Abstract
Research on psychological influences on physiology primarily focuses on biological responses during stressful challenges, and how those responses can become dysregulated with prolonged or repeated exposure to stressful circumstances. At the same time, humans spend considerable time recovering from those challenges, and a host of biological processes involved in restoration and repair take place during normal, non-stressed activities. We review restorative biological processes and evidence for links between psychosocial factors and several restorative processes including sleep, wound healing, antioxidant production, DNA repair, and telomerase function. Across these biological processes, a growing body of evidence suggests that experiencing negative emotional states, including acute and chronic stress, depressive symptoms, and individual differences in negative affectivity and hostility, can influence these restorative processes. This review calls attention to restorative processes as fruitful mechanisms and outcomes for future biobehavioral research.
Just as the constant increase of entropy is the basic law of the universe, so it is the basic law of life to be ever more highly structured and to struggle against entropy. Vaclav Havel, in an open letter to the General Secretary of the Czechoslovak Communist Party, 1975
Every single day, we withstand physical challenges and damage, from bombardment by solar radiation, exposure to toxins, to the repetitive strain of work-related tasks like lifting or typing. Further compounding the wear and tear that accompanies the physical demands of everyday life, three decades of research in health psychology and psychoneuroimmunology strongly suggests that psychosocial factors like stress, social isolation, and negative affect-related personality characteristics (i.e., antagonism, trait anxiety) contribute to physical damage (Glaser, Rabin, Chesney, Cohen, & Natelson, 1999; Miller, Chen, & Cole, 2009). A common theme across our conceptual understanding of how psychological stress influences health is entropy (disorder) – that biological systems can become dysregulated or disordered over time through prolonged or repeated exposure to stressful life events.
At the same time, all living organisms have the capacity to repair damage, and do so regularly by “struggling” against entropy to restore biological structures and functions. While restorative processes have received some attention in biobehavioral research, they have not received as much compared to processes involved in wear and tear. To bring attention to restorative processes in the links between psychosocial factors and health, we first define restorative processes, contrast them with biological processes that are typically associated with psychosocial factors, and outline a framework for understanding how psychosocial factors can impact restorative processes. We then highlight evidence for the role of psychosocial influences on several restorative processes. Finally, we suggest future directions for understanding how psychosocial factors impact restorative processes, and their relevance for understanding health and social and personality psychology.
Allostatic and restorative processes
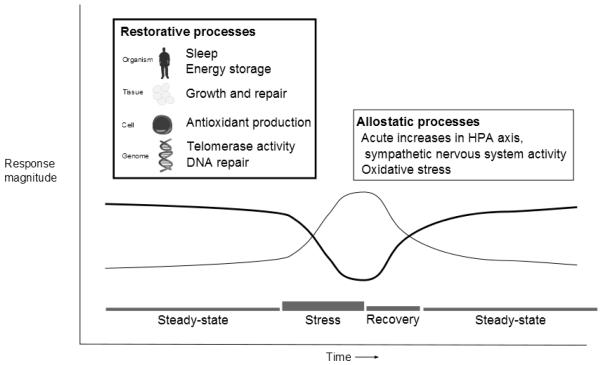
Restore is defined as “1. Give back, return; 2. To put or bring back into existence or use; and 3. To bring back to or put back into a former or original state: renew…” (Merriam-Webster, n. d.). Thus, restorative processes are involved in returning the organism to its original state prior to previous environmental challenges. Figure 1 shows examples of restorative biological processes across multiple levels of organization ranging from sleep to deoxyribonucleic acid (DNA) repair. In this review, we contrast restorative processes with allostatic processes, which have received the most empirical attention, also shown in Figure 1.
Figure 1.
Hypothetical relationship between allostatic and restorative processes as a function of psychological stress
Sterling and Eyer (1988) first described the concept of “allostasis” as a more refined definition of “homeostasis.” Homeostasis refers to “survival through staying the same,” a term initially defined in early physiology research as biological processes that were tightly regulated around a set point (Sterling & Eyer, 1988). For example, the degree of acidity/alkalinity of blood is maintained within a very narrow range (pH from 7.36 – 7.44, Collings, 2010). By comparison, we consume beverages with pH values ranging from 3 (fruit juices) to 7 (water). Allostasis means survival through change (Sterling & Eyer, 1988), and refers to biological processes with a broader range of function, such that survival depends on being able to change biological set points temporarily. For example, during infection, body temperature rises to create an environment that is inhospitable for infectious microorganisms, otherwise known as fever.
McEwen applied the allostasis concept to biological systems that respond to psychological and physical stress, particularly the neuroendocrine system (McEwen, 1998). For example, to survive a physical attack, the sympathetic nervous system and hypothalamic-pituitary-adrenal (HPA) axis release hormones that provide energy to the brain and enable physiological responses that support a fight or flight response (Figure 1). Over time, repeated exposures to adverse events and repeated activation of allostatic systems result in wear and tear across multiple biological systems. For example, the cardiovascular reactivity hypothesis posits that repeated increases in cardiac output and blood pressure in response to stressful or anger-provoking events leads to physical damage to blood vessels (e.g., shear stress on artery walls), which subsequently leads to processes that initiate and progress atherosclerosis (Lovallo & Gerin, 2003).
In contrast to biological processes that respond during challenge, many restorative processes respond following the cessation of environmental challenges, after the return of allostatic processes to steady-state or even quiescent levels. One reason for this is that during challenge, allostatic processes can inhibit certain restorative actions. For instance, as we describe later, elevated levels of norepinephrine and cortisol can inhibit DNA repair. That said, other restorative processes may persist during challenge, although we suggest that most are down-regulated in order for the organism to devote energy towards activities that help with adapting to short-term demands. One issue with this definition is that some challenges may never really end. For instance, we describe sleep as restorative, but in areas with heavy noise pollution or high crime those challenges can disrupt sleep. Indeed, the inability to return to a state where restorative processes can be initiated and allowed to persist may partly explain how chronic environmental conditions can negatively impact health.
The mechanisms through which psychosocial factors influence restorative processes involve mediators such as HPA axis hormones and autonomic nervous system output, which are also involved in allostatic processes (McEwen, 1998). Thus, the conceptual difference between allostatic and restorative processes involves: a) timeframe relative to challenge, and b) specificity to tissues. Most perspectives on behavioral medicine and health psychology regard the challenge or stressor as a key point of intersection between interpersonal (e.g., social conflict and support) and intraindividual (e.g., hostility, cognitive appraisals) factors and biological mechanisms that influence health. Importantly, characteristics of stressors, such as chronicity and uncontrollability, tend to predict larger psychological and physical impacts on the individual (Cohen, Kessler, & Underwood Gordon, 1995).
In terms of timing, Figure 1 shows that during challenge, allostatic processes are mobilized, and return to steady-state levels (normal, basal levels) following cessation of challenge (Seeman, Singer, Rowe, Horwitz, & McEwen, 1997). Figure 1 also shows that restorative processes are downregulated (partly or entirely) during challenge to redirect energy consumption towards allostatic processes, and return to pre-challenge states following the cessation of challenge. Allostatic processes have effects across a variety of tissues; for instance, cortisol has multiple effects on cells, tissues, and systems (Sapolsky, Romero, & Munck, 2000). Similarly, restorative processes like sleep and DNA repair have effects across a variety of tissues, while other processes like telomerase activity, which is most active in cells that have high turnover (e.g., immune cells and stem cells), and wound healing are restricted to specific cell and tissue types. In general, restorative processes are complementary to allostatic processes, and both contribute to long-term health outcomes.
