Abstract
In the present study by applying electron spin resonance-spin trapping method, when a high frequency (1650 kHz) ultrasound was irradiated to water dissolved with different gas molecules (O2, N2, Ar, Ne, He, and H2) at 25°C of water bulk temperature, free radical generation pattern differed dependently on the dissolved gas molecules. Only •OH was detected in the O2-dissolved water sample, and the amount of the radical was much greater than that determined in any of other gas-dissolved water samples. One of the possible reasons to explain why the •H radical was not detected in the O2-dissolved water is that the •H reacts with O2 to form •OOH. However, no electron spin resonance signals related to the adduct of not only 5,5-dimethyl-1-pyrroline-N-oxide but 5-(2,2-Dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide and •OOH were observed. In the H2-dissolved water, only •H was detected, suggesting that H2 reduces or neutralizes •OH. In the N2-disolved water, both •OH and •H were detected at comparable level. In the water samples dissolved with rare gases (Ar, Ne, and He), the amount of •H was almost double as compared with that of •OH, and both •OH and •H yields increased in the order Ar > Ne > He.
Keywords: water sonolysis, radical formation, electron spin resonance
Introduction
Ultrasound has been widely applied for clinical diagnosis, dental care and therapeutic tools.(1–7) Especially diagnostic devices are commonly used for tomography or distance measurement in internal medicine or obstetrics, by using ultrasound energy in the high frequency range at low intensity. In the field of ophthalmology for example, cataract surgeries are widely performed by using phacoemulsification and aspiration, which utilizes high-intensity ultrasound energy for the fragmentation and emulsification of the cataractous lens.(8)
Free radical formation by sonolysis of water has been considered to be generated by cavitation especially when ultrasound was irradiated with low frequency (from a few dozens to several hundred kHz).(8–10) Cavitation is a phenomenon which refers to the formation, growth, and collapse of small bubbles formed in liquids. During the process of cavitation, the extremely high temperature (several thousand degrees K) and high pressure (hundreds of atmospheres) of imploding cavitation bubbles lead to the thermal dissociation of water molecule, which in turn results in generation of •OH and •H.(11–13)
As reported in previous papers,(10,12) •OH yield produced by low-frequency (47 kHz) ultrasound was inversely proportional to the thermal conductivity of the dissolved rare gases. Furthermore, it was reported that when •OH yields were compared between 47 kHz and 400 kHz ultrasound in the presence of different rare gases, the amount of •OH produced by 400 kHz ultrasound was less inversely proportional to the thermal conductivity of the dissolved gas than that of •OH produced by 47 kHz,(9) suggesting that the temperature of the cavitation bubbles and •OH yield by ultrasound are strongly correlated. That is, the size of the cavitation bubbles produced by 400 kHz ultrasound is smaller than that by 47 kHz, and final temperature of the collapse by 400 kHz becomes relatively low, so that the difference of the final temperature induced by the differences of the thermal conductivities of rare gases decreases. In contrast, the formation mechanism of •OH by high frequency ultrasound (more than 1 MHz) has not been fully understood.
We have applied a free radical generation system in which ultrasound was irradiated at 1650 kHz to pure water to the basic studies such as a kinetic study of 5,5-dimethyl-1-pyrroline-N-oxide (DMPO)-OH, a spin adduct of DMPO and •OH, and an antimicrobial study.(14,15) In these studies, we could detect neither •H nor O2•− by applying an electron spin resonance (ESR)-spin trapping technique. In the present study, to elucidate the underlying mechanism of free radical formation by sonolysis with a high frequency (1650 kHz), we analyzed the free radicals (O2•−, •OH, and •H) generated by ultrasound irradiation to pure water containing a dissolved gas (O2, N2, Ar, Ne, He, or H2) by applying the ESR-spin trapping technique.
