Abstract
The total synthesis of the macrocyclic natural product engelhardione is reported. This effort led to the structural revision of the published structure of engelhardione to that of pterocarine. The revision reflects the change of the substitution pattern of one phenyl ether ring from the meta to the para position. To confirm, pterocarine (2) and its close regioisomer 3 were subsequently synthesized for comparison. Moreover, to the best of our knowledge, our synthesis of 1 represents the first example of a 14-membered macrocyclic diarylheptanoid with a meta-meta substitution pattern at the diphenyl ether moiety.
Keywords: Engelhardione, Pterocarine, Ullmann coupling, Claisen-Schmidt condensation, Macrocyclization, Sealed tube
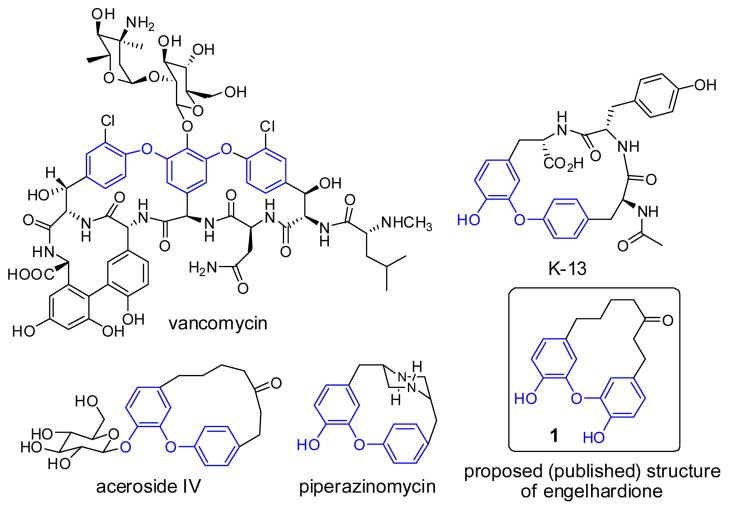
Engelhardione was originally isolated from the roots of Engelhardia roxburghiana (Juglandaceae).1 It belongs to a broad family of natural plant metabolites called diarylheptanoids, which are characterized by two phenolic aromatic rings linked by a linear seven-carbon aliphatic ketone chain to form a macrocyclic architecture.2 The macrocyclic diphenyl ether moiety is widely present in many naturally occurring molecules, including vancomycin, K-13, aceroside IV, piperazinomycin, and engelhardione (Fig. 1), which are important medicinal molecules with antibacterial and anticancer properties.3–5 Several plant species containing diarylheptanoids are widely used in traditional medicine.2
Figure 1.
Selected examples of macrocyclic natural products displaying a diaryl ether moiety.
Our interest in the synthesis of engelhardione arose from the following: engelhardione was reported to have potent in vitro activity against the Mycobacterium tuberculosis strain H37Rv (MIC = 0.2 μg/mL);1 and, despite its potent antituberculosis activity, no synthetic efforts towards engelhardione have been reported to date. Although several total syntheses of engelhardione-related natural products, including acerogenins, galeon, and pterocarine were reported,6–10 none of these analogs has been systematically evaluated for anti-tuberculosis activity. Furthermore, engelhardione has desirable and druglike Lipinski’s rule of 5 properties,11 including a low molecular weight of 312.36, 4 hydrogen bond acceptors, 2 hydrogen bond donors, and calculated LogP 3.18.12 Compared to the other members of diaryl ether natural products (Fig. 1), engelhardione possesses a relatively simple structure, and we envisioned that the synthesis should be quite achievable by adapting available synthetic schemes. To this end, engelhardione represents a valuable natural product lead for further investigation and evaluation.
Herein, we report the first total synthesis of engelhardione. Ultimately, our synthesis led to the structural revision of the proposed (published) structure 1 of engelhardione.
