Abstract
Facing a cancer diagnosis at any age is devastating. However, young cancer patients have the added burden that life-preserving cancer treatments, including surgery, chemotherapy, and radiotherapy, may compromise their future fertility. The possibility of reproductive dysfunction as a consequence of cancer treatment has a negative impact on the quality of life of cancer survivors. The field of oncofertility, which merges the clinical specialties of oncology and reproductive endocrinology, was developed to explore and expand fertility preservation options and to better manage the reproductive status of cancer patients. Fertility preservation for females has proved to be a particular challenge because mature female gametes are rare and difficult to acquire. The purpose of this article is to provide the gynecologist with a comprehensive overview of how cancer treatments affect the female reproductive axis, delineate the diverse fertility preservation options that are currently available or being developed for young women, and describe current measures of ovarian reserve that can be used pre- and post-cancer treatment. As a primary care provider, the gynecologist will likely interact with patients throughout the cancer care continuum. Thus, the gynecologist is in a unique position to join the oncofertility team in providing young cancer patients with up-to-date fertility preservation information and referrals to specialists.
Keywords: Young adult, adolescent, gynecologist, cancer, oncofertility, fertility preservation
There Is a Need for Oncofertility
Approximately 72,200 adolescents and young adults, defined as those 15–39 years of age, were diagnosed with cancer in 2006.1 Diverse types of cancers afflict adolescents and young adults, and cancer, in general, is the leading cause of disease-related deaths in this age group.1 Although medical advances have increased the survival rate from many cancers, the young adult population has not seen improvements in survival rates in the past 20 years compared with younger and older populations. 1,2 Nevertheless, adolescent and young adult men and women who survive their cancer are faced with the challenge of resuming their life with the same quality as prior to their cancer diagnosis. This goal is often difficult because a cancer diagnosis can interfere with the ability of these young adults to complete their education, develop a career, remain employed, or maintain relationships.3 In addition, cancer treatment can negatively and irreversibly alter several organ systems, thus decreasing a patient’s future quality of life in terms of health.4
Cancer treatments can compromise both male and female reproductive function and threaten future fertility.5–7 For males, the most common method of preserving fertility prior to cancer treatment is to cryopreserve sperm, which can be obtained non -invasively in most cases. Fertility preservation for adolescent and young adult females can be a greater challenge because of the rarity of female gametes and the difficulty in obtaining them. This article focuses on the adolescent and young adult female population and serves to provide gynecologists with a general but fundamental understanding of how common cancer therapies can threaten fertility, what fertility preservation options are available. and how reproductive function can be assessed post-cancer treatment.
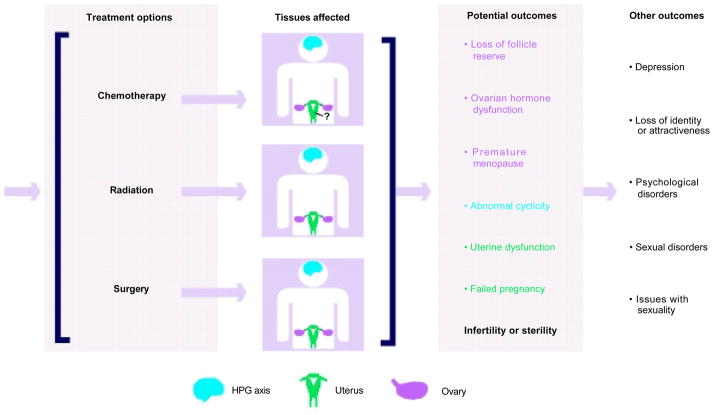
In adolescent and young adult females, cancer treatments can have a negative impact on nearly all aspects of the reproductive axis and lead to devastating conditions including premature menopause, premature ovarian failure, and infertility (see Figure 1).7 The possibility of reproductive dysfunction as a consequence of cancer therapy is devastating for women and is linked to increased depression, post-traumatic stress disorder, and a variety of other psychosocial problems.8–10 For example, a survey of 88 gynecologic cancer survivors showed that the levels of distress and depression coincided with the severity and number of menopausal symptoms experienced. 11 In another study, young adult women diagnosed with early-stage breast cancer reported that reproductive concerns led to consistent depressive symptoms.10 Furthermore, female cancer survivors show evidence of greater sexual dysfunction and lower physical quality of life compared with non-cancer-afflicted infertile women.9 Thus, there is a clear documented need to provide cancer patients with specific information and care regarding their reproductive function in light of their cancer diagnosis.
Figure 1. Cancer Treatments Have the Potential to Compromise Nearly All Aspects of the Reproductive Axis.
Common cancer treatments in response to a cancer diagnosis include chemotherapy, radiation, and surgery. The majority of these treatments can have direct or indirect effects on the ovary (pink), hypothalamic-pituitary-gonadal (HPG) axis (blue), and uterus (green) that ultimately compromise a patient’s fertility. It is not clear whether chemotherapy has an effect on the uterus. In addition to potential reproductive outcomes, these treatments can also have several measurable psychologic outcomes.
The field of oncofertility was developed in 2006 with the express purpose of preserving, expanding, and restoring the reproductive future of cancer patients whose treatment may have compromised their fertility.12 To ensure that these reproductive needs would be met, the Oncofertility Consortium was established and funded by a National Institutes of Health Roadmap Grant.13 The Oncofertility Consortium coordinates the work of clinicians, basic science researchers, social scientists, ethicists, and humanists so that breakthroughs at the bench can efficiently and safely transform patient care.13 National organisations including the American Society of Clinical Oncology (ASCO) and the American Society of Reproductive Medicine (ASRM) support oncofertility. ASCO and ASRM have issued guidelines that recommend that physicians make their patients aware of the threat to their fertility, provide them with fertility preservation options or refer them to reproductive specialists.14,15 The Oncofertility Consortium is meeting the urgent need of providing cancer patients with information and developing options concerning their fertility, but its continued success requires that clinicians, including gynecologists, provide their patients with a broad awareness of oncofertility and referrals to specialists.
Gynecologists Have a Unique Role in Oncofertility
Over the last several decades, the gynecologist has increasingly taken on the role of a primary care provider (PCP), and this shift in care has important implications for the emerging field of oncofertility.16,17 Once regarded as a medical and surgical specialty that provided women with specific gynecologic and reproductive services, gynecology now encompasses a comprehensive view of women ’s healthcare. In fact, gynecologists perform most of the general medical examinations for reproductive-aged women.16 It is also well-established among both patients and oncologists that patients rely heavily on PCPs during the cancer care continuum for pain management, coordination of referrals, general medical care, clinical decision-making, and provision of emotional support.18 As a PCP, the gynecologist has the potential to serve an invaluable role in oncofertility. Gynecologists can serve as patient advocates by providing their patients with knowledge, advice, and referrals concerning fertility preservation during all phases of cancer treatment.
Gynecologists need to educate their patients about oncofertility because, despite measures to introduce oncofertility into oncology settings, patients frequently report that they are not provided with ample information concerning fertility preservation.19 Consistent with these patient accounts, many oncologists report that they infrequently discuss fertility preservation with their patients or refer them to reproductive specialists.20–22 Oncologists cite several reasons for this gap in patient care.20,21 First, treating the cancer is their top priority, and they do not want to delay cancer therapy. Second, oncologists believe that discussing fertility adds stress to a patient’s situation, especially one with a poor prognosis. Third, oncologists express a lack of training in fertility risks and preservation options and discomfort in speaking to patients due to religious, language, and cultural barriers. Finally, oncologists are less likely to discuss fertility preservation if their patients are unmarried, already have a child, are homosexual, are too young, do not have insurance coverage, or may not have immediate access to reproductive services. The consequence of withholding such information from patients is measurable. Patients who do not receive adequate information concerning their reproductive options or outcomes report an increase in negativity and psychologic distress and a corresponding decrease in quality of life.9,19 Thus, it is critical that patients are provided with psychologic and medical support before, during, and after their cancer treatment. The gynecologist, as a PCP, is in a unique position to help patients do so.
