;137:209–214. doi: 10.1016/j.forsciint.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Boulton IC, Cooke JA, Johnson MS. Lesion scoring in field vole teeth: application to the biological monitoring of environmental fluoride contamination. Environ Monit Asses. 1999;55:409–422. doi: 10.1023/A:1005987016084. [DOI] [Google Scholar]
- Camargo JA. Fluoride toxicity to aquatic organisms: a review. Chemosphere. 2003;50:251–264. doi: 10.1016/S0045-6535(02)00498-8. [DOI] [PubMed] [Google Scholar]
- Choubisa SL. Dental fluorosis in domestic animals. Curr Sci. 2008;95:1674–1675. [Google Scholar]
- Churcher CS. Cranial variation in the North American red fox. J Mammal. 1960;4:349–360. doi: 10.2307/1377493. [DOI] [Google Scholar]
- Corsolini S, Focardi S, Leonzio C, Lovari S, Monaci F, Romeo G. Heavy metals and chlorinated hydrocarbon concentrations in the red fox in relation to some biological parameters. Environ Monit Assess. 1999;54:87–100. doi: 10.1023/A:1005974014029. [DOI] [Google Scholar]
- Dabkowska E, Machoy-Mokrzyńska A, Straszko J, Machoy Z, Samujło D. Temporal changes in the fluoride levels of jaws of European deer in industrial regions of Western Pomerania, Poland. Environ Geochem Health. 1995;17:155–158. doi: 10.1007/BF00661327. [DOI] [PubMed] [Google Scholar]
- Den Besten PK. Dental fluorosis: its use as a biomarker. Adv Dent Res. 1994;8:105–110. doi: 10.1177/08959374940080010201. [DOI] [PubMed] [Google Scholar]
- Dip R, Stieger C, Deplazes P, Hegglin D, Mueller U, Dafflon O, Koch H. Comparison of heavy metal concentration in tissues of red foxes from suburban and rural areas. Arch Environ Contam Toxicol. 2001;40:551–556. doi: 10.1007/s002440010209. [DOI] [PubMed] [Google Scholar]
- Eckerlin EH, Maylin GA, Krook L, Carmichael D. Ameliorative effects of reduced food-borne fluoride on reproduction in silver foxes. Cornell Vet. 1988;78:385–391. [PubMed] [Google Scholar]
- Ellenberg H (1991) Bioindicator and biological monitoring. In: Biological monitoring. Signal from the environment. Gate/GTZ, Braunschweig, pp 13–74
- ES WV (2009) Environmental state in West Pomerania Voivodship in 2008 [Stan środowiska w województwie zachodniopomorskim w 2008 roku]. www.wios.szczecin.pl [in Polish]
- Gołdyn B, Hromada MA, Surmacki A, Tryjanowski P. Habitat use and diet of the red fox Vulpes vulpes in an agricultural landscape in Poland. Zeitschr Jagwis. 2003;49:191–200. [Google Scholar]
- Gutiérrez-Salazar MP, Reyes-Gasga J. Microhardness and chemical composition of human tooth. Mater Res. 2003;6:367–373. doi: 10.1590/S1516-14392003000300011. [DOI] [Google Scholar]
- Gutowska I, Machoy Z, Machaliński B, Chlubek D. Living conditions of deer in the provinces of Western Pomerania and Lubuskie as revealed by mandibular content of fluoride, calcium, and magnesium. 1. Inter-relations between fluoride, calcium, and magnesium content in mandible. Ann Acad Med Stet (suppl) 2004;50:42–46. [PubMed] [Google Scholar]
- Harrison PTC. Fluoride in water: a UK perspective. J Fluor Chem. 2005;126:1448–1456. doi: 10.1016/j.jfluchem.2005.09.009. [DOI] [Google Scholar]
- Inkielewicz I, Czarnowski W, Krechniak J. Determination of fluoride in soft tissues. Fluoride. 2003;36:16–20. [Google Scholar]
- Inorganic Fluorides—PSL1 (1993) Canadian Environmental Protection Act. Environment Canada Health, Canada (www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/psl1)
- Jelenko I, Pokorny B. Historical biomonitoring of fluoride pollution by determining fluoride contents in roe deer (Capreolus capreolus L.) antlers and mandibles in the vicinity of the largest Slovene thermal power plant. Sci Total Environ. 2010;409:430–438. doi: 10.1016/j.scitotenv.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Kalisińska E, Lisowski P, Salicki W, Kucharska T, Kavetska K. Mercury in wild terrestrial carnivorous mammals from north-western Poland and unusual fish diet of red fox. Acta Theriol. 2009;54:345–356. doi: 10.4098/j.at.0001-7051.032.2008. [DOI] [Google Scholar]
- Kalisińska E, Palczewska M. Fluoride in the teeth of red fox Vulpes vulpes from western Pomerania. Ochr Środ Zas Nat. 2007;31:428–433. [Google Scholar]
- Kay CE, Tourangeau PC, Gordon CC. Fluoride levels in indigenous animals and plants collected from uncontaminated ecosystems. Fluoride. 1975;8:125–133. [Google Scholar]
- Kierdorf U, Kierdorf H, Sedlacek F, Fejerskov O. Structural changes in fluorosed dental enamel of reed deer (Cervus elaphus L.) from a region serve environmental pollution by fluorides. J Anat. 1996;188:183–195. [PMC free article] [PubMed] [Google Scholar]
