Abstract
Recent phylogenetic analyses imply a diphyly of tree sloths and a convergent evolution of their obligatory suspensory locomotion. In mammals the extrinsic shoulder musculature forms a ‘muscular sling’ to support the trunk in quadrupedal postures. In addition, the extrinsic pectoral muscles are responsible for moving the proximal forelimb elements during locomotion. Due to the inverse orientation of the body in regard to the gravitational force, the muscular sling as configured as in pronograde mammals is unsuited to suspend the weight of the thorax in sloths. We here review the muscular topography of the shoulder in Choloepus didactylus and Bradypus variegatus in the light of presumably convergent evolution to adapt to the altered functional demands of the inverse orientation of the body. In addition, we venture to deduce the effect of the shoulder musculature of C. didactylus during locomotion based on previously published 3D kinematic data. Finally, we assess likely convergences in the muscular topography of both extant sloth lineages to test the hypothesis that convergent evolution is reflected by differing morphological solutions to the same functional demands posed by the suspensory posture. Muscular topography of the shoulder in C. didactylus is altered from the plesiomorphic condition of pronograde mammals, whereas the shoulder in B. variegatus more closely resembles the general pattern. Overall kinematics as well as the muscles suitable for pro- and retraction of the forelimb were found to be largely comparable to pronograde mammals in C. didactylus. We conclude that most of the peculiar topography of extrinsic forelimb musculature can be attributed to the inverse orientation of the body. These characteristics are often similar in both genera, but we also identified different morphological solutions that evolved to satisfy the new functional demands and are indicative of convergent evolution. We suggest that the shared phylogenetic heritage canalized the spectrum of possible solutions to new functional demands, and digging adaptations of early xenarthrans posed morphological constraints that resulted in similar suspensory postures. The data of this study, including muscle maps, will be helpful to infer locomotor characteristics of fossil sloths.
Keywords: Bradypus, Choloepus, convergent evolution, locomotion, posture, shoulder, Xenarthra
Introduction
Recent morphological and molecular phylogenetic studies point to a long history of independent evolution of the two lines leading to extant tree sloths and only a distant relationship between the two remaining extant genera Choloepus and Bradypus (Höss et al. 1996; Delsuc et al. 2001; Greenwood et al. 2001; Poinar et al. 2003; Gaudin, 2004; Pujos et al. 2007). Some of the molecular phylogenetic studies even make use of ancient DNA recovered from extinct forms (Höss et al. 1996; Greenwood et al. 2001; Poinar et al. 2003). The results of these phylogenetic studies imply a convergent evolution of the quadrupedal suspensory posture and locomotion of modern sloths (Gaudin, 2004; Gaudin & McDonald, 2008).
In comparison with monotremes, the therian pectoral girdle is not rigid but extensively mobilized. A mobile and often even completely reduced clavicle represents the only remaining skeletal connection between the thorax and the forelimbs (e.g. Eaton, 1944). Hence, the extrinsic shoulder musculature has to fulfill two important roles during locomotion: (i) suspending the weight of the thorax between the forelimbs and (ii) moving the skeletal elements of the proximal forelimbs (scapula and humerus) (e.g. Jenkins & Weijs, 1979; Carrier et al. 2006). In pronograde therian mammals the former is achieved by the extrinsic shoulder musculature forming a ‘muscular sling’ (Davis, 1949), which is composed of three primary muscles: the m. rhomboideus, the m. serratus ventralis, and the m. pectoralis. Specifically, the m. serratus ventralis thoracis has been deduced from its attachment sites and orientation to predominantly or even entirely be responsible for the support of body mass at the forelimbs. This hypothesis has been experimentally validated in an electromyographic (EMG) study on trotting dogs (Carrier et al. 2006), an aclaviculate mammal adapted for sustained running. However, because of the lines of action of the muscles forming the ‘muscular sling’ these muscles are at least partly unsuited to support body mass at the forelimbs in the quadrupedal suspensory posture of sloths unless profound morphological changes would have taken place during the evolution of modern sloths (Fig. 1).
Fig. 1.
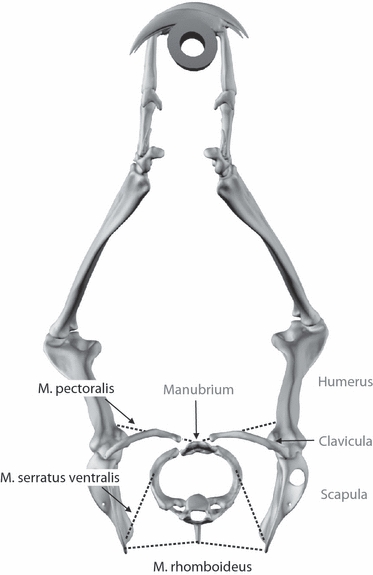
Frontal view of the orientation of the limbs in relation to the thorax in Choloepus didactylus reconstructed after x-ray images. Dotted lines represent primary muscles of the ‘muscular sling’ in the pectoral girdle of a generalized pronograde therian mammal. Only the first thoracic vertebra, rib, and the frontal part of the manubrium sterni are shown. There are no articulating surfaces in the sternoclavicular joint (see text). M. serratus ventralis and m. rhomboideus are unsuited to suspend the thorax between the limbs of C. didactylus.
