Abstract
This study evaluated the effects of the interaction of diabetes and a carbonyl iron supplemented on hepatic and pancreatic tissues, oxidative stress markers and liver peroxisome proliferator-activated receptor-α expressions. Hamsters were divided: Control which received a standard AIN 93 diet; Control Iron, composed of control animals that received a diet with 0.83% carbonyl iron; Diabetic, composed of animals that received a injection of streptozotocin (50 mg/kg, intraperitoneal) on day 35; and Diabetic Iron composed of streptozotocin treated animals that received a diet supplemented with carbonyl iron. Diabetes increased the glucose level and reduced triglycerides. Diabetic Iron group showed higher levels of glucose and serum triglycerides as compared to the Diabetic group. Diabetes decreased mRNA levels of peroxisome proliferator-activated receptor-α. Iron attenuated the diabetes induced down regulation of peroxisome proliferator-activated receptor-α mRNA. Moreover, diabetes increased carbonyl protein and decreased glutathione levels and catalase activity, while iron attenuated the increase in levels of carbonyl protein and attenuated the decrease in those of glutathione level and catalase activity. Histological analysis shows that supplementation iron caused an increase in the size of the islets in Control Iron. The results show that iron does not aggravated liver oxidant/antioxidant status and peroxisome proliferator-activated receptor-α expression in diabetic hamsters.
Keywords: carbonyl iron, streptozotocin, oxidative stress, hamsters, PPAR-α
Introduction
Diabetic mellitus, a chronic metabolic disorder, is one of the most important health problems in the world, especially in developing countries. It is characterized by imperfection in insulin secretion and insulin receptor or post receptor events with derangement in carbohydrate, protein and lipid metabolism and results in chronic hyperglycemia.(1) Chronic hyperglycemia, promotes glucotoxicity, and has negative impacts on various organs and tissues, such as pancreas, liver, kidneys, muscles, etc. Glucotoxicity eventually leads to progressive β-cell dysfunction, impaired insulin gene transcription and permanent β-cell loss due to apoptosis, ensuing in a vicious cycle with exasperation of the hyperglycemia.(2) Indeed, hyperglycemia is contemplated to generate reactive oxygen species through diverse pathways, such as mutilation of the redox equilibrium, augmentation of advanced glycation products, activation of protein kinase C or overproduction of mitochondrial superoxides that eventually leading to oxidative stress in a variety of tissues.(3) The vulnerability of each tissue to oxidative stress can vary depending upon their expressed antioxidant enzymes. In addition to the pancreatic β-cells, supraphysiological glucose is notorious to provoke oxidative stress in hepatocytes which can cause hepatic tissue damages.(4,5)
Dietary components can modify the redox status of an organism and appear as an important factor in controlling diabetes and its complications. Nutrients such as iron have a dual role, with the ability to increase or decrease the organism’s redox capacity. Iron, acting as a cofactor for many proteins, including antioxidant enzymes, is essential for cellular metabolism and aerobic respiration. However, in high concentrations it can lead to cellular toxicity and oxidative damage of cellular components due to its role in the formation of free radicals.(6,7)
Association between excessive systemic iron and diabetes was suggested by the observation that the incidence of diabetes is increased in classical hereditary hemochromatosis,(8) in which there is a large increase in iron pools. Epidemiological data show a positive correlation between body iron pools and development of glucose intolerance seen in both diabetes type 2(9–11) and gestational diabetes.(12) Recently, have been demonstrated, in a mouse model of hereditary hemochromatosis, that glucose uptake is increase in skeletal muscle but glucose oxidation is decreased and the ratio of fatty acid to glucose oxidation is increased, that may to contribute to the risk of diabetes with excessive tissue iron.(13)
Together, these observations suggest that there is a close relationship between iron and diabetes pathophysiology. However, not much data exist regarding this relationship in experimental models. Such models would allow better understanding of the mechanisms involved in this interaction and could contribute to a better control of diabetes and to a reduction of its complications when associated to iron supplementation.
To expand knowledge in between diabetes and iron, we sought to describe the effect of iron and diabetes on oxidative stress, liver histology, and lipid homeostase by studying the effects on peroxisome proliferator-activated receptor-α (PPAR-α) mRNA in liver of hamsters with a diet supplemented with carbonyl iron, an iron for oral use. A growing body of evidence supports the role of PPAR-α in the development of liver disease and as a putative target for the treatment of steatohepatitis. Disabling the PPAR-α gene is known to increase hepatic triglyceride accumulation, especially under conditions of fasting.(14–16) Pharmacological PPAR-α activation has been shown to lower hepatic triglyceride levels and effectively attenuate steatohepatitis.(17–19) Furthermore, diabetes can modulate PPARs through increased inflammatory cytokines and oxidative stress in the heart.(20) Thus it is possible to suppose that the interaction between iron and diabetes might modulate the PPAR-α expression in the liver and, as a consequence, the lipid homeostasis.
We also studied histologic caracteristics of pancreas sections. This study allowed us to analyze possible iron effects without excessive iron overload by using the hamster as a model, because this animal has a lipoprotein profile similar to that of humans. This allowed us to establish parallels between the alterations seen here and those known to occur in humans, making this a useful model to unravel the mechanisms involved in this relationship. This relationship is especially relevant in developing countries, which have an increasing incidence of diabetes as well as iron deficiency anemia(21) and, therefore, where an indiscriminate iron supplementation occurs.