Cancer Therapies can have a Negative Impact on all Aspects of the Reproductive Axis
In order to be autonomously fertile, a woman must have the following: a functioning neuroendocrine system that regulates the menstrual cycle and can maintain a pregnancy; a healthy pool of follicles that will grow in response to hormonal cues and produce mature and fertilisable gametes; and a receptive uterus that will support embryo implantation and fetal development to term. Cancer treatments, which typically rely on chemotherapy, radiotherapy, or surgery either in isolation or in combination, can compromise these three major components of the reproductive axis (see Figure 1). It is difficult to generalize how a specific cancer treatment will affect fertility. For example, a patient’s age, specific cancer type and fertility status prior to starting cancer treatment are important predictive factors of how devastating a treatment may be to fertility.23 Younger patients can tolerate larger doses of irradiation and chemotherapy compared with older patients before manifesting menopausal or infertility symptoms, likely because they have a larger starting follicle pool.24 The reproductive consequences of radiotherapy are highly dependent on the dose, site, and duration of exposure, frequency of treatments, and whether or not it is administered in isolation or in combination with chemotherapy.25,26 Similar to radiotherapy, the effects of chemotherapy are highly dependent on the type of drug used, the dose, the frequency, and the treatment duration.27
Despite the difficulty in predicting how cancer treatment will affect the reproductive axis, several main points can be made. The ovary, which in a young adult contains follicles in all stages of development, is highly susceptible to all forms of cancer therapy. It is generally accepted that women are born with a non-renewable pool of approximately two million primordial follicles, which decreases to 500,000 at the onset of menarche. By 37 years of age, this finite pool is only 25,000, and the onset of menopause occurs when this number reaches approximately 1,000.28 The number of primordial follicles dictates a woman’s reproductive lifespan, and any treatment that hastens the decline of the follicle pool will result in premature ovarian failure and menopause. Radiotherapy can damage follicles, targeting them for either repair or elimination.26 In general, actively dividing cells are more susceptible to radiation-induced death, and because oocytes in the young adult are arrested in prophase of meiosis I, they are more resistant to radiation than cells in mitosis. Primordial follicles, which are considered to be quiescent, appear to be more resistant to radiation compared with growing follicles.26 Nevertheless, the human oocyte is indeed sensitive to radiation therapy. The LD50, or the dose required to destroy 50 % of immature human oocytes, is less than 2Gy.29 Mathematical modeling predicts that the effective sterilizing dose of radiation is inversely correlated with age, such that at birth it is 20.3Gy and at 20 years of age it decreases to 16.5Gy.30 In addition to being radiosensitive, the ovary is also chemosensitive. Alkylating agents, especially cycl ophosphamide and busulfan, are more gonadotoxic compared with other chemotherapeutics, including platinum agents, plant alkaloids, and antimetabolites.27,31 Alkylating agents, which produce DNA breaks irrespective of cell-cycle stage, have a high risk for targeting primordial follicles for death and also of compromising stromal cell function. 32 Follicle destruction, whether by radiation- or chemotherapy-induced mechanisms, does not simply lead to gamete loss, but can also result in impaired ovarian hormone production and uterine dysfunction. Although follicles may resist cancer therapies, the ovarian reserve may be compromised and deplete early, resulting in premature menopause.
In addition to the ovary, the neuroendocrine axis, or the hypothalamic-pituitary-gonadal (HPG) axis, is another target of cancer therapies. The HPG axis controls the menstrual cycle and pregnancy by regulating the secretion of hormones including gonadotrophin-releasing hormone (GnRH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, progesterone, and prolactin. Radiation, particularly targeting the cranium, can cause altered hypothalamus and pituitary function.26,33,34 There is also evidence that hypothalamic dysfunction can occur after chemotherapy in the absence of cranial irradiation.35
The uterus functions primarily to support embryo implantation as well as fetal growth and development. Although chemotherapy alone does not appear to adversely affect the uterus, radiotherapy can have negative long-term consequences on the ability of the uterus to support a future pregnancy.25,36 Radiotherapy can reduce the uterine volume and elasticity, damage the uterine musculature and endometrium and decrease the vasculature.37–39 If women are able to conceive following radiotherapy, they have an increased risk for adverse pregnancy outcomes including higher incidence of miscarriage, placental abnormalities, pre-term birth, and delivery of low-birth-weight infants.38,40
Cancer therapies, as described above, can threaten fertility by depleting a woman’s ovarian reserve, thereby forcing her into premature menopause by altering the function of the HPG axis or by making her uterus inhospitable to an embryo. As important as it is to counsel patients that their fertility may be threatened by their cancer treatment, it is equally important to advise them that they may in fact never lose their fertility. Gynecologists should discuss contraception at the time of diagnosis because conceiving during cancer treatment can have negative consequences for the fetus and the patient. 41,42 Patients may also regain natural fertility during and post-treatment, and various tests of ovarian reserve are available to predict a patient’s fertility status at points throughout her treatment course (see Table 1). Patients should not assume that their cancer treatment has left them sterile, and appropriate contraceptive methods should be used if pregnancy is not intended. In addition to potentially altering fertility, cancer therapies can also diminish a woman’s sense of sexuality. For example, increased menopausal symptoms, including vaginal dryness and hot flushes, could lead to sexual dysfunction, and body changes due to surgery could lead to a loss of identity or attractiveness.9,43–45
Table 1.
Common Clinical Tests for Measuring Ovarian Reserve
| Fertility Test | Description | What it Measures | Additional Information |
|---|---|---|---|
| Hormone Tests | |||
| Anti-Müllerian hormone (AMH)85,88,90,92 | Blood test performed on any day of the menstrual cycle to measure AMH levels in the bloodstream |
|
This test is best performed in combination with antral follicle counts in the ovary |
| Day 3 estradiol120,121 | Blood test performed on day 3 of the menstrual cycle to measure estradiol levels in the bloodstream |
|
Always performed in combination with a Day 3 FSH test to reaffirm FSH results |
| Day 3 follicle-stimulating hormone (FSH)120,121 | Blood test performed on day 3 of the menstrual cycle to measure FSH levels in the body |
|
|
| Day 3 inhibin B121,122 | Blood test performed on day 3 of the menstrual cycle to measure Inhibin B levels in the body |
|
|
| Ultrasound Tests | |||
| Antral follicle counts (AFC)92,123–125 | Transvaginal ultrasound test in which antral follicles approximately 2–10 mm in diameter are counted | Correlates to the number of dormant primordial follicles remaining in the ovary | Best performed in combination with an AMH blood test |
| Ovarian volume123,126 | Transvaginal ultrasound test to measure the length, width, and depth of both ovaries | Ovarian size decreases with age, so a smaller ovary may indicate a smaller ovarian reserve |
|
| Uterine ultrasound24 | Ultrasound scan test is used to asses uterine size and shape, blood supply, and endometrial thickness | Problems with these uterine characteristics may impact implantation and the ability to conceive |
|
| Dynamic Tests | |||
| Clomiphene citrate challenge test127,128 | Patient is given clomiphene for five days to induce ovulation and then a blood test is performed to test levels of estradiol, FSH, and LH |
|
The clinical value of this test is controversial |
| Biological Markers | |||
| Menstruation97 | A patient’s return to or lack of menstruation post-treatment | May indicate that normal fertility has been restored if a patient begins to cycle post-treatment |
|
AFC = antral follicle count; AMH = anti-Müllerian hormone; FSH = follicle-stimulating hormone; LH = luteinizing hormone.
