Abstract
Although higher serum phosphate level is a risk factor for cardiovascular diseases in general population as well as chronic kidney disease patients, it has not been clarified whether higher phosphate can affect atherosclerotic plaque formation. In this study, we investigated the effect of prolonged-intake of different concentrations of phosphate on atherosclerosis formation using apolipoprotein E-deficient mice. Apolipoprotein E-deficient mice were fed with high fat diet including 0.6%, 1.2% or 1.8% phosphate. After 20-week treatment, atherosclerotic plaque formation in aorta in 1.8% phosphate diet group was unexpectedly less than that in the other groups. To elucidate mechanisms of suppression of plaque formation by high phosphate diet, we hypothesized that high phosphate diet may modify a profile of monocytes/macrophages suppressing plaque formation. We confirmed that elevated peripheral monocytes (CD11b+, F4/80+ cell numbers) in apolipoprotein E-deficient mice were decreased by feeding with 1.8% P diet. In addition, ex vivo study indicated that high dose of phosphate induced macrophage apoptosis. These observations suggest that excess phosphate intake decreased atherosclerosis formation, at least in part, by changing the profile of peripheral monocytes or inducing apoptosis of macrophages in apolipoprotein E-deficient mice.
Keywords: hyperphosphatemia, atherosclerogenesis, apoptosis, macrophage, chronic kidney disease
Introduction
Higher serum phosphate (P) level, even within the normal range, has been reported as a risk factor for cardiovascular diseases (CVDs) in recent epidemiological researches.(1,2) It has been thought to be a possible mechanism that excessive serum P can induce vascular calcification.(3–5) On the other hand, there are a few reports describing relationship between serum P levels and carotid intima-media thickness, which are used as an indicator of progression of atherosclerosis.(6–8) However, it is unknown whether hyperphosphatemia or high P diet intake can induce atherosclerosis or not.
Atherosclerosis is a chronic inflammatory disease in artery, and caused by complicated pathogenesis. Both endothelial dysfunction and infiltrating macrophages in sub intimal layer are primary pathogenic mechanisms for the development of atherosclerosis.(9) Indeed, reducing inflammation by nonsteroidal anti-inflammatory drugs can reduce plaque formation in early to intermediate stage of atherosclerosis.(10) To date, several animal models have been established to understand the relationship between risk factors and atherosclerogenesis. Hypercholesterolemic apolipoprotein E (ApoE)-deficient mouse is a useful model for studying about atherosclerosis. It has been reported that increased oxidative stress inactivated nitric oxide (NO) responsible for endothelial dysfunction.(11–13) Furthermore, inhibition of NO synthesis aggravated atherosclerotic plaque formation in ApoE-deficient mice.(14,15) In our previous study, we demonstrated that high dose P loading induced endothelial dysfunction by increasing oxidative stress and decreasing NO production ex vivo and in vitro.(16) Thus we hypothesized that higher P intake can increase serum P levels, and accelerate atherosclerotic plague formation by aggravating endothelial dysfunction in ApoE-deficient mice. In this study, we examined the effects of different amount of P intake on progression of atherosclerosis in ApoE-deficient mice.
Materials and Methods
Mice
5-week-old male C57BL/6.KOR/StmSlc-ApoEshl and C57BL/6 mice were obtained from Japan SLC (Shizuoka, Japan). After 1 week of adaptation, mice were divided and fed a western-type diet (20% Fat; 0.15% cholesterol (w/w)) containing 0.6% calcium, and 0.6%, 1.2% or 1.8% phosphorus (w/w) respectively for 7 weeks for flow cytometry or 20 weeks for atherosclerotic formation analysis. All procedures were approved by the guidelines for animal experimentation of the University of Tokushima.
Plasma and urinary analysis
A blood sample was obtained via abdominal inferior vena cava with heparin, immediately centrifuged at 10,000 rpm for 3 min at room temperature and the plasma was stored at −80°C until measurement. Urine was collected over a 24-h period and was also stored at −80°C until measurement. Total-Cholesterol (T-Cho), triglycerides (TG), inorganic P, Ca and creatinine levels in the plasma and/or urine were measured by using commercially available kits (Wako Pure Chemical Industries, Ltd., Osaka, Japan). ELISA was performed for measuring plasma FGF23 (KAINOS LABORATORIES, Inc., Tokyo, Japan), intact parathyroid hormone (iPTH) (Immunotopics International, San Clemente, CA) and MCP-1 (Thermo Fisher Scientific, Inc., Rockford, IL). Tissue plasminogen activator inhibitor-1 (tPAI-1), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were measured with a mouse adipokine plasma multiplex assay system (Millipore Japan, Tokyo, Japan). 1,25-dihydroxyvitamin D (1,25-(OH)2D) was measured by the radioreceptor assay (SRL, Inc., Tokyo, Japan).
