Abstract
Insulin resistance has been associated with the accumulation of fat within skeletal muscle fibers as intramyocellular lipid (IMCL). Here, we have examined in a cross-sectional study the interrelationships among IMCL, insulin sensitivity, and adiposity in European Americans (EAs) and African Americans (AAs). In 43 EA and 43 AA subjects, we measured soleus IMCL content with proton-magnetic resonance spectroscopy, insulin sensitivity with hyperinsulinemic–euglycemic clamp, and body composition with dual-energy X-ray absorptiometry. The AA and EA subgroups had similar IMCL content, insulin sensitivity, and percent fat, but only in EA was IMCL correlated with insulin sensitivity (r = −0.47, P < 0.01), BMI (r = 0.56, P < 0.01), percent fat (r = 0.35, P < 0.05), trunk fat (r = 0.47, P < 0.01), leg fat (r = 0.40, P < 0.05), and waist and hip circumferences (r = 0.54 and 0.55, respectively, P < 0.01). In a multiple regression model including IMCL, race, and a race by IMCL interaction, the interaction was found to be a significant predictor (t = 1.69, DF = 1, P = 0.0422). IMCL is related to insulin sensitivity and adiposity in EA but not in AA, suggesting that IMCL may not function as a pathophysiological factor in individuals of African descent. These results highlight ethnic differences in the determinants of insulin sensitivity and in the pathogenesis of the metabolic syndrome trait cluster.
INTRODUCTION
The prevalence of type 2 diabetes is increasing globally at an alarming rate (1), and this problem is particularly severe in populations of African descent. Results from the Atherosclerosis Risk in Communities (ARIC) study of over 10,000 Americans reveal that African American (AA) women and men have a 2.4-fold and 1.5-fold greater incidence of type 2 diabetes than their European-American (EA) counterparts (2), which is only partially related to the increased prevalence of obesity seen in AA, suggesting that other factors play a role (3).
Insulin resistance, a central pathophysiological feature in the initiation and progression toward type 2 diabetes, is associated with obesity; however, general adiposity can explain only a small percentage of the interindividual variability in insulin sensitivity observed in nondiabetic individuals (4). Total body fat distribution or lipid accumulation within muscle or hepatic cells can exist independently of generalized adiposity and can also contribute to insulin resistance (5–7). Several studies have found that insulin sensitivity is negatively associated with adipose stores in the abdominal region, particularly visceral adipose tissue (VAT), and this is consistent across age and ethnicity (8,9). Surprisingly, however, AA adults have significantly lower levels of VAT than their EA counterparts, despite having a higher prevalence of insulin resistance and type 2 diabetes. Studies of ethnic differences in fat distribution show that, even when controlling for total fat and BMI, AA women have significantly less VAT than EA women (10), which persists even after significant weight gain (11) and weight loss (12). Furthermore, AA youth carry less VAT than their EA peers, indicating that this difference is independent of age (13).
Similarly, both AA adolescents (13) and adults (14) have very little hepatic fat compared to EA. AA adults also have a substantially lower prevalence of nonalcoholic fatty liver disease than EA (15). Thus, neither visceral nor hepatic fat depots can explain the higher prevalence of insulin resistance observed in the AA population. However, it is possible that fat depots other than VAT and hepatic fat contribute to insulin resistance and greater type 2 diabetes prevalence among AA relative to EA.
Several studies have reported an inverse relationship between intramyocellular lipid (IMCL) and insulin sensitivity in inactive individuals, independent of measures of general adiposity in both animal (16) and human models (17). Furthermore, IMCL is elevated in patients with both type 1 and type 2 diabetes (18), as well as in populations that are prone to developing type 2 diabetes, such as Pima Indians (19) and offspring of parents with type 2 diabetes (6,18). Moreover, the improvement in insulin sensitivity observed after a short-term hypocaloric diet was explained by a decrease in IMCL rather than by changes in total adiposity in both nondiabetic patients and patients with type 2 diabetes (4). Similar improvements in insulin sensitivity were observed in parallel with IMCL depletion in morbidly obese subjects after surgical treatment of obesity (20). These findings highlight the importance of IMCL as a metabolically active fat depot that influences glucose metabolism independently of total body adiposity. The objective of this study was to determine whether racial differences exist in the relationships linking IMCL, insulin sensitivity, and body composition, including measurements of total and regional adiposity.
