Abstract
The development of a ‘click, click, cy-click’ process utilizing a double aza-Michael reaction to generate functionalized 1,2,5-thiadiazepane 1,1-dioxides is reported. Optimization in flow, followed by scale out of the inter-/intramolecular double aza-Michael addition has also been realized using a microwave-assisted, continuous flow organic synthesis platform (MACOS). In addition, a facile one-pot, sequential strategy employing in situ Huisgen cycloaddition post-double aza-Michael has been accomplished, and is applicable to library synthesis.
Keywords: double-aza-Michael; click; MACOS; flow; 1,2,5-thiadiazepane 1,1-dioxides
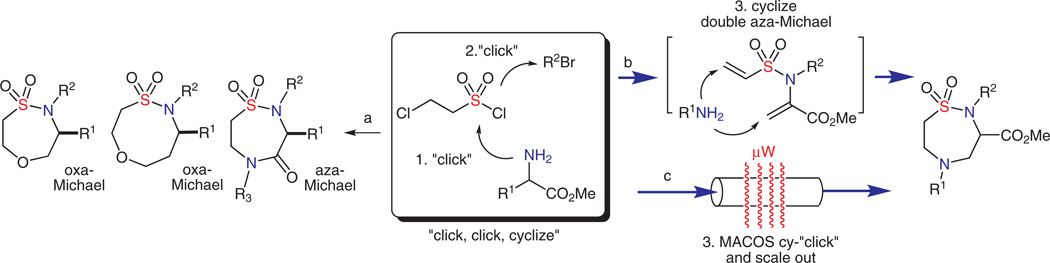
The development of facile, step-efficient methods to access new heterocycles is a key component in the drug-discovery process. Hetero-Michael reactions are efficient pathways that, historically, have been broadly used in synthesis, including several intramolecular examples to access a variety of heterocycles, namely, dioxolanes en route to 1,3-diols,1 azacycles,2 oxacycles,3 thiacycles,4 and bis-heterocycles.5 Recently, double aza-Michael reactions have been utilized as efficient means to install two N–C bonds in a single operation.6 Herein, we report a new approach termed ‘click, click, cy-click’ that utilizes two click reactions7 and a double aza-Michael reaction for the facile synthesis of 1,2,5-thiadiazepane 1,1-dioxides. Optimization using a microwave-assisted continuous flow synthetic (MACOS) platform, has transformed the non-click cyclization step into a facile one-minute (resident flow time) cyclization event (cy-click) that has also been scaled out.
Although not found in nature, sultams have emerged as privileged structures due to their extensive chemical and biological profiles.8,9 Recently, a number of reports of sultams have appeared demonstrating broad-spectrum bioactivity. Such reports include anti-HIV activity,10 antidepressant activity,11 and as inhibitors of RSV,12 selective tumor necrosis factor,13 and metalloproteinase.14
Previously, the inherent reactivity of vinyl sulfonamides was utilized in a ‘click, click, cyclize’ strategy for the facile construction of diverse sultam scaffolds via intramolecular oxa- and intermolecular aza-Michael reactions (Scheme 1, pathway a).3i,15 At the heart of this approach is the facile production of precursor tertiary vinyl sulfonamides by the use of two click reactions,7 namely, sulfonylation of in situ generated vinyl sulfonyl chloride, followed by alkylation of the resulting 2° sulfonamide and subsequent cyclization.
Scheme 1.
Hetero-Michael pathways using a ‘click, click, cyclize’ strategy
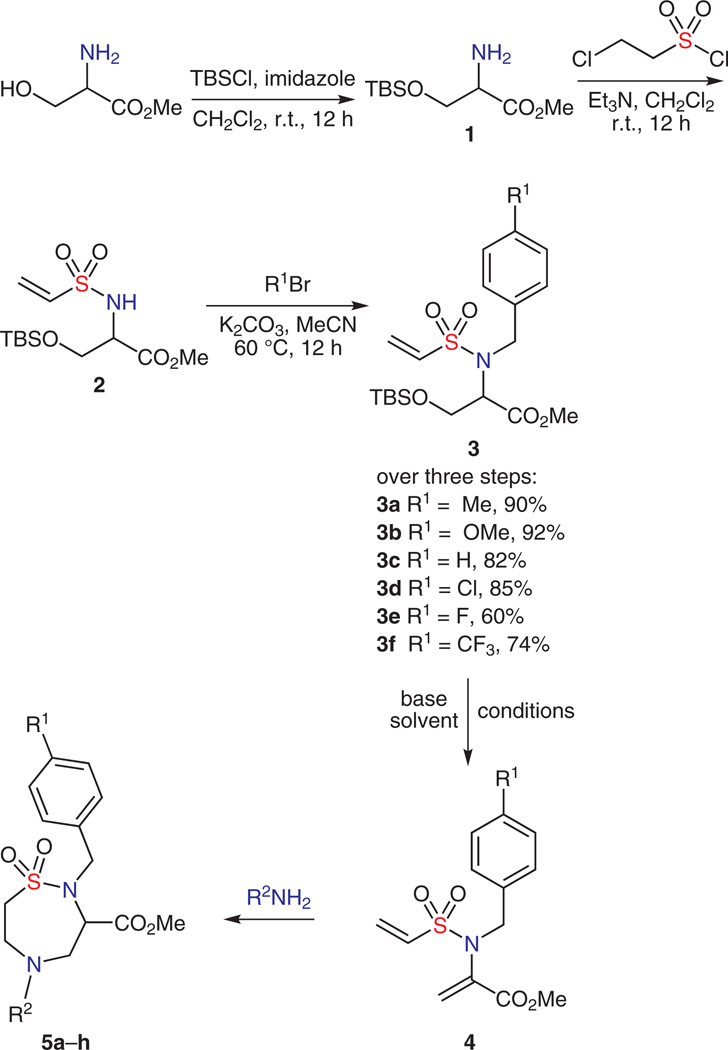
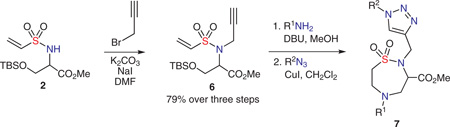
In this new report outlined in Scheme 1 (pathways b and c), an inter-/intramolecular double aza-Michael pathway serves as the cyclization step in a ‘click, click, cy-click’ sequence, and utilizes a TBS-protected serinol methyl ester in the initial sulfonylation step. Subsequent alkylation, followed by a one-pot, β-elimination/double aza-Michael reaction yields functionalized 1,2,5-thiadiazepane 1,1-dioxide scaffolds.
Overall, this facile approach utilizes the β-elimination of the alcohol in the serine-moiety present in 2 to generate a second Michael acceptor armed for the titled protocol. This later sequence has its roots in the biosynthesis of tryptophan, which utilizes tryptophan synthase, a pyridoxal phosphate-containing bifunctional enzyme (PLP), to catalyze an elimination/indole addition pathway on serine.16 Taken collectively, the route employs two click reactions and a double aza-Michael cyclization step that is optimized using a MACOS platform17 (cy-click).