en face aorta analysis
The aorta was perfused with phosphate buffered saline (PBS) from the left ventricle of the heart and used for en face aorta analysis. The aorta from iliac bifurcation to aortic arch was removed and external fatty deposits outside the aorta were completely removed. The aorta was cut open by longitudinally and fixed with 4% (w/v) formalin for 30 min. After Rinsing in distilled water and 60% (v/v) isopropanol, the specimen was stained with oil red O for 5 min, and then rinsed in 60% (v/v) isopropanol and distilled water. Picture was taken by TS-CA-200M camera (SUGITOH Co., Ltd., Tokyo, Japan). Image analysis was performed with Image Pro Plus (version 6.0; Media Cybernetics, Bethesda, MD). The amount of lipid deposits in each sample was measured relative to total area.
Flow cytometry
Anti-mouse Gr-1 (Ly-6G) antibody conjugated with eFluor®780, anti-CD11b (M1/70) antibody conjugated with PE(phycoerythrin)-APC-Cy7 and anti-F4/80 (BM8) antibody conjugated with eFluor®450 were obtained from eBioscience, Inc. (San Diego, CA). Peripheral blood leucocytes (500 µl whole blood per a mouse) were collected via inferior vena cava and erythrocytes were lysed. Samples were incubated with FcγIII/II receptor (2.4G2) antibody to block nonspecific antibody binding for 15 min at 4°C, stained with antibody mixture for 15 min at 4°C then stained for 7-amino-actinomycin D (7-AAD) (Sigma Aldrich Japan, Tokyo, Japan) to detect dead cells. Cells were analyzed on FACS Canto II (Japan Becton Dickinson, Tokyo, Japan). Up to 100,000 events were acquired.
Preparation of intraperitoneal macrophages
To collect the peritoneal macrophages, non-treatment C57BL/6 mice were injected i.p. with 4.05% (w/v) thioglycolate medium, and after 3 days, mice were euthanized and i.p. macrophages were collected with 0.9% (w/v) saline. After washing with saline, macrophages were centrifuged for 5 min at 1,000 rpm, resuspended with Dulbecco’s modified Eagle’s medium (DMEM) including 10% (v/v) of fetal calf serum (FCS) and seeded in 96-well plates (5 × 104 cells per well) for AlamarBlue assay and on coverslip in 24-well plates (5 × 105 cells per well) for TUNEL staining. After incubation in humidified 37°C, 5% (v/v) CO2 incubator for several hours, non-attached cells were removed then macrophages were used for each experiment.
AlamarBlue assay
Macrophages were starved fresh medium without FCS overnight and medium was changed 2 h before stimulation. To evaluate effect of phosphate concentrations in medium on cellular viability, macrophages were incubated with DMEM including 10% (v/v) of AlamarBlue reagent (Invitrogen Japan, Tokyo, Japan) for 4 h. We added appropriate amounts of sodium phosphate buffer (0.1 M Na2HPO4/NaH2PO4, pH 7.4). Delta absorbance (570–600 nm) was measured.
TUNEL staining
Apoptosis in situ detection kit (Wako) was used for TUNEL assay. Macrophages on coverslip were fixed with 4% (w/v) formalin and permeablized with permeablization solution (0.1% (w/v) sodium citric acid and 0.1% (v/v) Triton X-100). Applying TdT reaction solution for 10 min at 37°C, washing with PBS, peroxidase (POD)-conjugated antibody for 10 min at 37°C, washing with PBS then positive staining cells were visualized with diaminobenzidine (DAB) solution.
Statistical analysis
All data were expressed as the means ± SD. Statistical analysis was analyzed with student’s t test for non-parametric comparison between two groups, or analysis of variance (ANOVA) with post hoc test by Fisher’s protected least significant difference (PLSD) test for multiple comparisons. p<0.05 was considered significant.