METHODS AND PROCEDURES
Subject characteristics
The study subjects were recruited from advertisements and word-of-mouth referrals. Those meeting inclusion requirements and providing informed consent were sequentially enrolled and studied. However, an effort was made to have equal enrollment of European- and African-Americans such that only AAs were entered into the study after the full complement of EAs had been recruited. The final study group comprised 86 volunteers (43 EA and 43 AA) with ages between 21 and 59 years.
The clinical characteristics of the study group are listed in Table 1. Subjects were admitted to a metabolic ward, the Participant and Clinical Interaction Resources of the University of Alabama at Birmingham (UAB) Center for Clinical and Translational Science, where they received a eucaloric diet during the length of stay (3 days) comprising 20% protein, 30% fat, and 50% carbohydrate calories. Weight was stable (±3%) for at least 3 months before study, BMI was between 21 and 46, and none of the study subjects engaged in regular exercise. None of the volunteers had cardiovascular, renal, or hepatic disease, and all were chemically euthyroid. No subjects were ingesting pharmacological agents known to affect carbohydrate homeostasis, lipids, or body composition. Race was determined by self-report. Protocols were approved by the institutional review board, and written informed consent was obtained from every subject. Subjects underwent evaluation as described below.
Table 1.
Body composition and metabolic characteristics of study subjects by race
| Variable | EA adults
|
AA adults
|
P value |
|---|---|---|---|
| N = 43 (14 M/29 F) | N = 43 (14 M/29 F) | ||
| Age (years) | 39.0 ± 11 (20–60) | 37.6 ± 10 (22–56) | 0.64 |
| Weight (kg) | 83.8 ± 16 (61–136) | 90.5 ± 17 (65–132) | 0.06 |
| BMI (kg/m2)a | 29.3 ± 5.8 (21–46) | 31.8 ± 5.2 (22–43) | 0.04 |
| % Body fat (DXA)b | 38.8 ± 12 (7–55) | 39.9 ± 9.4 (16–53) | 0.64 |
| Trunk fat (kg)b | 16.8 ± 7.0 (4.2–36) | 18.2 ± 7.1 (6.3–41) | 0.35 |
| Leg fat (kg)b | 5.9 ± 2.6 (1.4–13) | 6.90 ± 2.6 (2.3–14) | 0.08 |
| Trunk/leg ratiob | 2.94 ± 0.7 (1.5–5.2) | 2.74 ± 0.6 (1.3–4.0) | 0.17 |
| Waist (cm)b | 93.5 ± 13 (74–131) | 98.1 ± 12 (75–122) | 0.10 |
| Hip (cm)b | 108 ± 13 (88–149) | 111 ± 9.6 (94–134) | 0.25 |
| Waist/hip ratiob | 0.86 ± 0.1 (0.7–1.0) | 0.88 ± 0.1 (0.7–1.1) | 0.28 |
| GDR (mg/kgLBM/min)c | 14.3 ± 4.2 (7.2–22) | 13.9 ± 3.3 (5.6–21) | 0.67 |
| Fasting insulin (μU/ml)d | 16.2 ± 14 (4.1–87) | 16.4 ± 12 (5.3–53) | 0.96 |
| Fasting glucose (mg/dl)e | 93.9 ± 8.4 (78–117) | 92.3 ± 10 (76–113) | 0.43 |
| IMCL (AU) | 2.39 ± 1.8 (0.2–7.1) | 2.61 ± 1.7 (0.4–7.1) | 0.57 |
Results are mean ± s.d. (range). N = 43 EA and 43 AA, unless specified otherwise. AA, African American; AU, arbitrary units; DXA, dual-energy X-ray absorptiometry; EA, European American; GDR, maximally stimulated glucose disposal rate; IMCL, intramyocellular lipid content.
Difference between EA and AA, P < 0.05.
N = 42 EA, 42 AA.
N = 39 EA, 38 AA.
N = 35 EA, 33 AA.
N = 43 EA, 41 AA.