Initial efforts focused on the preparation of vinyl sulfonamide 3, obtained in a simple three-step sequence from serine methyl ester (Scheme 2). Racemic serine methyl ester was protected as a TBS-ether,18 to which 2-chloroethanesulfonyl chloride was added, providing 2, which was subsequently benzylated under mild conditions to provide 3. A facile pathway to 5 was next envisioned to proceed via elimination of the TBS-ether moiety19,20 to generate 4, followed by a double aza-Michael reaction of an amine. Towards this goal, treatment of 3 with TBAF in tetrahydrofuran, in the absence of nucleophile, resulted in the production of elimination product 4 in 30% yield. Use of cesium carbonate in acetonitrile further promoted the elimination pathway to yield 4 in 91%. Addition of cyclopentylamine to 4 in acetonitrile gave 5 in moderate yield (Scheme 2).
Scheme 2.
The preparation of double aza-Michael precursors
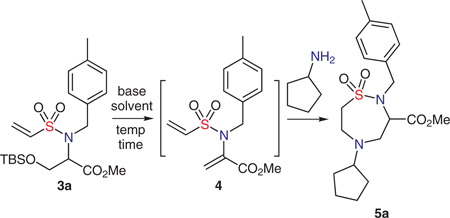
Further studies showed that while 4 can be isolated, a one-pot operation consisting of adding base and cyclopentylamine to a stirring solution of 3, directly afforded the double aza-Michael product 5a (Table 1). Optimization of various parameters, including solvent, temperature and base (equiv), revealed that cesium carbonate in acetonitrile at 40 °C gave nice conversion (Table 1, entries 1–6). However, use of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as base (Table 1, entries 7–10) improved the yield significantly, with optimized conditions established using 0.1 equivalent of DBU in methanol at 40 °C for one hour (entry 10).
Table 1.
Optimization of One-Pot Elimination and Double Aza-Michael Reactiona
 | ||||
|---|---|---|---|---|
| Entry | Base | Temp, time | Solvent | Yield (%)b |
| 1 | Cs2CO3 (1 equiv) | 40 °C, 12 h | MeCN | 50 |
| 2 | Cs2CO3 (1 equiv) | 40 °C, 12 h | CH2Cl2 | 44 |
| 3 | Cs2CO3 (1 equiv) | 40 °C, 12 h | MeOH | 0 |
| 4 | Cs2CO3 (1 equiv) | 60 °C, 12 h | MeCN | 43 |
| 5 | Cs2CO3 (2 equiv) | 60 °C, 12 h | MeCN | 32 |
| 6 | Cs2CO3 (1 equiv) | 40 °C, 12 h | DMF | 42 |
| 7 | DBU (0.5 equiv) | r.t., 12 h | MeCN | 23 |
| 8 | DBU (0.1 equiv) | 40 °C, 1 h | DMF | 30 |
| 9 | DBU (0.1 equiv) | r.t., 12 h | MeOH | 69 |
| 10 | DBU (0.1 equiv) | 40 °C, 1 h | MeOH | 75 |
A solution of compound 3a (1 equiv) and cyclopentylamine (1.1 equiv) in solvent (0.1 M) was added base and stirred at the indicated temperature and time.
Isolated yields following silica gel chromatography.
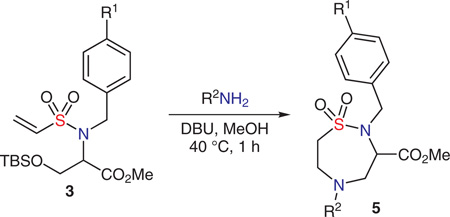
With the optimized conditions in hand, a demonstration set of 1,2,5-thiadiazepane 1,1-dioxides were synthesized using linear (Table 2, entries 1, 2, 5, and 6) as well as benzylamines (Table 2, entries 3, 4, and 7). Within the aliphatic amines, several linear amines worked well. Not surprisingly, more sterically encumbered tert-butylamine failed to undergo the double aza-Michael reaction, but instead simple Michael addition of methanol was obtained in roughly 40% yield.
Table 2.
Synthesis of 1,2,5-Thiadiazepane 1,1-Dioxides
 | ||||
|---|---|---|---|---|
| Entry | R1 | R2 | Product | Yield (%) |
| 1 | MeO | n-C8H17 | 5b | 65 |
| 2 | H | n-Bu | 5c | 74 |
| 3 | H | 4-FC6H4CH2 | 5d | 69 |
| 4 | Cl | 4-MeOC6H4CH2 | 5e | 82 |
| 5 | Cl | PhCH2CH2 | 5f | 77 |
| 6 | F | i-Bu | 5g | 65 |
| 7 | CF3 | Bn | 5h | 79 |
| 8a | Me | c-C5H9 | 5aa | 60a |
| 9a | H | n-Bu | 5ca | 71a |
Performed using the following MACOS conditions: 3a/3c (1.0 equiv), DBU (1.0 equiv), amine (1.2 equiv), i-PrOH (0.3 M), 75 µL/min, 100 °C, 130 W.
In efforts to utilize the double aza-Michael strategy in library synthesis, it was deemed imperative that the scaffold be synthesized efficiently and quickly on a larger scale. This seemed achievable by combining microwave heating and flow chemistry using the MACOS (microwave-assisted, continuous-flow organic synthesis) platform. Using this synthetic technique, one set of optimization experiments is all that is necessary to obtain, and in theory, any amount of desired product can be generated by scaling the synthesis out, rather than scaling it up, which involves extensive reaction reoptimization at each growing scale in the process.
The optimal bench conditions in Table 1 (entry 10) were initially employed in MACOS, resulting in a mixture with the mono aza-Michael product as the major component. A more complex product mixture was obtained when additional heat was applied in order to push the reaction in methanol. Use of higher-boiling n-butanol did provide the double aza-Michael product, but transesterification was also observed (5–15%). This problem was circumvented by the use of propan-2-ol leading to products 5a and 5c in 60% (0.5 g) and 71% (0.7 g), respectively (Table 2, entries 8 and 9). These proof-of-concept experiments illustrate that we can obtain any amount of product we require for library elaboration using scale-out in MACOS.
Successes with the double aza-Michael reaction led us to explore a sequential, one-pot process employing an additional in situ Huisgen 1,3-dipolar cycloaddition click reaction, to terminate the route.21 The propargyl-substituted sulfonamide was synthesized in 79% yield (over three steps) using potassium carbonate in the presence of sodium iodide in N,N-dimethylformamide. The double aza-Michael reaction was performed using the optimal bench conditions (Table 1, entry 10), and the resultant mixture was concentrated in the reaction vial and reconstituted in dichloromethane, after which the azide and copper(I) iodide were added, and stirred at room temperature for 16 hours. Efforts aimed at simplifying the procedure by using same solvent for both double aza-Michael and Huisgen cycloaddition reactions, either gave a lower yield (in CH2Cl2) or multiple products (in MeOH). While additional efforts continue toward this goal, a set of reactions was carried out using a sequential, one-pot procedure to afford the desired products in good yields (Table 3).
Table 3.