Diverse Fertility Preservation Options are Available
The fertility preservation option menu is constantly expanding as research breakthroughs are translated into clinical use (see Table 2 and Figure 2). Although there are many fertility preservation options, ranging from standard to experimental to theoretical, unique patient-intrinsic factors will dictate the best course of action. These factors include a patient’s age, ovarian reserve prior to the start of treatment, type of cancer and cancer therapy dose, duration, and timing.46 Despite the broad spectrum of options available to patients, embryo cryopreservation is the only established method recognized by the ASRM.15 In this method, initiated prior to cancer treatment, a woman typically undergoes hormone-induced hyperstimulation to recruit multiple follicles to grow and produce fertilisable eggs (see Table 2 and Figures 2 and 3). These eggs are then aspirated and fertilized using assisted reproductive technologies (ARTs) such as in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ISCI). Embryos are then cryopreserved by slow-freezing or vitrification technologies and stored for the patient’s future use.46,47 Following cancer treatment, these embryos can be thawed and transferred back to the patient’s uterus or to a surrogate’s uterus. Cryopreservation is generally successful. It is estimated that 3.5 million children worldwide have been born to date by ART procedures, and one-quarter of these have been generated following cryopreservation.48 Furthermore, the obstetric outcomes of children born following cryopreservation are comparable to those born following fresh IVF/intracytoplasmic sperm injection (ICSI) cycles, although long-term follow up child health studies are required.49
Table 2.
A Comprehensive Guide to Oncofertility Options for Young Adult Female Cancer Patients
| Oncofertilty Option | Procedure | 1* | 2* | 3* | 4* | 5* | 5* | Additional Considerations |
|---|---|---|---|---|---|---|---|---|
| Standard | ||||||||
| Ovarian transposition/oophoropexy102,103 | Surgery is performed to move the ovaries and fallopian tubes out of the radiation field | • | • |
|
||||
| Gonadal shielding104 | Shielding is used to minimize the exposure of reproductive organs to radiation | • | • | Only protects against irradiation | ||||
| Embryo cryopreservation46 |
|
• | • | • |
|
|||
| Investigational | ||||||||
| Egg cryopreservation46,55 |
|
• | • | • |
|
|||
| Oocyte cryopreservation105 |
|
• | • | • |
|
|||
| Isolation of an oocyte or an egg from a natural cycle106 |
|
• | • |
|
||||
| Isolation of oocytes from an ovarian biopsy107 | Oocytes are harvested from an ovarian biopsy | • | • |
|
||||
| Ovarian tissue cryopreservation followed by transplantation60,61,108 |
|
• | • |
|
||||
| Ovarian hormonal suppression109 | Patients are treated with gonadotrophin- releasing hormone analogs (GnRH) or oral contraceptives to keep the ovary in a hypogonadotropic environment | • | • |
|
||||
| In Development | ||||||||
| Ovarian tissue cryopreservation followed by in vitro follicle growth70–72,108,110 |
|
• | • |
|
||||
| Follicle isolation and cryopreservation72,111 |
|
• | • |
|
||||
| Transplantation of isolated follicles112 |
|
• | • |
|
||||
| Xenotransplantation of ovarian tissue or follicles113–116 |
|
• | • |
|
||||
| Use of fertoprotective drugs117,118 | Novel chemotherapeutics whose delivery mode is less gonadotoxic (ex. nanobins; our unpublished results) | • | ||||||
| Non-biological/Third Party | ||||||||
| Egg or embryo donor |
|
|
||||||
| Surrogate |
|
|
||||||
| Adoption119 | Patients can adopt a non-biological child (children) |
|
||||||
ART = assisted reproductive technology; ICSI = intracytoplasmic sperm injection; IVF = in vitro fertilisation; IVM = in vitro maturation.
Potentially delays cancer treatment >2 weeks
Potentially delays cancer treatment <2 weeks
Requires hyperstimulation
Requires sperm donor at time of procedure
Potentially preserves or restores natural reproductive function hormonal
Requires additional ART procedures to attempt pregnancy
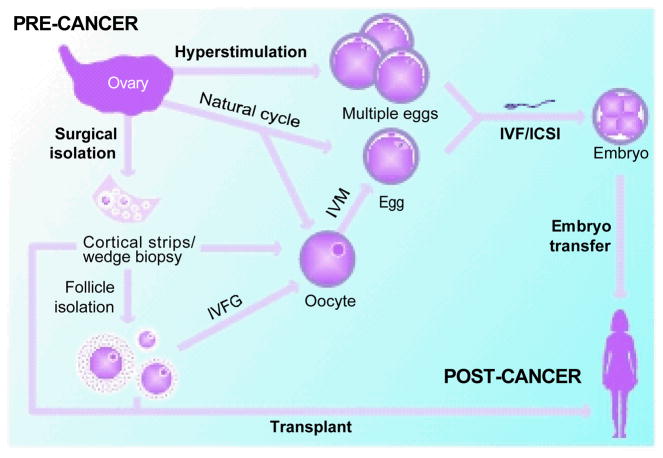
Figure 2. The Ovary Is a Robust Source of Tissue that Can Be Used for Numerous Fertility Preservation Options.
Female gametes can be obtained following hyperstimulation or natural cycle protocols. If oocytes are obtained, they can be in vitro matured (IVM) to produce an egg. Eggs can be fertilized using assisted reproductive (ART) procedures such as in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI), and embryos ultimately can be transferred to a recipient uterus. If gametes cannot be obtained, a patient’s ovarian tissue can be surgically removed. This ovarian tissue can be cryopreserved and later thawed and used for transplantation. Alternatively, follicles can be isolated from fresh or previously cryopreserved ovarian tissue. These follicles can be used for transplant, or they can be used for in vitro follicle growth (IVFG) to obtain oocytes that can be used for IVM. Although there are many potential fertility preservation options, patient-specific factors dictate which strategy will be employed. Furthermore, the fertility preservation methods presented here and in Table 1 range from standard to theoretical, and IVFG and follicle transplantation, for example, are still under development and not yet used clinically. It is important to note that methods exist to cryopreserve oocytes, eggs, embryos, follicles, and/or ovarian tissue, which can be stored for a patient’s future use.
Despite the general success of embryo cryopreservation, there are several drawbacks of this method in the context of oncofertility. This procedure requires time. An ART cycle can take anywhere between two and five weeks to complete, and this potential delay in cancer treatment may be detrimental for women with aggressive or advanced cancers.46 Embryo cryopreservation requires hyperstimulation regimens, which are contraindicated for women with hormone-sensitive cancers.50,51 This technique also presupposes that a patient is willing to use a sperm donor or has an existing partner who agrees to create embryos. Finally, there are myriad ethical and potential legal concerns surrounding the generation and cryopreservation of embryos that may or may not be used.52,53
With the exception of embryo cryopreservation, the ASRM maintains that all other fertility preservation methods are experimental and should be performed only in a research setting under institutional review board approval.15 However, the experimental nature of these alternative procedures perhaps should not be a barrier for use under specific medical circumstances, such as oncofertility, where they may be a patient’s only choice.54 The ovary, which contains follicles in different stages of development, is a robust source of tissue that can be used for numerous fertility preservation options (see Figures 2 and 3). For example, for women who want to maintain their reproductive autonomy and do not want to create embryos at the time of cancer diagnosis, egg cryopreservation is a potential option. In this technique, a woman undergoes hormone-induced hyperstimulation to recruit multiple follicles to grow, and the eggs are aspirated and cryopreserved for later fertilisation (see Figure 2).46,55 Accumulating data suggest that this procedure is a viable option, especially in an oncofertility setting.56 When performed at experienced fertility centers, the live birth rate using cryopreserved eggs is similar to that following conventional IVF using fresh eggs.55 It should also be emphasized that female cancer patients who seek fertility preservation are typically young. The average age of patients who have sought fertility preservation methods through the Oncofertility Consortium since 2007 is 27 (our unpublished data). Thus, the well-documented decline in egg quality and fertility that increases with advanced maternal age is unlikely to be an additional compounding factor for these young women.57–59
For women who cannot delay their cancer treatment or who cannot be exposed to supraphysiologic hormone levels, ovarian tissue cryopreservation is their only option. In this technique, ovarian tissue is removed and cortical strips are cryopreserved for future use (see Figure 2). The cortical strips can be thawed following cancer treatment and transferred back to the patient.60–62 Autotransplantation of cryopreserved ovarian tissue to orthotopic sites has successfully restored the hormonal function and reproductive potential of patients and has resulted in several live births to date.63–68 Nevertheless, ovarian tissue transplants have the inherent risk for reintroducing cancerous cells.69 To avoid this problem, follicles could instead be isolated from the cortical strips and grown in vitro to produce eggs that can be fertilized using ART procedures (see Figure 2).70 In vitro follicle growth (IVFG) technology is still being developed and is currently an active area of research.71–73 In addition to these fertility preservation methods, there are numerous others that are investigational, in development, or non-biological (see Table 2).