How might psychosocial factors influence restorative processes? As shown in Table 1, psychosocial factors could slow the rate of repair and rebuilding, which may not be fully complete by the time another challenge disrupts the organism, making cells or tissues unable to fully withstand a subsequent challenge. Psychosocial factors could also disrupt the integrity of materials used to restore function. Thus, even if rebuilding of tissues and cells occurs at a normative rate, the materials used for repair may not be of sufficient quality. For example, chronic stress may contribute to fragmented sleep – multiple awakenings during the night – which may impede other restorative processes that occur during sleep. Finally, psychosocial factors could disrupt normal restorative processes in a manner detrimental to the organism through overcompensation, such as increasing normal programmed cell death in a population of cells, scarring following wound healing, increasing preference for high sweet and fat foods, or shifting an individual’s circadian sleep-wake cycle.
Table 1.
Ways in which psychosocial factors may influence restorative processes
| Mechanism of influence | Example |
|---|---|
| Slowed rate of repair or rebuilding | Delays in wound healing Insulin resistance |
| Disrupted integrity of materials used to restore function |
Impaired telomerase activity Sleep disruption |
| Disrupted normative restorative processes – overcompensation |
Excessive cell death due to failed repair Scarring following wound healing Increased appetite and decreased metabolism Disrupted circadian sleep cycles |
The mediating pathways through which psychosocial factors influence restorative processes clearly involve steady-state levels of neuroendocrine hormones and neurotransmitters, which are the primary way the brain communicates with the body. Of particular interest should be physiological mechanisms that regulate growth, including the parasympathetic nervous system which is involved in “rest-and-digest” activities (Heijboer et al., 2006), and the insulin-like growth factor pathway, the central biological mechanism involved in regulating energy storage (Butler & Le Roith, 2001). In addition, the essential building blocks that are provided by diet and nutrition to promote growth are key mediators (Kiecolt-Glaser, 2010).
Our discussion of restorative processes shares conceptual overlap with the distinction between catabolic and anabolic hormones in the study of metabolism, which was extended by Epel and colleagues to how psychological factors can impact physiology (Epel, McEwen, & Ickovics, 1998). As we discuss in greater detail in the Appendix, anabolic hormones like growth hormone and insulin promote energy storage and growth. Thus, they are clearly primary mechanisms involved in restorative processes. That said, our emphasis is on activity at the level of target organs and tissues, in addition to production of anabolic hormones described by Epel and colleagues. Moreover, our framework focuses on processes that occur during an individual’s steady-state level of activity, presumably after the cessation of challenges.
Exemplar restorative processes at multiple levels
What follows are several restorative processes at different levels of analysis – from the entire organism to the genome within a single cell. We also discuss evidence for the influence of psychosocial factors on those processes, and review current research explaining the neuroendocrine mechanisms through which psychosocial factors impact the process of interest.
Organism level: Sleep
Sleep may be the one time in our lives that we are at steady-state activity, free of external challenges in the immediate environment. No “grand unified theory” explains why animals sleep, but one prominent cluster is restorative theories of sleep, which propose that biological processes in the brain and the body during sleep help sustain waking behavior (Zepelin, Siegel, & Tobler, 2005). Such processes include energy conservation, promoting new learning, and consolidating memories (Rechtschaffen, 1998; Zepelin, et al., 2005). Our focus is on the organism-wide biological processes involved in physical growth and restoration across multiple organ and tissue systems.
One of the key reasons sleep is described as physically restorative is its role in physical growth. Growth hormone (GH) is a key anabolic hormone, primarily produced during sleep (for more detail on GH and its anabolic functions, see the Appendix). Sleep begins with sleep onset, followed by several cycles of slow wave sleep (SWS) and rapid eye movement sleep during the night (Kryger, Roth, & Dement, 2005). During sleep onset, healthy young adults secrete a pulse of GH; among middle-age and older men, this sleep onset pulse of GH may be the only period of GH secretion during the day (Van Cauter & Copinschi, 2000). Among premenopausal women, GH pulses occur during the daytime in addition to sleep onset. GH production primarily occurs during SWS, the deepest stages of sleep that occur during the early portions of the night, accounting for approximately 70% of GH pulses (Van Cauter & Copinschi, 2000). Greater depth of SWS, indicated by longer duration and specific brain activity patterns make GH pulses more likely (Gronfier et al., 1996). SWS suppression, either experimentally or naturalistically in patients with sleep disorders is associated with lower nighttime GH production, which can be normalized with treatment (Van Cauter & Copinschi, 2000).
Sleep is also important for regulating energy metabolism and appetite (see Appendix for an overview of metabolism, also Morselli, Leproult, Balbo, & Spiegel, 2010). For example, healthy young men who were allowed four hours of time in bed per night (cf. their usual pattern of eight hours per night) for six days showed impaired ability to store glucose (increased insulin resistance, reduced glucose tolerance) compared to when the same men were allowed 12 hours of time in bed per night (Spiegel, Leproult, & Van Cauter, 1999). Moreover, depriving individuals of SWS for three consecutive nights (without any reduction in total amount of sleep) resulted in increased insulin resistance and impaired glucose tolerance (Tasali, Leproult, Ehrmann, & Van Cauter, 2008). Taken together, these studies suggest sleep impairment decreases the body’s ability to effectively store glucose, which is also the primary problem in type 2 diabetes.
Sleep disruptions can also alter endocrine signals that inform the brain about the energy state of the body (whether the body is sufficiently “fed”). Leptin, a hormone produced by fat cells, is a key substance along with insulin that provides information about stored energy levels to the brain (Schwartz, Woods, Porte Jr., Seeley, & Baskin, 2000). Low leptin levels signal inadequate energy stores, and lead to increased energy intake through stimulating appetite and other motivational mechanisms for eating, and decreased energy expenditure by reducing basal metabolism and physical activity. Ghrelin, an appetite-stimulating hormone produced in the stomach, normally increases just before a meal and decreases after eating. Both laboratory (Spiegel, Tasali, Penev, & Van Cauter, 2004) and epidemiological studies (Chaput, Despres, Bouchard, & Tremblay, 2007) show that short sleep duration leads to decreased circulating leptin and increased circulating ghrelin, which in the short-term should lead to increased appetite and energy intake. Thus, sleep loss leads to disruption in normal rebuilding processes (cannot effectively store glucose) and overcompensation (a stronger drive to eat).
Numerous psychosocial factors at multiple levels are potential pathways to sleep disruption. Potential influences range from individual differences in personality characteristics, such as conscientiousness (Gray & Watson, 2002), to relationship functioning (Troxel, Robles, Hall, & Buysse, 2007), to chronic stressors (Steptoe & Marmot, 2003). While few studies have explicitly linked these factors with the restorative processes involved in growth and energy metabolism during sleep, the existing data suggest that sleep disruptions may be a key pathway through which psychosocial factors influence health.
Tissue level: Wound healing
Wound healing is a cascade of multiple cellular events that is initiated by damage to the skin (Christian, Graham, Padgett, Glaser, & Kiecolt-Glaser, 2006; Singer & Clark, 1999). The primary function of the skin is to provide a protective barrier for internal tissues against the outside world through physical, chemical, and biological means (Elias, 2005). Even minor damage to the skin, such as the removal of cells in the upper layer of the epidermis through a carpet burn, initiates the cascade involved in repairing the skin barrier, and occurs immediately following damage to the skin. The first step in the cascade is an allostatic process designed to combat threat of infection through release of chemicals that prevent blood loss, followed by inflammatory signals to recruit immune cells to the site of injury and clear the wound site of invading microorganisms and debris. Following this inflammatory phase, which lasts hours to days after wounding, restorative processes begin with other cells rebuilding the supporting structures of the surrounding tissues. The final restorative phases, which occur in the days to months after wounding, involve reforming the structures that line the tissue, contraction of the wound, and maturation of the scar. Thus, the vast majority of events in wound healing occur in the hours, days, weeks, and months after wounding, well after the challenge that caused the wound has abated.