Materials and Methods
Reagents were purchased from the following sources: 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) from Labotec (Tokyo, Japan); hydrogen peroxide (H2O2) from Santoku Chemical Industries (Tokyo, Japan); 4-hydroxy-2,2,6,6-tetramethylpiperidine (TEMPOL) from Sigma Aldrich (St. Louis, MO). 5-(2,2-Dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) was synthesized according to the protocol described in the previous report.(16) All other reagents used were of analytical grade. O2, H2 and N2 gases were purchased from Japan Fine Products Co., Ltd. (Kawasaki, Japan), Ar and Ne gases from Japan Air Gases Co., Ltd. (Tokyo, Japan), and He gas from Union Helium Co., Ltd. (Kawasaki, Japan). The purities of these gases were 99.5–99.999%. An experimental device which generates 1650 ± 50 kHz ultrasound with an acoustic intensity of 50 W/cm2 was kindly supplied by Nimo Corporation (Nagoya, Japan). A glass tube (16 mm in diameter and 125 mm long) containing 400 µl of 445 mM DMPO dissolved in ultrapure water was placed in the device. Subsequently, each of six different gases was bubbled for 3 min, followed by ultrasound irradiation for 10 to 120 sec. In an experiment using water sample dissolved with O2, 27 mM CYPMPO was used as a spin trap alternative to DMPO. The temperature of the water was controlled by a thermo-regulated water bath. After ultrasound irradiation, the DMPO aqueous solution was transferred to a quartz cell for ESR spectrometry, and then an ESR spectrum was immediately recorded on an X-band ESR spectrometer (JES-FA-100, JEOL, Tokyo, Japan). The measurement conditions for ESR were as follows; field sweep, 330.50–340.50 mT; field modulation frequency, 100 kHz; field modulation width, 0.1 mT; amplitude, 20; sweep time, 2 min; time constant, 0.03 sec; microwave frequency, 9.421 GHz: microwave power, 4 mW. To calculate the concentrations of DMPO-H and DMPO-OH, 20 µM of TEMPOL was used as a standard sample for quantitative analysis, and the ESR spectrum of manganese (Mn2+) which was equipped in the ESR cavity was used as an internal standard. The concentrations of •H and •OH were determined using Digital Data Processing (JEOL, Tokyo, Japan) and the concentrations of DMPO-H and DMPO-OH were expressed in µM.
Dissolved O2 concentration was determined as in the following way. A beaker containing 100 ml of ultrapure water was placed in a homo-isothermal bath and the ultrapure water was bubbled with O2 gas. When the temperature of the ultrapure water stabilized, the dissolved O2 concentration was measured by a diaphragm electrode method using a Dissolved Oxygen Meter YSI-5100 (YSI InF, Yellow Spring, OH).
Results
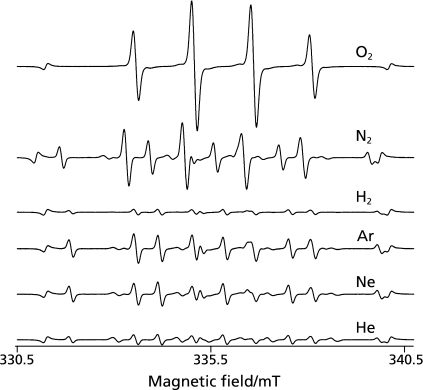
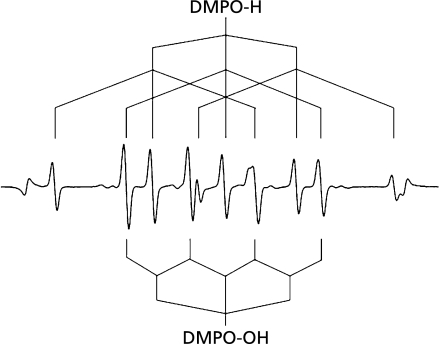
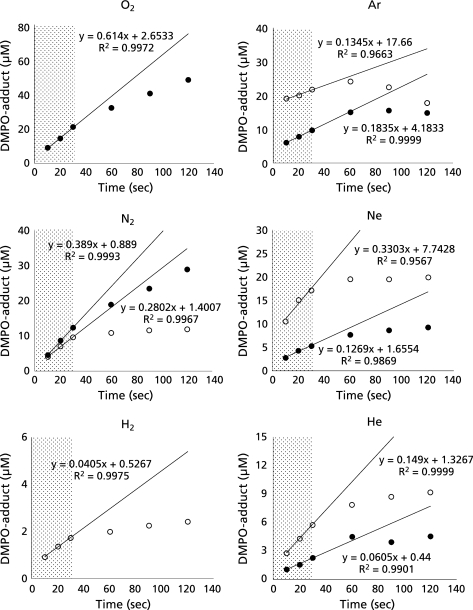