Regardless of which fertility preservation method is employed, patients should be counselled about the limitations and risks they may encounter. First, for example, ART procedures, which are broadly used in oncofertility, do not guarantee live births even in non-cancer patients. Currently, the cumulative live-birth rate following IVF is around 50 %.57 Second, there are potential risks associated with ART procedures in general. It has been suggested that singletons born following IVF may have an increased risk for low birth weight, prematurity, and perinatal mortality.74 ART procedures are also associated with a 10-fold increase in the number of multiple births compared with natural conceptions. 74 Furthermore, three imprinting disorders—Beckwith-Wiedemann syndrome, Angelman syndrome, and maternal hypomethylation syndrome—have been linked to ART procedures.75 Finally, patients who have undergone radiotherapy should be counselled adequately about the well -documented negative impact this treatment may have on the uterus and their pregnancy potential (see Figure 1). It is important to emphasize that although methods such as ovarian transposition and gonadal shielding exist to minimize the radiation impact to the ovary, they do not protect the uterus (see Table 2). Patients who have a damaged uterus may have to use a surrogate even if they have their own cryopreserved gametes or embryos or decide to use a donor.
There are ways to minimize the risk involved and to maximize the likelihood of success in fertility preservation. To increase the chances of a live birth following cancer treatment, patients should be counselled to seek fertility preservation and ART treatments at the most established institutions possible, as not all are equivalent (see Table 3; Assisted Reproductive Technology Resources). One of the reasons that cancer patients choose not to pursue fertility preservation is their fear of passing their cancer onto their offspring.76 However, only between 5 and 10 % of all cancers are hereditary.77 To avoid the risk for potentially transmitting gene mutations associated with inherited cancers, fertility preservation methods can be combined with pre-implantation genetic testing (PGT). PGT is a technique in which genetic testing is performed on a biopsy from a pre-implantation-stage embryo to ensure that only embryos free of the specific assayed mutation are transferred back to the uterus.78 PGT has been used to screen for several cancer predisposition syndromes, including adenomatous polyposis of the colon (APC), neurofibromatosis type 2 (NF2). and inherited breast cancer (BRCA1 and BRCA2).79 It is encouraging that children born to cancer survivors are not at increased risk for malformations or death compared with women without a cancer history; 80 however, they may be at increased risk for low birth weight and prematurity.80
Table 3.
Selected Patient and Provider Oncofertility Resources
| Patient and Provider Websites | ||||
|---|---|---|---|---|
| Organization/Site | Web Address | Purpose | Patient | Provider |
| MyOncofertility |
www.myoncofertility.org (English) es.myoncofertility.org (Spanish) |
Provides patients with information about fertility preservation options throughout cancer treatment | • | |
| The Oncofertility Consortium | oncofertility.northwestern.edu | Coordinates an interdisciplinary team of medical specialists, basic scientists and scholars to explore and expand the fertility preservation options for cancer patients | • | • |
| The Oncofertility Consortium Blog | blog.oncofertility.northwestern.edu | Provides synopses of recent news, information, and research related to oncofertility | • | • |
| FERTLINE | oncofertility.northwestern.edu fertline (001) 866 708 3378 |
Phoneline that connects patients and providers to one-on-one fertility preservation support | • | • |
| Fertile Hope | www.fertilehope.org | Provides reproductive information and support to cancer patients and survivors | • | • |
| Lance Armstrong Foundation: Female Infertility | www.livestrong.org/Get-HelpLearn-About-Cancer/Cancer-Support-Topics/Physical-Effects-Of-CancerFemale-Infertility | Identifies and addresses emotional and physical issues faced by cancer survivors | • | |
| I’m too young for this! | i2y.com | Disseminates age-appropriate information and support to young cancer patients at all stages of survivorship | • | |
| Imerman Angels | www.imermanangels.org | Provides personalized connections that enable 1-on-1 support among cancer fighters, survivors, and caregivers | • | |
| Center for Young/Women’s Health (Children’s Hospital Boston) | www.youngwomenshealth.org/gyn_menu.html#cancer | Helps teen girls and their parents, teachers, and healthcare providers improve their understanding of normal health and development, as well as of specific diseases and conditions | • | • |
| Hope for Two | www.pregnantwithcancer.org | Provides women diagnosed with cancer while pregnant with information, support, and hope | • | |
| Cancer and Careers | www.cancerandcareers.org | Provides tools and support to women in the workplace dealing with a cancer diagnosis | • | • |
| Be Bright Pink | www.bebrightpink.org | Provides education and support tools for high-risk individuals to take control of their breast and ovarian health | • | |
| PRISM (University of Chicago Medical Center) | www.uchospitals.edu/specialtiesobgyn/prism | Helps to identify, prevent, and treat sexual health problems in female cancer patients and survivors | • | |
| Cancer Legal Resource Center | www.disabilityrightslegalcenter.org/about/cancerlegalresource.cfm | Provides free and confidential information and resources on cancer- related legal issues to cancer survivors, care-givers, healthcare professionals, employers, and others coping with cancer | • | • |
| SaveMyFertility | http://savemyfertility.org | Provides cancer patients and families with information about fertility preservation and protecting hormonal health before, during, and after cancer treatment. This site also provides healthcare providers with pocket guides and patient fact sheets. Information from this website is available as an iPhone application (iSaveFertility) and can be downloaded free at http://itunes.apple.com/us/app/isavefertility/id436397171?mt=8&ls=1. | • | • |
| Fertility Preservation Practice Guidelines | |||
|---|---|---|---|
| Society | Web Address | Patient | Provider |
| ASCO (clinical guidelines) | www.asco.org/ascov2/Practice+&+Guidelines/Guidelines | • | |
| ASRM (Ethics Committee: Fertility Preservation and Reproduction in Cancer Patients) | www.asrm.org/publications/detail.aspx?id=613 | • | |
| Assisted Reproductive Technology Resources | ||||
|---|---|---|---|---|
| Resource | Web Address | Purpose | Patient | Provider |
| 2007 CDC ART Report | www.cdc.gov/ART/ART2007/ifct.htm | Provides a comparison of ART clinics based on types of ART used, patient diagnoses, success rate that each clinic reported and verified for 2007, and individual program characteristics | • | • |
| Society for Assisted Reproductive Technologies | www.sart.org | Provides and maintains a standard set of guidelines for ART practices in addition to helping patients locate and contact infertility clinics and view national and individual clinic IVF success rates | • | • |
ART = assisted reproductive technology.