The past 15 years of research clearly show that psychological stress can impact wound healing (Christian, et al., 2006). Stressors including laboratory tasks, marital conflict, academic exams, and chronic stressors are related to delayed skin barrier recovery and wound healing across a variety of different types of wounds (Ebrecht et al., 2004; Garg et al., 2001; Kiecolt-Glaser et al., 2005; Kiecolt-Glaser, Marucha, Malarkey, Mercado, & Glaser, 1995; Marucha, Kiecolt-Glaser, & Favagehi, 1998; Robles, 2007). A recent meta-analysis of 11 studies across different contexts and wound types showed that the relationship between stress and wound healing was a moderate effect size r = −.42 (Walburn, Vedhara, Hankins, Rixon, & Weinman, 2009). Thus far, most work has primarily been on psychological stressors, although recent work suggests that individual differences in trait positive affectivity (Robles, Brooks, & Pressman, 2009) and anger expression may also be related to wound healing (Gouin, Kiecolt-Glaser, Malarkey, & Glaser, 2008).
In terms of neuroendocrine mediators, the HPA axis, sympathetic nervous system, and other neuroendocrine mediators at the level of the skin play important roles that have not been fully delineated. In mice, increasing systemic corticosterone (the cortisol equivalent for mice) through chronic stressors delays wound healing, while blocking corticosterone during chronic stress normalizes wound healing (Padgett, Marucha, & Sheridan, 1998). Although pharmacological administration of high-potency corticosteroids, typically 30 times the potency of cortisol, delays wound healing in humans (Kao et al., 2003), the concept that endogenous levels of cortisol directly mediate links between psychological factors and wound healing has not been consistently demonstrated in humans (cf. Ebrecht, et al., 2004). The skin is also extensively innervated by the sympathetic nervous system (Arck, Slominski, Theoharides, Peters, & Paus, 2006), and heightened steady-state activity in the sympathetic nervous system could influence wound healing processes. Work in rodent models suggests that removing sympathetic input to the skin may accelerate wound healing, suggesting that sympathetic activation may delay wound healing (Souza, Cardoso, Amadeu, Desmouliere, & Costa, 2005). However, the role of sympathetic activity in wound healing has not been systematically documented in humans.
The primary complication to studying biological mediators of wound healing is that measuring biological processes that take place in the skin requires invasive tissue removal, which makes quantifying levels of hormones or neurotransmitters challenging in humans. Furthermore, many neuroendocrine hormones, neurotransmitters, and neuropeptides are actually produced by cells in the skin (Slominski & Wortsman, 2000). For example, hair follicles contain all the necessary molecular machinery to produce all the HPA axis hormones (Ito et al., 2005). Thus, mapping out the specific signaling pathways through which the brain can “talk” to the skin will require some understanding of the source of neuroendocrine signals. For example, if cortisol is a key mediator, is the cortisol coming from the adrenal gland or from local production by hair follicles? If the source is the latter, how does the brain send signals to hair follicles that lead to subsequent cortisol production? Moreover, most work to date has focused on the early inflammatory, allostatic phase of wound healing (cf. Yang, Bane, MacCallum, Kiecolt-Glaser, & Glaser, 2002), which makes sense because early delays in the process can significantly delay other components of the wound healing cascade. At the same time, the remodeling phases of wound healing are significantly longer than the early phase, taking place over weeks and months, leaving a larger window of time for influences from psychosocial factors. Thus, the key challenge for future work is incorporating non-invasive techniques for understanding how signals sent from the brain literally get under the skin, and sampling those signals at multiple points throughout the wound healing process.
Cellular and genomic levels: Oxidative stress, DNA repair, and telomerase
Oxidative stress
At the cellular level, a number of restorative processes are disrupted upon activation of the neuroendocrine stress response, particularly energy storage. This biochemical shift moves away from increasing energy reserves and repairing damage towards a preparatory response in anticipation of injury and high energy demands. Thus the stress response mobilizes the body’s resources and defenses by increasing circulating levels of stored energy in the form of lipids (fat) and glucose, the number of circulating cells (e.g., platelets), and energy expenditure in circulating cells.
One major consequence of these alterations is increases in oxidative stress, typically defined as an imbalance between oxidants and antioxidants, with more oxidants present than can be appropriately regulated by available antioxidants (for further discussion, see the Appendix, Table 2, and Finkel & Holbrook, 2000). Oxidants, often referred to as free radicals, can cause damage to DNA, proteins, and lipids, ultimately interfering with normal cellular processes. Some common sources of free radicals include normal cellular metabolic activity, inflammation, ultraviolet (UV) light, and environmental toxin exposure (e.g., cigarette smoke, pollution). As oxidant production is part of normal cellular activity, there are built in compensatory mechanism to protect from oxidant insult: Antioxidants, dietary and cellular derived (See Table 2 for examples), work to return harmful oxidants to a harmless state. Thus, antioxidants serve a restorative function by reducing oxidant load and preventing oxidative damage. The studies described below examine links between psychosocial factors, including negative emotional states and psychological stressors, to oxidative stress. We note that oxidative stress and antioxidant capacity can be measured in a variety of ways, and that while our focus conceptually is on the restorative role of antioxidants, this is typically measured through assessing the wear and tear caused by oxidative insult.
Table 2.
| Cellular and Dietary Derived Antioxidants and Their Sources. |
|---|
| Cellular Derived |
| Catalase (CAT) |
| Glutathione peroxidase (GPx) |
| Glutathione reductase (GR) |
| Glutathione S-transferase (GST) |
| Glutathione (GSH) |
| Superoxide Dismutase (SOD) |
| Dietary Derived | Common Sources |
|---|---|
| Alpha-tocopherol/Vitamin E | Found in nuts, seeds, olives, vegetable oils, and green leafy vegetables |
| Ascorbic acid (AsA)/Vitamin C |
Found in citrus fruits, strawberries, and kiwis |
| Beta-carotene/Vitamin A | Found in green, orange, or red fruits and vegetables such as butternut squash and carrots |
| Beta-cryptoxanthin | High levels found in citrus fruits, peaches, and apricots |
| Lutein | Found in green leafy vegetables, broccoli, brussel sprouts, peas, kiwi, and grapes |
| Lycopene | Found in tomatoes, but also in some other fruits and vegetables |
| Zeaxanthin | Found in green leafy vegetables, corn, seeds, tangerines, persimmons, and egg yolks |
| Zinc | Found in oysters, meats, nuts, legumes, and whole grains |
Note. This table is not a comprehensive list of all antioxidants nor does it list all sources of these dietary antioxidants. (For a more complete discussion of biomarkers of oxidative stress, readers are referred to the following excellent reviews: Cutler & Mattson, 2003; Griffiths et al., 2002; Loft et al., 2008).
Oxidative stress and negative emotional states
Negative emotional states including individual differences in hostility (Carroll et al., 2010; Ohira et al., 2008), depressive symptoms and clinical depression (Forlenza & Miller, 2006; Tsuboi, 2004), and experiencing psychological stress (Epel, Blackburn, & Lin, 2004; Irie, Asami, Nagata, Miyata, & Kasai, 2001; Sivonová et al., 2004) are related to elevations in oxidative stress. Interestingly, several experimental studies report inverse associations between positive affect and oxidative stress, including laughter (Atsumi, 2004), meditation practice (Schneider et al., 1998; Sharma et al., 2008; Sharma et al., 2003), and yogic breathing (Bhattacharya, Pandey, & Verma, 2002). Likewise, a handful of studies have documented decreases in oxidative stress after antidepressant treatment (Bilici, 2001; Cumurcu, Ozyurt, Etikan, Demir, & Karlidag, 2009; Khanzode, Dakhale, Khanzode, Saoji, & Palasodkar, 2003; Ozcan, Gulec, Ozerol, Polat, & Akyol, 2004), suggesting that reducing depressive symptoms also reduces oxidative stress. Paralleling the human studies, there is an extensive animal literature examining numerous indicators of oxidative stress under conditions of mild to extreme stress. Highlights from this literature suggest that when the stress is given repeatedly or for prolonged periods, there is an increase in oxidative damage (Das, Bandyopadhyay, Bhattacharjee, & Banerjee, 1997; Gumuslu, Sarikcioglu, Sahin, Yargicoglu, & Agar, 2002; Sahin & Gumuslu, 2007). This work parallels the findings from Dhabhar and others, which suggest acute episodes of stress may enhance certain aspects of immunity, while prolonged exposure has a significant suppressive effect on efficacy (for a review, see Dhabhar, 2009).