Sequential, One-Pot Elimination, Double Aza-Michael and Huisgen 1,3-Dipolar Cycloaddition
 | ||||
|---|---|---|---|---|
| Entry | R1 | R2 | Product | Yield (%) |
| 1 | n-Bu | 4-MeC6H4CH2 | 7a | 61 |
| 2 | n-Bu | 3-ClC6H4CH2 | 7b | 65 |
| 3 | n-Bu | 2-BrC6H4CH2 | 7c | 73 |
| 4 | Bn | 4-MeC6H4CH2 | 7d | 83 |
| 5 | Bn | 3-ClC6H4CH2 | 7e | 65 |
| 6 | Bn | 2-BrC6H4CH2 | 7f | 74 |
| 7 | HOCH2CH2 | 4-MeC6H4CH2 | 7g | 81 |
| 8 | HOCH2CH2 | 3-ClC6H4CH2 | 7h | 87 |
| 9 | HOCH2CH2 | 2-BrC6H4CH2 | 7i | 73 |
In conclusion, we have developed a double aza-Michael procedure using MACOS to synthesize 1,2,5-thiadiazepane 1,1-dioxides that involves minimal chromatography and is step-efficient. Application of flow chemistry for optimization and scale-out sets the stage for library synthesis. Further investigations have led to a sequential, one-pot elimination, double aza-Michael and Huisgen cycloaddition for the synthesis of triazolated 1,2,5-thiadiazepane 1,1-dioxides. Subsequent research will be focused on library synthesis using this protocol. All compounds synthesized have been submitted to the NIH Molecular Library Small Molecule Repository (MLSMR) for distribution within the MLSCN, which will allow for extensive biological screening.
All reactions were carried out without inert atmosphere. Stirring was achieved with oven-dried magnetic stir bars. Solvents were either purchased through Sigma-Aldrich or purified by passage through the Solv-Tek purification system employing activated Al2O3.22 Et3N was purified by passage over basic Al2O3 or distilled over CaH2 and stored over KOH. Flash column chromatography was performed with Sorbent Technologies (30930M-25, Silica Gel 60A, 40–63 µm). TLC was performed on silica gel 60F254 plates (EM-5717, Merck). Deuterated solvents were purchased from Cambridge Isotope laboratories. 1H, 13C NMR spectra were recorded on a Bruker DRX-400 spectrometer operating at 400 MHz, 100 MHz, respectively, as well as a Bruker DRX-500 spectrometer operating at 500 MHz, 125 MHz, respectively, and a Avance AV-III 500 with a dual carbon/proton (CPDUL) cryoprobe operating at 500 MHz, 125 MHz, respectively. High-resolution mass spectrometry (HRMS) measurements were obtained on a VG Instrument ZAB double-focusing mass spectrometer.
Generation of 1,2,5-Thiadiazepane 1,1-Dioxides 5 via Double Aza-Michael on Bench; General Procedure
To the tertiary sulfonamide 3 (0.2 mmol, 1 equiv) was added MeOH (0.1 M), amine (0.22 mmol, 1.1 equiv), and DBU (0.04 mmol, 0.2 equiv). The solution was stirred at 40 °C for 1 h, the solvent was evaporated and the crude product was purified by flash column chromatography on silica gel (hexane–EtOAc, 3:1).
Methyl 5-Cyclopentyl-2-(4-methylbenzyl)-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (5a)
Yield: 57 mg (75%); colorless oil.
FTIR (thin film): 2951, 2866, 1734, 1329, 1142 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.29 (d, J = 8.0 Hz, 2 H), 7.15 (d, J = 7.8 Hz, 2 H), 4.79 (d, J = 15.0 Hz, 1 H), 4.31 (d, J = 15.0 Hz, 1 H), 3.94–3.86 (m, 1 H), 3.60 (s, 3 H), 3.45 (s, 1 H), 3.43 (d, J = 3.3 Hz, 1 H), 3.23 (d, J = 4.3 Hz, 1 H), 3.22 (d, J = 4.1 Hz, 1 H), 3.11 (ddd, J = 14.8, 4.1, 4.1 Hz, 1 H), 3.01 (ddd, J = 14.7, 6.7, 6.7 Hz, 1 H), 2.76–2.67 (m, 1 H), 2.35 (s, 3 H), 1.67 (m, 4 H), 1.48–1.38 (m, 2 H), 1.35–1.20 (m, 2 H).
13C NMR (126 MHz, CDCl3): δ = 170.6, 137.8, 132.8, 129.2, 129.0, 63.3, 57.0, 55.0, 54.6, 53.6, 52.3, 48.7, 30.8, 30.2, 23.6, 23.5, 21.1.
HRMS (TOF MS ES+): m/z calcd for C19H29N2O4S (M + H)+: 381.1848; found: 381.1851.
Methyl 2-(4-Methoxybenzyl)-5-octyl-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (5b)
Yield: 57 mg (65%); colorless oil.
FTIR (thin film): 2951, 2928, 2854, 1736, 1514, 1329, 1248, 1142 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.34–7.29 (m, 2 H), 6.90–6.84 (m, 2 H), 4.67 (d, J = 14.9 Hz, 1 H), 4.34 (d, J = 14.9 Hz, 1 H), 3.90 (dd, J = 10.5, 6.5 Hz, 1 H), 3.81 (s, 3 H), 3.57 (s, 3 H), 3.47 (dd, J = 14.7, 10.6 Hz, 1 H), 3.36 (dd, J = 14.7, 6.5 Hz, 1 H), 3.26–3.22 (m, 2 H), 3.02 (dd, J = 7.8, 3.9 Hz, 2 H), 2.46–2.40 (m, 2 H), 1.41–1.17 (m, 12 H), 0.90 (t, J = 7.0 Hz, 3 H).
13C NMR (126 MHz, CDCl3): δ = 170.5, 159.4, 130.4, 127.8, 113.8, 57.1, 55.8, 55.3, 55.3, 54.8, 53.3, 52.3, 50.0, 31.8, 29.4, 29.3, 27.7, 27.1, 22.7, 14.1.
HRMS (TOF MS ES+): m/z calcd for C22H37N2O5S (M + H)+: 441.2423; found: 441.2419.
Methyl 2-Benzyl-5-butyl-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (5c)
Yield: 53 mg (74%); light yellow solid; mp 58 °C.
FTIR (thin film): 2954, 2931, 2869, 1737, 1454, 1330, 1143, 1072, 744, 703 cm−1.
1H NMR (300 MHz, CDCl3): δ = 7.38–7.36 (m, 2 H), 7.32–7.27 (m, 3 H), 4.72 (d, J = 15.3 Hz, 1 H), 4.32 (d, J = 15.0 Hz, 1 H), 3.93 (m, 1 H), 3.49 (s, 3 H), 3.43–3.27 (m, 2 H), 3.22–3.20 (m, 2 H), 2.99 (m, 2 H), 2.44 (t, J = 7.2 Hz, 2 H), 1.37–1.32 (m, 2 H), 1.26–1.21 (m, 2 H), 0.89 (t, J = 7.2 Hz, 3 H).
13C NMR (75 MHz, CDCl3): δ = 170.3, 135.9, 128.9, 128.4, 127.9, 57.4, 55.8, 55.2, 54.7, 53.7, 52.1, 49.3, 29.8, 20.1, 13.9.
HRMS (TOF MS ES+): m/z calcd for C17H26N2O4S (M)+: 354.1613; found: 354.1606.
Methyl 2-Benzyl-5-(4-fluorobenzyl)-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (5d)
Yield: 56 mg (69%); yellow oil.
FTIR (thin film): 2951, 2843, 1732, 1508, 1329, 1219, 1142, 771, 743, 704 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.44–7.35 (m, 5 H), 7.10–7.05 (m, 2 H), 7.02–6.94 (m, 2 H), 4.94 (d, J = 15.1 Hz, 1 H), 4.32 (d, J = 15.1 Hz, 1 H), 3.86 (dd, J = 10.5, 6.6 Hz, 1 H), 3.59–3.55 (m, 1 H), 3.57 (s, 3 H), 3.52–3.46 (m, 2 H), 3.34 (dd, J = 14.7, 6.6 Hz, 1 H), 3.25–3.22 (m, 2 H), 3.04–2.96 (m, 2 H).