Measures Exist to Assess Reproductive Function Pre- and Post-cancer Therapy
Ovarian reserve is defined as the functional potential of the ovary and reflects the number and quality of oocytes within the ovary.81 Being able to measure the ovarian reserve in an oncofertility setting is beneficial because doing so pre-cancer treatment can help predict how vulnerable a patient may be to cancer therapies and could dictate the need for fertility preservation options. Testing the ovarian reserve post -cancer therapy may indicate whether a potential for restored natural fertility exists or whether the patient must rely on previously performed fertility preservation methods. Although there is no single predictive marker of ovarian reserve, several tests have been developed (see Table 1).
Currently, serum anti-Müllerian hormone (AMH) levels combined with antral follicle count (AFC) serve as the most robust markers for evaluating ovarian reserve.82,83 AMH is a hormone that is produced by the granulosa cells in developing ovarian follicles. 84,85 AMH levels are not affected by menstrual cycle day, oral contraceptive use, or pregnancy.84 During natural aging, serum AMH levels decrease rapidly after 37 years, and this drop precedes but is related to the onset of menopause.86,87 A study that was performed in regularly menstruating women to test several hormonal markers of aging suggests that serum AMH is the most accurate marker in predicting the occurrence of the menopausal transition within four years.88
In addition to a woman’s age, serum AMH is also correlated inversely with the number of antral follicles, which can be determined by counting the number of 2–10 mm-diameter follicles using transvaginal ultrasound.87,89 A strong correlation between AMH levels and AFC has been well established in clinical studies.89–91 The number of small antral follicles is one of the best correlates to ovarian age with representation to ovarian reserve.89 It has also been shown that serum AMH levels and AFC, when analysed together, are indicative of primordial follicle number independent of age.92 Thus, it can be gathered that, in combination, serum AMH levels and AFC are strong correlates to ovarian age and the remaining ovarian reserve.
The use of serum AMH levels and AFC to test ovarian reserve has been effectively translated into the oncofertility setting, where various cancer treatments have been shown to compromise the follicle pool.93,94 Clinical studies have shown that serum AMH levels and AFC measured in cancer patients post-treatment were highly correlated to each other and significantly lower than in control non-cancer counterparts.23,91 In a recent longitudinal study, AFC, ovarian volume and levels of FSH, LH, estradiol, inhibin A and B, activin A, and AMH were compared between regularly menstruating breast cancer patients (22–42 years of age) and their non-cancer patient counterparts.95 The results of this study demonstrated that, although the breast cancer patients had normal ovarian reserve prior to cancer treatment, post-treatment a significant decrease in AFC and a drastic reduction in AMH levels occurred. Being able to correlate markers such as serum AMH levels and AFC with a diminished ovarian reserve is critical in the fertility preservation decision-making process. Performing these tests helps to assess the need for fertility intervention in patients who may be susceptible to early menopause or a decreased ovarian reserve both before and after treatment.
Although these tests are an appropriate measure of the remaining developing follicle pool, it is important to remember that they do not guarantee that pregnancy, the ultimate proof of fertility, will occur.81–83 While AMH plus AFC is one of the better combinations for prediction of ovarian reserve, a variety of other tests are commonly used to evaluate a woman’s fertility status (see Table 1).59,83 For example, additional hormonal assays include tests of day 3 estradiol, FSH, and inhibin B. Dynamic tests such as the clomiphene citrate challenge test can also be used to test a patient’s ability to ovulate. It is also of critical importance to counsel patients that a return to normal menstruation is not necessarily indicative of fertility, because there is still a possibility of anovulation or diminished ovarian reserve.96,97
Conclusion
Common cancer treatments have the potential to disrupt all parts of the female reproductive axis, thus leaving patients with side effects such as premature menopause or infertility. A threat to fertility on top of a cancer diagnosis can be devastating and greatly reduce a patient’s quality of life. Self-efficacy, which is defined as the extent to which an individual believes in his or her ability to organize and execute courses of action competently, greatly influences a young adult’s ability to deal with the challenges of cancer and maintain a positive quality of life.3 A patient’s confidence in his or her ability to acquire and understand medical information accurately contributes to overall self-efficacy. A recent online survey of young adult cancer patients demonstrated that more than 65 % had used or wanted information about infertility and options for having children.98,99
Oncofertility is an interdisciplinary field that was developed not only to explore and expand fertility preservation options for cancer patients but also to increase patient awareness of these options. Oncofertility requires a team-based approach to clinical care. In healthcare, a team is defined as a group of professionals who interact dynamically, interdependently, and adaptively toward the shared goal of assessing, planning, or carrying out patient care.100 At the simplest level, an oncofertility team consists of oncologists working closely with reproductive endocrinologists to define and implement the best treatment plan for the patient. In settings with established oncofertility programs, the team may be broader and include nurses, social workers, clinical psychologists, and patient navigators who help guide the patient through the fertility preservation process.101
As a PCP, the gynecologist also has the potential to be an essential part of an oncofertility team. Gynecologists are in a unique position because they are likely to interact with patients faced with a cancer diagnosis throughout their treatment and, most importantly, beyond. To be strong advocates for their patients, gynecologists can provide their patients with critical information regarding fertility preservation options. This can be streamlined with the help of the useful patient- and provider-focused websites and resources summarized in Table 3. In addition, gynecologists should consult with patients pre-, during, and post-treatment about their fertility status and refer them to reproductive endocrinologists as early as possible. Together with other oncofertility team members, gynecologists can help to vastly improve the quality of life of young adult cancer patients.
Acknowledgments
The authors thank Robin Skory, BS, for advice and useful discussions and the Oncofertility Consortium’s program director, Kate Timmerman, PhD, and patient navigator, Kristin Smith, BS, for help with compiling the list of patient- and provider-focused websites. This research was supported by grants from the National Institutes of Health (NIH) to the Oncofertility Consortium, grant number NIH RL1HD058295. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Footnotes
Disclosure: The authors have no conflicts of interest to declare.
References
- 1.NCI. A Snapshot of Adolescent and Young Adult Cancers. 2009 Available at: www.cancer.gov/aboutnci/servingpeople/aya-snapshot.pdf.