Materials and Methods
Animals and experimental design
Female Golden Syrian hamsters, weighing approximately 100 g, were obtained from the School of Nutrition of the Federal University of Ouro Preto and kept in collective cages in rooms with controlled temperature and humidity and with 12-h light and dark cycles. Hamsters received food and water ad libitum. Experiments were conducted according to the principles defined by the Brazilian College of Animal Experimentation.(22) Animal procedures were approved by the Ethics Committee of the Federal University of Ouro Preto. Hamsters were divided into four groups of eight animals each were normalized by weight. The control (C) group received the standard AIN-93 diet.(23) Control Iron group (CI) received the standard diet supplemented with 0.83% carbonyl iron according to Dabbagh et al.(24) Diabetic group (D) received an injection of streptozotocin (STZ) at 50 mg/kg, intraperitoneal, according to Iancu et al.,(25) on experimental day 35 and was kept on the standard diet. Diabetic Iron (DI) received the STZ injection and was kept on the standard diet supplemented with 0.83% carbonyl iron. Seven days after the STZ injection, glucose levels were measured, and only animals with glucose levels above 250 mg/dl remained in the experimental group. The total experimental time was 45 days.
Sample preparation
On experimental day 45, fasting hamsters were anesthetized and euthanized by an intraperitoneal injection of pentobarbital (Sigma, 60 mg/kg body weight). Blood samples were collected and centrifuged in 1.5 ml tubes for determination of serum components. Liver and pancreas were removed, weighed and stored either in liquid nitrogen or buffered formaldehyde for biochemical and histopathological analysis, respectively.
Assay methods
The concentration of serum iron was determined by spectrophotometric analysis using Labtest kit # 38 (Minas Gerais, Brazil) with a standard iron solution (500 µg/dl). Liver samples, 100 mg, were digested in 2 ml of nitric acid at 100°C. Excess acid was evaporated and iron levels were quantified by colorimetric analysis according to the Association of Official Analytical Chemistry (AOAC),(26) using the orthophenanthroline assay with an iron solution of 500 µg/dl as an external iron standard. Labtest kits # 53, 42, 99 and 19 were used to measure the activities of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum concentration of total proteins and albumin, respectively. Glucose and fructosamine concentrations and amylase activity were determined using Labtest kits # 84, 97 and 25 respectively. Triglycerides and total cholesterol concentrations were measured using Labtest kits # 59 and 60 with glycerol or cholesterol as standards, respectively. Following low density lipoprotein (LDL) and very low density lipoprotein (VLDL) precipitation with phosphotungstic acid and MgCl2, high density lipoprotein (HDL) cholesterol was measured in the supernatant using Labtest kit # 3. The remaining cholesterol fractions were calculated based on the difference between total and HDL cholesterol.
Histopathological evaluation
Liver and pancreas fragments not exceeding 4 mm in diameter were fixed in 10% formaldehyde solution and then dehydrated, diaphanized and embedded in paraffin. Paraffin sections of approximately 4 µm were obtained by sectioning embedded fragments on a rotary microtome. Sections were mounted on glass slides previously cleaned and degreased. Slides were stained with Hematoxylin and Eosin for visualization of histological changes and with Perls’ technique for determination of tissue iron pools. Digital morphometric analysis, conducted for determination of the average size and area of pancreatic islets as well as quantification of tissue iron pools, were done using a Leica DM5000 optical microscope with the Leica analysis software Qwin Plus.
Quantitative real-time RT-PCR assay
Trizol RNA extraction of liver, approximately 50 mg, was done using RNAgents® Promega-Total RNA Isolation System (Madison) according to the manufacturer’s recommendations. Total RNA concentration and purity was determined by spectrophotometric analysis at 260 and 280 nm on a NanoVue spectrophotometer (GE Healthcare, United Kingdom). cDNA was synthesized from total RNA using GeneAmp® RNA PCR (Applied Biosystems, Foster City). Briefly, cDNA was prepared in a 20 µl reaction using MultiScribeTM (50 U/µl) Reverse Transcriptase and Oligo d(T)16 primers (Applied Biosystems). Gene expression was analyzed using SYBR Green PCR Master Mix (Applied Biosystems). PPAR-α gene expression was normalized to that of β-actin. Primer sequences were as follows: F-TGTCGAATATGTGGGGACAA and R-AAACGGATTGCATTGTGTGA for PPAR-α and F-ACTGGCATTGTGATGGACTC and R-GTGGTGGTGAAGCTGTAGCC for β-actin. cDNA was amplified at 95–60°C for 40 cycles in an ABI 7300 Real-Time PCR instrument (Applied Biosystems). All samples were analyzed in triplicate. Measurement of relative PPAR distribution was done for each animal. Cycle Threshold values (ΔCT) were obtained by subtracting β-actin CT values from the respective PPAR CT values.
Activity of enzyme and antioxidant compound
Total glutathione concentration (GSH and GSSH) in liver homogenates was determined using Sigma Kit CS0260 (EUA). This assay utilizes a kinetic method to measure glutathione based on the reduction of DTNB (5,5'-dithio-bis-(2-nitrobenzoic) acid) to TNB, which can be spectrophotometrically determined at 412 nm. Sigma G4251 reduced glutathione was used as a standard.
Catalase activity was determined according to Aebi.(27) Briefly, 10 µl of tissue homogenate supernatant were added to a cuvette containing 2 ml of 50 mmol/l phosphate buffer (pH 7.2). The reaction was initiated by the addition of 1 ml of substrate (30 mM H2O2 in phosphate buffer). The rate of H2O2 decomposition was measured spectrophotometrically in 1-min intervals at 240 nm against a blank containing phosphate buffer instead of substrate. The decomposition of hydrogen peroxide was calculated using the molar extinction coefficient 39.41 l.mol−1/cm−1. One U of catalase is equivalent to the hydrolysis of 1 µmol of H2O2 per minute.