13C NMR (126 MHz, CDCl3): δ = 170.2, 162.1 (d, 1JC,F = 245.9 Hz), 135.9, 133.7 (d, 4JC,F = 3.1 Hz), 129.9 (d, 3JC,F = 8.0 Hz), 129.3, 128.7, 128.2, 115.4 (d, 2JC,F = 21.3 Hz), 59.1, 57.1, 55.7, 55.1, 53.6, 52.3, 49.7.
HRMS (TOF MS ES+): m/z calcd for C20H24FN2O4S (M + H)+: 407.1441; found: 407.1438.
Methyl 2-(4-Chlorobenzyl)-5-(4-methoxybenzyl)-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (5e)
Yield: 74 mg (82%); yellow oil.
FTIR (thin film): 2951, 2932, 2837, 1736, 1610, 1512, 1329, 1246, 1144, 1078, 1014, 837, 741 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.36 (s, 4 H), 7.10 (d, J = 8.6 Hz, 2 H), 6.86 (d, J = 8.7 Hz, 2 H), 4.77 (d, J = 15.3 Hz, 1 H), 4.36 (d, J = 15.3 Hz, 1 H), 3.88 (dd, J = 10.4, 6.7 Hz, 1 H), 3.82 (s, 3 H), 3.66 (d, J = 12.4 Hz, 1 H), 3.58 (d, J = 13.0 Hz, 1 H), 3.53 (s, 3 H), 3.45 (dd, J = 14.4, 10.5 Hz, 1 H), 3.32 (dd, J = 14.5, 6.6 Hz, 1 H), 3.25–3.14 (m, 2 H), 3.03–2.90 (m, 2 H).
13C NMR (126 MHz, CDCl3): δ = 170.0, 159.0, 134.6, 133.9, 130.4, 130.0, 129.7, 128.7, 113.9, 60.6, 57.7, 55.9, 55.3, 54.8, 52.9, 52.3, 49.5.
HRMS (TOF MS ES+): m/z calcd for C21H26ClN2O5S (M + H)+: 453.1251; found: 453.1242.
Methyl 2-(4-Chlorobenzyl)-5-phenethyl-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (5f)
Yield: 67 mg (77%); yellow oil.
FTIR (thin film): 2951, 2930, 2847, 1738, 1491, 1331, 1142, 1082, 1014, 752, 739, 700 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.36–7.30 (m, 2 H), 7.30–7.22 (m, 5 H), 7.19–7.14 (m, 2 H), 4.31 (d, J = 15.4 Hz, 1 H), 4.23 (d, J = 15.4 Hz, 1 H), 3.91 (dd, J = 10.5, 6.7 Hz, 1 H), 3.51 (s, 3 H), 3.48 (dd, J = 14.5, 10.6 Hz, 1 H), 3.39 (dd, J = 14.5, 6.7 Hz, 1 H), 3.24–3.19 (m, 2 H), 3.12–2.99 (m, 2 H), 2.95–2.83 (m, 2 H), 2.81– 2.73 (m, 2 H).
13C NMR (126 MHz, CDCl3): δ = 170.0, 139.4, 134.5, 133.7, 130.2, 128.6, 128.6, 128.5, 126.4, 58.2, 57.9, 56.2, 54.3, 52.9, 52.3, 49.9, 34.4.
HRMS (TOF MS ES+): m/z calcd for C21H26ClN2O4S (M + H)+: 437.1302; found: 437.1281.
Methyl 2-(4-Fluorobenzyl)-5-isobutyl-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (5g)
Yield: 48 mg (65%); colorless oil.
FTIR (thin film): 2955, 2870, 1738, 1510, 1329, 1221, 1146, 1078, 754 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.62–7.32 (m, 2 H), 7.19–6.91 (m, 2 H), 4.69 (d, J = 15.1 Hz, 1 H), 4.38 (d, J = 15.1 Hz, 1 H), 3.92 (dd, J = 10.6, 6.5 Hz, 1 H), 3.55 (s, 3 H), 3.48 (dd, J = 14.7, 10.7 Hz, 1 H), 3.33 (dd, J = 14.7, 6.5 Hz, 1 H), 3.28–3.22 (m, 2 H), 3.04–2.99 (m, 2 H), 2.28–2.18 (m, 2 H), 1.68–1.62 (m, 1 H), 0.86 (d, J = 6.6 Hz, 6 H).
13C NMR (126 MHz, CDCl3): δ = 170.2, 162.5 (d, 1JC,F = 246.7 Hz), 131.8 (d, 4JC,F = 3.2 Hz), 130.7 (d, 3JC,F = 8.2 Hz), 115.4 (d, 2JC,F = 21.5 Hz), 63.9, 57.4, 56.5, 54.9, 53.0, 52.3, 50.4, 26.8, 20.5, 20.5.
HRMS (TOF MS ES+): m/z calcd for C17H26FN2O4S (M + H)+: 373.1597; found: 373.1595.
Methyl 5-Benzyl-2-[4-(trifluoromethyl)benzyl]-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (5h)
Yield: 72 mg (79%); yellow oil.
FTIR (thin film): 2953, 2930, 2843, 1740, 1325, 1163, 1144, 1122, 1067, 735, 700 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.64 (d, J = 8.1 Hz, 2 H), 7.56 (d, J = 8.1 Hz, 2 H), 7.36–7.28 (m, 3 H), 7.25–7.21 (m, 2 H), 4.85 (d, J = 15.6 Hz, 1 H), 4.47 (d, J = 15.6 Hz, 1 H), 3.95 (dd, J = 10.4, 6.6 Hz, 1 H), 3.77 (d, J = 13.2 Hz, 1 H), 3.69 (d, J = 13.2 Hz, 1 H), 3.50 (s, 3 H), 3.49 (dd, J = 14.4, 10.5 Hz, 1 H), 3.35 (dd, J = 14.4, 6.6 Hz, 1 H), 3.29–3.19 (m, 2 H), 3.08–2.94 (m, 2 H).
13C NMR (126 MHz, CDCl3): δ = 169.8, 140.3, 138.0, 130.2 (q, 2JC,F = 32.5 Hz), 129.1, 128.6, 128.5, 127.8, 126.2 (q, 1JC,F = 272.1 Hz), 125.4 (q, 3JC,F = 3.7 Hz), 61.6, 58.0, 56.1, 54.7, 52.9, 52.3, 49.7.
HRMS (TOF MS ES+): m/z calcd for C21H24F3N2O4S (M + H)+: 457.1409; found: 457.1406.
Scale-Out Synthesis of 1,2,5-Thiadiazepane 1,1-Dioxides 5a and 5c Utilizing MACOS Flow Platform; General Procedure
A stock solution containing the respective sulfonamide 3a, 3c (2.4 or 2.8 mmol, 1.0 equiv), DBU (2.4 or 2.8 mmol, 1.0 equiv), and amine (2.8 or 3.3 mmol, 1.2 equiv) in i-PrOH (0.3 M) was prepared and loaded into Hamilton gas-tight syringe (10 mL). The tubing was primed with i-PrOH and the syringe was connected to the reactor system with the aid of Microtight™ fittings. A sealed collection vial was connected to the system, where a pressurized airline (75 psi) was attached to create backpressure. A Harvard 22 syringe pump was set to deliver the reaction solution at a rate of 75 µL/min. The single mode microwave was programmed to heat constantly with the power level controlled manually so as to keep the temperature constant at the specified levels (130 W, ~100 °C). The effluent from the reactor was fed into a sealed vial and analyzed directly by 1H NMR spectroscopy immediately after the reaction. The crude reaction mixture was collected and the product was purified by flash chromatography (EtOAc–n-pentane, 2:8) to afford the desired sultam 5a (534 mg, 60%, greenish oil) and 5c (700 mg, 71%, light yellow solid).