Furthermore, whereas in aclaviculate mammals movements of the scapula are mostly restricted to the parasagittal plane, scapular movements are much more complex in claviculate therians (Jenkins, 1974; Schmidt et al. 2002; Nyakatura & Fischer, 2010a). The orientation of the proximal forelimb elements relative to the thorax changes considerably during the contact phase of a limb. Thus different muscles of the ‘muscular sling’ could be suitable to support body weight at the forelimbs at different instants of the contact phase. Since sloths have highly mobile forelimbs, similar to primates (Grand, 1972; Mendel, 1985), the three-dimensional (3D) kinematic data of the shoulder region of sloths from high-speed x-ray recordings (Nyakatura et al. 2010; Nyakatura & Fischer, 2010a) provide an adequate basis to interpret the muscular topography of two-toed sloths (Xenarthra: Choloepus didactylus, Linné 1758) for improving our understanding of the function of the ‘muscular sling’ in sloths.
Descriptions of the myology of the shoulder in two- and three-toed sloths are often contradictory or incomplete and thus we find it necessary to review, confirm and extend available anatomical description of the shoulder girdle. We chose to use a three-step approach. First, we wanted to document myological topography of the extrinsic and intrinsic shoulder musculature of C. didactylus and B. variegatus and identify similarities and differences. Secondly, we wanted to use the in vivo three-dimensional kinematic data of skeletal shoulder elements during steady-state locomotion of C. didactylus to interpret the myology regarding its role in weight suspension of the thorax and movement of the proximal skeletal elements of the forelimbs in two-toed sloths. Finally, we wanted to identify possible convergent solutions to the functional demands in regard to the generation of propulsion and weight bearing at the shoulder in the two distantly related modern sloth lineages. Specifically, we here test the hypothesis that the proposed convergent evolution of similar functional capacity, i.e. quadrupedal suspensory locomotion, is reflected by functionally significant muscular specializations that are realized differently in the two modern lineages of tree sloths.
Materials and methods
All the experiments and procedures were registered with the Committee for Animal Protection of the State of Thuringia, Germany, and were conducted in accordance with its guidelines (Reg.-No.: 02-08/04).
Myological preparation
No sloths were sacrificed for this study. We received two frozen carcasses of adult female C. didactylus from zoos in Paris, France, and Dresden, Germany. Both specimens died a natural death and were available for the anatomical investigation. The subjects were formalin fixated after skin was removed. The specimen of Bradypus is from the anatomical collection of the Phyletisches Museum Jena (Mam 3842) and was conserved in alcohol. Unfortunately, the species name of the specimen as well as its place and date of origin was not documented, but the specimen was determined as B. variegatus according to the identification key of Wetzel (1985) based on the absence of foramina in the antero-dorsal nasopharynx of the skull and the suborbital stripe outlining paler color of the ocular area of the face. We carefully documented the different stages of the dissection with photographs and drawings. Muscles were identified using the criterion of innervation following nomenclature of Evans (1993) and according to earlier publications of myological description (C. didactylus: Humphry, 1869; Mackintosh, 1875; Lucae, 1882; Windle & Parsons, 1899; Miller, 1935; Mendel, 1985; B. variegatus: Humphry, 1869; Mackintosh, 1870; Windle & Parsons, 1899; Meincke, 1911; Miller, 1935).
X-ray motion analysis
The experimental setup for the high-speed x-ray motion analysis and the methodology of the x-ray reconstruction of moving morphology approach (XROMM; Brainerd et al. 2010) has been described extensively in previous publications (Nyakatura et al. 2010; Nyakatura & Fischer, 2010a,b;). In short, three C. didactylus were trained to move along a simulated branch (wooden pole 4 m long; 40 mm in diameter) at their preferred speeds using positive motivation via food rewards. Locomotion was filmed with two synchronized high-speed x-ray cameras and an additional standard light camera (also synchronized) filming the scene at 300 frames per second. The two x-ray cameras were oriented at the dorso-ventral and the lateral perspective and the covered 3D space was calibrated with a 20 × 12 cm calibration object that has metal beads inserted at regular intervals. To obtain three-dimensional kinematic data, two subjects were trained to move along a motorized version of the simulated branch. We made use of the non-invasive, markerless XROMM method termed ‘scientific rotoscoping’ (Gatesy et al. 2010). This method combines reconstructions of skeletal elements from CT bone scans and the high-speed x-ray videos to realistically animate and measure the kinematics of internal skeletal structures. Quantitative data is available in previous publications (Nyakatura et al. 2010; Nyakatura & Fischer, 2010a). We here will use qualitative description of the occurring movements based on the data of these studies.
Limitations of the data as collected and presented
Our data, i.e. an integration of a detailed anatomical investigation with high resolution 3D kinematics, does not provide direct evidence for muscle activity. Since the three C. didactylus individuals used for the in vivo motion analysis (Nyakatura et al. 2010; Nyakatura & Fischer, 2010a,b;) were valuable loans from the Dortmund Zoo, invasive EMG recordings to collect direct data were not aspired to in this study. Instead, we gathered substantial indirect evidence to deduce the role of muscles during locomotion using a non-invasive approach. To distinguish our dataset from direct measurements of muscle activity via EMG recordings, we here use the term muscle effect, which is deduced indirectly from the anatomy and observed 3D motion. Furthermore, we discuss whether a deduced effect is consistent with published data on muscle activity from other arboreal specialists. This means, we here venture a step of plausibility to address the question of which muscles are adequate to fulfill the two primary roles of the extrinsic shoulder musculature during quadrupedal suspensory locomotion in C. didactylus during the course of a step cycle. In depth motion analysis is necessary, because complex 3D movements at the pectoral girdle have been described not only for sloths (Nyakatura & Fischer, 2010a) but also for various other mammalian taxa including terrestrial species (Jenkins & Weijs, 1979) and arboreal specialists (e.g. Jenkins et al. 1978; Schmidt et al. 2002). Our non-invasive study of muscle effects is thus complementary to direct measurements of muscle activity via EMG.