Results
Effects of prolonged higher P intake on Ca-P and lipid metabolisms
ApoE-deficient mice were fed with western diet including 0.6% Ca, and 0.6%, 1.2% or 1.8% P for 20 weeks. In normal condition, serum Ca and P concentrations are strictly regulated by parathyroid hormone (PTH), Fibroblast growth factor 23 (FGF23) and 1,25-(OH)2D, thus excess P can be immediately excreted into the urine. To confirm effects of prolonged much amount of P intake on kidney function and mineral metabolism in ApoE-deficient mice, we assayed plasma and urinary concentrations of markers related on Ca and P metabolisms. All groups did not show any significant difference in plasma P, and plasma and urinary Ca levels while urinary P level corrected by creatinine in 1.8% P group was significantly higher than that in the other groups (Table 1). Plasma intact PTH level in 1.2% P group was significantly higher than that in 0.6% P group, and plasma FGF23 in 1.8% P group was significantly higher than that in the other groups but plasma 1,25-(OH)2D levels in all groups were not significantly different. Considering all various markers together, Ca and P metabolisms were normally regulated in ApoE-deficient mice in response to dietary P modifications.
Table 1.
Fasting blood and urine chemistry relating on P, Ca and lipid metabolisms, and kidney function in ApoE-deficient mice fed with high fat diet for 20 weeks. Plasma was collected after overnight fast. Urine was collected for 24 h. All urinary markers were normalized by creatinine. TG; triacylglyceride, T-Cho; total cholesterol, Crea; creatinine. Values shown are means ± SD. *p<0.05 vs 0.6% P, **p<0.05 vs 1.2% P.
| 0.6% P | 1.2% P | 1.8% P | ||
|---|---|---|---|---|
| Plasma | ||||
| P | (mmol/l) | 1.55 ± 0.17 | 1.52 ± 0.17 | 1.35 ± 0.24 |
| Ca | (mmol/l) | 2.13 ± 0.28 | 2.17 ± 0.25 | 2.15 ± 0.16 |
| Intact PTH | (pg/ml) | 26.8 ± 7.26 | 35.8 ± 17.3* | 31.3 ± 9.67 |
| FGF23 | (pg/ml) | 138 ± 23.6 | 216 ± 27.6 | 958 ± 197*,** |
| 1,25-(OH)2D | (pg/ml) | 119 ± 62.1 | 88.8 ± 32.5 | 51.5 ± 8.84 |
| TG | (mmol/l) | 1.51 ± 0.58 | 1.84 ± 0.38 | 1.76 ± 0.34 |
| T-Cho | (mmol/l) | 16.3 ± 3.85 | 17.5 ± 3.49 | 18.2 ± 4.01 |
| Urinary | ||||
| P | (mol/mol Crea) | 45.8 ± 11.2 | 85.6 ± 6.75* | 136 ± 18.4*,** |
| Ca | (mol/mol Crea) | 0.57 ± 0.23 | 0.55 ± 0.17 | 0.82 ± 0.45 |
As abnormal blood lipids are important risk factors for atherosclerosis, we examined effects of different amount of P intake on lipid metabolism. There was no significant difference among groups on plasma TG and T-Cho levels. Therefore, dietary P intake did not affect blood lipid concentrations in ApoE-deficient mice.
Atherosclerotic formation in aorta and atherosclerotic indicators in plasma
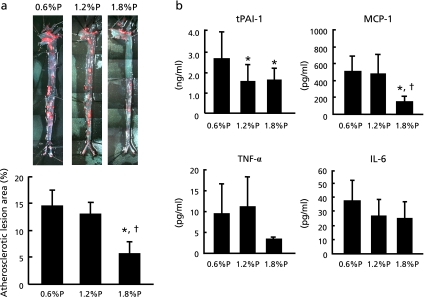
We evaluated atherosclerotic plaque formation by staining enface aorta with Oil red O. Despite our hypothesis which higher P intake can progress atherosclerotic formation, surprisingly, 1.8% P intake significantly suppressed the development of atherosclerotic plaque compared with lower P intake (Fig. 1a). Especially, lesions in thoracic and abdominal aorta decreased in 1.8% P group.
Fig. 1.