There are basically three different synthetic strategies to construct a macrocyclic diarylheptanoid bearing a diphenyl ether skeleton: (i) the dianion chemistry of methyl acetoacetate and the SNAr reaction for the diaryl ether formation reported by the Zhu group;6, 7 (ii) the modified Ullmann reaction and the Wittig reaction reported by the Nógrádi group;13 and, (iii) the classical Ullmann coupling using a Cu (II) catalyst and aldol condensation reported by the Natarajan8 and Jahng9, 10 groups. We chose to adapt the scheme of Jahng et al. for the synthesis of 1 because this classical macrocyclization procedure employing Cu (II) oxide and potassium carbonate has also been successfully used to synthesize the other members of naturally occurring macrocyclic ethers,14 and also, mild reaction conditions were used in the aldol and Claisen-Schmidt condensation reactions. More importantly, from a medicinal chemistry point of view, a range of diversified engelhardione analogs, such as benzylated and methylated engelhardione derivatives, can be sequentially generated. These analogs would be invaluable compounds for subsequent structure-activity relationships (SAR) studies.
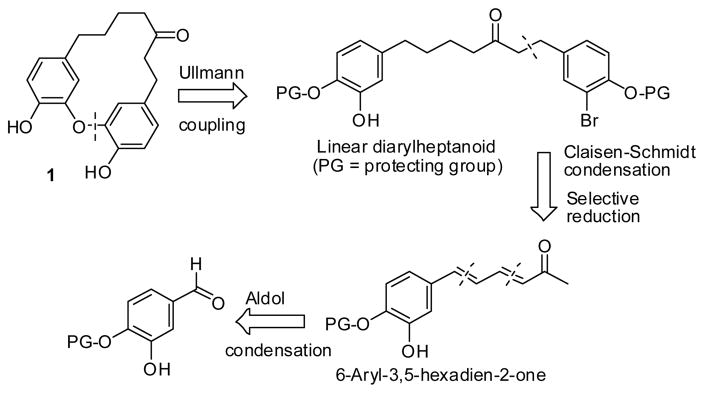
The retrosynthesis of 1 is shown in Scheme 1. Initial disconnection of the diphenyl ether bond generated the linear diarylheptanoid intermediate, and this macrocyclization would be achieved via intramolecular Ullmann coupling between the corresponding suitably protected phenol derivative and the aryl bromide. The overall synthetic strategy of the key linear 1,7-diaryl-3-ketone can be implemented using a series of cross aldol condensation reactions. The resulting diarylheptanoid would be further disconnected to the unsaturated α,β-conjugated olefin ketone 6-aryl-3,5-hexadien-2-one derivative following selective reduction and Claisen-Schmidt condensation, which can be further derived from the suitably substituted benzaldehyde.
Scheme 1.
Retrosynthesis of 1.
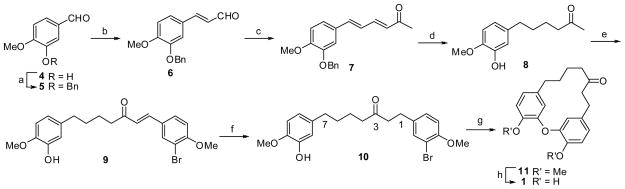
Synthesis of 1 is illustrated in Scheme 2. First, in the presence of potassium carbonate, the hydroxyl group of the commercially available 3-hydroxy-4-methoxybenzaldehyde 4 reacted with benzyl bromide in methanol to give the benzyl protected ether derivative 5 in quantitative yield. The successive aldol condensations with acetaldehyde and then acetone yielded the substituted cinnamaldehyde derivative 6 and 3,5-hexadien-2-one 7, respectively. Next, the α,β-conjugated olefin ketone 7 underwent selective palladium/carbon (Pd/C)-catalyzed hydrogenation in methanol to afford the debenzylated and Schmidt condensation of 8 with 3-bromo-4-methoxy-benzaldehyde was performed to generate the conjugated diarylhepten-3-one 9. Final chemoselective reduction of the olefin 9 was achieved using 10% Pd/C in the presence of diphenylsulfide16 to yield the desired 1,7-diphenylheptan-3-one derivative 10 in 90% yield.17
Scheme 2.