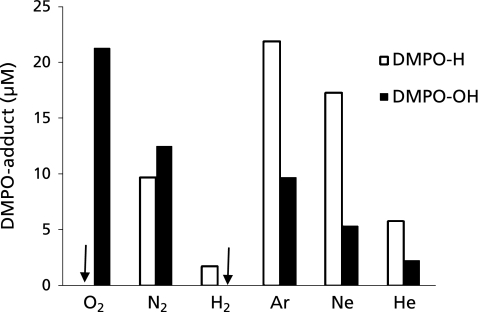
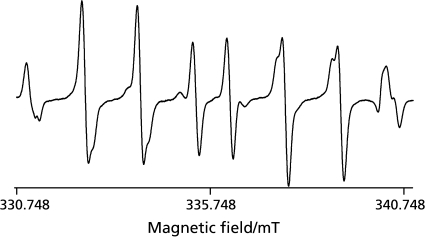
When ultrasound at 1650 kHz was irradiated to ultrapure water samples dissolved with either of six different gases in the presence of 445 mM DMPO at 25 ± 5°C of the water bulk temperature, the ESR signals of DMPO-spin adducts were observed (Fig. 1). In the water samples dissolved with N2, Ar, Ne and He, the signals consists of quartet segments (intensity ratio, 1:2:2:1) and triplet of triplet segments (intensity ratio, 1:1:2:1:2:1:2:1:1) were observed. As shown in the representative ESR spectrum of the Ar-dissolved water irradiated by ultrasound (Fig. 2), the former segments were assigned to DMPO-OH, a spin adduct derived from •OH (hyperfine coupling constant, aN = 1.49; aH = 1.49 mT). The latter segments were assigned to DMPO-H, a spin adduct derived from •H (hyperfine coupling constant, aN = 1.63; aH = 2.25 mT) as reported in a previous study.(17) In the water samples dissolved with O2 and H2, the ESR-signal of only DMPO-OH and that of only DMPO-H were observed, respectively (Fig. 1). Since the formation and decay of the spin adducts would affect the yield of the spin adducts, time course changes in the concentrations of the spin adducts generated for 10 to 120 sec were monitored (Fig. 3). In any of the gas-saturated water samples, both DMPO-OH and DMPO-H increased with time up to certain time points, and a linear relationship with a correlation coefficient of over 0.99 between the concentration of each DMPO-adduct and the irradiation time was observed up to 30 sec. Based on the results shown in Fig. 3, the yields of DMPO-OH and DMPO-H obtained after irradiation for 30 sec were compared among the water samples (Fig. 4). Only •OH (as indicated by DMPO-OH) was detected in the O2-dissolved water and the amount of the radical was much greater than that determined in any of the other gas-dissolved water samples, and only •H (as indicated by DMPO-H) was detected in the H2-disiolved water and the amount of the radical was less than that in the N2-disolved water. As for •H yields in the presence of rare gases, the amount of •H was double or more as compared with that of •OH. Emphasizing a focus on the effect of dissolved rare gases on the radical formation, both •OH and •H yields increased in the order Ar > Ne > He (Fig. 4). To further examine whether •H was not generated in the water sample saturated with O2, CYPMPO was used as an alternative spin trapping agent. Fig. 5 shows the representative ESR spectrum of sonolyzed water sample in the presence of O2 and CYPMPO. The ESR signal was proved to be CYPMPO-OH since the hyperfine coupling constants aN = 1.39; aH = 1.39 mT; ap = 5.04 mT were identical to those reported in a previous study,(16) whilst no ESR signals related to the adducts of CYPMPO and •OOH were observed.