Obviously this study would greatly benefit if the motion analysis could have been performed for both genera. But Bradypus is not held captive in Europe and was not available for the x-ray motion analysis. Therefore, interpretation of muscle effects is restricted to two-toed sloths.
Results
Morphology
We here describe the morphology of the pectoral girdle in C. didactylus and compare it to B. variegatus. Often we found published literature incomplete or contradictory. In these cases we report opposing views.
Skeletal anatomy of pectoral girdle
The thorax of C. didactylus has 23–24 ribs, of which 12 are connected directly to the sternum. In the cranial direction the thorax tapers considerably. The well developed clavicle does not have an articulating facet on its sternal end. Rather, the sterno-clavicular joint is composed of connective tissue between clavicle and manubrium sterni (see below). As described earlier, this joint allows virtually all degrees of freedom when the clavicle is stripped of attaching muscles (Mendel, 1985). The slightly curved clavicle articulates to the scapula in a small notch at the widely flared acromial process. Fused to the coracoid process, the acromion forms an arch that is extended cranially beyond the humeral head. It provides prominent attachment sites for the m. trapezius, m. subclavius, and m. deltoideus. The scapula is thin and the spina scapulae is short and does not reach the vertebral border. The caudal border is characterized by a broad attachment site for the well developed m. teres major. The scapula is comparatively small (Nyakatura & Fischer, 2010a). The gleno-humeral joint is marked by a shallow fossa glenoidalis and round humeral head. There is a considerable incongruity between both articulating surfaces.
The thorax of B. variegatus has 14 ribs, of which 11–12 are connected directly to the sternum. As in C. didactylus the thorax of B. variegatus tapers considerably in cranial direction. The clavicle is rudimentary and covers only half of the distance from the acromion to the manubrium, and, as in C. didactylus, is connected to it by a ligament. In contrast to C. didactylus the coracoid process is not fused to the acromion in B. variegatus. Instead, another ligament connects the ends of these to bony elements (coraco-acromial ligament). The scapula of B. variegatus is comparable to that of C. didactylus in that it is also small, the spina does not quite extend to the vertebral border, and there is a prominent attachment site of the m. teres major. As in C. didactylus there is considerable incongruity between the articulating surfaces of the fossa glenoidalis and the humeral head.
Extrinsic shoulder musculature
In C. didactylus the m. trapezius originates along the mid-line of the neck between the occiput and an aponeurosis at the eighth thoracic spine. The cervical and thoracic portions can be separated due to a large intramuscular hiatus of the dorsal fascia in the area dorsal to the fossa infraspinata of the scapula. M. trapezius pars thoracis inserts via the dorsal fascia along the proximal spina scapulae and parts of the margo dorsalis of the scapula, whereas the pars cervicis inserts along the whole length of the short spina scapulae, the flared acromion and dorso-lateral surface of the clavicle (Fig. 2).
Fig. 2.
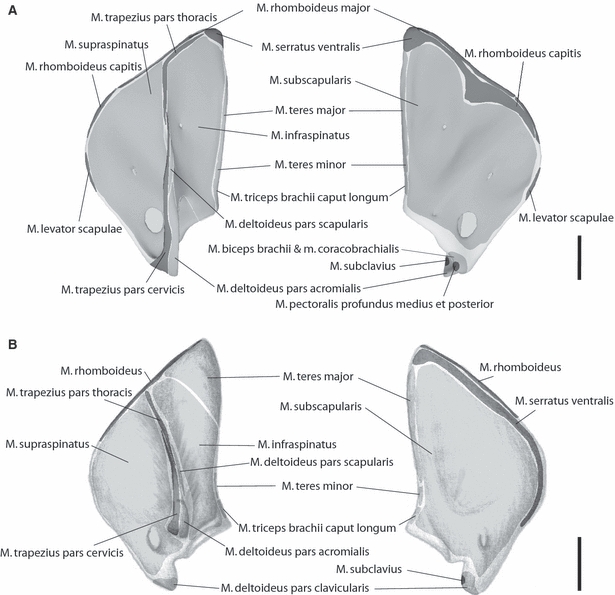
Muscle maps of the shoulder musculature attaching to the scapula. (a) Choloepus didactylus; (b) Bradypus variegatus; left: lateral aspect of a left scapula; right: medial aspect of a left scapula. Dark grey: attachment areas of extrinsic shoulder musculature; light grey: attachment areas of intrinsic shoulder musculature. Scale bar = 2 cm. Further explanations see text.
In accordance with the description of Windle & Parsons (1899), in our specimen the m. trapezius of B. variegatus has a similar topography to that described here for C. didactylus, but does not reach the occiput. A cervical portion can also be distinguished from a thoracic one by a large intra-muscular hiatus. The origin of the muscle extends from the lower five cervical spines to the sixth thoracic spine and it inserts along the whole length of the spina scapulae, the coraco-acromial ligamentum and the cranial aspect of the short clavicle. In contrast, Miller (1935) found no cervical portion in her specimen. In accordance with the specimens studied by Windle & Parsons (1899) and Mackintosh (1870), we found that the most anterior fibers continued into the clavicular head of the m. deltoideus (see below).