- Kierdorf H, Kierdorf U, Richards A, Sedlacek F. Disturbed enamel formation in wild boars (Sus scrofa L.) from fluoride polluted areas in Central Europe. Anat Rec. 2000;259:12–24. doi: 10.1002/(SICI)1097-0185(20000501)259:1<12::AID-AR2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Li DS, Cutress TW, Pearce EIF, Coote GE. Effects of arsenic or/and fluoride on mineralized tissues of the rat. Fluoride. 1996;29:156–162. [Google Scholar]
- Macdonald DW, Reynolds JC. Red fox Vulpes vulpes Linnaeus, 1758. In: Sillero-Zubiri C, Hoffmann M, Macdonald DW, editors. Canids: foxes, wolves, jackals and dogs. Status survey and conservation action plan. IUCN/SSC Canid Specialist Group. Switzerland and Cambridge: Gland; 2004. pp. 129–136. [Google Scholar]
- Machoy Z, Dabkowska D, Samujło D, Ogoński T, Raczyński J, Gębczyńska Z. Relationships between fluoride content in bones and the age in European elk (Alces alces L.) Comp Biochem Physiol. 1995;3C:117–120. doi: 10.1016/0742-8413(95)00012-v. [DOI] [PubMed] [Google Scholar]
- Macicek P, Krook LP. Fluorosis in horses drinking artificially fluoridated water. Fluoride. 2008;41:177–183. [Google Scholar]
- Ozsvath DL. Fluoride and environmental health: a review. Rev Environ Sci Biotech. 2009;8:59–79. doi: 10.1007/s11157-008-9136-9. [DOI] [Google Scholar]
- Qin C, Brunn JC, Jones J, George A, Ramachandran A, Gorski JP, Butler WT. A comparative study of sialic acid-rich proteins in rat bone and dentin. Eur J Oral Sci. 2001;109:133–141. doi: 10.1034/j.1600-0722.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- Richter H, Kierdorf U, Richards A, Kierdorf H. Dentin abnormalities in cheek teeth of wild red deer and roe deer from a fluoride-polluted area in Central Europe. Ann Anat. 2010;192:86–95. doi: 10.1016/j.aanat.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Saiani RA, Porto IM, Marcantonio E, Cury JA, de Sousa FB, Gerlach RF. Morphological characterisation of rat incisor fluorotic lesions. Arch Oral Biol. 2009;54:1008–1015. doi: 10.1016/j.archoralbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Sidorovich VE, Sidorovich A, Izotova IV. Variations in the diet and population density of the red fox Vulpes vulpes in the mixed woodlands on northern Belarus. Mamm Biol. 2006;71:74–89. doi: 10.1016/j.mambio.2005.12.001. [DOI] [Google Scholar]
- Suttie JS, Dickie R, Clay AB, Nielsen P, Mahan WE, Baumann DP, Hamilton RJ. Effects of fluoride emissions from modern primary aluminium smelter on a local population of white-tailed deer (Odocoileus virginianus) J Wildl Dis. 1987;23:135–143. doi: 10.7589/0090-3558-23.1.135. [DOI] [PubMed] [Google Scholar]
- Tataruch F, Kierdorf H. Mammals as biomornitors. In: Markert AA, Breure AM, Zechmeister HG, editors. Bioindicators and biomonitors. Amsterdam: Elsevier; 2004. pp. 737–772. [Google Scholar]
- Telesiński A, Śnioszek M. Bioindicators of environmental pollution with fluorine. Bromatol Chem Toksykol. 2009;42:1148–1154. [Google Scholar]
- Verkerk RHJ. The paradox of overlapping micronutrient risks and benefits obligates risk/benefit analysis. Toxicology. 2010;278:27–38. doi: 10.1016/j.tox.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Vieira AP, Mousny M, Maia R, Hancock R, Everett ET, Grynpas MD. Assessment of teeth as biomarkers for skeletal fluoride exposure. Osteoporos Int. 2005;16:1576–1582. doi: 10.1007/s00198-005-1870-z. [DOI] [PubMed] [Google Scholar]
- Vieira AP, Hancock R, Maia R, Limeback H, Grynpas MD. Is fluoride concentration in dentin and enamel a good indicator of dental fluorosis? J Dent Res. 2004;83:76–80. doi: 10.1177/154405910408300115. [DOI] [PubMed] [Google Scholar]
- Walton KC. Fluoride in fox bone near an aluminium reduction plant in Anglesy, Wales, and elsewhere in the United Kingdom. Environ Pollut B. 1984;7:273–280. doi: 10.1016/0143-148X(84)90042-9. [DOI] [Google Scholar]
- Walton KC. Environmental fluoride and fluorosis in mammals. Mamm Rev. 1988;18:77–90. doi: 10.1111/j.1365-2907.1988.tb00077.x. [DOI] [Google Scholar]
- Weinstein LH, Davison A. Fluorides in the environment. Oxford: CABI Publishing; 2004. [Google Scholar]
- Zakrzewska H. Evaluation of some biochemical parameters in blood serum and bones of sheep Western Pomerania regions of various contamination degree. Ann Acad Med Stet. 1996;10:105–125. [Google Scholar]
- Zakrzewska H, Machoy-Mokrzyńska A, Materny M, Gutowska I, Machoy Z. Estimation of fluoride distribution in the mandible and teeth of the red deer (Cervus elaphus L.) from industrially polluted areas in Poland. Arch Oral Biol. 2005;50:309–316. doi: 10.1016/j.archoralbio.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Zemek F, Herman M, Kierdorf H, Kierdorf U, Sedlácek F. Spatial distribution of dental fluorosis in roe deer (Capreolus capreolus) from North Bohemia (Czech Republic) and its relationships with environmental factors. Sci Total Environ. 2006;370:491–505. doi: 10.1016/j.scitotenv.2006.04.027. [DOI] [PubMed] [Google Scholar]