Fig. 1.
Representative ESR signals of DMPO-spin adducts generated by ultrasound irradiation at 1650 kHz to ultrapure water dissolved with a particular gas for 1 min.
Fig. 2.
Representative ESR signals of DMPO-spin adducts generated by ultrasound irradiation at 1650 kHz to water dissolved with Ar gas for 1 min. The signals are assigned to DMPO-H (a spin adduct of DMPO and •H) and DMPO-OH (a spin adduct of DMPO and •OH).
Fig. 3.
Time course changes in the concentrations of the spin adducts generated by ultrasound irradiation to ultrapure water in the presence of different dissolved gases at 25°C. Each value represents the mean of duplicate determinations. Solid circle (I) and open circle (○) indicate DMPO-OH and DMPO-H, respectively.
Fig. 4.
Generation of •H and •OH as indicated by spin adducts, DMPO-H and DMPO-OH, by ultrasound irradiation to ultrapure water for 30 sec in the presence of different dissolved gases at 25°C. Each value represents the mean of duplicate determinations. ↓: not detected.
Fig. 5.
Representative ESR signal of CYPMPO-spin adduct generated by ultrasound irradiation at 1650 kHz to ultrapure water dissolved with O2 gas for 60 sec. The signal is assigned to CYPMPO-OOH (a spin adduct of CYPMPO and •OH).
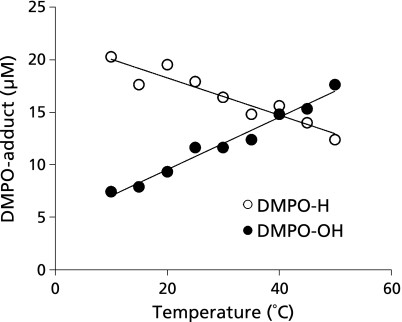
Then, to examine the kinetics of •H and •OH, ESR analysis of the radicals from the water samples sonolyzed for 60 sec in the presence of either Ar or N2 was performed at different temperatures. As shown in Fig. 6, the yield of •OH as indicated by DMPO-OH derived from sonolysis of water in the presence of Ar increased with increasing water bulk temperature, whilst the yield of •H as indicated by DMPO-H decreased. The similar phenomenon was observed in the sonolysis of water dissolved with N2 (data not shown).
Fig. 6.
Generation of •H and •OH as indicated by spin adducts, DMPO-H and DMPO-OH, by ultrasound irradiation to ultrapure water for 60 sec in the presence of Ar at different water bulk temperatures. Each value represents the mean of duplicate determinations.
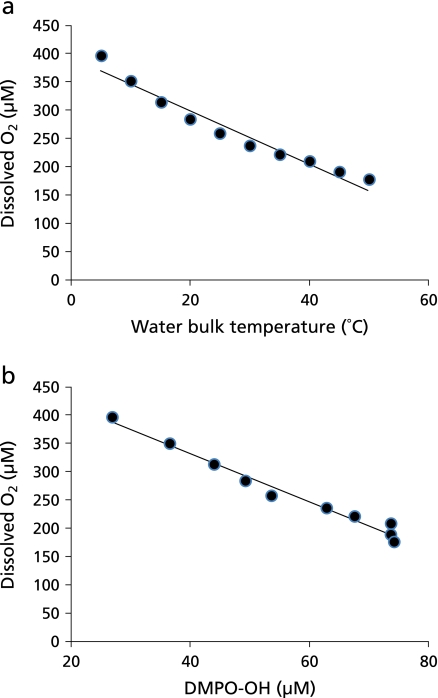
To discuss the radical formation mechanism in water sonolysis with the frequency of 1650 kHz in the presence of O2, relation among water bulk temperature, dissolved O2, and •OH yield was evaluated. As shown in Fig. 7a, increasing the water temperature led to dissolved O2 concentration decreased. Similarly, •OH yield as indicated by the amount of DMPO-OH increased with decreasing dissolved O2 concentration (Fig. 7b).