Protein oxidation analysis
Carbonyl protein was measured by using the modified method of Levine.(28) In brief, 0.5 ml of supernatant was placed in two tubes. Then 0.5 ml of 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2.5 M HCl was added to one of the tubes, while 0.5 ml of HCl (2.5 mM) was added to the other. Tubes were then left for 30 min at room temperature. Samples were vortex-mixed every 15 min. Then 0.5 ml of trichloroacetic acid (TCA) (20%, wt/vol) was added, and the tubes were left on ice for 5 min followed by centrifugation for 10 min at 5.000 g, to collect the protein precipitates. The pellets were then washed two times with 1 ml of ethanol/ethyl acetate (1:1, vol/vol). The final precipitates were dissolved in 1 ml of SDS 6%, followed by centrifugation for 10 min, at 10.000 g. Absorbance of the sample was measured at 370 nm. The carbonyl content was calculated based on the molar extinction coefficient of DNPH (22000 mol−1/cm−1) and expressed as nmol/mg of protein. The content of total liver protein was determined according to Lowry et al.(29)
Statistical analysis
Data were analyzed by the Kolmogorov–Smirnov test. All data, except for the expression of PPAR-α, were found to follow a normal distribution and were analyzed by the bivariate analysis of variance (two-way ANOVA). Classification factors were diabetes and iron, as well as their interaction. Whenever an effect was found for one of the factors, we used the univariate variance test (one-way ANOVA) with Tukey post test to determine the differences between groups. The data for PPAR-α expression were analyzed by Kruskal Wallis test. Results were expressed as mean ± standard deviation.
Results
Iron status, biochemical indicators of hepatic function and body and liver weights
The concentration of iron in the serum was twice as high in animals from the CI group as compared to animals in the C group (Table 1). This result is in contrast to animals in group DI, whose serum iron concentrations were comparable to those of animals in group C. Surprisingly, animals of group D had higher concentrations of serum iron than those in group C. Iron levels in the liver were also analyzed, and, in this case, we found that animals whose diet was supplemented with carbonyl iron, namely groups CI and DI, had iron levels equivalent to 6- and 4.4-fold those of group C, respectively. We found that hamsters with STZ-induced diabetes presented reduced body weight as compared to the control group. This weight loss was more pronounced in the DI group (Table 1). Liver weight was also decreased in diabetic animals. To determine whether iron excess and/or diabetes caused hepatic injury, ALT and AST activities and albumin and total protein concentrations were measured. ALT activity was higher in hamsters in group D as compared to those in group C. However AST activity was higher in animals receiving iron supplementation, i.e., groups DI and CI. The concentration of total proteins in serum was lower in both groups of diabetic hamsters, namely D and DI. The concentration of albumin did not vary with any of the analyzed variables, remaining similar in all groups.
Table 1.
Iron status, biochemical indicators of hepatic function, body and liver weights, glycemic and lipids profile of hamsters
| Variables | Experimental groups |
||||||
|---|---|---|---|---|---|---|---|
| Control | Control + Iron | Diabetes | Diabetes + Iron | ANOVA (p values) |
|||
| Iron | Diabetes | Iron × Diabetes | |||||
| Laboratory Data | |||||||
| Serum Iron µmol/l | 36.45 ± 5.34c | 73.34 ± 9.89a | 52.71 ± 5.57b | 40.06 ± 7.68c | <0.00001 | 0.001 | <0.00001 |
| Liver Iron µmol | 0.69 ± 0.24c | 4.38 ± 0.22a | 0.83 ± 0.11c | 3.09 ± 0.65b | <0.00001 | 0.417 | <0.0001 |
| ALT U/ml | 44.55 ± 10.26b | 61.16 ± 13.87b | 115.12 ± 58.39a | 70.75 ± 21.65a.b | 0.219 | 0.004 | 0.019 |
| AST U/ml | 47.66 ± 5.2b | 55.37 ± 12.87a.b | 49.29 ± 6.53b | 64.68 ± 13.33a | 0.0037 | 0.153 | 0.241 |
| Albumin µmol/l | 302.2 ± 43.66 | 315.18 ± 18.39 | 298.09 ± 9.65 | 292.07 ± 18.17 | 0.674 | 0.172 | 0.336 |
| Total Protein g/l | 30.13 ± 3.13a | 31.33 ± 2.31a | 26.32 ± 2.09b | 25.51 ± 1.1b | 0.761 | <0.00001 | 0.248 |
| Glucose mmol/l | 7.07 ± 1.15c | 7.15 ± 0.99c | 18.44 ± 4.94b | 25.15 ± 1.96a | 0.0003 | <0.00001 | 0.007 |
| Fructosamine µmol/l | 9.35 ± 1.71c | 11.72 ± 2b.c | 16.86 ± 1.9a | 14.34 ± 2.6a.b | 0.618 | <0.00001 | 0.003 |
| Amylase U/dl | 770.59 ± 5.67a | 735.95 ± 21.3b | 731.09 ± 7.37b | 726.47 ± 5.37b | 0.0001 | <0.0001 | 0.001 |
| Cholesterol mmol/l | 3.03 ± 0.44a | 3.49 ± 0.59a | 2.89 ± 0.4a | 1.91 ± 0.33b | 0.192 | <0.00001 | 0.0001 |
| Triglycerides mmol/l | 2.49 ± 0.49a.b | 1.94 ± 0.62b | 2.28 ± 0.35b | 3.39 ± 0.93a | 0.356 | 0.0049 | 0.001 |
| HDL mmol/l | 1.57 ± 0.22a | 1.84 ± 0.38a | 0.93 ± 0.11b | 0.88 ± 0.11b | 0.293 | <0.00001 | 0.05 |
| Other Fractions mmol/l | 1.46 ± 0.3b.c | 1.64 ± 0.58a.b | 2.04 ± 0.36a | 1.03 ± 0.31c | 0.02 | 0.555 | 0.0004 |
| Anthropometric Data | |||||||
| Final Body Weight g | 130.88 ± 12.99a.b | 136.11 ± 14.25a | 113.55 ± 17.92b.c | 105.13 ± 14.08c | 0.893 | <0.0001 | 0.177 |
| Autopsy Data | |||||||
| Liver Weight g | 4.36 ± 0.36a | 4.91 ± 0.69a | 3.61 ± 0.62b | 3.49 ± 0.43b | 0.0938 | <0.00001 | 0.221 |
ALT, alanine aminotransferase; ANOVA, analysis of variance; AST, aspartate aminotransferase; HDL, high-density lipoprotein; ¶ Values are shown as the mean ± standard deviation (n = 8). Data were analyzed by bivariate ANOVA analysis. When one of the factors was significant (p<0.05) univariate analysis with Tukey post test was performed to determine specific differences between means. Statistical differences are shown by different superscript letters.