Triazolated 1,2,5-Thiadiazepane 1,1-Dioxides 7; General Procedure
To the tertiary sulfonamide 6 (0.2 mmol, 1 equiv) was added MeOH (0.1 M), amine (0.24 mmol, 1.2 equiv), and DBU (0.04 mmol, 0.2 equiv). The solution was stirred at 40 °C for 1 h. The solvent was removed under a Techne sample concentrator. The residue was further dried under high vacuum pump for 2 h. To the residue was added CH2Cl2 (0.1 M), azide (0.4 mmol, 2 equiv), CuI (0.04 mmol, 0.2 equiv) and stirred overnight. The solvent was removed under a Techne sample concentrator and the crude product was purified by flash column chromatography on silica gel (hexane–EtOAc, 2:1).
Methyl 5-Butyl-2-{[1-(4-methylbenzyl)-1H-1,2,3-triazol-4-yl]methyl}-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (7a)
Yield: 45 mg (61%); yellow oil.
FTIR (thin film): 2953, 2930, 1747, 1329, 1144, 779, 758 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.61 (s, 1 H), 7.18 (s, 4 H), 5.47 (s, 2 H), 4.74 (d, J = 15.9 Hz, 1 H), 4.50 (d, J = 15.9 Hz, 1 H), 4.08 (t, J = 8.5 Hz, 1 H), 3.54 (s, 3 H), 3.43 (d, J = 8.6 Hz, 2 H), 3.24–3.13 (m, 2 H), 3.07–2.93 (m, 2 H), 2.52–2.39 (m, 2 H), 2.35 (s, 3 H), 1.43–1.33 (m, 2 H), 1.33–1.20 (m, 2 H), 0.91 (t, J = 7.2 Hz, 3 H).
13C NMR (126 MHz, CDCl3): δ = 170.3, 144.2, 138.7, 131.5, 129.8, 128.1, 123.2, 58.2, 55.8, 54.7, 54.4, 54.0, 52.2, 49.8, 45.7, 29.8, 21.1, 20.2, 13.9.
HRMS (TOF MS ES+): m/z calcd for C21H32N5O4S (M + H)+: 450.2175; found: 450.2160.
Methyl 5-Butyl-2-{[1-(3-chlorobenzyl)-1H-1,2,3-triazol-4-yl]methyl}-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (7b)
Yield: 51 mg (65%); colorless oil.
FTIR (thin film): 2953, 2860, 1745, 1327, 1143, 775 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.70 (s, 1 H), 7.37–7.29 (m, 2 H), 7.25 (s, 1 H), 7.18–7.15 (m, 1 H), 5.50 (s, 2 H), 4.73 (d, J = 15.9 Hz, 1 H), 4.54 (d, J = 15.9 Hz, 1 H), 4.11 (t, J = 8.5 Hz, 1 H), 3.57 (s, 3 H), 3.46–3.40 (m, 2 H), 3.19 (t, J = 5.2 Hz, 2 H), 3.05–2.96 (m, 2 H), 2.56–2.43 (m, 2 H), 1.46–1.35 (m, 2 H), 1.34–1.22 (m, 2 H), 0.92 (t, J = 7.3 Hz, 3 H).
13C NMR (126 MHz, CDCl3): δ = 170.2, 144.7, 136.6, 135.0, 130.4, 129.0, 128.0, 126.0, 123.5, 58.5, 55.8, 54.9, 54.4, 53.5, 52.3, 49.8, 45.8, 29.8, 20.2, 14.0.
HRMS (TOF MS ES+): m/z calcd for C20H29ClN5O4S (M + H)+: 470.1629; found: 470.1630.
Methyl 2-{[1-(2-Bromobenzyl)-1H-1,2,3-triazol-4-yl]methyl}-5-butyl-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (7c)
Yield: 63 mg (73%); yellow oil.
FTIR (thin film): 2953, 2930, 2860, 1747, 1329, 1144, 756 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.73 (s, 1 H), 7.62 (dd, J = 7.9, 1.1 Hz, 1 H), 7.32 (td, J = 7.5, 1.2 Hz, 1 H), 7.24 (td, J = 7.8, 1.8 Hz, 1 H), 7.18 (dd, J = 7.6, 1.5 Hz, 1 H), 5.66 (s, 2 H), 4.78 (d, J = 15.9 Hz, 1 H), 4.52 (d, J = 15.9 Hz, 1 H), 4.11 (t, J = 8.5 Hz, 1 H), 3.59 (s, 3 H), 3.44 (d, J = 8.6 Hz, 2 H), 3.26–3.14 (m, 2 H), 3.00 (dt, J = 9.1, 3.3 Hz, 2 H), 2.53–2.36 (m, 2 H), 1.43–1.33 (m, 2 H), 1.30–1.20 (m, 2 H), 0.90 (t, J = 7.3 Hz, 3 H).
13C NMR (126 MHz, CDCl3): δ = 170.3, 144.1, 134.0, 133.3, 130.5, 130.4, 128.3, 123.7, 123.5, 58.3, 55.8, 54.7, 54.5, 53.9, 52.4, 49.9, 45.6, 29.8, 20.2, 14.0.
HRMS (TOF MS ES+): m/z calcd for C20H29BrN5O4S (M + H)+: 514.1124; found: 514.1134.
Methyl 5-Benzyl-2-{[1-(4-methylbenzyl)-1H-1,2,3-triazol-4-yl]methyl}-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (7d)
Yield: 67 mg (83%); yellow oil.
FTIR (thin film): 2951, 2924, 1745, 1329, 1142, 735 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.61 (s, 1H), 7.36–7.28 (m, 3H), 7.23 (d, J = 7.0 Hz, 2H), 7.20–7.12 (m, 4H), 5.50 (s, 2H), 4.89 (d, J = 15.9 Hz, 1H), 4.49 (d, J = 15.9 Hz, 1H), 4.08 (dd, J = 10.1, 6.9 Hz, 1H), 3.67 (d, J = 13.4 Hz, 1H), 3.59 (d, J = 13.4 Hz, 1H), 3.54 (s, 3H), 3.50 (dd, J = 14.7, 10.1 Hz, 1H), 3.44 (dd, J = 14.7, 6.9 Hz, 1H), 3.24–3.09 (m, 2H), 3.05–2.90 (m, 2H), 2.34 (s, 3H).
13C NMR (126 MHz, CDCl3): δ = 170.2, 144.1, 138.8, 138.0, 131.5, 129.8, 128.6, 128.5, 128.1, 127.6, 123.2, 59.2, 58.1, 55.9, 54.5, 54.1, 52.3, 49.1, 45.5, 21.1.
HRMS (TOF MS ES+): m/z calcd for C24H29N5O4S + Na (M + Na)+: 506.1838; found: 506.1838.
Methyl 5-Benzyl-2-{[1-(3-chlorobenzyl)-1H-1,2,3-triazol-4-yl]methyl}-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (7e)
Yield: 55 mg (65%); colorless oil.