Regarding neuroendocrine mediators, elevations in oxidized lipids after in vivo administration of norepinephrine (Aizawa et al., 2002), or corticosterone (H. Lin, Decuypere, & Buyse, 2004), and exposure of cells to norepinephrine or cortisol resulting in increased oxidants, decreased antioxidant enzyme availability, and increased damage to DNA (Flint, Baum, Chambers, & Jenkins, 2007; McIntosh, Cortopassi, & Sapolsky, 1998; Srivastava et al., 2007). Furthermore, prolonged exposure to neuroendocrine mediators disrupts cellular function and increase cell death via excess oxidative stress (Sapolsky, et al., 2000; Singh, Xiao, Remondino, Sawyer, & Colucci, 2001). In sum, animal models of stress and in vitro studies of intracellular events of stress hormone exposure offer substantial evidence that stress disrupts the normal oxidative balance towards a pro-oxidant environment, ultimately influencing the amount of damage that will need to be repaired.
DNA repair
A recent review of biobehavioral influences in cancer points to research showing that stress hormones may directly alter the microenvironment of tumor cells (Antoni et al., 2006). Our current discussion of restorative processes highlights one pathway through which stress hormones can contribute to changes in the cellular environment that may lead to tumor formation or cancer progression, DNA repair. DNA repair is by nature a restorative process. After insult by damaging agents such as UV light, chemicals, nuclear radiation, or intra- or extra-cellularly derived oxidants has occurred, the cell must be able to repair DNA to avoid mutating and becoming potentially cancerous. Although this restorative process has vast implications for cancer research, few studies have explicitly examined psychosocial influences on DNA repair. Rather than studying repair of existing DNA damage, studies specifically examining the efficacy of DNA repair often observe changes in repair activity or the rate of repair after exposure to external agents that induce damage (e.g., UV light, hydrogen peroxide).
Of the few studies to date that have assessed the impact of psychosocial factors on DNA repair in humans, the findings are mixed. In psychiatric patients, high self-reported distress was related to lower repair capacity compared to low distress patients (Kiecolt-Glaser, Stephens, Lipetz, Speicher, & Glaser, 1985), while among healthy medical students exam stress increased DNA repair capacity (Cohen, Marshall, Cheng, Agarwal, & Wei, 2000; Forlenza, Latimer, & Baum, 2000). Paralleling the human findings, animal research also reports increased and decreased DNA repair, with a short duration restraint stressor in mice increasing expression of DNA repair genes (Flint et al., 2005) while a repeated stressor over 24 hours decreasing repair enzyme activity in rats (Glaser, Thorn, Tarr, Kiecolt-Glaser, & D’Ambrosio, 1985). These findings, although preliminary, suggest that again the duration of exposure to adverse circumstances may influence whether there is an upregulation of repair, when stress is short lived and there is immediate demand for repair, or downregulation when stress is prolonged.
Interestingly, direct exposure to neuroendocrine mediators of the stress response (glucocorticoids and norepinephrine) appear to decrease the rate of repair of exogenously damaged DNA (Flint, et al., 2007), although whether this pathway exists across all cell types and tissue is not clear. Moreover, although DNA repair pathways might be compromised during psychosocial stress, the unrepaired damage does not necessarily lead to cancer, but rather a combination of events are required for cancer to develop (for a review, see Antoni, et al., 2006)
Telomere repair
Chromosomes, which are organized structures formed by our DNA, are capped at the end by telomeres (Blackburn, 2000). Telomere length is thought of as a biomarker of cell aging, such that short telomere length indicates an older cellular age (a more detailed discussion can be found in the Appendix and J. Lin, Epel, & Blackburn, 2008). Using telomere length as a marker of cell age, recent research has identified several psychosocial factors that are correlated with shorter telomere length, including individual psychological stress (Damjanovic et al., 2007; Epel, et al., 2004; Parks et al., 2009), mood disorders (Lung, Chen, & Shu, 2007; Simon et al., 2006), pessimistic disposition (O’Donovan et al., 2009), childhood adversity (Kananen et al., 2010; Kiecolt-Glaser et al., 2011; Tyrka et al., 2010), and indicators of lower socioeconomic position (Carroll, 2010; Cherkas et al., 2006).
Although telomere length in part reflects cell replication history and is shortened more rapidly under conditions of high oxidative stress (See Appendix for further detail), recent evidence suggests other factors influence telomere length, notably telomerase activity (Blackburn, 2000; von Zglinicki, 2002). Telomerase is an enzyme that recaps and elongates shortened telomeres, and appears to be important in preventing, and possibly even reversing, cell senescence (Blackburn, 2000; Jaskelioff et al., 2010). Thus, telomerase activity may be an important restorative process through which psychosocial and behavioral factors operate at the level of DNA. Preliminary evidence suggests lower telomerase activity in immune cells is associated with greater stress burden among younger female caregivers (Epel, et al., 2004), but not among older caregivers (Damjanovic, et al., 2007), lower telomerase in those with higher neuroendocrine activity and cardiovascular reactivity (Epel et al., 2006), and a downregulation of telomerase expression in cells exposed to cortisol (Choi, Fauce, & Effros, 2008). In addition, to date only one intervention reports observing changes in telomerase activity, with an increase observed in the group with a comprehensive lifestyle change involving improved diet, more exercise, and improvements in stress management (Ornish et al., 2008). More recently, higher telomerase activity was observed among subjects after attending a 3-month meditation retreat compared to control subjects; however, there was no pre-intervention measure of telomerase, limiting conclusions as to the efficacy of meditation to activate telomerase (Jacobs et al., 2010 Oct 29).
Summary at the cellular and genomic level
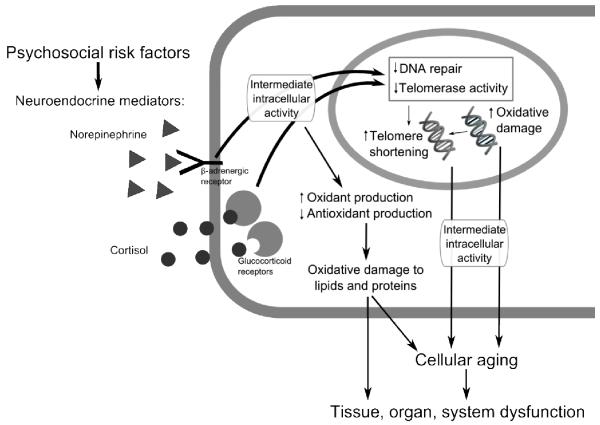
Growing evidence suggests that psychosocial risk factors may influence restorative processes at the cellular level, including changing metabolic activity that increases oxidant load, utilizing available reserves and decreasing antioxidant enzyme expression that decreases defenses against oxidative insults, and altering the rate of biological aging through both increases in oxidative damage and decreases in telomerase repair activity (Figure 2). As mentioned previously, hormones and neurotransmitters mediate relationships between psychosocial factors and restorative processes at the cellular and genomic level are, and of particular relevance are the HPA axis and sympathetic nervous system. Figure 2 highlights how hormones like glucocorticoids and norepinephrine bind to their receptors, which initiates a cascade of multiple intermediate events inside the cell. Many of the intermediate intracellular events are “second messenger” systems which relay signals from receptors to target molecules inside the cell, such as DNA inside the nucleus (Neves, Ram, & Iyengar, 2002). Although the molecular and chemical pathways in these systems are well beyond the scope of this review, many hormones rely on second messenger pathways to alter cellular function. Indeed, second messenger pathways are becoming increasingly studied in biobehavioral research (Bierhaus et al., 2003; Cole et al., 2007; Pace, Hu, & Miller, 2007). The downstream effects of stress hormones binding to their receptors, causing changes to intracellular activity are hypothesized to ultimately contribute to cellular aging and organ dysfunction.