Glycemic and lipid profile
To determine the effects of iron on glucose and lipid homeostasis, the glycemic and lipid profiles of all groups were analyzed (Table 1). As expected, animals that received STZ injections had increased glucose and fructosamine levels. Glucose levels in animals of group D were 2.6 times higher than those in group C, whereas fructosamine levels were 1.8 times higher in group D than in group C. Amylase activity, on the other hand, was decreased by 5.1% in group D as compared to group C. The administration of excess iron potentiated the changes in the glycemic profile caused by diabetes. Animals of group DI had glucose levels 3.56 times higher than those in group C. Amylase activity was further decreased by 5% in group DI. The concentration of serum triglycerides was higher in group DI than in groups D and CI, with no significant difference noted between these and group C. Hamsters of group DI showed decreased total serum cholesterol as compared to the other groups. Data analysis of HDL cholesterol concentration showed that this was also decreased in animals of groups DI and D. The later showed an increase in the other cholesterol fractions that was not seen in group DI.
Histological analysis
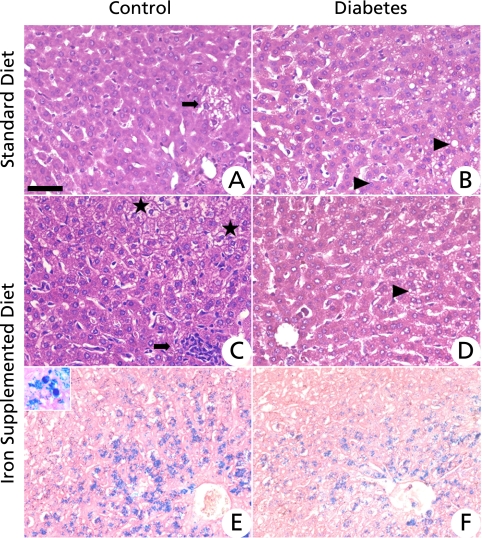
Histological analysis showed that, overall, the liver of animals in group C was normal. There were some ballooned hepatocytes that had large and clear cytoplasm (Fig. 1A), indicating the presence of a hydropic degenerative process. These are generally associated with inflammatory processes with a predominance of mononuclear cells, mainly macrophages. This inflammatory process occurred in the presence of normal levels of iron stores. Animals of the CI group showed a moderate presence of ballooned hepatic cells, which were diffuse throughout the parenchymal tissue. This presentation is generally associated with granulomatous inflammatory foci, and tissue iron stores were located primarily in hepatocytes and Kupfer cells (Fig. 1 C and E).
Fig. 1.
Photomicrographs of histological liver sections. (A) Control group showing normal histology with a small number of cells undergoing a degenerative process (arrow). (B) Diabetic group featuring some hepatic cells with microvesicular cytoplasm indicative of the steatosis process (arrowheads). (C) Control group receiving the diet supplemented with iron (group CI) featuring ballooned hepatic cells (stars) suggesting a hydropic degenerative process and focal granulomatous inflammation (arrow). (D) Diabetic group receiving a diet supplemented with iron showing histology similar to B. Arrowhead indicates cells with cytoplasmic microvesicles. (E) Control group receiving a diet supplemented with iron and (F) diabetic group receiving a diet supplemented with iron and featuring iron deposits in hepatocytes. Insert showing citoplasmatic iron store in hepatocyte (head arrow) and Kupffer cell (star) in ×1110 magnification A, B, C and D—Hematoxylin & Eosin staining. E and F—Perls staining. Bar = 50 µM.
The most important lesion found in animals of groups D and DI was the presence of numerous hepatic cells with multiple intracytoplasmic vesicles presenting as a negative image on HE staining, which is a morphologic indication of multilocus steatosis. In animals of group D, steatosis was mild, located in hepatic portal regions (Fig. 1B) and was associated with the presence of small inflammatory foci without the presence of tissue iron stores. In animals of the DI group, the steatosis was also mild, but in this case, it was diffused throughout all areas of the liver and was associated with small inflammatory foci and tissue iron pools (Fig. 1 D and F). Histological analysis of Perls for C and D groups showed no iron deposits.