Insulin sensitivity
In vivo insulin sensitivity was assessed using the euglycemic–hyperinsulinemic glucose clamp technique at a maximally effective steady-state serum insulin concentration as previously described (21). Briefly, after a 12-h fast, a catheter was inserted into the brachial vein to administer insulin, glucose, and KPO4. A dorsal hand vein was cannulated in a retrograde manner and kept in a warming device (65 °C) to provide arterialized venous blood for sampling. To maximally stimulate skeletal muscle glucose uptake and suppress hepatic glucose production, we administered regular insulin (Humulin; Eli Lilly, Indianapolis, IN) at a rate of 200 mU·m−2·min−1, producing a mean steady-state insulin concentration of 3,480 ± 138 pmol/l, which is maximally effective for suppressing hepatic glucose production and reflects maximally stimulated skeletal muscle glucose uptake (22). Serum glucose was clamped at 5.0 mmol/l for at least 3 h, and maximal glucose uptake for each individual was calculated from the mean glucose infusion rate over the final three 20-min interval. Whole-body glucose uptake was calculated based on the glucose infusion rate corrected for changes in the glucose pool size, assuming a distribution volume of 19% body weight and a pool fraction of 0.65. Glucose uptake was normalized per kilogram lean body mass (excluding bone mass) determined by dual-energy X-ray absorptiometry scanning to yield the glucose disposal rate per kilogram of lean body mass (GDR). Lower GDR values indicate greater insulin resistance. Of the 86 subjects tested, nine subjects (five AA and four EA females) were missing GDR data because of mechanical difficulties with intravenous filtration, difficulties with blood draw, or patient request to discontinue test.
IMCL
IMCL was quantified using 1H magnetic resonance spectroscopy. 1H magnetic resonance spectroscopy is a reliable and noninvasive technique for measuring IMCL (23). It correlates with fluorescence microscopy of Oil Red O staining, however, it does not provide information about lipid compartmentalization within the muscle cell (24).
All subjects were studied on a Philips 3T system (Philips Medical Systems, Best, The Netherlands). IMCL was measured using a point-resolved spectroscopy single voxel acquisition sequence with a commercially provided 1H transmit/receive torso phased-array coil (Philips Medical Systems). Legs were positioned inside this commercial torso coil with knees extended and ankles secured in a neutral position. The water-suppressed point-resolved spectroscopy voxels (1 cm × 1 cm × 1 cm voxel size) were positioned in the soleus muscle in areas that avoid fascia, vascular structures, and gross marbling. All point-resolved spectroscopy acquisitions utilized the following parameters to collect the IMCL data: repetition time = 2000 ms, echo time = 40 ms, and 128 signal averages. Separate non-water–suppressed spectra were also collected with the same repetition time/echo time but only 16 signal averages as an amplitude reference. All IMCL and internal water spectra were corrected for T1 and T2 relaxation as well as normalized for point-resolved spectroscopy voxel sizes and number of signal averages.
Spectra were analyzed by fitting the peak positions and areas through time domain fitting using Java-Based Magnetic Resonance User Interface (jMRUI) (25). IMCL content in the soleus spectra was fit using previously published fitting models and sets of prior knowledge information (23). All peak areas in this study are expressed in arbitrary units/pixel area relative to internal water. Although we cannot absolutely quantify our lipid measurements with these methods, the use of the internal water as an intensity reference allow us to determine differences in lipid contents among subjects.
Anthropometric and body composition measurements
BMI was calculated as body weight in kilograms divided by the square of height in meters (kg/m2). Fat distribution was assessed by waist and hip circumferences (cm) using a tension-controlled tape measure by Novel Products (Rockton, IL). Dual-energy X-ray absorptiometry scanning was performed using Prodigy (GE Medical Systems LUNAR, Madison, WI) with the use of software version 6.10.029 (enCORE 2002) and provided body composition measures including total body fat, trunk fat, leg fat, percentage of body fat, and lean body mass independent of bone mass.
Statistical analyses
Differences between AA and EA in variables of interest were compared using univariate ANOVA. Relationships among IMCL and different metabolic and anthropometric variables in both ethnic groups were examined using Pearson correlation coefficients and were controlled for age and gender.
Multiple regression analysis was used to determine whether the relationship between GDR and IMCL varies between races. Anthropometric measures were not included in the regression model because of the collinearity of the anthropometric measures and other predictors. Missing data were handled by pairwise deletion. Analyses were performed using SPSS 17.0 for Windows (SPSS, Chicago, IL) and differences were accepted as significant at P < 0.05.
In the initial multiple regression between IMCL, race, their interaction, and GDR, residual plots indicated that homoscedasticity was most likely violated. To alleviate this problem, we imposed a heteroscedasticity consistent covariance matrix (HC2 method in Proc Reg).
RESULTS
General characteristics of study subjects
The subject characteristics are summarized in Table 1. As shown in this table, BMI was slightly higher in the AA group compared with EA, but no significant differences existed for insulin sensitivity, IMCL, body weight, percent fat, trunk fat, or waist or hip circumferences. Thus, although the groups differed slightly in BMI, EA and AA were well matched for overall age, percent fat, IMCL, and insulin sensitivity.