Fig. 7.
Relation among water bulk temperature, dissolved O2, and •OH generation by ultrasound irradiation for 60 sec. a, Relation between the concentration of dissolved O2 and water bulk temperature. b, Relation between the concentration of •OH as indicated by DMPO-OH and the concentration of dissolved O2. Each value represents the mean of duplicate determinations.
Discussion
In the present study where water was exposed to ultrasound irradiation at 1650 kHz, the pattern of radical formation was different among the dissolved gases. Of the dissolved gases, only •OH was detected in the water sample under O2 and the amount of the radical was much greater than that determined in any of the other gas; -dissolved water samples (Figs. 1, 3 and 4). Two possible reasons can be considered to explain why the •H radical was not detected in the O2-dissolved water: (i) the formation of •H was suppressed and (ii) the •H reacted with O2 to form •OOH. Regarding the latter case, it was reported that when aqueous DMPO solutions were sonicated during air bubbling or in the presence of air, only the ESR signal due to DMPO-OH adduct, but not that due to DMPO-H, was observed.(18) To explain this phenomenon, the authors speculated that O2 scavenges •H in the cavitation bubbles. In the present study, however, no ESR signals related to the adducts of the DMPO and •OOH were observed, suggesting that the formation of •H might be suppressed. To further confirm the absence of •OOH, an additional experiment with CYPMPO as a spin trap, which reacts with O2•− with a rate constant that is superior to DMPO, and the half-life of the corresponding spin trapped adduct is considered longer,(16,19) was conducted. (Fig. 5). As is the case with DMPO, no ESR signals related to the adduct of CYPMPO and •OOH were observed. If the formation of •H is suppressed under the O2-saturated condition, the reaction between •H and •OH to form H2O is also suppressed because of •H depletion, which in turn makes the concentration of •OH increase. Meanwhile, in another point view in which the •OH yield was much greater under O2 than that determined under any of the other gasses, following reactions can be considered as proposed by Uddin et al.(20)
H2O → •H + •OH
•H + O2 → •OH + O
O + •OH → •OOH
In this case, O2 reacts with •H to form •OH, indicating that the presence of dissolved O2 in water sonolysis gives additional amount of •OH. However, a question is still remained as described above. That is, no ESR signals related to the adduct of DMPO and •OOH were observed. In addition, the results of the experiments, in which the relation among water bulk temperature, dissolved O2, and •OH yield was evaluated (Fig. 7 a and b), revealed that the •OH yield decreases with increasing dissolved O2 concentration, suggesting that the reaction ”•H + O2 → •OH + O” seems to be controversial. Of the effects of different gases, the •OH yield in the water sonolysis under N2 was almost equal to that under Ar (Figs. 3 and 4). In a study where 50 kHz ultrasound-induced ubiquinol formation in aqueous solutions of ubiquinone was studied in the presence of different gases,(21) the yield of ubiquinone ring radical, which was formed by addition of •OH (or •H) to the 5,6 double bond of ubiquinone, decreased when aqueous solution of ubiquinone was saturated with N2 instead of Ar. In this case, it is assumed that the temperature of collapsing cavitation bubbles is much lower in N2- than in Ar-saturated solution.(12) In the present study, •OH yields from N2- and Ar-dissolved water samples were almost the same (Figs. 3 and 4), suggesting that collapsing cavitation bubbles might not be implicated in the phenomenon. As for H2-dissolved water sample, since it was reported that H2 reduces or neutralizes •OH,(22,23) it seems to be natural that DMPO-OH was not detected in the water sonolysis under H2 (Figs. 3 and 4). Concerning the focus on the effect of dissolved rare gases on the radical formation, both •OH and •H yielded in the order Ar > Ne > He (Fig. 4). Fukuda et al.