Synthesis of 1. Reagents and conditions: (a) PhCH2Br, K2CO3, MeOH, reflux, 4 h, 100%; (b) CH3CHO, 10% NaOH, EtOH, rt, 3 h, 18%; (c) acetone, 10% NaOH, rt, overnight, 95%; (d) 10% Pd/C, H2, MeOH, rt, 4 h, 94%; (e) 3-Br-4-MeOC6H3CHO, 10% NaOH, EtOH, rt, overnight, 64%; (f) 10% Pd/C, H2, Ph2S (0.05 equiv), CHCl3, 7 h, 90%; (g) CuO, K2CO3, pyridine, 175 °C, 4.5 h, 52%; (h) AlCl3, CH2Cl2, reflux, 20 h, 31%.
With the linear building block 10 in hand, Ullmann macrocyclization was subsequently performed to generate the 14-membered macrocyclic diphenyl ether product 11. To screen the experimental conditions, conversion of 10 to 11 was examined using a variety of Cu reagents, bases, and solvent systems and the results are shown in Table 1. According to the procedure reported by Jahng and co-workers,10 when CuO was used and the reaction was conducted in pyridine using K2CO3 as base, the macrocyclic product 11 was first provided in 25% yield after 20 h (Table 1, entry 1). It should be noted, by comparing entries 1 and 2, that the same reaction without N2 yielded a similar result. Further inspired by the work of the Natarajan group,8 a sealed pressure tube was then employed in an attempt to accelerate the reaction and to improve the yield. In addition, a systematic optimization was performed to evaluate the effect of temperature on the reaction yield. When the experiment was conducted at 130 °C in a sealed tube, no reaction occurred after 20 h based on HPLC analysis of the reaction mixture (entry 4). A more encouraging result was obtained after the temperature was increased to 150 °C, when the reaction was complete in 12 h, affording the macrocyclic product 11 in 71% yield (entry 5). As expected, when the temperature was raised to 175 °C, a significant decrease in reaction time was observed; after 4.5 h the reaction went to completion to afford 11 in 52% yield. Under the same reaction conditions with N2 atmosphere, a comparable yield of 51% was obtained (entries 6 and 7). Increasing the reaction temperature further to 200 °C resulted in a much shorter reaction time (1 h), nevertheless, a lower yield (44%) was also observed due to the formation of polymeric by-products.
Table 1.
Optimization of intramolecular macrocyclic Ullmann reaction of 10 to 11a
| Entry | Cu reagent (equiv) | Base (equiv) | Solvent | Reaction condition | T (°C) | Conc. (M) | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | CuO (2.5) | K2CO3 (1.0) | Pyridine | Reflux under N2 | 130 | 0.02 | 20 | 25 |
| 2 | CuO (2.5) | K2CO3 (1.0) | Pyridine | Reflux | 130 | 0.02 | 25 | 25 |
| 3 | CuO (2.5) | K2CO3 (1.0) | Pyridine | Reflux | 150 | 0.02 | 20 | 33 |
| 4 | CuO (2.5) | K2CO3 (1.0) | Pyridine | Sealed tube | 130 | 0.02 | 20 | NR |
| 5 | CuO (2.5) | K2CO3 (1.0) | Pyridine | Sealed tube | 150 | 0.02 | 12 | 71 |
| 6 | CuO (2.5) | K2CO3 (1.0) | Pyridine | Sealed tube | 175 | 0.02 | 4.5 | 52 |
| 7 | CuO (2.5) | K2CO3 (1.0) | Pyridine | Sealed tube under N2 | 175 | 0.02 | 4.5 | 51 |
| 8 | CuO (2.5) | K2CO3 (1.0) | Pyridine | Sealed tube under N2 | 200 | 0.02 | 1 | 44 |
| 9 | CuO (2.5) | K2CO3 (1.0) | Pyridine | Sealed tube | 175 | 0.004 | 50 | 59 |
| 10 | CuBr-SMe2 (10) | NaH (2.0) | 1,4-Dioxane | Reflux under N2 | 130 | 0.004 | 46 | NRe |
| 11f | CuI (0.2) | CsCO3 (2.0) | 1,4-Dioxane | Reflux under N2 | 100 | 0.05 | 16.5 | NR |
Experiments were performed using 0.08 mmol of 10 except entry 9 (0.016 mmol of 10 was used) and monitored by HPLC of the reaction mixture.
Experiments were performed under N2 atmosphere except entries 2–6 and 9.
Isolated yield based on flash column chromatography.
NR indicates that no product 11 was detected by HPLC at the end of the experiment.