In C. didactylus, m. rhomboideus is divisible into two parts. M. rhomboideus major arises from the last cervical and first three thoracic vertebrae. It inserts along the whole margo vertebralis of the scapula and is made up of parallel fibers that span the length of the muscle (Fig. 2). Anterior to this muscle we find a thin occipital portion (m. rhomboideus capitis) that was also present in the specimens studied by Lucae (1882) and Windle & Parsons (1899). It has its origin at the occiput and inserts at the superior angle of the shoulder blade. A third distinct portion under the m. rhomboideus major as described by Mackintosh (1875) is not found in our individuals, in agreement with Lucae (1882).
In our specimen of B. variegatus no occipital portion of the m. rhomboideus is present. This is in line with the findings of Mackintosh (1870) and Windle & Parsons (1899).
In C. didactylus, m. latissimus dorsi (Fig. 5) arises from the fourth to seventh thoracic vertebrae, from the 11th to 15th ribs, and from the fascia lumbodorsalis. The muscle runs over the dorsal side of the scapula and the long fibers converge towards the insertion. The attachment is on the medial side of the proximal humerus in a broad tendon distal to the tuberculum minus and medial to the well separated attachment site of the m. teres major (Fig. 3).
Fig. 5.

Superficial muscles of the shoulder region of Choloepus didactylus (A) and Bradypus variegatus (B). Ventral aspect. 1: M. pectoralis superficialis anterior; 2: M. pectoralis superficialis posterior; 3: M. pectoralis profundus medius; 4: M. pectoralis profundus posterior; 5: M. latissimus dorsi; 6: M. dorso-epitrochlearis; 7: M. deltoideus; 8: M. sternomastoideus; 9: M. cleidomastoideus; 10: M. deltoideus pars clavicularis; 11: M. deltoideus pars acromialis; 12: M. biceps brachii anterior belly; 13: M. biceps brachii caudal belly; 14: Common tendon of 2, 11, 12; 15: M. coracobrachialis; 16: M. serratus ventralis; 17: M. pectoralis superficialis; 18: M. triceps brachii caput mediale.
Fig. 3.

Muscle maps of shoulder muscles attaching to the humerus. (A) Choloepus didactylus; (B) Bradypus variegatus; left: caudal aspect; right: cranial aspect. Dark grey: extrinsic shoulder musculature; light grey: intrinsic shoulder musculature. Scale bar: 2 cm. For frther explanations see text.
Similarly, the m. latissimus dorsi of our B. variegatus has its origin from the posterior half of the thoracic spines, the fascia lumbodorsalis, and the fifth to 12th ribs. It attaches to its common site at the medial side of the proximal humerus. As in C. didactylus, the attachment is via a broad tendon which is separated from the fleshy insertion of the m. teres major.
In C. didactylus, proximal to the insertion of the m. latissimus dorsi its fibers are inseparable from those of the m. dorso-epitrochlearis (Fig. 5). This comparable large parallel-fibered muscle runs medially alongside the much more slender caput longum of m. triceps brachii on the dorsal side of the upper arm and inserts on the epicondylus medialis into the anterior ridge of the supracondyloid foramen. Due to its fleshy origin from the m. latissimus dorsi, this muscle was identified as m. latissimus dorsi pars brachialis by Lucae (1882) and m. latissimo-olecranalis by Windle & Parsons (1899). However, it is innervated by nervus radialis and can thus be hypothesized to be a derivate of the m. triceps brachii.
Although present, the same muscle is much more slender in B. variegatus. As there is no supracondyloid foramen, the muscle inserts at the supracondylar ridge. Our specimen is in line with that of Meincke (1911) but, in contrast, Mackintosh (1875) notes that the muscle originates at the triceps.
The m. pectoralis in C. didactylus is characterized by a complicated architecture (Fig. 5). We find five clearly separated portions, all innervated by branches of n. pectoralis. Superficially, m. pectoralis superficialis anterior arises from the cranial tip of the manubrium sterni and m. pectoralis superficialis posterior originates adjacent to the first, slightly more caudal from the manubrium sterni and most cranial sternum. The former inserts in a short broad tendon on the tuberculum majus and along the proximal part of the pectoral crest of the humerus directly underneath the clavicular part of the m. deltoideus (Fig. 3). It was termed m. pectoralis minor by Lucae (1882), m. pectoralis major by Miller (1935), and was not described by Mackintosh (1875). M. pectoralis superficialis posterior, composed of long parallel fibers, runs medially down the upper arm and eventually forms a common tendon with the m. deltoideus pars acromialis. This tendon subsequently joins the anterior belly of m. biceps brachii and inserts on the tuberositas of the proximal radius (Fig. 4). This superficial portion of the m. pectoralis was identified in all earlier descriptions and has also been termed bicipital head or gladiolar head of m. pectoralis by Mackintosh (1875) and Mendel (1985), respectively. We here refer to the general terminology used by Evans (1993). Beneath the m. pectoralis superficialis, we find three layered portions of the m. pectoralis profundus, in agreement with the observation of Miller (1935). The most superficial of which, the m. pectoralis profundus anterior, originates underneath the m. pectoralis superficialis posterior on the manubrium sterni and cranial sternum and attaches to the pectoral crest under m. pectoralis superficialis anterior, but extends more distally (to about half the length of the humerus). Its fibers are aligned in parallel. The m. pectoralis profundus medius has the broadest area of origin (the whole length of the sternum); its cranial origin lies underneath the origin of m. pectoralis profundus anterior, and its fibers converge distally. The muscle attaches more proximally than the former with a short broad tendon on the proximal pectoral crest and the tuberculum majus of the humerus, on the shoulder joint capsule underneath the m. pectoralis superficialis anterior, and at the processus coracoideus of the scapula (Figs 2 and 3). The deepest layer, the m. pectoralis profundus posterior, arises from the eighth to 11th ribs, runs as a slender muscle underneath m. pectoralis profundus medius and joins its inserting tendon on the proximal side. The three parts of the m. pectoralis profundus have different orientations, from approximately medio-lateral (m. pectoralis profundus anterior) to almost cranio-caudal (m. pectoralis profundus posterior).