(9) reported that the amount of •OH formed by sonication of water at either 47 kHz or 400 kHz was inverse order of the thermal conductivity of rare gases. That is, •OH yield increased in the order Ar > Ne > He, which was in agreement with not only our results but the previous study.(12) Thus, the result of the present study might also be dependent on the cavitation phenomenon, in which the lower the thermal conductivity is, the higher the temperature of cavitation collapse is. In a point of view that the ratios of •H and •OH were near 2:1 under any of Ar, Ne, and He atmosphere, the result is hard to be interpreted by the cavitation phenomenon where •H and •OH are assumed to be formed at a ratio of 1:1 via thermal dissociation of water led by the extremely high temperature and high pressure of imploding cavitation bubbles. Regarding the point that DMPO-H detected was more than twice of DMPO-OH in the water samples in the presence of rare gasses, one of the possibilities is that hydrated electron (eaq-) increases the level of •H. It was postulated that in the core of a cavitation bubble, the concentration of electrons upon electric breakdown can be high enough and, thus, can lead to the formation of eaq-.(24) Then, the resultant eaq- could increase the level of •H via following reaction.
eaq- + H2O → OH− + •H or eaq- + H+ → •H
However, Gutierréz and Henglein(25) proposed that eaq- is formed when •H is generated in strongly alkaline solutions as the pK value of the equilibrium ”•H + OH− ⟺ H2O + eaq-” is 9.8, so that the reaction does not seem to contribute significantly at neutral pH as in the present study. At present, since we could not reach the firm conclusion, further study is required to elucidate the fundamental mechanism by which the DMPO-H detected is more than twice of DMPO-OH in water saturated with rare gases.
If radical formation observed in the present study is mediated by cavitation, the yield of •H was supposed to increase as did •OH, because sonolysis of water to •H and •OH caused by cavitation is an endothermic reaction. However, the yield of •OH from the water sonolysis under either Ar or N2 increased with water bulk temperature, whilst the yield of •H decreased (Fig. 6). To explain this phenomenon, the following reactions are considered to proceed dependently on the water bulk temperature.
•H + •H → H2
Regarding the reaction, Rassokhin et al.(26) reported that H2 formation rate increased with increasing water bulk temperature from 5 to 50°C when water was irradiated by ultrasound (724 kHz, 50 W) under an Ar atmosphere. Meanwhile, it seems natural that •OH yield increases with water bulk temperature. Indeed, Uddin et al.(20) assumed that •OH yield increases from sonolysis of water with increasing water bulk temperature. In their study where sonolysis of bisphenol A, a representative endocrine disrupting chemical, was studied at different temperatures (5 to 60°C) in aqueous solutions under various gases at 489 kHz, the degradation rate increased with water bulk temperature under O2 and air up to 50 and 40°C, respectively. Under Ar and N2, the degradation rate also increased slightly with the temperature up to 40°C. Since the increase in degradation rate was cancelled by adding tert-butyl alcohol, a •OH scavenger, they assumed that •OH is responsible for temperature effects on sonolysis of bisphenol A. As for the relation among the water bulk temperature, dissolved O2 concentration and DMPO-OH yield (Fig. 7), decreasing the dissolved O2 concentration increased the DMPO-OH yield. Thus, a question, for instance, is raised that why the concentration of DMPO-OH was low in N2-saturated water which was an extreme case of low O2 condition. As described above, if •H formation is suppressed under O2-saturated condition, •OH fails to react with •H, resulting in an elevated level of •OH. In this case, it seems to be natural that the •OH yield under O2 is higher than that under N2, even though N2-saturated water is an extreme case of low O2 condition. From the viewpoint of N2, one of the possibilities is the following reactions by which N2 is supposed to react with •OH, resulting in a reduced level of •OH.