FTIR (thin film): 2951, 2833, 1747, 1327, 1142, 737 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.70 (s, 1 H), 7.38–7.24 (m, 8 H), 7.16 (d, J = 7.1 Hz, 1 H), 5.52 (s, 2 H), 4.88 (d, J = 16.0 Hz, 1 H), 4.54 (d, J = 15.9 Hz, 1 H), 4.11 (dd, J = 10.1, 7.0 Hz, 1 H), 3.72 (d, J = 13.3 Hz, 1 H), 3.64 (d, J = 13.5 Hz, 1 H), 3.56 (s, 3 H), 3.51 (dd, J = 14.9, 10.2 Hz, 1 H), 3.45 (dd, J = 14.7, 7.0 Hz, 1 H), 3.23–3.12 (m, 2 H), 3.05–2.94 (m, 2 H).
13C NMR (126 MHz, CDCl3): δ = 170.1, 144.6, 138.0, 136.5, 135.1, 130.4, 129.0, 128.6, 128.5, 128.0, 127.6, 126.0, 123.5, 59.5, 58.5, 56.0, 54.4, 53.5, 52.4, 49.2, 45.6.
HRMS (TOF MS ES+): m/z calcd for C23H26ClN5O4S + Na (M + Na)+: 526.1292; found: 526.1281.
Methyl 5-Benzyl-2-{[1-(2-bromobenzyl)-1H-1,2,3-triazol-4-yl]methyl}-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (7f)
Yield: 69 mg (74%); yellow oil.
FTIR (thin film): 2951, 2841, 1744, 1329, 1142, 737 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.74 (s, 1 H), 7.62 (d, J = 7.8 Hz, 1 H), 7.32 (m, 4 H), 7.25–7.17 (m, 4 H), 5.69 (s, 2 H), 4.93 (d, J = 15.9 Hz, 1 H), 4.52 (d, J = 15.9 Hz, 1 H), 4.11 (dd, J = 10.1, 6.9 Hz, 1 H), 3.67 (d, J = 13.4 Hz, 1 H), 3.60 (d, J = 13.4 Hz, 1 H), 3.59 (s, 3 H), 3.52 (dd, J = 14.8, 10.2 Hz, 1 H), 3.46 (dd, J = 14.9, 7.1 Hz, 1 H), 3.26–3.11 (m, 2 H), 3.06–2.92 (m, 2 H).
13C NMR (126 MHz, CDCl3): δ = 170.2, 144.0, 138.0, 133.9, 133.3, 130.5, 130.4, 128.6, 128.5, 128.2, 127.6, 123.7, 123.5, 59.1, 58.2, 55.9, 54.6, 54.0, 52.4, 49.2, 45.4.
HRMS (TOF MS ES+): m/z calcd for C23H26BrN5O4S + Na (M + Na)+: 570.0787; found: 570.0790.
Methyl 5-(2-Hydroxyethyl)-2-{[1-(4-methylbenzyl)-1H-1,2,3-triazol-4-yl]methyl}-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (7g)
Yield: 58 mg (81%); yellow oil.
FTIR (thin film): 3381, 2951, 2926, 1740, 1329, 1142, 779, 758 cm−1.
1H NMR (400 MHz, acetone-d6): δ = 8.00 (s, 1 H), 7.27 (d, J = 7.7 Hz, 2 H), 7.19 (d, J = 7.6 Hz, 2 H), 5.57 (s, 2 H), 4.85 (d, J = 15.9 Hz, 1 H), 4.44 (d, J = 16.0 Hz, 1 H), 4.17 (dd, J = 10.4, 6.9 Hz, 1 H), 3.53 (s, 3 H), 3.50–3.28 (m, 6 H), 3.19–3.04 (m, 2 H), 3.04–2.92 (m, 1 H), 2.52 (s, 2 H), 2.31 (s, 3 H).
13C NMR (126 MHz, acetone-d6): δ = 171.2, 144.5, 138.8, 134.0, 130.2, 128.9, 124.6, 60.7, 58.1, 57.3, 57.1, 55.4, 54.0, 52.2, 51.2, 45.8, 21.0.
HRMS (TOF MS ES+): m/z calcd for C19H27N5OS + Na (M + Na)+: 460.1631; found: 460.1632.
Methyl 2-{[1-(3-Chlorobenzyl)-1H-1,2,3-triazol-4-yl]methyl}-5-(2-hydroxyethyl)-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (7h)
Yield: 66 mg (87%); yellow oil.
FTIR (thin film): 3433, 2951, 2928, 1742, 1329, 1142, 777 cm−1.
1H NMR (400 MHz, acetone-d6): δ = 8.12 (s, 1 H), 7.45–7.30 (m, 4 H), 5.67 (s, 2 H), 4.86 (d, J = 16.0 Hz, 1 H), 4.47 (d, J = 16.0 Hz, 1 H), 4.20 (dd, J = 10.4, 7.0 Hz, 1 H), 3.56 (s, 3 H), 3.52–3.42 (m, 4 H), 3.40–3.32 (m, 1 H), 3.20–3.05 (m, 2 H), 3.03–2.94 (m, 1 H), 2.56 (t, J = 4.8 Hz, 2 H), 2.09 (s, 1 H).
13C NMR (126 MHz, acetone-d6): δ = 171.2, 144.7, 139.3, 134.9, 131.4, 129.1, 128.8, 127.4, 125.0, 60.7, 58.3, 57.5, 57.1, 55.4, 53.4, 52.2, 51.2, 45.8.
HRMS (TOF MS ES+): m/z calcd for C18H24ClN5O5S + Na (M + Na)+: 480.1084; found: 480.1088.
Methyl 2-{[1-(2-Bromobenzyl)-1H-1,2,3-triazol-4-yl]methyl}-5-(2-hydroxyethyl)-1,2,5-thiadiazepane-3-carboxylate 1,1-Dioxide (7i)
Yield: 62 mg (73%); yellow oil.
FTIR (thin film): 3385, 2926, 2930, 1734, 1327, 1142, 760 cm−1.
1H NMR (400 MHz, acetone-d6): δ = 8.08 (s, 1 H), 7.68 (d, J = 8.5 Hz, 1 H), 7.41 (t, J = 7.4 Hz, 1 H), 7.32 (dd, J = 10.4, 4.7 Hz, 1 H), 7.22 (d, J = 7.5 Hz, 1 H), 5.74 (s, 2 H), 4.89 (d, J = 16.0 Hz, 1 H), 4.48 (d, J = 15.9 Hz, 1 H), 4.26–4.15 (m, 1 H), 3.59 (s, 3 H), 3.56–3.35 (m, 6 H), 3.18–3.09 (m, 2 H), 3.03–2.96 (m, 1 H), 2.56 (s, 2 H).
13C NMR (126 MHz, acetone-d6): δ = 171.2, 136.0, 133.9, 131.2, 131.2, 129.6, 129.1, 125.2, 123.8, 60.7, 58.2, 57.4, 57.1, 55.4, 54.1, 52.3, 51.2, 45.8.
HRMS (TOF MS ES+): m/z calcd for C18H24BrN5O5S + Na (M + Na)+: 524.0579; found: 524.0587.
Supplementary Material
Acknowledgment
Financial support of this work was provided by the Institute of General Medical Sciences (P50-GM069663 and P41-GM076302), NIH K-INBRE funds (D.B., P20 RR016475), and the University of Kansas for an Undergraduate Research Award (D.B.), and is gratefully acknowledged.