Fig. 4.
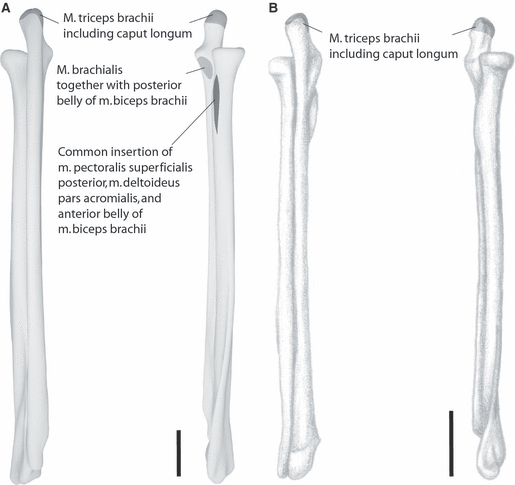
Muscle maps of shoulder muscles attaching to the forearm. (A) Choloepus didactylus; (B) Bradypus variegatus; left: caudal aspect; right: cranial aspect. Dark grey: extrinsic shoulder musculature; light grey: intrinsic shoulder musculature. Scale bar: 2 cm. For further explanations see text.
In Bradypus the m. pectoralis is less complex. In our specimen we discerned three portions. Superficially, the m. pectoralis superficialis originates from the whole length of the sternum including the manubrium. The fibers of this relatively flat sheet converge and insert along the pectoral crest at the proximal half of the humerus. It is important to note that although no insertion at the forearm is present as described above for C. didactylus, the m. pectoralis superficialis exchanges some fibers with the anterior belly of the m. biceps brachii. M. pectoralis profundus comprises two portions in B. variegatus. Cranially, the m. pectoralis profundus anterior originates at the manubrium and attaches deep to the superficial sheet at the proximal pectoral crest. The m. pectoralis profundus posterior (named m. pectoralis quartus by Mackintosh, 1870 and Meincke, 1911) arises from the sixth to 10th ribs laterally from the m. pectoralis profundus anterior, but ventral to the m. lattisimus dorsi. In contrast to Humphry (1869), we did not find it originating from the m. rectus abdominis. It attaches proximal and deep to the other pectoral portions at the tuberculum majus of the humerus and the shoulder joint capsule. As in C. didactylus, the portion that arises most caudally has the most proximal insertion.
In C. didactylus, the m. serratus ventralis can be differentiated into a cervical and a thoracic part. The origin of the cervical part extends from the third to seventh (and last) cervical transverse processes, whereas the thoracic part arises from the first eight ribs. Both parts insert on the margo vertebralis of the scapula. Our results correspond to Windle & Parsons (1899), but a cervical part was not found in the specimen of Miller (1935).
In B. variegatus, the m. serratus ventralis can also be differentiated into a cervical and a thoracic part. The cervical part, however, is weakly developed and has its origin at the transverse processes of the three most caudal cervical vertebrae. The thoracic part arises from the first eight ribs as in C. didactylus. Both parts attach to the whole length of the vertebral border of the scapula. Miller (1935) did not mention a cervical part in the description of her specimen.
M. levator scapulae is a thin slender muscle that arises from the processus mastoideus and attaches to the margo cranialis of the scapula in C. didactylus (Fig. 2). We did not find a corresponding muscle in B. variegates, in accordance with the earlier studies (Mackintosh, 1870; Windle & Parsons, 1899; Miller, 1935).
In C. didactylus, a slender m. subclavius originates on the sternal end of the first rib and attaches to the inner border of the acromio-clavicular joint (Fig. 2). The muscle is also present in B. variegatus. It has the same origin, but inserts at the coracoid process and at the under side of the clavicle.
In C. didactylus, m. sternomastoideus and m. cleidomastoideus attach to the processus mastoideus and expand to the sterno-clavicular articulation and the clavicle, respectively (Fig. 5). We did not find these muscles separated in our specimen of B. variegatus. The muscle exchanges some fibers with the m. pectoralis superficialis. We could not determine the attachment site of the muscle due to damage of the specimen.
Intrinsic shoulder musculature
Although it was found to be restricted to its clavicular origin by Miller (1935), we found a m. deltoideus separable into the usual three parts in C. didactylus, in agreement with Mackintosh (1875) and Lucae (1882): partes clavicularis, acromialis, and scapularis. Whereas the clavicular and scapular parts have the common attachment sites at the deltoid crest of the humerus (Fig. 3), the m. deltoideus acromialis forms a common tendon with the m. pectoralis superficialis posterior and more distally joins the anterior belly of m. biceps brachii to have a common insertion on the radius (Fig. 4).
In B. variegatus, it is more difficult to distinguish into the three parts of the m. deltoideus arising from the spina scapulae, the acromion, and the clavicle and coraco-acromial ligament, respectively. The most anterior fibers of the clavicular part continue into the cervical part of the m. trapezius. These were recognized as m. cephalo-humeral by Windle & Parsons (1899) and have also been described by Mackintosh (1870) and Meincke (1911). It is important to note that the acromial part of the m. deltoideus exchanges fibers with anterior belly of the m. biceps brachii.