79% of 10 was recovered after column chromatography.
0.3 equiv of N,N-dimethyl glycine was used as additive, which has been reported to promote the intermolecular diphenyl ether reaction.19
The effect of concentration was then evaluated. A 59% yield was obtained in a dilute solution in pyridine (0.004 M) in notably prolonged time (50 h) (entry 9). Finally, attempts to conduct the experiment in milder conditions by employing alternative Cu reagents, including CuBr-SMe218 (entry 10) or CuI and N,N-dimethyl glycine as an additive19 (entry 11) failed to afford the cyclic product 11 in our experiments. Overall, considering the reaction time and yield, we chose the conditions (CuO, K2CO3, 0.02 M, sealed tube, 175 °C, 4.5 h; entry 6) as the optimum experimental conditions for the macrocyclization step. Final O-demethylation of 11 by AlCl3 in CH2Cl2 at reflux afforded 1 in 31% yield.20
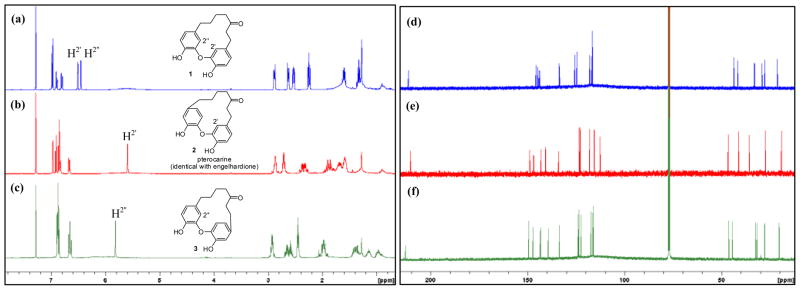
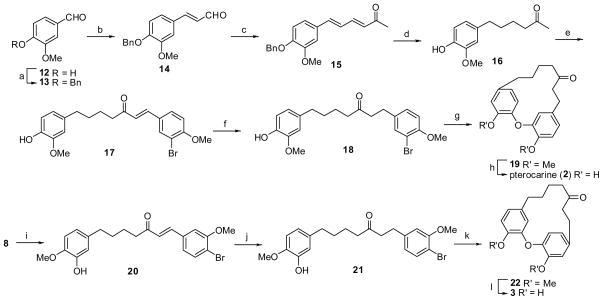
After completing the synthesis of 1, we were surprised to find that both 1H and 13C NMR data of our synthetic engelhardione were not consistent with those of the reported engelhardione, although the 1H and 13C NMR spectra of both samples were recorded in CDCl3 at 400 and 100 MHz, respectively. In particular, we did not observe the previously reported aromatic proton peak at 5.57 ppm (1H, d, J = 2.0 Hz)1 in the 1H NMR spectrum of our synthetic sample (Fig. 2a). After further reviewing the literature, it became evident that this characteristic signal appeared to indicate that the diphenyl ether moiety of the macrocyclic diarylheptanoid was connected at meta and para positions, respectively. The mass spectra of 1 and reported engelhardione showed the two compounds had the same molecular weight. Therefore, instead of the meta-meta linked diphenyl ether core structure as originally proposed, we hypothesized that the published structure of engelhardione should possess the meta and para-linked macrocyclic architecture, which could be compound 2 or 3, as illustrated in Scheme 3. Compound 2 was originally reported as pterocarine21 and its total synthesis has been published.9 After comparing the published 1H and 13C NMR data of engelhardione and pterocarine, we found that the reported spectroscopic data of engelhardione were, indeed, consistent with those of pterocarine. To further confirm our hypothesis, pterocarine (2) and its close regioisomer 3 were next synthesized following the same synthetic strategy (Scheme 3). Synthesis of 2 was achieved starting from 3-methoxy-4-hydroxybenzaldehyde 12, followed by benzylation, aldol condensation reactions, and selective hydrogenations to generate 1,7-diphenylheptan-3-one variant 18, followed by the macrocyclic Ullmann condensation and O-demethylation to afford pterocarine (2). Accordingly, Claisen-Schmidt condensation of 8 with 4-bromo-3-methoxybenzaldehyde followed by chemoselective reduction, macrocyclization, and demethylation gave the regioisomer 3.