- 2.Couzin J. Oncology. In their prime, and dying of cancer. Science. 2007;317:1160–62. doi: 10.1126/science.317.5842.1160. [DOI] [PubMed] [Google Scholar]
- 3.Zebrack B, Hamilton R, Smith AW. Psychosocial outcomes and service use among young adults with cancer. Semin Oncol. 2009;36:468–77. doi: 10.1053/j.seminoncol.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Freyer DR. Transition of care for young adult survivors of childhood and adolescent cancer: rationale and approaches. J Clin Oncol. 2010;28(32):4810–18. doi: 10.1200/JCO.2009.23.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dohle GR. Male infertility in cancer patients: Review of the literature. Int J Urol. 2010;17:327–31. doi: 10.1111/j.1442-2042.2010.02484.x. [DOI] [PubMed] [Google Scholar]
- 6.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–11. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt K, Larsen E, Andersen C. Risk for ovarian failure and fertility preserving methods in girls and adolescents with a malignant disease. BJOG. 2010;117(2):163–74. doi: 10.1111/j.1471-0528.2009.02408.x. [DOI] [PubMed] [Google Scholar]
- 8.Kazak AE, Derosa BW, Schwartz LA, et al. Psychological outcomes and health beliefs in adolescent and young adult survivors of childhood cancer and controls. J Clin Oncol. 2010;28:2002–7. doi: 10.1200/JCO.2009.25.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter J, Raviv L, Applegarth L, et al. A cross-sectional study of the psychosexual impact of cancer-related infertility in women: third-party reproductive assistance. J Cancer Surviv. 2010;4:236–46. doi: 10.1007/s11764-010-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorman JR, Malcarne VL, Roesch SC, et al. Depressive symptoms among young breast cancer survivors: the importance of reproductive concerns. Breast Cancer Res Treat. 2010;123:477–85. doi: 10.1007/s10549-010-0768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter J, Chi DS, Brown CL, et al. Cancer-related infertility in survivorship. Int J Gynecol Cancer. 2010;20:2–8. doi: 10.1111/IGC.0b013e3181bf7d3f. [DOI] [PubMed] [Google Scholar]
- 12.Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat Res. 2007;138:3–11. doi: 10.1007/978-0-387-72293-1_1. [DOI] [PubMed] [Google Scholar]
- 13.Woodruff TK. The Oncofertility Consortium—addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7:466–75. doi: 10.1038/nrclinonc.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 15.Medicine ECotASfR. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622–8. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Hill LD, Erickson K, Holzman GB, et al. Practice trends in outpatient obstetrics and gynecology: findings of the Collaborative Ambulatory Research Network, 1995–2000. Obstet Gynecol Surv. 2001;56:505–16. doi: 10.1097/00006254-200108000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Dickerson VM. The evolution of women’s primary health care in California: the role of the obstetrician/gynecologist. Prim Care Update Ob Gyns. 2001;8:116–21. doi: 10.1016/s1068-607x(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 18.Smith G, Toonen T. The role of the primary care physician during the active treatment phase. Prim Care. 2009;36(4):685–702. doi: 10.1016/j.pop.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Rosen A, Rodriguez-Wallberg KA, Rosenzweig L. Psychosocial distress in young cancer survivors. Semin Oncol Nurs. 2009;25:268–77. doi: 10.1016/j.soncn.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Forman EJ, Anders CK, Behera MA. A nationwide survey of oncologists regarding treatment-related infertility and fertility preservation in female cancer patients. Fertil Steril. 2010;94(5):1652–6. doi: 10.1016/j.fertnstert.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Quinn GP, Vadaparampil ST, King L, et al. Impact of physicians’ personal discomfort and patient prognosis on discussion of fertility preservation with young cancer patients. Patient Educ Couns. 2009;77:338–43. doi: 10.1016/j.pec.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Quinn GP, Vadaparampil ST, Lee J-H, et al. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol. 2009;27:5952–7. doi: 10.1200/JCO.2009.23.0250. [DOI] [PubMed] [Google Scholar]
- 23.Rosendahl M, Andersen CY, la Cour Freiesleben N, et al. Dynamics and mechanisms of chemotherapy-induced ovarian follicular depletion in women of fertile age. Fertil Steril. 2010;94:156–66. doi: 10.1016/j.fertnstert.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 24.Bath LE, Wallace WHB, Critchley HOD. Late effects of the treatment of childhood cancer on the female reproductive system and the potential for fertility preservation. BJOG. 2002;109:107–14. doi: 10.1111/j.1471-0528.2002.t01-1-01007.x. [DOI] [PubMed] [Google Scholar]
- 25.Critchley HO. Factors of importance for implantation and problems after treatment for childhood cancer. Med Pediatr Oncol. 1999;33:9–14. doi: 10.1002/(sici)1096-911x(199907)33:1<9::aid-mpo3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Ogilvy-Stuart AL, Shalet SM. Effect of radiation on the human reproductive system. Environ Health Perspect. 1993;101(Suppl 2):109–16. doi: 10.1289/ehp.93101s2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oktay K, Sönmezer M. Chemotherapy and amenorrhea: risks and treatment options. Curr Opin Obstet Gynecol. 2008;20:408–15. doi: 10.1097/GCO.0b013e328307ebad. [DOI] [PubMed] [Google Scholar]
- 28.Faddy MJ, Gosden RG, Gougeon A, et al. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–6. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 29.Wallace WHB, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod. 2003;18:117–21. doi: 10.1093/humrep/deg016. [DOI] [PubMed] [Google Scholar]
- 30.Wallace WHB, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738–44. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 31.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7:535–43. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 32.Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer. 2007;110:2222–9. doi: 10.1002/cncr.23071. [DOI] [PubMed] [Google Scholar]
- 33.Agha A, Sherlock M, Brennan S, et al. Hypothalamic-pituitary dysfunction after irradiation of nonpituitary brain tumors in adults. J Clin Endocrinol Metab. 2005;90:6355–60. doi: 10.1210/jc.2005-1525. [DOI] [PubMed] [Google Scholar]
- 34.Constine LS, Woolf PD, Cann D, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328:87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 35.Rose SR, Schreiber RE, Kearney NS, et al. Hypothalamic dysfunction after chemotherapy. J Pediatr Endocrinol Metab. 2004;17:55–66. doi: 10.1515/jpem.2004.17.1.55. [DOI] [PubMed] [Google Scholar]
- 36.Green DM, Hall B, Zevon MA. Pregnancy outcome after treatment for acute lymphoblastic leukemia during childhood or adolescence. Cancer. 1989;64:2335–9. doi: 10.1002/1097-0142(19891201)64:11<2335::aid-cncr2820641124>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Critchley HOD, Bath LE, Wallace WHB. Radiation damage to the uterus — review of the effects of treatment of childhood cancer. Hum Fertil (Camb) 2002;5:61–6. doi: 10.1080/1464727022000198942. [DOI] [PubMed] [Google Scholar]
- 38.Critchley HOD, Wallace WHB. Impact of cancer treatment on uterine function. J Natl Cancer Inst Monographs. 2005;34:64–8. doi: 10.1093/jncimonographs/lgi022. [DOI] [PubMed] [Google Scholar]
- 39.Wo J, Viswanathan A. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol. 2009;73(5):1304–12. doi: 10.1016/j.ijrobp.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norwitz ER, Stern HM, Grier H, Lee-Parritz A. Placenta percreta and uterine rupture associated with prior whole body radiation therapy. Obstet Gynecol. 2001;98(5 Pt 2):929–31. doi: 10.1016/s0029-7844(01)01435-1. [DOI] [PubMed] [Google Scholar]
- 41.Yang D, Hladnik L. Treatment of acute promyelocytic leukemia during pregnancy. Pharmacotherapy. 2009;29:709–24. doi: 10.1592/phco.29.6.709. [DOI] [PubMed] [Google Scholar]
- 42.Vinatier E, Merlot B, Poncelet E, et al. Breast cancer during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009;147:9–14. doi: 10.1016/j.ejogrb.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 43.Sadovsky R, Basson R, Krychman M, et al. Cancer and sexual problems. J Sex Med. 2010;7(1 Pt 2):349–73. doi: 10.1111/j.1743-6109.2009.01620.x. [DOI] [PubMed] [Google Scholar]