In C. didactylus the m. supraspinatus arises from the fossa supraspinata and attaches to the tuberculum majus. M. infraspinatus originates from the fossa infraspinata and the spina scapulae and inserts distally adjacent to the aforementioned muscle on the tuberculum majus of the humerus as well (Figs 2 and 3). M. subscapularis, arising from the fossa subscapularis of the scapula, attaches to the tuberculum minus. Mackintosh (1875) noted that its weight is about the same combined weight of m. supraspinatus and m. infraspinatus (Figs 2 and 3). These muscles are also present in a similar manner in B. variegatus.
In C. didactylus the m. biceps brachii arises in a single long flat tendon from the processus coracoideus of the scapula and splits into an anterior and a posterior belly (Fig. 5). The anterior belly joins the common tendon of m. deltoideus and m. pectoralis superficialis posterior and attaches to the tuberositas on the proximal radius (Fig. 4). The posterior belly fuses with m. brachialis and attaches to the ulna (Fig. 4). In B. variegatus, no coracoidal head is present and the origin is only on the humerus. We include it here because the m. pectoralis superficialis and the m. deltoideus pars acromialis both exchange fibers with the anterior belly, which inserts on the inner side of the forearm at the ulna.
M. teres major is relatively large in C. didactylus, arises at the margo caudalis of the scapula, and inserts on the crista tuberculi minoris medial to the attachment site of m. latissimus dorsi (Fig. 3). In B. variegatus, this muscle is even more prominent. It has its origin not only on the margo caudalis of the scapula but also on the posterior fossa infraspinata. As in C. didactylus it inserts on the crista tuberculi minoris medial to the latissimus dorsi.
M. teres minor was not recognized by Mackintosh (1875),Lucae (1882) or Miller (1935) in C. didactylus, and was suggested to be fused to m. infraspinatus (Mackintosh, 1875). However, we found this small muscle arising from the distal margo caudalis of the scapula innervated by n. axillaris. It runs superficial to the long head of m. triceps brachii and inserts distal to m. infraspinatus on the tuberculum majus of the humerus (Fig. 3). It is also found in our specimen of B. variegatus in accordance with Meincke (1911) and Miller (1935), although it was not mentioned by Mackintosh (1870).
Caput longum of m. triceps brachii of C. didactylus as well as B. variegatus originates on the distal margo caudalis of the scapula adjacent to the origin of m. teres minor (Fig. 2). It runs laterally to the m. latissimus dorsi and m. dorso-epitrochlearis into a common strong fascia with the medial and lateral head, which both originate from the humerus. The fascia attaches to the short olecranon (Figs 4 and 6).
Fig. 6.

Superficial muscles of the shoulder region of Choloepus didactylus (A) and Bradypus variegatus (B). Lateral aspect. 1: M. trapezius pars cervicis; 2: M. trapezius thoracis; 3: M. latissimus dorsi; 4: M. pectoralis profundus posterior; 5: M. pectoralis superficialis; 6: M. teres major; 7: M. deltoideus pars scapularis; 8: M. deltoideus pars acromialis; 9: M. dorso-epitrochlearis; 10: M. triceps brachii caput longum; 11: M. deltoideus; 12: M. biceps brachii.
In C. didactylus, m. coracobrachialis maintains its usual attachment sites on the coracoid and the humerus (Figs 2 and 3). A second distinct portion, mentioned by Lucae (1882), is not present in our specimens, in accordance with observations of Humphry (1869), Mackintosh (1875) and Miller (1935). The presence and sites of origin and attachment are also found in our specimen of B. variegatus.
3-D kinematics of the shoulder in C. didactylus
In depth quantification of the 3D kinematics at the shoulder are presented elsewhere (Nyakatura & Fischer, 2010a). We here provide a qualitative description of the movements as a basis for the interpretation of the effect individual muscles have during locomotion.
At touch down of a forelimb, the scapula is protracted and positioned dorsally so that it lies dorsally on the thorax with the glenoid fossa facing towards the ear (Fig. 7). The shoulder joint is lower than the sternum (i.e. further away from the support). It is extended and the humerus is thus protracted. However, humeral retraction started briefly prior to touch down. The clavicle is rotated cranially and at the same time points somewhat dorsally.
Fig. 7.
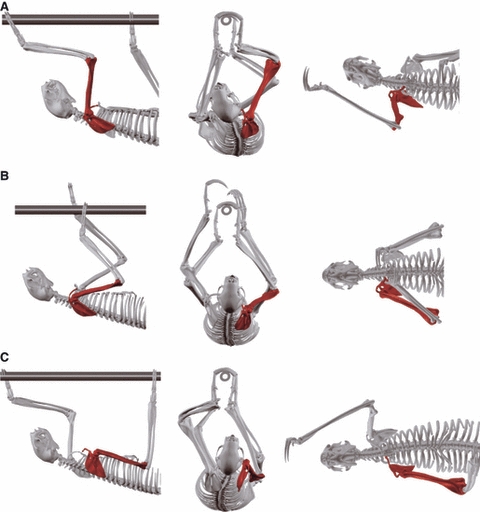
3D kinematics of the shoulder region in sloths animated according to high-speed x-ray recordings in lateral, frontal and ventral view. (A) touch down; (B) mid-contact; (C) lift off. For further explanations see text.