Relationships between IMCL, metabolism, and body composition
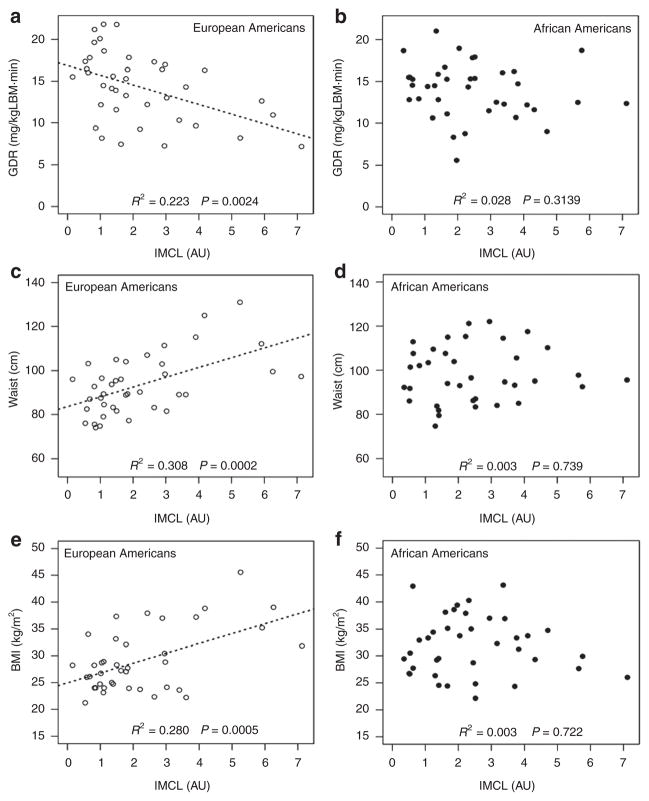
Correlations between IMCL, GDR, and body composition parameters were controlled for age and gender and are listed in Table 2. In EA, IMCL was widely related to measures of generalized and regional adiposity and insulin sensitivity. In contrast, in AA, IMCL was not correlated with any of the variables measured. IMCL was inversely associated with GDR and positively related to waist circumference, BMI, and percent fat in EA, whereas these relations were not statistically significant in AA (Figure 1).
Table 2.
Pearson correlation coefficients relating IMCL, GDR, and metabolic parameters in European-American and African-American adults
| Variable | EA
|
AA
|
||||||
|---|---|---|---|---|---|---|---|---|
| IMCL | N | GDR | N | IMCL | N | GDR | N | |
| IMCL (AU) | — | 43 | −0.47** | 39 | — | 43 | −0.17 | 38 |
| GDR (mg/kgLBM/min) | −0.47** | 39 | — | 39 | −0.17 | 38 | — | 38 |
| BMI (kg/m2) | 0.53** | 43 | −0.35* | 39 | 0.04 | 43 | −0.42** | 38 |
| % Body fat (DXA) | 0.31* | 38 | −0.33* | 39 | 0.00 | 42 | −0.13 | 37 |
| Trunk fat (kg) | 0.43** | 38 | −0.39* | 39 | −0.12 | 42 | −0.38* | 37 |
| Leg fat (kg) | 0.37** | 38 | −0.23 | 39 | −0.11 | 42 | −0.20 | 37 |
| Trunk/leg ratio | 0.20 | 38 | −0.40* | 39 | 0.06 | 42 | −0.37* | 37 |
| Waist (cm) | 0.53** | 38 | −0.41** | 39 | 0.04 | 42 | −0.48** | 37 |
| Hip (cm) | 0.51** | 38 | −0.35* | 39 | 0.00 | 42 | −0.17 | 37 |
| Waist/hip ratio | 0.25 | 38 | −0.29 | 39 | 0.14 | 42 | −0.51** | 37 |
Significant values are in boldface. Estimates and significance tests are controlled for age and gender.
AA, African American; AU, arbitrary units; DXA, dual-energy X-ray absorptiometry; EA, European American; GDR, maximally stimulated glucose disposal rate; IMCL, intramyocellular lipid content.
P ≤ 0.05,
P ≤ 0.01.
Figure 1.
Relationships between intramyocellular lipid ( IMCL) and insulin resistance, waist circumference, and BMI in American adults of European-American (EA) and African-American (AA) descent. The figures show correlations between IMCL as measured by proton-nuclear magnetic resonance spectroscopy and (a,b) glucose disposal rate (GDR) assessed using the euglycemic–hyperinsulinemic glucose clamp technique, (c,d) waist circumference, and (e,f) BMI in EA and AA, respectively. Empty circles represent EA values and filled circles represent AA values. Race was determined by self-report. AU, arbitrary units.