Figure 2.
Summary of restorative processes at the cellular and genomic level
In sum, although several aspects of this cellular environment are associated with psychosocial factors, and mounting animal and in vitro evidence suggests a causal pathway, to date the majority of human research is correlational and limited in its ability to test mediation by behavioral and biological factors. Thus extensive prospective and experimental research is needed to identify the causal pathways in these psychosocially altered cellular restorative processes.
Restorative processes summary
Restorative processes exist at the organism, tissue, cellular, and genomic level and can be disrupted by psychological challenges. Most of the work described above focused on psychological stressors, though some work suggests that individual differences in trait dispositions, such as hostility, may play an important moderating role. Across these processes, external challenges disrupt restorative processes via the mediators that are typically studied in biobehavioral research, including the HPA axis and autonomic nervous system. Importantly, many disruptions in restorative processes occur during steady-state activity, with effects persisting well after the termination of the allostatic response to environmental challenge.
Future directions in understanding restorative processes for social and personality psychology
Restorative processes have received less attention in the broader biobehavioral literature, particularly because of the well-deserved emphasis on allostatic responses to environmental challenges. At the same time, humans spend much of their time not facing challenges – for instance, we spend on average one-third of our lives asleep. Thus, biological activities involved in growth and repair during baseline or steady-state conditions, when individuals are not facing environmental challenges, may be just as important for mental and physical health as the biological activities involved in coping with environmental challenges.
We encourage researchers interested in the intersection between social and personality factors, biology, and health to consider incorporating restorative processes as a potential outcome in future work for several reasons. The factors that promote restorative processes at the organism, tissue, cellular, and genomic level are strongly influenced by individual differences and social psychological processes. Behavioral factors that promote restorative processes include adequate diet (Halliwell, 1996) and exercise (Emery, Kiecolt-Glaser, Glaser, Malarkey, & Frid, 2005). At the level of an individual’s socioemotional functioning, positive emotions and facets of positive functioning likely play a role in promoting restoration (Bower, Low, Moskowitz, Sepah, & Epel, 2007; Pressman & Cohen, 2005). At the group level, animal research suggests that social integration is clearly an important promoter of growth and repair (Detillion, Craft, Glasper, Prendergast, & DeVries, 2004; Glasper & DeVries, 2005).
We should also note that psychological and restorative processes, much like psychological and allostatic processes, influence each other in a bidirectional manner. For example, energy storage is a key function of restorative processes, and the inability to replenish energy stores can have detrimental effects on psychological self-regulation. Gailliot and Baumeister (2007) suggest that blood glucose is a key component of self-control, and demonstrated in experimental studies that attempts at self-control, such as maintaining attention on one stimulus in the face of multiple stimuli, can decrease blood glucose levels. Moreover, self-control attempts predict poorer performance on later self-control tasks and less willingness to help others, which can be restored by increasing blood glucose levels (DeWall, Baumeister, Gailliot, & Maner, 2008; Gailliot et al., 2007). In another example, sleep deprivation can disrupt emotion regulation (assessed through pupillary reactivity to unpleasant pictures) (Franzen, Buysse, Dahl, Thompson, & Siegle, 2009). Interestingly, although sleep disturbances are symptoms of disorders such as depression, sleep disturbances themselves may be risk factors for depression (Weissman, Greenwald, Nino-Murcia, & Dement, 1997), further suggesting a role for sleep in preserving emotion regulation.
A key unanswered question for future research is whether restorative processes can be “enhanced” above normal levels of functioning. Epel and colleagues proposed that although exposure to repeated stressors likely contributes to cumulative damage in many, it may in others induce physical (and psychological) thriving when individuals are able to show rapid psychological and physical adaptation to familiar stressors (e.g., viewing the same stressor as a challenge rather than a threat; rapid decreases in SNS activation following subsequent exposures to the same stressor)(Epel, et al., 1998). Similarly, Dhabhar and colleagues suggest that in the short-term, exposure to certain types of stressors may enhance some arms of the immune response, while repeated exposures may be immunosuppressive (Dhabhar, 2010). Moreover, individual differences in personality, early experience, and learned coping responses may also influence the likelihood of successful adaptation and thriving (Bolger & Zuckerman, 1995; Taylor, Repetti, & Seeman, 1997). These biological changes were described as increasing “physiological resilience” that would not have been present before the occurrence of the stressor.
If physical thriving or enhanced restorative functions were possible, the psychosocial factors that originally impaired functioning could have the opposite effects. For example, rather than slowing the rate of repair, physical thriving may involve either speeding the rate of repair, or increasing the available time for “restorative breaks from stress”(Epel, et al., 1998, p. 310) - allowing more time for repair. However, while the normal ranges for many restorative processes are known, such as insulin sensitivity or sleep parameters, other restorative processes like repair following oxidative stress, telomerase activity, and wound healing do not have known ranges.
For restorative processes, especially those with unknown normal measurement ranges, determining whether psychosocial factors can causally enhance repair and rebuilding will necessitate laboratory-based experimental manipulations and intervention studies. The physical thriving framework suggests relaxation could provide the necessary “restorative break” and increase activity of allostatic mediators that promote growth and repair, namely the parasympathetic nervous system (Epel, et al., 1998). Moreover, given that the integrity of materials may be an important component to restoring function, comprehensive lifestyle interventions that incorporate diet, nutrition, exercise, and coping may be another potential set of interventions that could enhance restoration (Ornish et al., 2008). At the same time, given that diet, nutrition, and exercise play important roles across various restorative processes, controlling for those factors and/or examining such behavioral factors as mediators will be important directions for future work.
Enhanced restorative processes and physical thriving fit with the larger concept of resilience, a person’s capacity to recover from and continue in the face of adversity (Zautra, 2005). Clearly resilience operates at multiple levels, from optimistic personalities to optimal telomerase activity. While much of the emphasis in biobehavioral research on resilience involves measuring processes that help maintain survival by initiating biological changes, humans also spend significant time and energy rebounding from those changes. Restorative processes are yet another avenue through which the mind communicates with the body to aid in the struggle against entropy.
Acknowledgments
This research was supported by Research Grant 9333 from the William T. Grant Foundation and NIH grant R21-AG032494 to the first author, and training grant T32-MH19925 and the Cousins Center for Psychoneuroimmunology. We would like to thank Teresa Seeman and Donald Lamkin for their helpful comments on earlier versions of this manuscript.
Appendix
A brief primer on cell biology and metabolism relevant to restorative processes
Technological advances in the biological sciences are allowing biobehavioral scientists to explore biological processes at increasingly finer levels of analysis, particularly those that take place within cells. In this Appendix we briefly review some basic concepts in cell biology that become important for understanding both allostatic and restorative processes.
Cell structure, metabolism, and oxidative stress
All tissues in the body are composed of cells, which contain many different structures and molecules with multiple functions, including organelles, proteins, and DNA. The DNA within the nucleus of a cell is a repeat sequence of nucleic acid, which is wound into large structures called chromosomes. DNA is the primary template for the creation of specialized proteins that conduct normal cellular activities, including energy metabolism.