Figure 2.
1H and 13C NMR spectra (recorded at 400 and 100 MHz in CDCl3, respectively) of 1, pterocarine (2), and 3.
Scheme 3.
Synthesis of pterocarine (2) and regioisomer 3. Reagents and conditions: (a) PhCH2Br, K2CO3, MeOH, reflux, 4 h, 100%; (b) CH3CHO, 10% NaOH, EtOH, rt, 3 h, 20%; (c) Acetone, 10% NaOH, rt, overnight, 81%; (d) 10% Pd/C, H2, MeOH, rt, 2 h, 92%; (e) 3-Br-4-MeOC6H3CHO, 10% NaOH, EtOH, rt, 9.5 h, 52%; (f) 5% Pd/C, H2, Ph2S (0.05 equiv), CHCl3, 18 h, 78%; (g) CuO, K2CO3, pyridine, 175 °C, 5 h, 54%; (h) AlCl3, CH2Cl2, reflux, 25 h, 62%; (i) 4-Br-3-MeOC6H3CHO, 10% NaOH, EtOH, rt, 3.5 h, 41%; (j) 5% Pd/C, H2, Ph2S (0.05 equiv), 18 h, 80%; (k) CuO, K2CO3, pyridine, 175 °C, 5 h, 58%; (l) AlCl3, CH2Cl2, reflux, 11 h, 42%.
Macrocyclic compounds 1–3 were fully characterized by mass spectrometry, 1H and 13C NMR, and 2D HSQC, HMBC, and COSY spectroscopy (See supporting information). Their complete 1H and 13C NMR spectra are shown in Fig. 2a–f. The spectroscopic data of our synthetic pterocarine (2) were identical with those of the originally reported engelhardione1 as well as natural and synthetic pterocarine.9, 21 Moreover, on the basis of these spectra, interestingly, we noted that minor structural changes in the substitution patterns at the two aromatic rings result in dramatic differences in their respective NMR spectra. Most notably, for meta and para connected pterocarine (2) and regioisomer 3, high-field shifts of H-2′ (δ = 5.59 ppm, d, J = 1.7 Hz) of 2 (Fig. 2b) and H-2″ (δ = 5.82 ppm, d, J = 2.0 Hz) of 3 (Fig. 2c) were observed due to the anisotropic effect of the adjacent aromatic rings, as previously reported.6, 7, 10 In contrast, the resonances of these aromatic protons H-2′ and H-2″ from the meta-meta diaryl ether-linked 1 (Fig. 2a) are at 6.51 and 6.45 ppm with a coupling constant value of 1.9 Hz, respectively. Differences of the chemical shifts of the aliphatic protons of the heptan-3-one chain among 1–3 were also observed (Fig. 2a–c). Our data suggest that these macrocyclic molecules display a high degree of conformational flexibility in solution. Further evidence was demonstrated from the 1H NMR data of 19, which was recorded in both non-polar CDCl3 and polar DMSO-d6 for comparison. We noted that the signals of the geminal protons in the heptyl chain of 19 merged together in DMSO-d6 compared to those signals recorded in CDCl3, additionally, changes of chemical shifts from the aromatic protons were also observed (See supporting information).
In conclusion, we report the first synthesis of engelhardione and this effort led to the structural revision of this macrocyclic natural product.22 The correct structure of the previously reported engelhardione should be that of pterocarine (2). To confirm, 2 and its close regioisomer 3 were also synthesized. The published spectroscopic data of engelhardione were in full agreement with those of pterocarine. Biological studies of these macrocyclic compounds and syntheses of their structural analogs are currently ongoing and will be reported in due course.
Supplementary Material
Acknowledgments
We thank UH Hilo College of Pharmacy for the start-up funding, and this work is also in part supported by INBRE program P20 RR016467 awarded by NCRR. We thank Dr. Philip Williams at University of Hawai’i at Manoa for performing high-resolution mass spectrometric analysis of compound 11. We also thank Prof. Ih-Sheng Chen for providing NMR spectra of engelhardione.