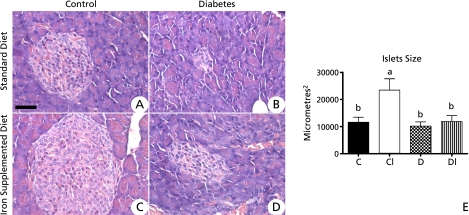
Pancreatic analysis showed that animals in group CI had pancreatic islets one fold higher than animals in group C. However, animals in both groups D and DI showed average size of pancreatic islets equal as compared to those in group C (Fig. 2). Total percentage of the pancreatic area occupied by islets was 8.23% for group C and 10.24% for group CI, both of which were significantly higher than those observed for groups D and DI, in which the percentage was 2.98% and 4.82%, respectively (Fig. 2). Histological studies showed no changes in cell profiles in any of the pancreatic regions. Furthermore, no tissue iron pools in all groups analyzed was seen.
Fig. 2.
Photomicrographs of histological pancreatic sections. (A) Control group. (B) Diabetic group. Note the decrease of the size of pancreatic islets as compared to the control group. (C) Control group receiving the iron supplemented diet and showing significant increase of pancreatic islets as compared to all other groups. (D) Diabetic group receiving iron supplemented diet. Note the decreased size of pancreatic islet as compared to the control group. Hematoxylin & Eosin staining. Bar = 50 µM. Figure E- Average size of pancreatic islets from hamsters fed standard diet (C), diet supplemented with carbonyl iron (CI), diabetes (D) and diabetes supplemented with carbonyl iron (DI)*.
* Values are mean ± standard deviation (n = 6). Data were analyzed by bivariate ANOVA analysis. When one of the factors was significant (p<0.05), univariate analysis with Tukey post test was performed to determine specific differences between means. Statistical differences are shown by different superscript letters.
Antioxidant status
Results of these analyses are shown in Table 2. We found that diabetes reduced the antioxidant status in the liver, as it caused a decrease of 8.5% and 33% in hepatic glutathione and catalase activities, respectively. However, an interaction of excess iron with diabetes, led to an increase in this antioxidant status as seen by the activities of both glutathione and catalase in the liver.
Table 2.
Hamster antioxidant and carbonyl protein levels
| Variables | Experimental Groups |
||||||
|---|---|---|---|---|---|---|---|
| Control | Control + Iron | Diabetes | Diabetes + Iron | ANOVA (p values) |
|||
| Iron | Diabetes | Iron × Diabetes | |||||
| Liver glutathione nmoles/ml | 40.43 ± 4.81a | 40.12 ± 1.61a | 34.77 ± 3.06b | 40.01 ± 5.47a | 0.146 | 0.0383 | 0.097 |
| Liver catalase µmol/mg protein | 18.30 ± 3.19a | 16.75 ± 3.274a | 12.16 ± 1.34b | 17.67 ± 2.36a | 0.104 | 0.01 | 0.002 |
| Liver carbonyl protein nmol/mg protein | 2.25 ± 1.2b | 3.24 ± 1a.b | 3.74 ± 0.76a | 3.18 ± 0.63a.b | 0.685 | 0.108 | 0.0445 |
¶ Values are shown as the mean ± standard deviation (n = 8). Data were analyzed by bivariate ANOVA analysis. When one of the factors was significant (p<0.05) univariate analysis with Tukey post test was performed to determine specific differences between means. Statistical differences are shown by different superscript letters.
Liver carbonyl protein
As shown in Table 2, levels of carbonyl protein in the liver of animals from group D were found to be 41% higher than those in group C. Animals in group DI had carbonyl protein levels equal to those in group C.
PPAR-α/β-actin relative levels in the liver
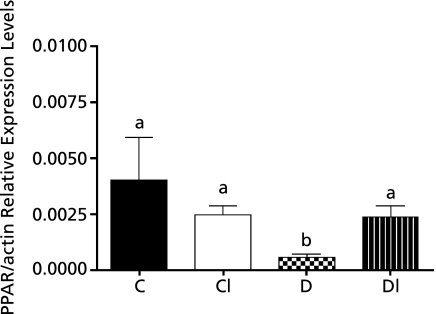
Fig. 3 shows qPCR analysis of the expression of PPAR-α in the liver of diabetic hamsters and in the liver of those whose diets were supplemented with excess iron. PPAR-α mRNA levels were significantly lower (85%) in group D as compared to group C. However, excess iron did not alter the expression of PPAR-α mRNA.
Fig. 3.
Levels of relative PPAR-α/β-actin expression in the liver of hamsters offered the standard diet (C), the C diet supplemented with carbonyl iron (CI) and diabetic hamsters offered the standard diet (D) and diabetic hamsters offered the C diet supplemented with carbonyl iron (DI)*.
* Values are mean ± standard deviation (n = 4). Data were analyzed by the Kruskal-Wallis test. Statistical differences are shown by different letters.