Energy metabolism involves chemical reactions that support life, and are usually divided into two processes: Anabolic and catabolic. At the cellular level, anabolic processes involve the building of larger, complex molecules out of smaller ones, such as storing glucose (a primary source of energy) in the form of glycogen. Catabolic processes refer to breaking down larger, complex molecules into smaller, simpler ones to release energy for the organism’s use, such as breaking down glucose to release energy for use by a cell. Just like burning fossil fuels provides energy but also creates harmful pollutants, normal catabolic processes within a cell create cellular “pollutants” including reactive oxygen species (ROS) and reactive nitrogen species (RNS), which can damage the basic building blocks of the cell (DNA, proteins, lipids). Although antioxidants are intended to prevent excess oxidative damage within the cell, oxidants often cause damage, with accumulation of this damage ultimately interfering with normal cellular functions and causing oxidative stress. Numerous biomarkers are used to determine the extent of oxidative stress in biological samples including measuring oxidant damage to DNA (i.e. 8-OHdG, comet assay), lipids, LDL cholesterol (i.e. F2-isoprostanes, MDA, oxidized LDL), or proteins (carbonyls). Oxidative stress can also be measured by assessing tissue or circulating levels of oxidants or antioxidants (i.e. beta-carotene, ascorbic acid, vitamin E, superoxide dismutase (SOD), glutathione peroxidase (GPx), etc.), or by examining the capacity of tissue or serum samples to reduce a known amount of oxidant to a harmless state (total antioxidant capacity). Readers interested in a more thorough understanding of the numerous markers of oxidative stress are encouraged to read reviews by Griffiths et al., 2002, Cutler & Mattson, 2003, and Loft et al., 2008.
Cell replication, telomeres, and telomerase
Every tissue of the body is made up of cells with specific functions for that environment. Cells that sustain damage from excess demands and oxidative insults and cannot be repaired (e.g. restored) will stop functioning properly and often die, protecting against cancerous growth. Here, new cells are produced to replace non-functional or dead cells through a process of renewal; one way the body replaces dead or non-functional cells is for healthy cells to divide. When cells divide, the cell must replicate its genome in order to create identical daughter cells with identical DNA. With every cell division and genome replication, a small piece at the ends of each chromosome is lost. To prevent this loss from causing damage to DNA that is involved in the building of proteins, a majority of cells contain a protective, repeat sequence of DNA that “caps” the end of each chromosome known as a telomere (Blackburn, 2000). The telomere protects the vital genetic material from deteriorating during cell division. Telomeres themselves shorten over time with each successive cell division, and eventually reach a critical length that signals the cell to stop dividing, a point known as cellular senescence. While the cell does not always die, its function is altered. Thus, “older” cells have shorter telomere length and increased likelihood of entering senescence (an aged state). Over time, if the population of cells in a particular tissue shifts towards greater senescent cell ratios, the overall health of the system and specific tissues diminishes, as a decrease in the ability to replicate and renew non-functional or dead cells ultimately affects the efficacy of that tissue or cell population to perform its functions (i.e., immunosenescence associated with compromised immunity).
Interestingly, oxidative stress can accelerate telomere erosion and cause a more rapid cellular aging (von Zglinicki, 2002), and also contributes to cellular senescence through mechanisms that do not involve activation of cellular arrest via critically short telomeres - when accumulated oxidative damage is too high (Sikora et al., 2010). As the telomeric region of the chromosome is more susceptible to damage from oxidative insults, some suspect that oxidative damage may be a larger source of telomere erosion than replicative history, tracking the oxidative history of the cell (Monaghan, 2010; von Zglinicki, 2002). As we discuss in the article, telomerase is an enzyme that lengthens the telomere and helps in maintaining the cap on the end of the chromosome. Telomerase is active in cells that undergo frequent division, including cells of the immune system, skin, intestinal lining, and hair follicles. More active telomerase is thought to lengthen the life of the cell and ultimately slow the rate of biological aging (Blackburn, 2000; Lin, Epel & Blackburn, 2008). Although still unclear, several lines of research hypothesize that increases in telomerase may ultimately lengthen human lifespan, renewing interest in a “fountain of youth”. One recent finding documents the renewal potential of telomerase using a mouse model of telomerase insufficiency. Here, tissue degeneration as the result of aging cells was given renewed life upon exposure to telomerase, with reversals in neuronal degeneration and organ dysfunction (e.g. intestinal, spleen, and testicular functions) (Jaskelioff et al., 2011).
Metabolism at the organism level
Anabolic and catabolic processes happen at the level of the cells, tissues, and the entire organism. To orchestrate metabolism across the whole organism, we produce hormones that regulate anabolic and catabolic processes. One of the central anabolic, growth-promoting hormones is growth hormone, the primary determinant of body size in children. Growth hormone acts through its actions on other growth-related hormones and peptides, most notably insulin-like growth factor (Butler & Le Roith, 2001). In combination with insulin-like growth factors, growth hormone induces the production of proteins and uptake of amino acids (the essential building blocks for all cells) in muscle. Another key anabolic hormone is insulin, which stimulates cells in the body to capture glucose outside the cell and store it in the form of glycogen. Catabolic hormones, which stimulate energy expenditure, include hormones produced by the sympathetic nervous system (epinephrine, norepinephrine) and other hormones involved in energy release (cortisol, glucagon) (Epel et al., 1998).
Contributor Information
Theodore F. Robles, Department of Psychology, University of California, Los Angeles
Judith E. Carroll, Carroll, Cousins Center for Psychoneuroimmunology, Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles.
References
- Aizawa T, Ishizaka N, Usui SI, Ohashi N, Ohno M, Nagai R. Angiotensin II and catecholamines increase plasma levels of 8-epi-prostaglandin F(2alpha) with different pressor dependencies in rats. Hypertension. 2002;39:149–154. doi: 10.1161/hy1201.097301. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nature Reviews Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. Neuroimmunology of stress: skin takes center stage. Journal of Investigative Dermatology. 2006;126:1697–1704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi T. Pleasant feeling from watching a comical video enhances free radical-scavenging capacity in human whole saliva. Journal of Psychosomatic Research. 2004;56:377–379. doi: 10.1016/S0022-3999(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Pandey US, Verma NS. Improvement in oxidative status with yogic breathing in young healthy males. Indian Journal of Physiology and Pharmacology. 2002;46:349–354. [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilici M. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. Journal of Affective Disorders. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Bolger N, Zuckerman A. A framework for studying personality in the stress process. Journal of Personality and Social Psychology. 1995;69:890–902. doi: 10.1037//0022-3514.69.5.890. [DOI] [PubMed] [Google Scholar]
- Bower JE, Low CA, Moskowitz JT, Sepah S, Epel ES. Benefit finding and physical health: Positive psychological changes and enhanced allostasis. Social and Personality Psychology Compass. 2007;2:223–244. [Google Scholar]
- Butler AA, Le Roith D. Control of growth by the somatropic axis: Growth hormone and the insulin-like growth factors have related and independent roles. Annual Review of Physiology. 2001;63:141–164. doi: 10.1146/annurev.physiol.63.1.141. [DOI] [PubMed] [Google Scholar]
- Carroll JE. The association of affective, behavioral, and cognitive components of hostility with telomere length, a marker of biological aging. Doctoral dissertation, University of Pittsburgh; Pittsburgh, PA: 2010. [Google Scholar]
- Carroll JE, Marsland AL, Jenkins F, Baum A, Muldoon MF, Manuck SB. A urinary marker of oxidative stress covaries positively with hostility among midlife community volunteers. Psychosomatic Medicine. 2010;72:273–280. doi: 10.1097/PSY.0b013e3181d0d72b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring, Md.) 2007;15:253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JPS, G. L, Kimura M. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain, Behavior, and Immunity. 2008;22:600–605. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2006;13:337–346. doi: 10.1159/000104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biology. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Underwood Gordon L, editors. Measuring stress: A guide for health and social scientists. Oxford; New York: 1995. [Google Scholar]
- Cohen L, Marshall GD, Cheng L, Agarwal SK, Wei Q. DNA repair capacity in healthy medical students during and after exam stress. Journal of Behavioral Medicine. 2000;23:531–544. doi: 10.1023/a:1005503502992. [DOI] [PubMed] [Google Scholar]