Atherosclerotic lesion area and plasma cytokine levels in ApoE-deficient mice fed with high fat diet for 20 weeks. (a) Whole aortas were stained with Oil red O to reveal lipid deposits and atherosclerotic lesion area was quantitated as the ratio of the total lipid deposits area to the total surface area. (b) Tissue plasminogen activator inhibitor 1 (tPAI-1), monocyte chemotactic protein-1 (MCP-1), Tumor necrosis factor-α (TNF-α), Interleukin-6 (IL-6) were measured as atherosclerotic indicators. Values shown are means ± SD. *p<0.05 vs 0.6% P, †p<0.05 vs 1.2% P.
To elucidate the mechanism of suppression of atherosclerosis formation in 1.8% P group, we analyzed plasma tissue plasminogen activator inhibitor-1 (tPAI-1), monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) concentrations. Plasma tPAI-1 levels were significantly decreased in 1.2% P and 1.8% P groups compared with 0.6% P group (Fig. 1b). Plasma MCP-1 levels were significantly decreased in 1.8% P group compared with the other groups. Plasma TNF-α and IL-6 did not show any significant difference among all groups.
Dietary phosphate affected peripheral blood monocytes in vivo
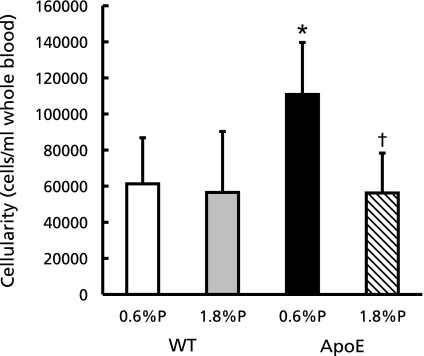
As shown above, high P diet intake unexpectedly suppressed atherosclerosis formation. Next we investigated effect of dietary P intake on peripheral blood monocytes, because monocyte recruitment is a primary event for atherosclerogenesis. We fed ApoE-deficient and C57BL/6, as a control, mice with high fat diet including 0.6% or 1.8% phosphorus for 7 weeks and the blood leukocytes were analyzed by flow cytometry. Intraperitoneal CD11b positive and F4/80 positive cells, which indicate monocytes, were significantly increased in ApoE-0.6% P group than C57BL/6-0.6% P (WT-0.6% P) group (Fig. 2). On the other hand, the cell numbers in ApoE-1.8% P group were reduced as same as that in WT-0.6% P group.
Fig. 2.
F4/80 positive cell numbers in peripheral leukocytes of WT (C57BL/6) and ApoE-deficient mice fed with high fat diet for 7 weeks. Peripheral leukocytes were labeled with anti-CD11b antibody conjugated with PE-Cy7 and anti-F4/80 antibody conjugated with eFluor®450 to determine the number of monocytes and analyzed by flow cytometry. Values shown are means ± SD. *p<0.05 vs WT-0.6% P, †p<0.05 vs ApoE-0.6% P.
Effects of P intake on intraperitoneal macrophage apoptosis in vitro
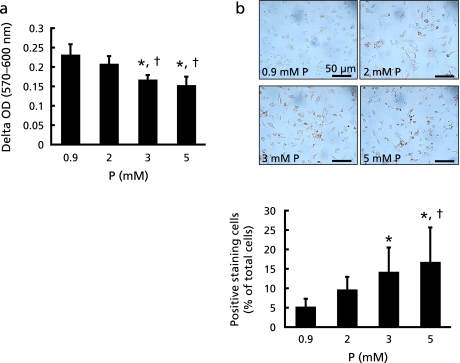
As reason for alteration of peripheral blood monocyte cellularity, we took into considerations of effects of dietary P intake on haematocyte differentiation and/or monocyte apoptosis. In this study, we investigated whether dietary P intake can induce monocyte apoptosis; because there are several reports which higher dose P stimulation can induce apoptosis in some cell lines, such as endothelial cells, osteoblasts and chondrocytes.(17–19) First, we used AlamarBlue reagent to assess whether extracellular P concentrations can affect macrophage viability. High P loading dose-dependently decreased the metabolic activity of thioglycolate-elicited macrophages (Fig. 3a). Since ApoE-deficient mice show increased oxidative stress,(20) thioglycolate-elicited macrophages were stimulated by high P loading under oxidative stress condition, and apoptosis was analyzed by TUNEL staining to confirm whether high concentrations of P can stimulate macrophage apoptosis. Macrophage apoptosis was enhanced by high P loading under oxidative stress (Fig. 3b).
Fig. 3.