Footnotes
Supplementary data (experimental procedures for the synthesis of compounds 1–22, copies of 1H and 13C NMR spectra, HPLC chromatographs for compounds 1–22, and HSQC, HMBC, and COSY spectra of macrocyclic compounds 1–3, 11, 19, and 22) associated with this article can be found, in the online version, at
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Lin WY, Peng CF, Tsai IL, Chen JJ, Cheng MJ, Chen IS. Planta Med. 2005;71:171. doi: 10.1055/s-2005-837786. [DOI] [PubMed] [Google Scholar]
- 2.Keserü GM, Nogradi M. In: Studies in Natural Products Chemistry. Atta-Ur-Rahman, editor. Vol. 17. Elsevier Science; 1995. p. 357. [Google Scholar]
- 3.Ishida J, Kozuka M, Wang H-K, Konoshima T, Tokuda H, Okuda M, Yang Mou X, Nishino H, Sakurai N, Lee K-H, Nagai M. Cancer Lett. 2000;159:135. doi: 10.1016/s0304-3835(00)00538-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee KS, Li G, Kim SH, Lee CS, Woo MH, Lee SH, Jhang YD, Son JK. J Nat Prod. 2002;65:1707. doi: 10.1021/np0201063. [DOI] [PubMed] [Google Scholar]
- 5.Nomura M, Tokoroyama T, Kubota T. Phytochemistry. 1981;20:1097. [Google Scholar]
- 6.Gonzalez GI, Zhu J. J Org Chem. 1997;62:7544. [Google Scholar]
- 7.Gonzalez GI, Zhu J. J Org Chem. 1999;64:914. doi: 10.1021/jo981844s. [DOI] [PubMed] [Google Scholar]
- 8.Kishore Kumar GD, Natarajan A. Tetrahedron Lett. 2008;49:2103. [Google Scholar]
- 9.Wang Q, Son JK, Jahng Y. Synth Commun. 2007;37:675. [Google Scholar]
- 10.Jeong BS, Wang Q, Son JK, Jahng Y. Eur J Org Chem. 2007;2007:1338. [Google Scholar]
- 11.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv Drug Deliver Rev. 2001;46:3. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 12.Calculated using Advanced Chemistry Development (ACD/Labs) Software V8.19 for Solaris (© 1994-2011 ACD/Labs).
- 13.Keserü GM, Nógrádi M, Szöllösy Á. Eur J Org Chem. 1998;1998:521. [Google Scholar]
- 14.Evano G, Blanchard N, Toumi M. Chem Rev. 2008;108:3054. doi: 10.1021/cr8002505. [DOI] [PubMed] [Google Scholar]
- 15.When chloroform was initially used as solvent, the reaction was complete after 2.5 h. However, 19% of over-reduced secondary alcohol by-product was produced based upon HPLC and 1H NMR monitoring. We found, when methanol was used as solvent, the over-reduction of the carbonyl group can be sufficiently suppressed for this reaction.
- 16.Mori A, Mizusaki T, Miyakawa Y, Ohashi E, Haga T, Maegawa T, Monguchi Y, Sajiki H. Tetrahedron. 2006;62:11925. [Google Scholar]
- 17.Using the procedure reported by Sajiki and co-workers,16 the addition of 0.05 equiv of diphenylsulfide as catalyst poison proved to be effective to achieve chemoselective hydrogenolysis between olefin and ketone.
- 18.Boger DL, Yohannes D, Zhou J, Patane MA. J Am Chem Soc. 1993;115:3420. [Google Scholar]
- 19.Ma D, Cai Q. Org Lett. 2003;5:3799. doi: 10.1021/ol0350947. [DOI] [PubMed] [Google Scholar]
- 20.The reaction proceeded smoothly during the first 20 h to give a mixture of desired product 1 (54%), partially demethylated intermediates (32%) with one methoxy group intact, and unreacted starting material (14%) (characterized by MS, 1H NMR, and HPLC). However, no further reaction was detected after 20 h.
- 21.Liu H, Cui C, Cai B, Gu Q, Zhang D, Zhao Q, Guan H. Chinese Chem Lett. 2005;16:215. [Google Scholar]
- 22.When we contacted Prof. Ih-Sheng Chen for advice regarding the structure revision of engelhardione to pterocarine, we were informed that the incorrect structural elucidation has also been found very recently in his laboratory.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.