IMCL remained negatively correlated with GDR in EA (r = −0.36, P = 0.03) and not in AA (r = −0.17, P = 0.33), when the relationship was controlled for BMI. The correlations in EA between percent fat, trunk fat, leg fat, waist circumference, and hip circumference with IMCL or GDR were reduced to nonsignificant levels when BMI was controlled. This result is predictable based on the collinearity of BMI to the other measures of adiposity. The correlations between GDR and trunk-to-leg fat ratio in EA and waist-to-hip ratio in AA changed slightly and remained significant, whereas the correlation between GDR and waist-to-hip ratio in EA was reduced to a nonsignificant level.
Self-reported race was found to significantly affect the relationship between IMCL and GDR (race by IMCL interaction; t = 1.69, DF = 1, P = 0.04), although the individual race variable was not significant (t = −1.51, DF = 1, P = 0.11).
DISCUSSION
The purpose of this study was to assess racial differences in the relationships among IMCL, insulin sensitivity, and adiposity in AA and EA adults. In particular, we (26,27) and others (2,3,28) have reported racial differences in the metabolic syndrome trait cluster, however, no data have addressed whether IMCL is a determinant of insulin resistance in AA adults as has been reported in EA. To our knowledge, this is the first study to definitively assess the role of ethnicity on the relationship between IMCL, insulin sensitivity, and body composition in EA and AA adults. We assessed insulin sensitivity as GDR using the gold-standard hyperinsulinemic–euglycemic clamp, IMCL content by proton-magnetic resonance spectroscopy, and total and regional body composition by dual-energy X-ray absorptiometry and circumferences. Mean values for adiposity, body composition, IMCL, and insulin sensitivity were equivalent in our EA and AA subgroups; however, we found marked racial differences in the relationships between IMCL and metabolic and adiposity parameters.
When we analyzed the main effects of IMCL and ethnicity, we found no evidence of an ethnic difference in insulin sensitivity, which is consistent with previous studies using the euglycemic clamp method of measuring insulin sensitivity (22,29). When we accounted for the interaction between IMCL and ethnicity, however, our data revealed an ethnic difference in insulin sensitivity (GDR) which is dependent upon IMCL content. We found that, at lower IMCL levels, AAs and EAs have similar levels of insulin sensitivity. At higher levels of IMCL, AAs are respectively more insulin sensitive.
IMCL was significantly and negatively related to insulin sensitivity in EA independent of BMI, supporting previous findings on the relationship between insulin sensitivity and IMCL (19,30). This is also consistent with a previous study in lean nondiabetic offspring of patients with type 2 diabetes which showed that insulin-resistant offspring have a substantially higher IMCL content than insulin-sensitive offspring matched for age, sex, BMI, percent fat, waist-to-hip ratio, and physical fitness (6). In AA, on the other hand, IMCL was not correlated with insulin sensitivity.
In EA, IMCL was extensively correlated with BMI and regional measures of adiposity including waist circumference, trunk fat, and leg fat. IMCL was not related to measures of adiposity in AA. The difference shown in Figure 1c,d between the IMCL and waist circumference relationships in AA as compared to EA is striking. In EA, IMCL is highly correlated with waist circumference, but this relationship is not present in AA. It is interesting to note that no differences were seen in IMCL content between AA and EA. The finding that IMCL is related to waist circumference and other measures of obesity in EA but not AA, combined with the reduced VAT and liver fat accumulation that is reported in AA (11–13), indicates that the shuttling of fat to ectopic stores in response to increased obesity and insulin resistance may not occur in AA, in contrast to that which is observed in EA. It is important to note that the correlations between IMCL and adiposity measures reduced to nonsignificant levels when BMI was controlled (data not shown). This result is expected, given the high degree of collinearity between BMI and other measures of adiposity, and does not change our interpretation of the correlations.