Effects of P on thioglycolate-elicited macrophages. (a) Thioglycolate-elicited intraperitoneal macrophages from WT mice were stimulated with different concentrations of P, and their cellular metabolic activity was measured with AlamarBlue reagent. Metabolic activity of cells was expressed as delta OD (570–600 nm). (b) Cells were stimulated with different concentrations of P under oxidative stress condition (0.05 mM hydrogen peroxide). Apoptotic cells were detected by TUNEL. TUNEL positive staining cell numbers were corrected by total cell numbers in the field of vision. Values shown are means ± SD. *p<0.05 vs 0.9 mM, †p<0.05 vs 2 mM.
1.8% P diet intake decreased F4/80+, Gr-1+cells in peripheral blood in ApoE-deficient mice
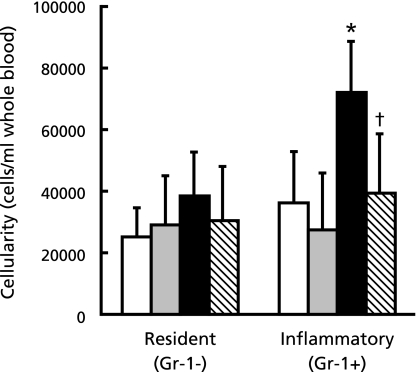
Activation of monocytes plays an important role in the development of atherosclerosis. Peripheral leukocytes from 7-week fed ApoE-deficient and C57BL/6 mice were analyzed by flow cytometry. We found that CD11b positive, F4/80 positive and Gr-1 positive cells (including inflammatory monocytes) were 2-fold as large as Gr-1 negative cells (resident monocytes) in ApoE-0.6% P group but not the other groups (Fig. 4).
Fig. 4.
Gr-1 (LY-6G) positive or negative cell numbers in peripheral leukocytes of WT (C57BL/6) and ApoE-deficient mice fed with high fat diet for 7 weeks. Peripheral leukocytes were labeled with anti-CD11b antibody conjugated with Cy7, anti-F4/80 antibody conjugated with eFluor®450 and anti-Gr-1 (LY-6G) antibody conjugated with eFluor®780 to determine the number of resident monocytes (Gr-1 negative) and inflammatory monocytes (Gr-1 positive) and analyzed by flow cytometry. Open column; WT-0.6% P, dotted column; WT-1.8% P, closed column; ApoE-0.6% P, hatched column; ApoE-1.8% P. Values shown are means ± SD. *p<0.05 vs WT-0.6% P, †p<0.05 vs ApoE-0.6% P.
Discussion
Inhibition of NO production aggravated atherosclerosis in ApoE-deficient mice.(14,15) Excess P loading can induce endothelial dysfunction by decreasing endothelial nitric oxide production,(16) increasing oxidative stress,(16,17) or inducing apoptosis.(17) Therefore, we hypothesized that P loading can synergistically aggravated atherosclerosis in ApoE-deficient mice, however, excessive P intake ameliorated atherosclerosis in aorta in ApoE-deficient mice, unexpectedly.
We considered that the unexpected effect of high P loading on the development of atherosclerosis must be independent on endothelial dysfunction. Because, there was no significant difference between 1.8% P diet and 0.6% P diet groups in acetylcholine-dependent vasodilation of thoracic aortic ring in ApoE-deficient mice (our unpublished observation). The observation suggests that endothelial function in ApoE-deficient mice has been impaired as shown in previous study,(21) and dietary P intake did not additionally affect endothelial function. Generally, recruitment and migration of monocytes across endothelium are very important events as much as endothelial dysfunction in atherosclerosis formation. Liu et al. reported that reduction of macrophage apoptosis accelerated atherosclerosis.(22) In addition, other researchers demonstrated that macrophage apoptosis in early stage of atherosclerosis development was very efficient and diminished plaque formation but not advanced stage.(23–25) Therefore, we investigated the effect of high P loading on monocytes/macrophages.
In this study, we demonstrated that peripheral CD11b+, F4/80+ cell numbers were decreased by 1.8% P intake compared with 0.6% P in ApoE-deficient mice and high concentrations of dietary P induced thioglycolate-elicited macrophage apoptosis under oxidative stress condition. These effects suggest that the effect of excess P intake on peripheral blood would be anti-atherosclerogenesis by inducing monocyte/macrophage apoptosis under oxidative condition.