- 44.Fobair P, Spiegel D. Concerns about sexuality after breast cancer. Cancer J. 2009;15:19–26. doi: 10.1097/PPO.0b013e31819587bb. [DOI] [PubMed] [Google Scholar]
- 45.Carter J, Rowland K, Chi D, et al. Gynecologic cancer treatment and the impact of cancer-related infertility. Gynecol Oncol. 2005;97:90–95. doi: 10.1016/j.ygyno.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 46.Ata B, Chian R-C, Tan SL. Cryopreservation of oocytes and embryos for fertility preservation for female cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:101–12. doi: 10.1016/j.bpobgyn.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Loutradi KE, Kolibianakis EM, Venetis CA, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril. 2008;90:186–93. doi: 10.1016/j.fertnstert.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 48.ICMART. International Committee Monitoring ART: Presentation of preliminary data for 2004. 24th Annual Meeting of the ESHRE; Barcelona, Spain. 2008. [Google Scholar]
- 49.Wennerholm U, Soderstrom-Anttila V, Bergh C. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod. 2009;24(9):2158–72. doi: 10.1093/humrep/dep125. [DOI] [PubMed] [Google Scholar]
- 50.Oktay K, Buyuk E, Davis O, et al. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod. 2003;18:90–95. doi: 10.1093/humrep/deg045. [DOI] [PubMed] [Google Scholar]
- 51.Hulvat MC, Jeruss JS. Maintaining fertility in young women with breast cancer. Curr Treat Options Oncol. 2009;10:308–17. doi: 10.1007/s11864-010-0116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crockin SL. Legal issues related to parenthood after cancer. J Natl Cancer Inst Monographs. 2005;34:111–13. doi: 10.1093/jncimonographs/lgi024. [DOI] [PubMed] [Google Scholar]
- 53.Dolin G, Roberts DE, Rodriguez LM, Woodruff TK. Medical hope, legal pitfalls: potential legal issues in the emerging field of oncofertility. Cancer Treat Res. 2010;156:111–34. doi: 10.1007/978-1-4419-6518-9_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campo-Engelstein L. Consistency in insurance coverage for iatrogenic conditions resulting from cancer treatment including fertility preservation. J Clin Oncol. 2010;28:1284–6. doi: 10.1200/JCO.2009.25.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93:391–6. doi: 10.1016/j.fertnstert.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 56.Noyes N, Boldt J, Nagy ZP. Oocyte cryopreservation: is it time to remove its experimental label? J Assist Reprod Genet. 2010;27:69–74. doi: 10.1007/s10815-009-9382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moragianni VA, Penzias AS. Cumulative live-birth rates after assisted reproductive technology. Curr Opin Obstet Gynecol. 2010;22:189–92. doi: 10.1097/GCO.0b013e328338493f. [DOI] [PubMed] [Google Scholar]
- 58.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Broekmans FJ, Kwee J, Hendriks DJ, et al. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 60.Donnez J, Jadoul P, Squifflet J, et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:87–100. doi: 10.1016/j.bpobgyn.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 61.von Wolff M, Donnez J, Hovatta O, et al. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy—a technique in its infancy but already successful in fertility preservation. Eur J Cancer. 2009;45:1547–53. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 62.Sonmezer M, Oktay K. Orthotopic and heterotopic ovarian tissue transplantation. Best Pract Res Clin Obstet Gynaecol. 2010;24:113–26. doi: 10.1016/j.bpobgyn.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Roux C, Amiot C, Agnani G, et al. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93:2413, e2415–19. doi: 10.1016/j.fertnstert.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 64.Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 65.Meirow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–21. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 66.Demeestere I, Simon P, Emiliani S, et al. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist. 2007;12:1437–42. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- 67.Andersen CY, Rosendahl M, Byskov AG, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–72. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- 68.Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008;359:2617–18. doi: 10.1056/NEJMc0804321. [DOI] [PubMed] [Google Scholar]
- 69.Meirow D, Hardan I, Dor J, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23:1007–13. doi: 10.1093/humrep/den055. [DOI] [PubMed] [Google Scholar]
- 70.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–46. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu M, Barrett SL, West-Farrell E, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–40. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103:378–86. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu M, West-Farrell ER, Stouffer RL, et al. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–94. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schieve LA, Rasmussen SA, Buck GM, et al. Are children born after assisted reproductive technology at increased risk for adverse health outcomes? Obstet Gynecol. 2004;103:1154–63. doi: 10.1097/01.AOG.0000124571.04890.67. [DOI] [PubMed] [Google Scholar]
- 75.Amor DJ, Halliday J. A review of known imprinting syndromes and their association with assisted reproduction technologies. Hum Reprod. 2008;23:2826–34. doi: 10.1093/humrep/den310. [DOI] [PubMed] [Google Scholar]
- 76.Schover LR. Motivation for parenthood after cancer: a review. J Natl Cancer Inst Monogr. 2005;34:2–5. doi: 10.1093/jncimonographs/lgi010. [DOI] [PubMed] [Google Scholar]
- 77.Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol. 2005;23:276–92. doi: 10.1200/JCO.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 78.Simpson JL. Preimplantation genetic diagnosis at 20 years. Prenat Diagn. 2010;30:682–95. doi: 10.1002/pd.2552. [DOI] [PubMed] [Google Scholar]
- 79.Spits C, De Rycke M, Van Ranst N, et al. Preimplantation genetic diagnosis for cancer predisposition syndromes. Prenat Diagn. 2007;27(5):447–56. doi: 10.1002/pd.1708. [DOI] [PubMed] [Google Scholar]
- 80.Mueller BA, Chow EJ, Kamineni A, et al. Pregnancy outcomes in female childhood and adolescent cancer survivors: a linked cancer-birth registry analysis. Arch Pediatr Adolesc Med. 2009;163:879–86. doi: 10.1001/archpediatrics.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maheshwari A, Fowler P, Bhattacharya S. Assessment of ovarian reserve—should we perform tests of ovarian reserve routinely? Hum Reprod. 2006;21:2729–35. doi: 10.1093/humrep/del188. [DOI] [PubMed] [Google Scholar]
- 82.Jayaprakasan K, Campbell B, Hopkisson J, et al. A prospective, comparative analysis of anti-Müllerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93:855–64. doi: 10.1016/j.fertnstert.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 83.Domingues TS, Rocha AM, Serafini PC. Tests for ovarian reserve: reliability and utility. Curr Opin Obstet Gynecol. 2010;22:271–6. doi: 10.1097/GCO.0b013e32833b4f5c. [DOI] [PubMed] [Google Scholar]
- 84.La Marca A, Volpe A. Anti-Müllerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf) 2006;64:603–10. doi: 10.1111/j.1365-2265.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- 85.Kwee J, Schats R, McDonnell J, et al. Evaluation of anti-Müllerian hormone as a test for the prediction of ovarian reserve. Fertil Steril. 2008;90:737–43. doi: 10.1016/j.fertnstert.2007.07.1293. [DOI] [PubMed] [Google Scholar]
- 86.de Vet A, Laven JS, de Jong FH, et al. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–62. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 87.van Disseldorp J, Faddy MJ, Themmen AP, et al. Relationship of serum antimullerian hormone concentration to age at menopause. J Clin Endocrinol Metab. 2008;93:2129–34. doi: 10.1210/jc.2007-2093. [DOI] [PubMed] [Google Scholar]
- 88.van Rooij IA, Tonkelaar I, Broekmans FJ, et al. Anti-mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601–6. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- 89.Haadsma ML, Bukman A, Groen H, et al. The number of small antral follicles (2–6 mm) determines the outcome of endocrine ovarian reserve tests in a subfertile population. Hum Reprod. 2007;22:1925–31. doi: 10.1093/humrep/dem081. [DOI] [PubMed] [Google Scholar]