Waist circumference was significantly correlated with insulin sensitivity in both EA and AA, but overall percent fat was not correlated with insulin sensitivity in either group. When controlling for BMI, the correlation between waist circumference and insulin sensitivity diminished, however trunk-to-leg fat ratio in EA and waist-to-hip ratio in AA were significantly correlated with insulin sensitivity after controlling for BMI. These findings indicate that insulin sensitivity has a stronger relationship with central adiposity than it has with general adiposity. Our data are consistent with previous findings that central adiposity plays a substantial role in insulin sensitivity and cardiometabolic disease risk across ethnicities (27). In 12,814 AA and EA men and women participating in the ARIC study, waist circumference was found to be predictive of developing type 2 diabetes over the 9-year study across both ethnicities and both genders (31). From the same study, it was reported that BMI and waist-to-hip ratio explain 39.9% of the difference in relative risk of type 2 diabetes between AA and EA (2). Waist circumference, more so than percent fat, was also found to be highly correlated with several metabolic syndrome measures in both AA and EA adults (32). Furthermore, a study spanning Europeans and African-Caribbeans found that waist circumference had the highest impact among several metabolic measures on glucose tolerance (33). A possible explanation for this finding is that waist circumference reflects VAT mass, which is known to relate highly to insulin resistance, even in AA, who have been shown to store less visceral fat than EA (11); however, subcutaneous abdominal fat has also been strongly and independently correlated to insulin sensitivity in AA (34). Therefore, while mounting evidence links central adiposity to insulin resistance and the metabolic syndrome trait cluster in AA, it is uncertain whether this relationship is predominantly driven by VAT or subcutaneous adipose tissue or a combination of the two. Since insulin sensitivity was associated with central adiposity, but not with overall percent body fat in both of our groups, it is tempting to argue that the relationship is driven more by VAT than subcutaneous adipose tissue in both EA and AA, although this is merely speculative because VAT was not assessed in our groups.
The reasons that AA and EA exhibit different relationships between IMCL and insulin sensitivity are unknown. One possible explanation is that intramuscular lipid could be compartmentalized differently in AA vs. EA, as has been observed in endurance athletes vs. type 2 diabetic individuals. Indeed, IMCL accumulation occurs in the skeletal muscle of endurance-trained individuals and is associated with insulin sensitivity in this group (35). Muscle from endurance-trained athletes displays a storage pattern characterized by lipid in droplets adjacent to the mitochondria, presumably providing the athlete with an enhanced ability to utilize the lipid as substrate during training. Skeletal muscle of type 2 diabetics, on the other hand, contains more subsarcolemmal lipid and this accumulation is inversely associated with insulin sensitivity (36). Whether different compartmentalization of lipids in EA vs. AA could explain the ethnic differences we have observed in the relationship between IMCL and insulin sensitivity remains to be determined.
Another possible explanation is a difference in skeletal muscle fiber type between AA and EA. Insulin resistance has recently been related to a higher IMCL content in type I (oxidative) muscle fibers more so than in type II (glycolytic) muscle fibers (37). Furthermore, AA women were found to have a higher percentage of type II muscle fibers than their EA counterparts. However, this difference was found to be related more to increased general adiposity in this group than to ethnic differences (38). Our groups did not differ in adiposity and therefore should not be expected to have differences in muscle fiber type. Nevertheless, more studies analyzing histochemical properties of skeletal muscle in AA vs. EA are needed.
Yet another ethnic difference that may impact insulin sensitivity relates to substrate oxidation. Reduced fatty acid oxidation has been observed in obese AA women, compared to EA women, and is related to reduced insulin sensitivity (39). Moreover, metabolic inflexibility in substrate use has been reported in healthy premenopausal AA women, compared to EA women (40). Therefore, the differing relationship between IMCL and insulin sensitivity in EA and AA groups may be due to differences in substrate flux within skeletal muscle.
A limitation of this study is that the subjects were not assessed for aerobic capacity. Maximal aerobic capacity has been shown to be an important determinant of tibialis IMCL. While VO2max was not significantly related to soleus IMCL, an interaction effect was observed between soleus IMCL, VO2max, and GDR (17). VO2max data was not available on our subjects; however, all subjects were screened to be completely sedentary and involved in no regular physical activity or planned exercise. Therefore, it is unlikely that aerobic fitness could entirely explain the differences we have found in the relationships between soleus IMCL and insulin sensitivity in EA and AA.
Taken together, our results suggest that, in EA, IMCL is a fat depot that closely relates to insulin sensitivity as well as to generalized and central adiposity. In AA, however, central adiposity is more closely related to insulin resistance. These data indicate that IMCL is a determinant of insulin resistance in EA but exists largely independent of insulin resistance in AA. Clearly, skeletal muscle insulin resistance is less dependent upon IMCL accumulation in AA. In both AA and EA, however, central adiposity is associated with insulin resistance and confers increased risk of cardiometabolic disease. These differences in metabolic and body composition traits and their associations with insulin resistance point to potential racial differences in the pathogenesis of the metabolic syndrome. Specifically, IMCL may serve as a less relevant pathophysiological role in the development of insulin resistance and the metabolic syndrome trait cluster in individuals of African descent. Large-scale clinical trials that include analyses for aerobic capacity, VAT, and hepatic fat are needed to further assess the specific contributions of ectopic fat to insulin resistance in individuals of African descent.