Recent studies reported that blood monocytes include two functional subsets, inflammatory and resident monocytes.(26–28) Despite no significant difference between atherosclerosis formation at the time of 7-week feeding with 0.6% or 1.8% P diet (data were not shown), excess P intake reduced only inflammatory monocytes in peripheral blood. However, we could not determine whether P induced apoptosis in inflammatory monocytes or affected monocytes subsets. The changed profile of monocytes may account for the decreases in inflammatory and atherosclerogenic factors such as tPAI-1, MCP-1, TNF-α in ApoE-deficient mice fed with 1.8% P diet. In addition, the decreases in those inflammatory and atherosclerogenic factors may have a protective effect on atherosclerogenesis by suppressing endothelial dysfunction. Because previous studies have reported that deficiency of MCP-1, TNF-α or PAI-1 reduced atherosclerosis formation,(29–32) and have indicated that these cytokines play an important role in plaque progression. Thus, these possible mechanisms could be underlying on the decrease in atherosclerotic plaque formation in ApoE-deficient mice fed with 1.8% P diet.
This study has some limitations. At least, we cannot exclude the effects of high P intake on the development of atherosclerosis other than that on monocytes/macrophages. For instance, FGF23 may be a possible candidate. In 1.8% P intake group, plasma FGF23 levels were very high compared with the other groups. FGF23 is responsible for P and vitamin D metabolisms and known as a predictor of chronic kidney disease.(33) Recent studies have reported that FGF23 null mice were smaller in kidney size and occurred vascular calcification(34,35) whereas FGF23 overexpression prevented progression of chronic kidney failure in rats.(36) In this study, we could not investigate the effects of FGF23 on the development of atherosclerosis. To understand the effect of high P loading on the development of atherosclerosis, further study must be needed.
High P diet intake can induce endothelial dysfunction and must be a risk factor for atherosclerosis in normal condition. On the other hand, high P diet intake may prevent atherosclerosis formation in enhanced atherosclerogenic condition such as ApoE-deficient mice. Although we cannot fully understand the role of high P loading in vascular disease model such as ApoE-deficient mice at this time, the actual mechanism must be complicated. Epidemiologically, whereas hyperphosphatemia is positively associated with cardiovascular event in general population as well as CKD patients,(1,27) it can be negatively associated with blood pressure.(1,37) We have no direct evidence, however, such contradictory effects of high P diet intake may help us to understand the complicated pathogenesis of cardiovascular disease in CKD patients.
In conclusion, prolonged excess P intake unexpectedly can ameliorate atherosclerosis formation in ApoE-deficient mice, at least in part, by changing the profile of peripheral inflammatory monocytes. To understand the role of high P diet intake in atherosclerogenesis in more detail, pathogenic conditions, the difference of tissue or organ, treatment period and dose, etc. should be considered in further investigations.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (B) (Y.T., E.T.), for Young Scientists (A) (Y.T.), Exploratory Research (Y.T.) from the Ministry of Education, Culture, Sports, Science, and Technology in Japan, and also was supported by the Kidney Foundation (JKFB09-15) (Y.T.). All authors declare no conflict of interest.
Abbreviations
- ApoE
apolipoprotein E
- CKD
chronic kidney disease
- DMEM
Dulbecco’s modified Eagle’s medium
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal bovine serum
- FGF23
fibroblast growth factor 23
- IL-6
interleukin-6
- MCP-1
monocyte chemoattractant protein-1
- NO
nitric oxide
- 1,25-(OH)2D
1,25-dihydroxyvitamin D
- P
phosphate
- tPAI-I
tissue plasminogen activator inhibitor-1
- PBS
phosphate buffered saline
- PTH
parathyroid hormone
- T-Cho
total cholesterol
- TG
triglycerides
- TNF-α
tumor necrosis factor-α
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
References
- 1.Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G, ; Cholesterol And Recurrent Events Trial Investigators Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 3.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 4.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol. 2004;15:2959–2964. doi: 10.1097/01.ASN.0000145894.57533.C4. [DOI] [PubMed] [Google Scholar]
- 5.Son BK, Kozaki K, Iijima K, et al. Statins protect human aortic smooth muscle cells from inorganic phosphate-induced calcification by restoring Gas6-Axl survival pathway. Circ Res. 2006;98:1024–1031. doi: 10.1161/01.RES.0000218859.90970.8d. [DOI] [PubMed] [Google Scholar]