Discussion
Iron treatment increased serum and tissue iron levels. Surprisingly, although both control and diabetics groups have received the same treatment with carbonyl iron, apparently, animals that received both treatments presented an alteration in iron homeostasis, leading to a lower concentration of iron in serum and liver. It is known that the amount of plasma iron is determined by a regulated liberation of iron from most cells of the body. Macrophages, intestinal enterocytes, and hepatocytes have a particularly important role in this process, and this cellular efflux is modulated by liver hepcidin.(30) Fernandez Real et al.(31) verified that hepcidin levels increased significantly in patients with type 2 diabetes. Our data suggest that the interaction between iron supplementation and diabetes triggers changes in iron homeostasis, contributing to a lower absorption, resulting in lower iron levels in serum and liver. This effect can also be attributed to insulin since in vitro data suggest that it is capable of redistributing the cellular pool of transferring receptors, increasing the proportion at the cell surface, leading to increased cellular iron uptake in adipose tissue and the liver.(32) In our experimental model STZ destroys the beta cells, promoting insulin deficiency, which may have caused a smaller deposit of iron in the DI group as compared to CI. Our data on glycemic and lipids profile, besides the histological aspect of the pancreas showed a detrimental interaction between diabetes and iron. These data support those from epidemiological studies which show a correlation between increased iron pools and diabetes. On the other hand, the histological aspect of the liver in group DI followed the same profile of D group and the liver antioxidant levels were increased as compared to group D. Our results show the different tissue vulnerability to ferro-diabetes interaction. Damage through reactive oxygen species is determined not only by the generation of free oxygen radicals but also by the antioxidant defense status of the cell. This is why different organs can exhibit considerable differences in their susceptibility towards cytotoxic damage. Lenzen et al.(33) showed that in pancreatic islets very low levels of gene expression of all antioxidant enzymes are observe compared to liver. Further, seem that the higher expression of PPAR-α and lower oxidative stress played an important role in hepatic integrity. The histological data obtained here indicate that our model of iron supplementation caused less damage to the liver, as we did not see any fibrosis, thereby suggesting preservation of the metabolic functions, what was further supported by total protein and albumin concentrations. When we consider the activity of ALT, a marker of liver function, the DI group showed intermediate levels between C and D. This corroborates with data of oxidative stress and PPAR-α expression in the liver, indicating that the interaction of iron with diabetes reversed damage caused by the disease. As for AST activity, it was shown to be increased in the animals that received iron supplementation, whether they were diabetic or not. An increase in the concentration of AST may reflect damage to other organs such as muscle, since it is not distributed in various tissues. The data of activity of these enzymes also allow us to suppose that the interaction between iron and diabetes promoted different effects on the different tissues, possibly because the susceptibility of these tissues to oxidative stress is different.
In the present study we have found differential expression of liver PPAR-α among diabetic and iron-induced diabetic hamsters. PPAR-α was expressed at relatively lower levels in the diabetic animals. Previous studies have shown that the expression of PPAR-α can be regulated by diabetes. Marcill et al.(34) showed that oxidative stress decreases PPAR-α expression in macrophages of diabetics animals. Furthermore, Wang et al.(35) investigated the expression of PPAR-α in aorta, renal cortex and retina of diabetic rats and found it to be decreased in all studied tissues, besides Li et al.(36) having shown that type 2 diabetic hamsters also had decreased expression of this mRNA in the liver. It has been previously indicated that oxidative stress increases during diabetes(37,38) and contributes to down-regulation of PPAR-α in atrial myocytes both in vitro(39) and in vivo.(20) In the present study, diabetes significantly increased oxidative stress as evaluated by increasing carbonyl protein and decreasing glutathione levels and catalase activity, but was attenuated in iron-treated diabetic hamsters. The effectiveness of iron-diabetes to attenuate oxidative stress might be due to the greater induction of NF-E2-related factor 2 (Nrf2) target genes. This activation induces the production of antioxidant compounds and enzymes.(40,41) Tanaka et al.(42) have demonstrated that coordinated induction of Nrf2 target genes protects against iron nitrilotriacetate (FeNTA)-induced nephrotoxicity. Our results concerning antioxidants suggest that the interaction between diabetes and iron alters the redox balance and accounts for increased antioxidant levels, thus contributing to a higher expression of PPAR-α mRNA.
Reduced expressions of PPAR-α have been associated with lipid accumulation.(43) Increased concentration of triglycerides and reduced HDL levels are key characteristics of dyslipidemia in diabetes.(44) Previous studies have shown that excess iron can increase the concentration of triglycerides in the circulation.(45) Although increases in LDL-cholesterol and triglycerides are common in diabetic dyslipidemia, we did not observe changes in the levels of these metabolites in our diabetes model, probably due to the fact that in STZ-induced diabetes there is a reduction in insulin secretion.(46) When iron was associated with diabetes, lower cholesterol and higher triglyceride levels were observed. Our data suggest that the alterations in serum lipid homeostasis in group DI are independent of PPAR-α expression because the levels of expression of this mRNA were comparable to the group C and higher than the D groups; this was possibly due to the strong influence of insulin deficiency. HMG-CoA reductase, a key enzyme in the synthesis of cholesterol, has its activity stimulated by this hormone, thus lower insulin levels would lead to less cholesterol synthesis, justifying our data for cholesterol. Lower activity of lipoprotein lipase can contribute to higher levels of triglycerides because this enzyme is essential to the removal of triglycerides from the blood stream and further degradation and it is attenuated in insulin deprivation. Furthermore, although there is no significant difference between body weight of animals in groups D and DI, there is a tendency of group DI to have lower body weight than group D. When we analyzed glucose levels of these two groups it was observed that they were higher in the DI group, suggesting a reduced production of insulin, which can lead to increased lipolysis and proteolysis in this group, leading to greater weight loss in the DI animals as compared to the DI group.