- Collings JL. Acid-base disorders. In: Marx JA, Hockenberger RS, Walls RM, Adams JG, Barsan WG, Biros MH, Danzl DF, Gausche-Hill M, Ling J, Newton EJ, editors. Rosen’s emergency medicine. 7th ed Vol. 2. Mosby Elsevier; Philadelphia: 2010. [Google Scholar]
- Cumurcu BE, Ozyurt H, Etikan I, Demir S, Karlidag R. Total antioxidant capacity and total oxidant status in patients with major depression: Impact of antidepressant treatment. Psychiatry & Clinical Neuroscience. 2009;63:639–645. doi: 10.1111/j.1440-1819.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Mattson MP. Measuring oxidative stress and interpreting its clinical relevance for humans. In: Cutler RG, Rodriguez H, editors. Critical Reviews of Oxidative Stress and Aging: Advances in Basic Science, Diagnostics and Intervention. World Scientific Publishing Co.; Singapore: 2003. pp. 131–164. [Google Scholar]
- Damjanovic A, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimers disease patients. Journal of Immunology. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK. Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radical Biology and Medicine. 1997;23:8–18. doi: 10.1016/s0891-5849(96)00547-3. [DOI] [PubMed] [Google Scholar]
- Detillion CE, Craft TK, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Baumeister RF, Gailliot MT, Maner JK. Depletion makes the heart grow less helpful: Helping as a function of self-regulatory energy and genetic relatedness. Personality and Social Psychology Bulletin. 2008;34:1653–1662. doi: 10.1177/0146167208323981. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrecht M, Hextall J, Kirtley L, Taylor A, Dyson M, Weinman J. Perceived stress and cortisol levels predict speed of wound healing in healthy male adults. Psychoneuroendocrinology. 2004;29:798–809. doi: 10.1016/S0306-4530(03)00144-6. [DOI] [PubMed] [Google Scholar]
- Elias PM. Stratum corneum defensive functions: An integrated view. Journal of Investigative Dermatology. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- Emery CF, Kiecolt-Glaser JK, Glaser R, Malarkey WB, Frid DJ. Exercise accelerates wound healing among healthy older adults: A preliminary investigation. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2005;60(11):1432–1436. doi: 10.1093/gerona/60.11.1432. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn E, Lin J. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Science. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Epel ES, McEwen BS, Ickovics JR. Embodying psychological thriving: Physical thriving in response to stress. Journal of Social Issues. 1998;54:301–322. [Google Scholar]
- Finkel T, Holbrook NJ. Biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Flint MS, Baum A, Chambers WH, Jenkins FJ. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology. 2007;32:470–479. doi: 10.1016/j.psyneuen.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Flint MS, Carroll JE, Jenkins FJ, Chambers WH, Han ML, Baum A. Genomic profiling of restraint stress-induced alterations in mouse T lymphocytes. Journal of Neuroimmunology. 2005;167:34–44. doi: 10.1016/j.jneuroim.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Forlenza MJ, Latimer JJ, Baum A. The effects of stress on DNA repair capacity. Psychology and Health. 2000;15:881–891. doi: 10.1080/08870440008405589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosomatic Medicine. 2006;68:1–7. doi: 10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ, Dahl RE, Thompson W, Siegle GJ. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biological Psychology. 2009;80:300–305. doi: 10.1016/j.biopsycho.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailliot MT, Baumeister RF. The physiology of willpower: Linking blood glucose to self-control. Personality and Social Psychology Review. 2007;11:303–327. doi: 10.1177/1088868307303030. [DOI] [PubMed] [Google Scholar]
- Gailliot MT, Baumeister RF, DeWall CN, Maner JK, Plant EA, Tice DM. Self-control relies on glucose as a limited energy source: Willpower is more than a metaphor. Journal of Personality and Social Psychology. 2007;92:325–336. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- Garg A, Chren M, Sands LP, Matsui MS, Marenus KD, Feingold KR. Psychological stress perturbs epidermal permeability barrier homeostasis. Archives of Dermatology. 2001;137:53–59. doi: 10.1001/archderm.137.1.53. [DOI] [PubMed] [Google Scholar]
- Glaser R, Rabin B, Chesney M, Cohen S, Natelson B. Stress-induced immunomodulation: implications for infectious diseases? JAMA: The Journal of the American Medical Association. 1999;281(24):2268–2270. doi: 10.1001/jama.281.24.2268. [DOI] [PubMed] [Google Scholar]
- Glaser R, Thorn BE, Tarr KL, Kiecolt-Glaser JK, D’Ambrosio SM. Effects of stress on methyltransferase synthesis: an important DNA repair enzyme. Health Psychology. 1985;4:403–412. doi: 10.1037//0278-6133.4.5.403. [DOI] [PubMed] [Google Scholar]
- Glasper ER, DeVries AC. Social structure influences effects of pair-housing on wound healing. Brain, Behavior, and Immunity. 2005;19:61–68. doi: 10.1016/j.bbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Kiecolt-Glaser JK, Malarkey WB, Glaser R. The influence of anger expression on wound healing. Brain, Behavior, and Immunity. 2008;22:699–708. doi: 10.1016/j.bbi.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EK, Watson D. General and specific traits of personality and their relation to sleep and academic performance. Journal of Personality. 2002;70:177–206. doi: 10.1111/1467-6494.05002. [DOI] [PubMed] [Google Scholar]
- Griffiths HR, Møller L, Bartosz G, Bast A, Bertoni-Freddari C, Collins A. Biomarkers. Molecular Aspects of Medicine. 2002;23:101–208. doi: 10.1016/s0098-2997(02)00017-1. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Luthringer R, Follenius M, Schaltenbrand N, Macher JP, Muzet A. A quantitative evaluation of the relationships between growth hormone secretion and delta wave electroencephalographic activity during normal sleep and after enrichment in delta waves. Sleep. 1996;19:817–824. doi: 10.1093/sleep/19.10.817. [DOI] [PubMed] [Google Scholar]
- Gumuslu S, Sarikcioglu SB, Sahin E, Yargicoglu P, Agar A. Influences of different stress models on the antioxidant status and lipid peroxidation in rat erythrocytes. Free Radical Research. 2002;36:1277–1282. doi: 10.1080/1071576021000016508. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Antioxidants in human health and disease. Annual Review of Nutrition. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- Heijboer AC, Pilj H, Van den Hoek AM, Havekes LM, Romijn JA, Corssmit EPM. Gut-brain axis: Regulation of glucose metabolism. Journal of Neuroendocrinology. 2006;18:883–894. doi: 10.1111/j.1365-2826.2006.01492.x. [DOI] [PubMed] [Google Scholar]
- Irie M, Asami S, Nagata S, Miyata M, Kasai H. Relationships between perceived workload, stress and oxidative DNA damage. International Archives of Occupational and Environmental Health. 2001;74:153–157. doi: 10.1007/s004200000209. [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB Journal. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell D. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2010 Oct 29; doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2010;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvisaari J, Lönnqvist J, Peltonen L. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PloS one. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: Inhibition of epidermal lipid synthesis accounts for functional abnormalities. Journal of Investigative Dermatology. 2003;120:456–464. doi: 10.1046/j.1523-1747.2003.12053.x. [DOI] [PubMed] [Google Scholar]
- Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Report. 2003;8:365–370. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK. Stress, food, and inflammation: Psychoneuroimmunology and nutrition at the cutting edge. Psychosomatic Medicine. 2010;72:365–369. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Stephens RE, Lipetz PD, Speicher CE, Glaser R. Distress and DNA repair in human lymphocytes. Journal of Behavioral Medicine. 1985;8:311–320. doi: 10.1007/BF00848366. [DOI] [PubMed] [Google Scholar]
- Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. Saunders; New York: 2005. [Google Scholar]
- Lin H, Decuypere E, Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus) Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 2004;139:737–744. doi: 10.1016/j.cbpc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lin J, Epel ES, Blackburn EH. Telomeres, telomerase, stress, and aging. In: Berntson GG, Cacioppo JT, editors. Handbook of neuroscience for the behavioral sciences. Wiley; Hoboken, NJ: 2008. [Google Scholar]
- Loft S, Danielsen PH, Mikkelsen L, Risom L, Forchhammer L, Møller P. Biomarkers of oxidative damage to DNA and repair. Biochemical Society Transactions. 2008;36:1071–1076. doi: 10.1042/BST0361071. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Gerin W. Psychophysiological reactivity: Mechanisms and pathways to cardiovascular disease. Psychosomatic Medicine. 2003;65:36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- Lung FW, Chen NC, Shu BC. Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatric Genetics. 2007;17:195–199. doi: 10.1097/YPG.0b013e32808374f6. [DOI] [PubMed] [Google Scholar]
- Marucha PT, Kiecolt-Glaser JK, Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosomatic Medicine. 1998;60:362–365. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;388:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McIntosh LJ, Cortopassi KM, Sapolsky RM. Glucocorticoids may alter antioxidant enzyme capacity in the brain: kainic acid studies. Brain Research. 1998;791:215–222. doi: 10.1016/s0006-8993(98)00104-8. [DOI] [PubMed] [Google Scholar]
- Merriam-Webster (n. d.). Restore, from http://www.merriam-webster.com/dictionary/restore.