The first half of the support phase mainly involves humeral retraction while the scapula remains approximately at its position of initial contact. Humeral retraction is completed by 60% of the contact phase. In the second half of the contact phase scapula retraction is the predominant motion, whereas the shoulder joints remains flexed at approximately 80–90°. At the same time the scapula rotates from its dorsal position at touch down to a more strictly lateral position at lift off. Scapular retraction positions the shoulder joint at the same height as the sternum at lift off or even higher (i.e. closer to the support). Additionally, there is a small translation of the scapula along the thoracic wall facilitated by a ligamentous connection of the clavicle to the sternum (Nyakatura & Fischer, 2010a). The clavicle points laterally at lift off, forming an angle of approximately 90° to the sagittal plane. The glenoid fossa faces ventrally at lift off.
Discussion
The effect of shoulder muscles in C. didactylus during weight support
Due to the inverse orientation of the body in regard to the gravitational force during locomotion, the m. serratus ventralis and the m. rhomboideus (except for a short period prior to lift off) are unsuited to support weight at the forelimbs in C. didactylus. The ability of other muscles to fulfill this role greatly depends on the orientation of the forelimb at different instants of the contact phase. At touch down the fossa glenoidalis has a dorsal position in relation to the manubrium sterni and the upper arm is orientated nearly vertically. The scapula is pulled dorsal by the cervical m. trapezius and m. rhomboideus. Weight transmission to the limb after touch down leads to moments that tend to extend the elbow and shoulder joints. Intrinsic limb musculature is primed for powerful flexion of the shoulder and elbow joints to counteract gravity-induced extension (Mendel, 1985). However, in the early contact phase the m. pectoralis superficialis posterior and m. dorso-epitrochlearis (extrinsic shoulder muscles) are best suited to support the weight of the thorax at the forelimbs. Both muscles are characterized by long parallel fibers and have a nearly vertical orientation at this instant of the step cycle. M. latissimus dorsi may also help to keep the shoulder flexed, but has a less advantageous lever arm at the beginning of the contact phase. The ‘anti-extensors’ mm. teres major and dorso-epitrochlearis have the paramount responsibility to counteract extension at the shoulder joint. Their role is comparable to the emphasized flexor groups at the elbow in suspended quadrupeds (cf. Fujiwara et al. 2011). Furthermore, these muscles likely are the functional analogues of suspensory quadrupedalism to ‘anti-flexor’ muscles such as the long head of m. triceps brachii in pronograde mammals considering the importance of maintaining the zig-zag configuration of the leg for locomotion (Goslow et al. 1981; Fischer & Blickhan, 2006). M. trapezius also has the orientation necessary for effective weight transmission between limb and thorax (i.e. its insertion is more ventral than its origin) in the two-toed sloth. Later in contact phase the glenoid cavity is moved ventro-caudally; m. pectoralis superficialis posterior and m. dorso-epitrochlearis become more and more unsuited to suspend the weight of the thorax at the forelimbs due to progressive humeral retraction at the shoulder joint. In relation to the scapular retraction, the shoulder joint is positioned ventral to the sternum in late contact phase and now the m. pectoralis superficialis anterior is best suited for the task of weight support. M. trapezius and, prior to lift off, also m. rhomboideus major have suitable orientations to fulfill this role, too.
The effect of shoulder muscles in C. didactylus during quadrupedal suspensory locomotion
Recruitment of different muscles at the shoulder region during quadrupedal locomotion is well studied in aclaviculate mammals like cats (e.g. English, 1978) and dogs (e.g. Tokuriki, 1973a,b, 1974; Carrier et al. 2006, 2008), in which shoulder movements are largely confined to the parasagittal plane (Boczek-Funcke et al. 1996). EMG data for selected muscles acting on the pectoral girdle of claviculate arboreal quadrupedal primates are also published for four lemurid species, spider monkeys, howling monkeys, and wooly monkeys, as well as for the more terrestrial vervets and patas monkeys (Stern et al. 1980; Konstant et al. 1982; Larson & Stern, 1989; Schmitt et al. 1994; Larson & Stern, 2007). Moreover, data on forelimb muscle activation in gibbons are available (Jungers & Stern, 1980). Jenkins & Weijs (1979) comprehensively studied the functional morphology of the claviculate Virginia opossum. We will here discuss these data in the context of the observed kinematics and muscular morphology of C. didactylus.
In C. didactylus, the early contact phase is characterized by a constant position of the scapula. The mm. rhomboideus capitis et major, levator scapulae, and the cervical part of m. trapezius are suitable to pull the trunk and thereby keep the scapula in the protracted dorsal position during the early contact phase. Data for the cranial m. trapezius in spider monkeys during quadrupedal locomotion (Konstant et al. 1982), which have a generally more dorsal orientation of the scapula (Jenkins et al. 1978), were consistent with a function as assumed for C. didactylus.