Footnotes
Supporting Information for this article is available online at http://www.thieme-connect.com/ejournals/toc/synthesis.
References
- 1.(a) Oriez R, Prunet J. Tetrahedron Lett. 2010;51:256. [Google Scholar]; (b) Chandrasekhar S, Rambabu C, Shyamsunder T. Tetrahedron Lett. 2007;48:4683. [Google Scholar]; (c) Li M, O’Doherty GA. Org. Lett. 2006;8:6087. doi: 10.1021/ol062595u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Evans DA, Gauchet-Prunet JA. J. Org. Chem. 1993;58:2446. [Google Scholar]
- 2.(a) Ying Y, Kim H, Hong J. Org. Lett. 2011;13:796. doi: 10.1021/ol103064f. [DOI] [PubMed] [Google Scholar]; (b) Fustero S, Monteagudo S, Sánhez-Roselló M, Flores S, Barrio P, del Pozo C. Chem. Eur. J. 2010;16:9835. doi: 10.1002/chem.201000615. [DOI] [PubMed] [Google Scholar]; (c) Cai Q, Zheng C, You S-L. Angew. Chem. Int. Ed. 2010;49:8666. doi: 10.1002/anie.201003919. [DOI] [PubMed] [Google Scholar]; (d) Ghorai MK, Halder S, Das RK. J. Org. Chem. 2010;75:7061. doi: 10.1021/jo101680f. [DOI] [PubMed] [Google Scholar]; (e) Fustero S, Moscardó J, Sánchez-Roselló M, Rodríguez E, Barrio P. Org. Lett. 2010;12:5494. doi: 10.1021/ol102341n. [DOI] [PubMed] [Google Scholar]; (f) Che C, Li S, Jiang X, Quan J, Lin S, Yang Z. Org. Lett. 2010;12:4682. doi: 10.1021/ol1020477. [DOI] [PubMed] [Google Scholar]; (g) Fustero S, Catalán S, Sánchez-Roselló M, Simón-Fuentes A, del Pozo C. Org. Lett. 2010;12:3484. doi: 10.1021/ol101318t. [DOI] [PubMed] [Google Scholar]; (h) Priebbenow DL, Henderson LC, Pfeffer FM, Stewart SG. J. Org. Chem. 2010;75:1787. doi: 10.1021/jo902652h. [DOI] [PubMed] [Google Scholar]; (i) Comesse S, Sanselme M, Daïch A. J. Org. Chem. 2008;73:5566. doi: 10.1021/jo702752w. [DOI] [PubMed] [Google Scholar]; (j) Takasu K, Nishida N, Tomimura A, Ihara M. J. Org. Chem. 2005;70:3957. doi: 10.1021/jo050261x. [DOI] [PubMed] [Google Scholar]; (k) Gan Z-H, Reddy PT, Quevillon S, Couve-Bonnaire S, Arya P. Angew. Chem. Int. Ed. 2005;44:1366. doi: 10.1002/anie.200462298. [DOI] [PubMed] [Google Scholar]; (l) Carlson EC, Rathbone LK, Yang H, Collett ND, Carter RG. J. Org. Chem. 2008;73:5155. doi: 10.1021/jo800749t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Suwa T, Shibata I, Nishino K, Baba A. Org. Lett. 1999;1 [Google Scholar]
- 3.(a) Fang L, Chen Y, Huang J, Liu L, Quan J, Li C-C, Yang Z. J. Org. Chem. 2011;76:2479. doi: 10.1021/jo102202t. [DOI] [PubMed] [Google Scholar]; (b) Bates RW, Song P. Synthesis. 2010:2935. [Google Scholar]; (c) Rao GS, Sudhakar N, Rao BV, Basha SJ. Tetrahedron: Asymmetry. 2010;21:1963. [Google Scholar]; (d) Enders D, Wang C, Greb A. Adv. Synth. Catal. 2010;352:987. [Google Scholar]; (e) Wang H-F, Cui H-F, Chai Z, Li P, Zheng C-W, Yang Y-Q, Zhao G. Chem. Eur. J. 2009;15:13299. doi: 10.1002/chem.200902303. [DOI] [PubMed] [Google Scholar]; (f) Reyes E, Talavera G, Vicario JL, Badía D, Carrillo L. Angew. Chem. Int. Ed. 2009;48:5701. doi: 10.1002/anie.200901333. [DOI] [PubMed] [Google Scholar]; (g) Wang L-J, Liu X-W, Dong Z-H, Fu X, Feng X-M. Angew. Chem. Int. Ed. 2008;47:8670. doi: 10.1002/anie.200803326. [DOI] [PubMed] [Google Scholar]; (h) Bates RH, Shotwell JB, Roush WR. Org. Lett. 2008;10:4343. doi: 10.1021/ol801852j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Zhou A, Hanson PR. Org. Lett. 2008;10:2951. doi: 10.1021/ol8009072. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Saito N, Ryoda A, Nakanishi W, Kumamoto T, Ishikawa T. Eur. J. Org. Chem. 2008:2759. [Google Scholar]; (k) Baker-Glenn C, Hodnett N, Reiter M, Ropp S, Ancliff R, Gouverneur V. J. Am. Chem. Soc. 2005;127:1481. doi: 10.1021/ja043925d. [DOI] [PubMed] [Google Scholar]; (l) Betancort JM, Martín VS, Padrón JM, Palazón JM, Ramírez MA, Soler MA. J. Org. Chem. 1997;62:4570. [Google Scholar]; (m) Bhaket P, Morris K, Stauffer CS, Datta A. Org. Lett. 2005;7:875. doi: 10.1021/ol0473290. [DOI] [PubMed] [Google Scholar]; (n) Volz N, Bröhmer MC, Toräng J, Nieger M, Bräse S. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2009;48:1699. [Google Scholar]; (o) Nicolaou KC, Li A. Angew. Chem. Int. Ed. 2008;47:6579. doi: 10.1002/anie.200802632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Yu C, Zhang Y, Song A, Ji Y, Wang W. Chem. Eur. J. 2011;17:770. doi: 10.1002/chem.201002384. [DOI] [PubMed] [Google Scholar]; (b) Wang XF, Hua Q-L, Cheng Y, An X-L, Yang Q-Q, Chen J-R, Xiao W-J. Angew. Chem. Int. Ed. 2010;49:8379. doi: 10.1002/anie.201004534. [DOI] [PubMed] [Google Scholar]; (c) Enders D, Schmid B, Erdmann N, Raabe G. Synthesis. 2010:2271. [Google Scholar]; (d) Tan DQ, Martin KS, Fettinger JC, Shaw JT. Proc. Natl. Acad. Sci. U.S.A. 2011;108:6781. doi: 10.1073/pnas.1015261108. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) González-López M, Shaw JT. Chem. Rev. 2009;109:164. doi: 10.1021/cr8002714. and references contained therein. [DOI] [PubMed] [Google Scholar]
- 5.(a) Mendoza A, Pardo P, Rodríguez F, Fañanás FJ. Chem. Eur. J. 2010;16:9758. doi: 10.1002/chem.201001109. [DOI] [PubMed] [Google Scholar]; (b) Choi PJ, Rathwell DCK, Brimble MA. Tetrahedron Lett. 2009;50:3245. [Google Scholar]; (c) Rauhala V, Nättinen K, Rissanen K, Koskinen AMP. Eur. J. Org. Chem. 2005:4119. [Google Scholar]; (d) Forsyth CJ, Hao J-L, Aiguade J. Angew. Chem. Int. Ed. 2001;40:3663. doi: 10.1002/1521-3773(20011001)40:19<3663::aid-anie3663>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; (e) Hao J-L, Forsyth CJ. Tetrahedron Lett. 2002;43:1. [Google Scholar]; (f) Aiguade J, Hao J-L, Forsyth CJ. Org. Lett. 2001;3:979. [PubMed] [Google Scholar]; (g) Cordeiro A, Jimeno ML, Maestro MA, Camarasa M-J, Quesada E, San-Félix AJ. Org. Chem. 2007;72:9713. doi: 10.1021/jo701775a. [DOI] [PubMed] [Google Scholar]
- 6.For recent examples of double aza-Michael reactions, see: Liu S-W, Hsu H-C, Chang C-H, Tsai H-HG, Hou D-R. Eur. J. Org. Chem. 2010:4771. Han JH, Choi J, Jun YM, Lee BM, Kim BH. Heterocycles. 2010;81:317. Enders D, Narine AA, Toulgoat F, Biscchops T. Angew. Chem. Int. Ed. 2008;47:5661. doi: 10.1002/anie.200801354. Krishna PR, Sreeshailam A. Tetrahedron Lett. 2007;48:6924. Yun H, Gagnon A, Danishefsky SJ. Tetrahedron Lett. 2006;47:5311. Rulev AY, Yenil N, Pesquet A, Oulyadi H, Maddaluno J. Tetrahedron. 2006;62:5411. Rosiak A, Hoenke C, Christoffers J. Eur. J. Org. Chem. 2007:4376. Zou W, Sandbhor M, Bhasin M. J. Org. Chem. 2007;72:1226. doi: 10.1021/jo062057v.