- 90.Fanchin R, Schonauer LM, Righini C, et al. Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323–7. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 91.Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010;94:638–44. doi: 10.1016/j.fertnstert.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 92.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–5. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 93.Lie Fong S, Lugtenburg PJ, Schipper I, et al. Anti-müllerian hormone as a marker of ovarian function in women after chemotherapy and radiotherapy for haematological malignancies. Hum Reprod. 2008;23:674–8. doi: 10.1093/humrep/dem392. [DOI] [PubMed] [Google Scholar]
- 94.Lie Fong S, Laven JSE, Hakvoort-Cammel FGAJ, et al. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Müllerian hormone. Hum Reprod. 2009;24:982–90. doi: 10.1093/humrep/den487. [DOI] [PubMed] [Google Scholar]
- 95.Singh K, Muttukrishna S, Stein R. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer. 2007;96(12):1808–16. doi: 10.1038/sj.bjc.6603814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu B, Douglas N, Ferin MJ, et al. Changes in markers of ovarian reserve and endocrine function in young women with breast cancer undergoing adjuvant chemotherapy. Cancer. 2010;116:2099–2105. doi: 10.1002/cncr.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnston RJ, Wallace WHB. Normal ovarian function and assessment of ovarian reserve in the survivor of childhood cancer. Pediatr Blood Cancer. 2009;53:296–302. doi: 10.1002/pbc.22012. [DOI] [PubMed] [Google Scholar]
- 98.Zebrack B. Information and service needs for young adult cancer survivors. Support Care Cancer. 2009;17:349–57. doi: 10.1007/s00520-008-0469-2. [DOI] [PubMed] [Google Scholar]
- 99.Zebrack B. Information and service needs for young adult cancer patients. Support Care Cancer. 2008;16:1353–60. doi: 10.1007/s00520-008-0435-z. [DOI] [PubMed] [Google Scholar]
- 100.Xyrichis A, Ream E. Teamwork: a concept analysis. J Adv Nursing. 2008 Jan;61(2):232–41. doi: 10.1111/j.1365-2648.2007.04496.x. [DOI] [PubMed] [Google Scholar]
- 101.Gardino SL, Jeruss JS, Woodruff TK. Using decision trees to enhance interdisciplinary team work: the case of oncofertility. J Assist Reprod Genet. 2010;27:227–31. doi: 10.1007/s10815-010-9413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Terenziani M, Piva L, Meazza C, et al. Oophoropexy: a relevant role in preservation of ovarian function after pelvic irradiation. Fertil Steril. 2009;91:935, e915–36. doi: 10.1016/j.fertnstert.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 103.Williams RS, Littell RD, Mendenhall NP. Laparoscopic oophoropexy and ovarian function in the treatment of Hodgkin disease. Cancer. 1999;86:2138–42. [PubMed] [Google Scholar]
- 104.Bardo DM, Black M, Schenk K, Zaritzky MF. Location of the ovaries in girls from newborn to 18 years of age: reconsidering ovarian shielding. Pediatr Radiol. 2009;39:253–9. doi: 10.1007/s00247-008-1094-4. [DOI] [PubMed] [Google Scholar]
- 105.Cao YX, Chian RC. Fertility preservation with immature and in vitro matured oocytes. Semin Reprod Med. 2009;27:456–64. doi: 10.1055/s-0029-1241055. [DOI] [PubMed] [Google Scholar]
- 106.Chian RC, Buckett WM, Abdul Jalil AK, et al. Natural-cycle in vitro fertilization combined with in vitro maturation of immature oocytes is a potential approach in infertility treatment. Fertil Steril. 2004;82:1675–8. doi: 10.1016/j.fertnstert.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 107.Huang JY, Tulandi T, Holzer H, et al. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation and vitrification: an additional strategy of fertility preservation. Fertil Steril. 2008;89:567–72. doi: 10.1016/j.fertnstert.2007.03.090. [DOI] [PubMed] [Google Scholar]
- 108.Smitz J, Dolmans MM, Donnez J, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oktay K, Sonmezer M, Oktem O, et al. Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist. 2007;12:1055–66. doi: 10.1634/theoncologist.12-9-1055. [DOI] [PubMed] [Google Scholar]
- 110.Amorim CA, Van Langendonckt A, David A, et al. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod. 2009;24:92–9. doi: 10.1093/humrep/den343. [DOI] [PubMed] [Google Scholar]
- 111.Barrett SL, Shea LD, Woodruff TK. Noninvasive index of cryorecovery and growth potential for human folliclesin vitro. Biol Reprod. 2010;82:1180–89. doi: 10.1095/biolreprod.109.082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dolmans MM, Martinez-Madrid B, Gadisseux E, et al. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction. 2007;134:253–62. doi: 10.1530/REP-07-0131. [DOI] [PubMed] [Google Scholar]
- 113.Dolmans M-M, Yuan WY, Camboni A, et al. Development of antral follicles after xenografting of isolated small human preantral follicles. Reprod Biomed Online. 2008;16:705–11. doi: 10.1016/s1472-6483(10)60485-3. [DOI] [PubMed] [Google Scholar]
- 114.Dath C, Van Eyck AS, Dolmans MM, et al. Xenotransplantation of human ovarian tissue to nude mice: comparison between four grafting sites. Hum Reprod. 2010;25:1734–43. doi: 10.1093/humrep/deq131. [DOI] [PubMed] [Google Scholar]
- 115.Kim SS, Kang HG, Kim NH, et al. Assessment of the integrity of human oocytes retrieved from cryopreserved ovarian tissue after xenotransplantation. Hum Reprod. 2005;20:2502–8. doi: 10.1093/humrep/dei099. [DOI] [PubMed] [Google Scholar]
- 116.Kim SS, Soules MR, Battaglia DE. Follicular development, ovulation, and corpus luteum formation in cryopreserved human ovarian tissue after xenotransplantation. Fertil Steril. 2002;78:77–82. doi: 10.1016/s0015-0282(02)03144-8. [DOI] [PubMed] [Google Scholar]
- 117.Ahn RW, Chen F, Chen H, et al. A novel nanoparticulate formulation of arsenic trioxide with enhanced therapeutic efficacy in a murine model of breast cancer. Clin Cancer Res. 2010;16:3607–17. doi: 10.1158/1078-0432.CCR-10-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen H, Pazicni S, Krett NL, et al. Coencapsulation of arsenic- and platinum-based drugs for targeted cancer treatment. Angew Chem Int Ed Engl. 2009;48:9295–9. doi: 10.1002/anie.200903655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gardino SL, Russell AE, Woodruff TK. Adoption after cancer: adoption agency attitudes and perspectives on the potential to parent post-cancer. Cancer Treat Res. 2010;156:153–70. doi: 10.1007/978-1-4419-6518-9_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sills ES, Alper MM, Walsh APH. Ovarian reserve screening in infertility: practical applications and theoretical directions for research. Eur J Obstet Gynecol Reprod Biol. 2009;146:30–36. doi: 10.1016/j.ejogrb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 121.Coccia ME, Rizzello F. Ovarian reserve. Ann N Y Acad Sci. 2008;1127:27–30. doi: 10.1196/annals.1434.011. [DOI] [PubMed] [Google Scholar]
- 122.Seifer DB, Scott RT, Jr, Bergh PA, et al. Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin B before a rise in day 3 follicle-stimulating hormone. Fertil Steril. 1999;72:63–5. doi: 10.1016/s0015-0282(99)00193-4. [DOI] [PubMed] [Google Scholar]
- 123.Hendriks DJ, Kwee J, Mol BWJ, et al. Ultrasonography as a tool for the prediction of outcome in IVF patients: a comparative meta-analysis of ovarian volume and antral follicle count. Fertil Steril. 2007;87:764–75. doi: 10.1016/j.fertnstert.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 124.Muttukrishna S, McGarrigle H, Wakim R, et al. Antral follicle count, anti-mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? BJOG. 2005;112:1384–90. doi: 10.1111/j.1471-0528.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 125.Bancsi LFJMM, Broekmans FJM, Eijkemans MJC, et al. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328–36. doi: 10.1016/s0015-0282(01)02983-1. [DOI] [PubMed] [Google Scholar]
- 126.Syrop CH, Willhoite A, Van Voorhis BJ. Ovarian volume: a novel outcome predictor for assisted reproduction. Fertil Steril. 1995;64:1167–71. doi: 10.1016/s0015-0282(16)57979-5. [DOI] [PubMed] [Google Scholar]
- 127.Hendriks DJ, Broekmans FJM, Bancsi LFJMM, et al. Repeated clomiphene citrate challenge testing in the prediction of outcome in IVF: a comparison with basal markers for ovarian reserve. Hum Reprod. 2005;20:163–9. doi: 10.1093/humrep/deh553. [DOI] [PubMed] [Google Scholar]
- 128.Ragni G, Chiaffarino F, Scarduelli C, et al. The clomiphene citrate challenge test (CCCT) in women with elevated basal FSH: biological significance and predictive value. Eur J Obstet Gynecol Reprod Biol. 2008;141:44–8. doi: 10.1016/j.ejogrb.2008.06.009. [DOI] [PubMed] [Google Scholar]