- Miller GE, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JMG, Doll R. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-[kappa]B signaling. Biological Psychiatry. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Practices and Research Clinical Endocrinology and MEtabolism. 2010;24:687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G-protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Lin J, Dhabhar FS, Wolkowitz O, Tillie JM, Blackburn E. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain, Behavior, and Immunity. 2009;23:446–449. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira T, Hozawa A, Iribarren C, Daviglus ML, Matthews KA, Gross MD. Longitudinal association of serum carotenoids and tocopherols with hostility: the CARDIA Study. American Journal of Epidemiology. 2008;167:42–50. doi: 10.1093/aje/kwm267. [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncology. 2008;9:1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. International Clinical Psychopharmacology. 2004;19:89–95. doi: 10.1097/00004850-200403000-00006. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain, Behavior, and Immunity. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain, Behavior, and Immunity. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiology, Biomarkers, & Prevention. 2009;18:551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131(6):925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A. Current perspectives on the function of sleep. Perspectives in Biology and Medicine. 1998;41:359–390. doi: 10.1353/pbm.1998.0051. [DOI] [PubMed] [Google Scholar]
- Robles TF. Stress, social support, and delayed skin barrier recovery. Psychosomatic Medicine. 2007;69:807–815. doi: 10.1097/PSY.0b013e318157b12e. [DOI] [PubMed] [Google Scholar]
- Robles TF, Brooks KP, Pressman SD. Trait positive affect buffers the effects of acute stress on skin barrier recovery. Health Psychology. 2009;28:373–378. doi: 10.1037/a0014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Gumuslu S. Immobilization stress in rat tissues: alterations in protein oxidation, lipid peroxidation and antioxidant defense system. Comparative Biochemistry and Physiology: Toxicology & Pharmacology. 2007;144:342–347. doi: 10.1016/j.cbpc.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero M, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schneider RH, Nidich SI, Salerno JW, Sharma HM, Robinson CE, Nidich RJ. Lower lipid peroxide levels in practitioners of the Transcendental Meditation program. Psychosomatic Medicine. 1998;60:38–41. doi: 10.1097/00006842-199801000-00008. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JH, Horwitz RI, McEwen BS. Price of adaptation - allostatic load and its health consequences: MacArthur Studies of Successful Aging. Archives of Internal Medicine. 1997;157:2259–2268. [PubMed] [Google Scholar]
- Sharma H, Datta P, Singh A, Sen S, Bhardwaj NK, Kochupillai V. Gene expression profiling in practitioners of Sudarshan Kriya. Journal of Psychosomatic Research. 2008;64:213–218. doi: 10.1016/j.jpsychores.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Sharma H, Sen S, Singh A, Bhardwaj NK, Kochupillai V, Singh N. Sudarshan Kriya practitioners exhibit better antioxidant status and lower blood lactate levels. Biological Psychology. 2003;63:281–291. doi: 10.1016/s0301-0511(03)00071-1. [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biological Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RAF. Cutaneous wound healing. New England Journal of Medicine. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS. Adrenergic regulation of cardiac myocyte apoptosis. Journal of Cellular Physiology. 2001;189:257–265. doi: 10.1002/jcp.10024. [DOI] [PubMed] [Google Scholar]
- Sivonová M, Zitnanová I, Hlincíková L, Skodácek I, Trebatická J, Duracková Z. Oxidative stress in university students during examinations. Stress. 2004;7:183–188. doi: 10.1080/10253890400012685. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocrine Reviews. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Souza BR, Cardoso JF, Amadeu TP, Desmouliere A, Costa AM. Sympathetic denervation accelerates wound contraction but delays reepithelialization in rats. Wound Repair and Regeneration. 2005;13:498–505. doi: 10.1111/j.1067-1927.2005.00070.x. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Penev P, Van Cauter E. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of Internal Medicine. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Chandrasekar B, Gu Y, Luo J, Hamid T, Hill BG. Downregulation of CuZn-superoxide dismutase contributes to beta-adrenergic receptor-mediated oxidative stress in the heart. Cardiovascular Research. 2007;74:445–455. doi: 10.1016/j.cardiores.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. Burden of psychosocial adversity and vulnerability in middle age: associations with biobehavioral risk factors and quality of life. Psychosomatic Medicine. 2003;65:1029–1103. doi: 10.1097/01.psy.0000097347.57237.2d. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of life stress, cognition, and health. John Wiley & Sons; New York: 1988. pp. 629–649. [Google Scholar]
- Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Repetti RL, Seeman TE. Health psychology: What is an unhealthy environment and how does it get under the skin? Annual Review of Psychology. 1997;43:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- Troxel WM, Robles TF, Hall M, Buysse DJ. Marital quality and the marital bed: Examining the covariation between relationship quality and sleep. Sleep Medicine Reviews. 2007;11:389–404. doi: 10.1016/j.smrv.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi H. Depressive symptoms are independently correlated with lipid peroxidation in a female population: Comparison with vitamins and carotenoids. Journal of Psychosomatic Research. 2004;56:53–58. doi: 10.1016/S0022-3999(03)00567-1. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: Preliminary support for an effect of early stress on cellular aging. Biological Psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Copinschi G. Interrelationships between growth hormone and sleep. Growth Hormone and IGF Research. 2000;10(Supplement B):S57–62. doi: 10.1016/s1096-6374(00)80011-8. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends in Biochemical Sciences. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Walburn J, Vedhara K, Hankins M, Rixon L, Weinman J. Psychological stress and wound healing in humans: a systematic review and meta-analysis. Journal of Psychosomatic Research. 2009;67:253–271. doi: 10.1016/j.jpsychores.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. General Hospital Psychiatry. 1997;19:245–250. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- Yang EV, Bane CM, MacCallum RC, Kiecolt-Glaser JK, Glaser R. Stress-related modulation of matrix metalloproteinase expression. Journal of Neuroimmunology. 2002;133:144–150. doi: 10.1016/s0165-5728(02)00270-9. [DOI] [PubMed] [Google Scholar]
- Zautra AJ. Emotions, stress, and health. Oxford University Press; New York: 2005. [Google Scholar]
- Zepelin H, Siegel JM, Tobler I. Mammalian sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. 5th ed Saunders; New York: 2005. pp. 91–100. [Google Scholar]