- 7.Click chemistry is a chemical philosophy introduced by K. Barry Sharpless in 2001 that describes chemistry tailored to generate substances quickly and reliably by joining small units together, see: Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5.
- 8.(a) Austin CP. Curr. Opin. Chem. Biol. 2003;7:511. doi: 10.1016/s1367-5931(03)00083-8. [DOI] [PubMed] [Google Scholar]; (b) Hopkins AL, Groom CR. Nat. Rev. Drug Discov. 2002;1:727. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]; (c) Drews J. Nat. Biotechnol. 1996;14:1516. doi: 10.1038/nbt1196-1516. [DOI] [PubMed] [Google Scholar]
- 9.(a) Drews J. Science. 2000;287:1960. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]; (b) Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Curr. Med. Chem. 2003;10:925. doi: 10.2174/0929867033457647. [DOI] [PubMed] [Google Scholar]
- 10.Arranz ME, Díaz JA, Ingate ST, Witvrouw M, Pannecouque C, Balzarini J, De Clercq E, Vega S. Bioorg. Med. Chem. 1999;7:2811. doi: 10.1016/s0968-0896(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 11.Giannotti D, Viti G, Sbraci P, Pestellini V, Volterra G, Borsini F, Lecci A, Meli A, Dapporto P, Paoli P. J. Med. Chem. 1991;34:1356. doi: 10.1021/jm00108a018. [DOI] [PubMed] [Google Scholar]
- 12.Combrink KD, Gulgeze HB, Thuring JW, Yu K-L, Civiello RL, Zhang Y, Pearce BC, Yin Z, Langley DR, Kadow KF, Cianci CW, Li Z, Clarke J, Genovesi EV, Medina I, Lamb L, Yang Z, Zadjura L, Krystal M, Meanwell NA. Bioorg. Med. Chem. Lett. 2007;17:4784. doi: 10.1016/j.bmcl.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 13.Cherney RJ, Duan JJ, Voss ME, Chen L, Wang L, Meyer DT, Wasserman ZR, Hardman KD, Li R-Q, Covington MB, Qian M, Mandlekar S, Christ DD, Trzaskos JM, Newton RC, Magolda RL, Wexler RR, Decicco CP. J. Med. Chem. 2003;46:1811. doi: 10.1021/jm020475w. [DOI] [PubMed] [Google Scholar]
- 14.Cheng M, De B, Pikul S, Almstead NG, Natchus MG, Anastasio MV, McPhail SJ, Snider CE, Taiwo YO, Chen L, Dunaway CM, Gu F, Dowty ME, Mieling GE, Janusz MJ, Wang-Weigand S. J. Med. Chem. 2000;43:369. doi: 10.1021/jm990366q. [DOI] [PubMed] [Google Scholar]
- 15.(a) Fenster E, Long TR, Zang Q, Hill D, Neuenswander B, Lushington GH, Zhou A, Santini C, Hanson PR. ACS Comb. Sci. 2011;13:244. doi: 10.1021/co100060x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhou A, Rayabarapu D, Hanson PR. Org. Lett. 2009;11:531. doi: 10.1021/ol802467f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barends TRM, Dunn MF, Schlichting I. Curr. Opin. Chem. Biol. 2008;12:593. doi: 10.1016/j.cbpa.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 17.For prior applications of MACOS technologies, please see: Shore G, Yoo W-J, Li C-J, Organ MG. Chem. Eur. J. 2010;16:126. doi: 10.1002/chem.200902396. Ullah F, Samarakoon T, Rolfe A, Kurtz RD, Hanson PR, Organ MG. Chem. Eur. J. 2010;16:10959. doi: 10.1002/chem.201001651. Shore G, Organ MG. Chem. Commun. 2008:838. doi: 10.1039/b715709f. Shore G, Organ MG. Chem. Eur. J. 2008;14:9641. doi: 10.1002/chem.200801610. Shore G, Morin S, Mallik D, Organ MG. Chem. Eur. J. 2008;14:1351. doi: 10.1002/chem.200701588. Bremner WS, Organ MG. J. Comb. Chem. 2007;9:14. doi: 10.1021/cc060130p. Shore G, Morin S, Organ MG. Angew. Chem. Int. Ed. 2006;45:2761. doi: 10.1002/anie.200503600. Comer E, Organ MG. J. Am. Chem. Soc. 2005;127:8160. doi: 10.1021/ja0512069.
- 18.Novachek KA, Meyers AI. Tetrahedron Lett. 1996;37:1743. [Google Scholar]
- 19.For examples of direct elimination of serinol silyl ether, see: Monteiro LS, Kołomańska J, Suarez AC. Eur. J. Org. Chem. 2010;35:6731. Falb E, Bechor Y, Nudelman A, Hassner A, Albeck A, Gottlieb HE. J. Org. Chem. 1999;64:498.
- 20.For examples of direct elimination of Boc-protected serinol esters, see: Gembus V, Marsais F, Levacher V. Synlett. 2008:1463. Varnes JG, Lehr GS, Moore GL, Hulsizer JM, Albert JS. Tetrahedron Lett. 2010;51:3756. Ramesh R, De K, Chandrasekaran S. Tetrahedron. 2007;63:10534. Ferreira PMT, Monteiro LS, Pereira G, Ribeiro L, Sacramento J, Silva L. Eur. J. Org. Chem. 2007;35:5934.
- 21.For the recent review on Cu-catalyzed azide-alkyne cycloaddition, see: Meldal M, Tornøe CW. Chem. Rev. 2008;108:2952. doi: 10.1021/cr0783479. and references cited therein.
- 22.Grubbs RH, Rosen RK, Timmers FJ. Organometallics. 1996;15:1518. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.