Our data support those from epidemiological studies which show a correlation between increased iron pools and diabetes. It has been reported in clinical studies that increased iron levels in serum and specific tissues may increase the incidence of type 2 diabetes(32,47) and gestational diabetes.(48) Iron depletion has been demonstrated to be beneficial in coronary artery responses, endothelial dysfunction, insulin secretion, insulin action, and metabolic control in type 2 diabetes.(49) Our results show that iron supplemented in diabetes type I model increased serum concentrations of glucose. The interaction between iron and diabetes promoted different effects on the liver and pancreas, possibly because the susceptibility of these tissues to oxidative stress is different. Besides, for the first time, we have shown that PPAR-α expression is differentially expressed in diabetic and iron supplemented-diabetic hamsters livers.
Acknowledgments
This study was supported by the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG, Minas Gerais, Brazil n CDS-APQ 01126-08) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil; no. 475823/2007-9).
We thank Dr. Maria Terezinha Bahia (Chagas’ Disease Laboratory, Federal University of Ouro Preto) for the use of the real time PCR ABI 7300 equipment (Applied Biosystems). We are grateful to Rinaldo Cardoso dos Santos for suggestions and careful review of the manuscript.
Abbreviations
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- AST
aspartate aminotransferase
- AOAC
Association of Official Analytical Chemists
- CI
Control iron group
- CT
Cycle Threshold
- DNPH
2,4-Dinitrophenylhydrazine
- DTNB
5,5-dithio-bis-(2-nitrobenzoic) acid
- DI
Diabetes iron group
- FeNTA
iron-nitrilotriacetate
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- MDA
malondialdehyde
- Nrf2
NF-E2-related factor-2
- PPAR
Peroxisome proliferator-activated receptor
- ROS
Reactive oxygen species
- STZ
streptozotocin
- TCA
trichloroacetic acid
- TNB
2-nitro-5-thiobenzoic acid
- VLDL
very low density lipoprotein
References
- 1.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 2.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 3.Spitaler MM, Graier WF. Vascular targets of redox signalling in diabetes mellitus. Diabetologia. 2002;45:476–494. doi: 10.1007/s00125-002-0782-0. [DOI] [PubMed] [Google Scholar]
- 4.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 5.Stadler K, Jenei V, von Bölcsházy G, Somogyi A, Jakus J. Role of free radicals and reactive nitrogen species in the late complications of diabetes mellitus in rats. Orv Hetil. 2004;145:1135–1140. [PubMed] [Google Scholar]
- 6.Dunford HB. Free radicals in iron-containing systems. Free Radic Biol Med. 1987;3:405–421. doi: 10.1016/0891-5849(87)90019-0. [DOI] [PubMed] [Google Scholar]
- 7.Leonarduzzi G, Scavazza A, Biasi F, et al. The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor beta1 expression in the macrophage lineage: a link between oxidative injury and fibrosclerosis. FASEB J. 1997;11:851–857. doi: 10.1096/fasebj.11.11.9285483. [DOI] [PubMed] [Google Scholar]
- 8.McClain DA, Abraham D, Rogers J, et al. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia. 2006;49:1661–1669. doi: 10.1007/s00125-006-0200-0. [DOI] [PubMed] [Google Scholar]
- 9.Barbieri M, Ragno E, Benvenuti E, et al. New aspects of the insulin resistance syndrome: impact on haematological parameters. Diabetologia. 2001;44:1232–1237. doi: 10.1007/s001250100634. [DOI] [PubMed] [Google Scholar]
- 10.Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22:1978–1983. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- 11.Salonen JT, Tuomainen TP, Nyyssönen K, Lakka HM, Punnonen K. Relation between iron stores and non-insulin dependent diabetes in men: case-control study. BMJ. 1998;317:727. doi: 10.1136/bmj.317.7160.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lao TT, Tam KF. Maternal serum ferritin and gestational impaired glucose tolerance. Diabetes Care. 1997;20:1368–1369. doi: 10.2337/diacare.20.9.1368. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Jones D, Luo B, et al. Iron overload and diabetes risk: a shift from glucose to Fatty Acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes. 2011;60:80–87. doi: 10.2337/db10-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costet P, Legendre C, Moré J, Edgar A, Galtier P, Pineau T. Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J Biol Chem. 1998;273:29577–29585. doi: 10.1074/jbc.273.45.29577. [DOI] [PubMed] [Google Scholar]
- 15.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci USA. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 18.Kersten S. Peroxisome proliferator activated receptors and lipoprotein metabolism. PPAR Res. 2008;2008:132960. doi: 10.1155/2008/132960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakhshandehroo M, Sanderson LM, Matilainen M, et al. Comprehensive analysis of PPARalpha-dependent regulation of hepatic lipid metabolism by expression profiling. PPAR Res. 2007;2007:26839. doi: 10.1155/2007/26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee TI, Kao YH, Chen YC, Pan NH, Chen YJ. Oxidative stress and inflammation modulate peroxisome proliferator-activated receptors with regional discrepancy in diabetic heart. Eur J Clin Invest. 2010;40(8):692–699. doi: 10.1111/j.1365-2362.2010.02318.x. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization, author. Geneva. 2001. Iron deficiency anemia: assessment, prevention and control—a guide for programme managers. [Google Scholar]