Acknowledgments
We thank the research volunteers for their participation in this study. This work was supported from grants from the National Institutes of Health (DK-038765, DK-083562, PO1 HL-55782), the T32 training grant (HL-007457) entitled “Mechanisms of Hypertension and Cardiovascular Diseases” (PI: S. Oparil), the T32 training grant (HL-072757) entitled “UAB Statistical Genetics Post-Doctoral Training Program” (PI: D.B.A.), and by the Merit Review program of the Department of Veterans Affairs. We also acknowledge support from the UAB Center for Clinical and Translational Science (UL1 RR025777), and core facility support from the Clinical Nutrition Research Unit (P30-DK56336), and the Diabetes Research and Training Center (P60 DK079626). We further acknowledge Dr. Jan den Hollander at UAB for his assistance with IMCL measurements.
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 3.Shai I, Jiang R, Manson JE, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29:1585–1590. doi: 10.2337/dc06-0057. [DOI] [PubMed] [Google Scholar]
- 4.Lara-Castro C, Newcomer BR, Rowell J, et al. Effects of short-term very low-calorie diet on intramyocellular lipid and insulin sensitivity in nondiabetic and type 2 diabetic subjects. Metab Clin Exp. 2008;57:1–8. doi: 10.1016/j.metabol.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furler SM, Poynten AM, Kriketos AD, et al. Independent influences of central fat and skeletal muscle lipids on insulin sensitivity. Obes Res. 2001;9:535–543. doi: 10.1038/oby.2001.70. [DOI] [PubMed] [Google Scholar]
- 6.Jacob S, Machann J, Rett K, et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48:1113–1119. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- 7.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 8.Wagenknecht LE, Langefeld CD, Scherzinger AL, et al. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2003;52:2490–2496. doi: 10.2337/diabetes.52.10.2490. [DOI] [PubMed] [Google Scholar]
- 9.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes. 1997;46:456–462. doi: 10.2337/diab.46.3.456. [DOI] [PubMed] [Google Scholar]
- 10.Gower BA, Weinsier RL, Jordan JM, Hunter GR, Desmond R. Effects of weight loss on changes in insulin sensitivity and lipid concentrations in premenopausal African American and white women. Am J Clin Nutr. 2002;76:923–927. doi: 10.1093/ajcn/76.5.923. [DOI] [PubMed] [Google Scholar]
- 11.Lara-Castro C, Weinsier RL, Hunter GR, Desmond R. Visceral adipose tissue in women: longitudinal study of the effects of fat gain, time, and race. Obes Res. 2002;10:868–874. doi: 10.1038/oby.2002.119. [DOI] [PubMed] [Google Scholar]
- 12.Weinsier RL, Hunter GR, Gower BA, et al. Body fat distribution in white and black women: different patterns of intraabdominal and subcutaneous abdominal adipose tissue utilization with weight loss. Am J Clin Nutr. 2001;74:631–636. doi: 10.1093/ajcn/74.5.631. [DOI] [PubMed] [Google Scholar]
- 13.Liska D, Dufour S, Zern TL, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS ONE. 2007;2:e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 16.Kuhlmann J, Neumann-Haefelin C, Belz U, et al. Intramyocellular lipid and insulin resistance: a longitudinal in vivo 1H-spectroscopic study in Zucker diabetic fatty rats. Diabetes. 2003;52:138–144. doi: 10.2337/diabetes.52.1.138. [DOI] [PubMed] [Google Scholar]
- 17.Thamer C, Machann J, Bachmann O, et al. Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab. 2003;88:1785–1791. doi: 10.1210/jc.2002-021674. [DOI] [PubMed] [Google Scholar]
- 18.Perseghin G, Lattuada G, Danna M, et al. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab. 2003;285:E1174–E1181. doi: 10.1152/ajpendo.00279.2003. [DOI] [PubMed] [Google Scholar]
- 19.Pan DA, Lillioja S, Kriketos AD, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 20.Greco AV, Mingrone G, Giancaterini A, et al. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes. 2002;51:144–151. doi: 10.2337/diabetes.51.1.144. [DOI] [PubMed] [Google Scholar]
- 21.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222–234. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- 22.Weiss R, Dziura JD, Burgert TS, et al. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia. 2006;49:571–579. doi: 10.1007/s00125-005-0109-z. [DOI] [PubMed] [Google Scholar]