H2O → •OH + •H
N2 + •OH → N2O + •H
N2O + O → 2NO
The reactions were reported to take place when sonication is carried out during air injection.(27,28)
Results obtained in the present study suggest that free radical generation pattern induced by 1650 kHz ultrasound in the presence of different gases gives informative findings on the sonolysis of water. It is well known that sonolysis of water by a low frequency ultrasound results in •H and •OH formation via thermal dissociation of water led by the extremely high temperature (several thousand degrees K) and high pressure (hundreds of atmospheres) of imploding cavitation bubbles.(11–13) In the present study applying sonolysis of water induced by a high frequency ultrasound, some results are hard to be interpreted by cavitation phenomenon. To elucidate the underlying mechanism of radical formation in the sonolysis of water, a comparative study between sonolysis of water by 1650 kHz ultrasound and that by a low frequency ultrasound will be conducted.
Abbreviations
- CYPMPO
5-(2,2-Dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- ESR
electron spin resonance
- TEMPOL
4-hydroxy-2,2,6,6-tetramethylpiperidine
References
- 1.Matsumoto Y, Horiike S, Sakagami J, et al. Early ultrasonographic diagnosis and clinical follow-up of hepatic veno-occlusive disease after allogeneic bone marrow transplantation in a patient with acute lymphoblastic leukemia. Intern Med. 2009;48:831–835. doi: 10.2169/internalmedicine.48.1404. [DOI] [PubMed] [Google Scholar]
- 2.Geddes DT. The use of ultrasound to identify milk ejection in women - tips and pitfalls. Int Breastfeed J. 2009;4:5. doi: 10.1186/1746-4358-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinkala E, Gray S, Zulu I, et al. Clinical and ultrasonographic features of abdominal tuberculosis in HIV positive adults in Zambia. BMC Infect Dis. 2009;9:44. doi: 10.1186/1471-2334-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira RB, Marchesan M, Silva-Sousa YT, Sousa-Neto M. Effectiveness of root canal debris removal using passive ultrasound irrigation with chlorhexidine digluconate or sodium hypochlorite individually or in combination as irrigants. J Contemp Dent Pract. 2008;9:68–75. [PubMed] [Google Scholar]
- 5.Josgrilberg EB, Guimarães Mde S, Pansani CA, Cordeiro Rde C. Influence of the power level of an ultra-sonic system on dental cavity preparation. Braz Oral Res. 2007;21:362–367. doi: 10.1590/s1806-83242007000400014. [DOI] [PubMed] [Google Scholar]
- 6.Maestroni U, Ziveri M, Azzolini N, et al. High Intensity Focused Ultrasound (HIFU): a useful alternative choice in prostate cancer treatment. Preliminary results. Acta Biomed. 2008;79:211–216. [PubMed] [Google Scholar]
- 7.Mahoney CM, Morgan MR, Harrison A, Humphries MJ, Bass MD. Therapeutic ultrasound bypasses canonical syndecan-4 signaling to activate rac1. J Biol Chem. 2009;284:8898–8909. doi: 10.1074/jbc.M804281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi H. Free radical development in phacoemulsification cataract surgery. J Nippon Med Sch. 2005;72:4–12. doi: 10.1272/jnms.72.4. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda S, Ogawa R, Kondo T. Frequency dependence of free radical formation by ultrasound in the presence of different rare gases. Jpn J Appl Phys. 2001;40:3493–3494. [Google Scholar]