- 6.Ishimura E, Taniwaki H, Tabata T, et al. Cross-sectional association of serum phosphate with carotid intima-medial thickness in hemodialysis patients. Am J Kidney Dis. 2005;45:859–865. doi: 10.1053/j.ajkd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Onufrak SJ, Bellasi A, Shaw LJ, et al. Phosphporus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis. 2008;199:424–431. doi: 10.1016/j.atherosclerosis.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Ruan L, Chen W, Srinivasan SR, Xu J, Toprak A, Berenson GS. Relation of serum phosphorus levels to carotid intima-media thickness in asymptomatic young adults (from the Bogalusa Heart Study) Am J Cardiol. 2010;106:793–797. doi: 10.1016/j.amjcard.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 10.Hamaguchi M, Seno T, Yamamoto A, et al. Loxoprofen sodium, a non-selective NSAID, reduces atherosclerosis in mice by reducing inflammation. J Clin Biochem Nutr. 2010;47:138–147. doi: 10.3164/jcbn.10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 12.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 13.d’Uscio LV, Baker TA, Mantilla CB, et al. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1017–1022. doi: 10.1161/01.atv.21.6.1017. [DOI] [PubMed] [Google Scholar]
- 14.Kauser K, Cunha VD, Fitch R, Mallari C, Rubanyi GM. Role of endogenous nitric oxide in progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol. 2000;278:H1679–H1685. doi: 10.1152/ajpheart.2000.278.5.H1679. [DOI] [PubMed] [Google Scholar]
- 15.Kuhlencordt PJ, Gyurko R, Han F, et al. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart dissease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 16.Shuto E, Taketani Y, Tanaka R, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–1512. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Marco GS, Hausberg M, Hillebrand U, et al. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am J Physiol Renal Physiol. 2008;294:F1381–F1387. doi: 10.1152/ajprenal.00003.2008. [DOI] [PubMed] [Google Scholar]
- 18.Meleti Z, Shapiro IM, Adams CS. Inorganic phosphate induces apoptosis of osteoblast-like cells in culture. Bone. 2000;27:359–366. doi: 10.1016/s8756-3282(00)00346-x. [DOI] [PubMed] [Google Scholar]
- 19.Mansfield K, Rajpurohit R, Shapiro IM. Extracellular phosphate ions cause apoptosis of terminally differentiated epiphyseal chondrocytes. J Cell Physiol. 1999;179:276–286. doi: 10.1002/(SICI)1097-4652(199906)179:3<276::AID-JCP5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Praticò D, Tangirala RK, Rader DJ, Rokach J, FitzGerald GA. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- 21.Deckert V, Lizard G, Duverger N, et al. Impairment of endothelium-dependent arterial relaxation by high-fat feeding in ApoE deficient mice: toward normalization by human ApoA-I expression. Circulation. 1999;100:1230–1235. doi: 10.1161/01.cir.100.11.1230. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis; the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 24.Stoneman V, Braganza D, Figg N, et al. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautier EL, Huby T, Witztum JL, et al. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 26.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 27.Sunderkötter C, Nikolic T, Dillon MJ, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 28.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 29.Gosling J, Slaymaker S, Gu L, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Russo HM, Eitzman DT. Monocyte chemoattractant protein-1 deficiency protects against visceral fat-induced atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1151–1158. doi: 10.1161/ATVBAHA.110.205914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brånén L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-α reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 32.Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D. Plasminogen activator inhibitor-1 deficiency protects against atherosclerosis progression in the mouse carotid artery. Blood. 2000;96:4212–4215. [PubMed] [Google Scholar]
- 33.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 34.Razzaque MS, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant. 2005;20:2032–2035. doi: 10.1093/ndt/gfh991. [DOI] [PubMed] [Google Scholar]
- 35.Stubbs JR, Liu S, Tang W, et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18:2116–2124. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 36.Kusano K, Saito H, Segawa H, Fukushima N, Miyamoto K. Mutant FGF23 prevents the progression of chronic kidney disease but aggravates renal osteodystrophy in uremic rats. J Nutr Sci Vitamminol (Tokyo) 2009;55:99–105. doi: 10.3177/jnsv.55.99. [DOI] [PubMed] [Google Scholar]
- 37.Elliott P, Kesteloot H, Appel LJ, et al. Dietary phosphorus and blood pressure-international study of macro- and micro-nutrients and blood pressure. Hypertension. 2008;51:669–675. doi: 10.1161/HYPERTENSIONAHA.107.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]