- 23.Larson-Meyer DE, Smith SR, Heilbronn LK, et al. Look AHEAD Adipose Research Group. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14:73–87. doi: 10.1038/oby.2006.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Bock K, Dresselaers T, Kiens B, et al. Evaluation of intramyocellular lipid breakdown during exercise by biochemical assay, NMR spectroscopy, and Oil Red O staining. Am J Physiol Endocrinol Metab. 2007;293:E428–E434. doi: 10.1152/ajpendo.00112.2007. [DOI] [PubMed] [Google Scholar]
- 25.Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 26.Lara-Castro C, Doud EC, Tapia PC, et al. Adiponectin multimers and metabolic syndrome traits: relative adiponectin resistance in African Americans. Obesity (Silver Spring) 2008;16:2616–2623. doi: 10.1038/oby.2008.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gower BA, Ard JD, Hunter GR, Fernandez J, Ovalle F. Elements of the metabolic syndrome: association with insulin sensitivity and effects of ethnicity. Metab Syndr Relat Disord. 2007;5:77–86. doi: 10.1089/met.2006.0027. [DOI] [PubMed] [Google Scholar]
- 28.Taylor H, Liu J, Wilson G, et al. Distinct component profiles and high risk among African Americans with metabolic syndrome: the Jackson Heart Study. Diabetes Care. 2008;31:1248–1253. doi: 10.2337/dc07-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Guerra N, Arslanian S. Skeletal muscle lipid content and insulin sensitivity in black versus white obese adolescents: is there a race differential? J Clin Endocrinol Metab. 2010;95:2426–2432. doi: 10.1210/jc.2009-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White LJ, Ferguson MA, McCoy SC, Kim HW, Castellano V. Cardiovascular/non-insulin-dependent diabetes mellitus risk factors and intramyocellular lipid in healthy subjects: a sex comparison. Metab Clin Exp. 2006;55:128–134. doi: 10.1016/j.metabol.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 31.Stevens J, Couper D, Pankow J, et al. Sensitivity and specificity of anthropometrics for the prediction of diabetes in a biracial cohort. Obes Res. 2001;9:696–705. doi: 10.1038/oby.2001.94. [DOI] [PubMed] [Google Scholar]
- 32.Shen W, Punyanitya M, Chen J, et al. Waist circumference correlates with metabolic syndrome indicators better than percentage fat. Obesity (Silver Spring) 2006;14:727–736. doi: 10.1038/oby.2006.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riste L, Khan F, Cruickshank K. High prevalence of type 2 diabetes in all ethnic groups, including Europeans, in a British inner city: relative poverty, history, inactivity, or 21st century Europe? Diabetes Care. 2001;24:1377–1383. doi: 10.2337/diacare.24.8.1377. [DOI] [PubMed] [Google Scholar]
- 34.Tulloch-Reid MK, Hanson RL, Sebring NG, et al. Both subcutaneous and visceral adipose tissue correlate highly with insulin resistance in african americans. Obes Res. 2004;12:1352–1359. doi: 10.1038/oby.2004.170. [DOI] [PubMed] [Google Scholar]
- 35.Dubé JJ, Amati F, Stefanovic-Racic M, et al. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–E888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen J, Mogensen M, Vind BF, et al. Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E706–E713. doi: 10.1152/ajpendo.00692.2009. [DOI] [PubMed] [Google Scholar]
- 37.Coen PM, Dubé JJ, Amati F, et al. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes. 2010;59:80–88. doi: 10.2337/db09-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanner CJ, Barakat HA, Dohm GL, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282:E1191–E1196. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- 39.Cortright RN, Sandhoff KM, Basilio JL, et al. Skeletal muscle fat oxidation is increased in African-American and white women after 10 days of endurance exercise training. Obesity (Silver Spring) 2006;14:1201–1210. doi: 10.1038/oby.2006.137. [DOI] [PubMed] [Google Scholar]
- 40.Berk ES, Kovera AJ, Boozer CN, Pi-Sunyer FX, Albu JB. Metabolic inflexibility in substrate use is present in African-American but not Caucasian healthy, premenopausal, nondiabetic women. J Clin Endocrinol Metab. 2006;91:4099–4106. doi: 10.1210/jc.2005-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]