- 22.Colégio Brasileiro de Experimentação Animal, author. COBEA. 1991. Princípios éticos na experimentação animal do Colégio Brasileiro de Experimentação Animal. [Google Scholar]
- 23.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 24.Dabbagh AJ, Mannion T, Lynch SM, Frei B. The effect of iron overload on rat plasma and liver oxidant status in vivo. Biochem J. 1994;300:799–803. doi: 10.1042/bj3000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iancu RI, Haliga R, Luca V, Stitt PA, Mocanu V. Modulation of the platelet function by diet supplemented with flaxseed and vitamin E in diabetic hamsters. Rev Med Chir Soc Med Nat Iasi. 2006;110:962–967. [PubMed] [Google Scholar]
- 26.Association of Official Analytical Chemists, author. AOAC. Washington: 1980. Official methods of analysis. [Google Scholar]
- 27.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 28.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 29.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Darshan D, Anderson GJ. Interacting signals in the control of hepcidin expression. Biometals. 2009;22:77–87. doi: 10.1007/s10534-008-9187-y. [DOI] [PubMed] [Google Scholar]
- 31.Fernández-Real JM, Equitani F, Moreno JM, Manco M, Ortega F, Ricart W. Study of circulating prohepcidin in association with insulin sensitivity and changing iron stores. J Clin Endocrinol Metab. 2009;94:982–988. doi: 10.1210/jc.2008-1211. [DOI] [PubMed] [Google Scholar]
- 32.Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB. The role of iron in type 2 diabetes in humans. Biochim Biophys Acta. 2009;1790:671–681. doi: 10.1016/j.bbagen.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 34.Marcil V, Delvin E, Sané AT, Tremblay A, Levy E. Oxidative stress influences cholesterol efflux in THP-1 macrophages: role of ATP-binding cassette A1 and nuclear factors. Cardiovasc Res. 2006;72:473–482. doi: 10.1016/j.cardiores.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, Gao L, Gong B, et al. Tissue-specific expression of PPAR mRNAs in diabetic rats and divergent effects of cilostazol. Can J Physiol Pharmacol. 2008;86:465–471. doi: 10.1139/y08-043. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Liu X, Zhu H, et al. Insulin resistance in insulin-resistant and diabetic hamsters (Mesocricetus auratus) is associated with abnormal hepatic expression of genes involved in lipid and glucose metabolism. Comp Med. 2009;59:449–458. [PMC free article] [PubMed] [Google Scholar]
- 37.Karacay O, Sepici-Dincel A, Karcaaltincaba D, et al. A quantitative evaluation of total antioxidant status and oxidative stress markers in preeclampsia and gestational diabetic patients in 24–36 weeks of gestation. Diabetes Res Clin Pract. 2010;89:231–238. doi: 10.1016/j.diabres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Lai MH. Antioxidant effects and insulin resistance improvement of chromium combined with vitamin C and e supplementation for type 2 diabetes mellitus. J Clin Biochem Nutr. 2008;43:191–198. doi: 10.3164/jcbn.2008064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee TI, Kao YH, Chen YC, Chen YJ. Proinflammatory cytokine and ligands modulate cardiac peroxisome proliferator-activated receptors. Eur J Clin Invest. 2009;39:23–30. doi: 10.1111/j.1365-2362.2008.02062.x. [DOI] [PubMed] [Google Scholar]
- 40.Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. J Neurosci Res. 2005;79:157–165. doi: 10.1002/jnr.20280. [DOI] [PubMed] [Google Scholar]
- 41.McCord JM, Edeas MA. SOD, oxidative stress and human pathologies: a brief history and a future vision. Biomed Pharmacother. 2005;59:139–142. doi: 10.1016/j.biopha.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka Y, Aleksunes LM, Goedken MJ, et al. Coordinated induction of Nrf2 target genes protects against iron nitrilotriacetate (FeNTA)-induced nephrotoxicity. Toxicol Appl Pharmacol. 2008;231:364–373. doi: 10.1016/j.taap.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chinetti-Gbaguidi G, Fruchart JC, Staels B. Role of the PPAR family of nuclear receptors in the regulation of metabolic and cardiovascular homeostasis: new approaches to therapy. Curr Opin Pharmacol. 2005;5:177–183. doi: 10.1016/j.coph.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Syvänne M, Taskinen MR. Lipids and lipoproteins as coronary risk factors in non-insulin-dependent diabetes mellitus. Lancet. 1997;350:SI20–SI23. doi: 10.1016/s0140-6736(97)90024-6. [DOI] [PubMed] [Google Scholar]
- 45.Silva M, Silva ME, de Paula H, Carneiro CM, Pedrosa ML. Iron overload alters glucose homeostasis, causes liver steatosis, and increases serum triacylglycerols in rats. Nutr Res. 2008;28:391–398. doi: 10.1016/j.nutres.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Elsner M, Guldbakke B, Tiedge M, Munday R, Lenzen S. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia. 2000;43:1528–1533. doi: 10.1007/s001250051564. [DOI] [PubMed] [Google Scholar]
- 47.Zheng Y, Li XK, Wang Y, Cai L. The role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: therapeutic effects by chelators. Hemoglobin. 2008;32:135–145. doi: 10.1080/03630260701727077. [DOI] [PubMed] [Google Scholar]
- 48.Afkhami-Ardekani M, Rashidi M. Iron status in women with and without gestational diabetes mellitus. J Diabetes Complications. 2009;23:194–198. doi: 10.1016/j.jdiacomp.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Jiang R, Ma J, Ascherio A, Stampfer MJ, Willett WC, Hu FB. Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: a prospective cohort study. Am J Clin Nutr. 2004;79:70–75. doi: 10.1093/ajcn/79.1.70. [DOI] [PubMed] [Google Scholar]