- 10.Kondo T, Gamson J, Mitchell JB, Riesz P. Free radical formation and cell lysis induced by ultrasound in the presence of different rare gases. Int J Radiat Biol. 1988;54:955–962. doi: 10.1080/09553008814552351. [DOI] [PubMed] [Google Scholar]
- 11.Makino K, Mossoba MM, Riesz P. Chemical effects of ultrasound on aqueous solutions. Evidence for •OH and •H by spin trapping. J Am Chem Soc. 1982;104:3537–3539. [Google Scholar]
- 12.Riesz P, Kondo T. Free radical formation induced by ultrasound and its biological implications. Free Radic Biol Med. 1992;13:247–270. doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M, Chiba K, Li P. Free-radical generation from collapsing microbubbles in the absence of a dynamic stimulus. J Phys Chem B. 2007;111:1343–1347. doi: 10.1021/jp0669254. [DOI] [PubMed] [Google Scholar]
- 14.Iwasawa A, Saito K, Mokudai T, Kohno M, Ozawa T, Niwano Y. Fungicidal action of hydroxyl radicals generated by ultrasound in water. J Clin Biochem Nutr. 2009;45:214–218. doi: 10.3164/jcbn.08-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura K, Kanno T, Ikai H, et al. Reevaluation of quantitative ESR spin trapping analysis of hydroxyl radical by applying sonolysis of water as a model system. Bull Chem Soc Jpn. 2010;3:1037–1046. [Google Scholar]
- 16.Kamibayashi M, Oowada S, Kameda H, et al. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) Free Radic Res. 2006;40:1166–1172. doi: 10.1080/10715760600883254. [DOI] [PubMed] [Google Scholar]
- 17.Buettner GR. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med. 1987;3:259–303. doi: 10.1016/s0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- 18.Makino K, Mossoba MM, Riesz P. Chemical effects of ultrasound on aqueous solutions. Formation of hydroxyl radicals and hydrogen atoms. J Phys Chem. 1983;87:1369–1377. [Google Scholar]
- 19.Saito K, Takahashi M, Kamibayashi M, Ozawa T, Kohno M. Comparison of superoxide detection abilities of newly developed spin traps in the living cells. Free Radic Res. 2009;43:668–676. doi: 10.1080/10715760902988850. [DOI] [PubMed] [Google Scholar]
- 20.Uddin MH, Hatanaka S, Hyayshi S. Effects of aqueous temperature on sonolysis of bisphenol A: Rate constants increasing with temperature under oxygen. J Chem Eng Jpn. 2009;42:303–308. [Google Scholar]
- 21.Kondo T, Riesz P. Sonolysis of ubiquinone in aqueous solutions. An EPR spin-trapping study. Int J Radiat Biol. 1996;69:113–121. doi: 10.1080/095530096146246. [DOI] [PubMed] [Google Scholar]
- 22.Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O–) in Aqueous Solution. J Phy. Chem Ref Data. 1988;17:513–886. [Google Scholar]
- 23.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 24.Margulis MA. Sonochemistry and cavitation. Luxembourg: Gordon and Breach Publishers; 1995. pp. 353–363. [Google Scholar]
- 25.Gutierréz M, Henglein A. H atom reactions in the sonolysis of aqueous solutions. J Phys Chem. 1987;91:6687–6690. [Google Scholar]
- 26.Rassokhin DN, Kovalev GV, Bugaenko LT. Temperature effect on the sonolysis of methanol/water mixtures. J Am Chem Soc. 1995;117:344–347. [Google Scholar]
- 27.Petrier C, Micolle M, Merlln G, et al. Characteristics of pentachlorophenate degradation in aqueous solution by means of ultrasound. Environ Sci Technol. 1992;26:1639–1642. [Google Scholar]
- 28.Hart EJ, Henglein A. Sonolytic decomposition of nitrous oxide in aqueous solution. J Phys Chem. 1986;90:5992–5995. [Google Scholar]