Flexion in the gleno-humeral joint during early contact is likely be realized by the strong m. teres major and m. dorso-epitrochlearis. The long head of m. triceps brachii also contributes to shoulder joint flexion, especially if the elbow joint angle is held constant against gravity-induced extension (see below). M. latissimus dorsi and m. pectoralis have an orientation suitable for humeral retraction. Because of a more distal insertion on the pectoral crest of the humerus, m. pectoralis superficialis anterior (but not m. pectoralis superficialis posterior) is better suited to retract the humerus in the early contact phase than m. pectoralis profundus. Larson & Stern (2007) recorded only minimal activation by the mm. latissimus dorsi et pectoralis; they concluded that these muscles have a reduced role in body progression due to a functional differentiation between fore- and hindlimbs in primates. Instead, these authors interpreted the activity pattern of the m. pectoralis to involve the production of adducting forces at the shoulder. Due to the medial to lateral orientation of the m. pectoralis superficialis anterior the same role of this muscle is likely present in Choloepus. In contrast, in spider, woolly and howling monkeys the cranial m. pectoralis ‘fires’ throughout contact phase during pronograde quadrupedalism, whereas the caudal m. pectoralis is active in the first half of the contact phase (Stern et al. 1980). Both parts were found by these authors to be propulsive. Mm. latissimus dorsi et pectoralis are also recruited throughout almost all the contact phase in the opossum (Jenkins & Weijs, 1979). We assume the m. pectoralis superficialis anterior and especially the mm. latissimus dorsi et dorso-epitrochlearis effectively retract the humerus given the importance of humeral retraction for sloth forelimb stride length (Nyakatura et al. 2010). As previously proposed by Fischer (1998) for the hyrax, the combined effect of mm. supraspinatus, infraspinatus et subscapularis presumably guides humeral retraction at the gleno-humeral joint in the scapular plane and minimizes humeral abduction in terrestrial mammals. Synchronous activation of these three muscles in the opossum during the contact phase (Jenkins & Weijs, 1979) as well as synchronous recruitment of mm. supraspinatus et infraspinatus in vervets (Larson & Stern, 1989) seems to corroborate this notion. In Choloepus the weight of the body will tend to force the humerus into adduction. Therefore it is likely that these short scapulo-humeral muscles help to keep the humerus in its slightly abducted position.
With increasing gleno-humeral flexion the different portions of m. pectoralis become unsuited to further retract the forelimb at the humerus. Since the portion that originates most caudally (m. pectoralis profundus posterior) attaches most proximally at the shoulder, it is suitable to continue forelimb retraction until lift-off. After gleno-humeral flexion has ceased, mm. pectoralis profundus medius et posterior and m. latissimus dorsi further retract the limb in the second half of forelimb contact. The semi-circular motion described by the glenoid cavity also leads to a more ventral position of the glenoid at lift off, i.e. this movement could be mostly passive by making use of the inverse orientation of the body to gravity. The mode of progression during contact phase would thus be a pull due to humeral retraction in the first half of contact and a mainly passive scapular retraction in the second half of contact provoked by gravity pulling at the trunk.
This pattern would be consistent with the anterior/posterior-directed substrate reaction forces measured during upside-down locomotion of Loris (Ishida et al. 1990). These authors showed that during this locomotion, the limbs exert a pulling force in the first half of contact phase in contrast to the pushing force usually obtained in the second half of the stance of pronograde quadrupedalism (Ishida et al. 1990). In this scenario, it would be necessary to maintain the scapular pivot at a relatively constant position. Although Schmitt et al. (1994) did not find the m. serratus ventralis involved in scapular rotation in vervets, English (1978) and Jenkins & Weijs (1979) postulated that this task is realized by the combined effect of the cervical part of m. trapezius, which attaches ventral to the scapular pivot, and the cervical part of m. serratus ventralis, which attaches dorsal to it. Both muscles are suited to maintain the scapular pivot at its position during ventro-caudal displacement of the fossa glenoidalis by counteracting the forelimb retractors (cf. Fischer, 1998).
After lift off, the scapula is protracted and the gleno-humeral joint continues to extend. In contrast to pronograde locomotion the limb does not need to be flexed at the gleno-humeral and elbow joints (to be lifted off the ground), but is extended to protract the hand above the support. Effective scapular protractors are the cervical part of m. trapezius, m. levator scapulae, and m. rhomboideus capitis. M. cleidomastoideus is also suitable to protract the shoulder as long as the head is stabilized in its position by coactivation of other neck muscles. In the early swing phase, the m. pectoralis superficialis anterior could also effectively protract the shoulder due to the transversal orientation of the muscle and the shoulder's position caudal to the manubrium sterni at this instant of the step cycle. Thus, the m. pectoralis superficialis anterior probably contributes to pro- and retraction depending on the position of the limb, as was also inferred from the recruitment patterns of the m. pectoralis superficialis transversus in trotting dogs (Carrier et al. 2006). Action of the m. pectoralis superficialis posterior, m. deltoideus acromialis, and m. biceps brachii, which all insert in a common tendon on the forearm, protract the whole limb and extend the glenohumeral joint at early swing phase. During forelimb protraction, the scapular pivot is stabilized by the thoracic parts of m. trapezius and m. serratus ventralis. The m. subclavius was found to decelerate the protraction of the limb prior to touch down in quadrupedal locomotion of spider monkeys (Konstant et al. 1982). We deduce a similar effect of this muscle in C. didactylus due to the comparable topography and kinematics.
Convergent morphological solutions to new functional demands
When modern tree sloths adopted the entirely aboreal lifestyle they adapted to the necessity of locomotion on supports that have a small diameter relative to the thorax by evolving the typical upside-down posture and locomotion. But the inverse orientation of the body with regard to gravity is associated with new functional demands posed on the locomotor apparatus. If the proposed diphyletic origin and convergent evolution of upside-down posture in both extant sloth lineages (Gaudin, 2004) has led to any functionally significant morphological specializations, these should be present in both lineages. A clear indication of convergent evolution would be a morphologically different solution to the same problem posed by the new functional demand (here the inverse orientation of the body in regard to the force of gravity). However, the phylogenetic heritage of a structure tends to canalize the possible spectrum of solutions to problems posed by changing functional demands (e.g. Sudhaus, 2006). Hence, the more distantly related and thereby less phylogenetic heritage is shared, the more different solutions to changing functional demands can be expected to be realized by evolution.
The adoption of quadrupedal suspensory locomotion of C. didactylus has been shown to be characterized by a plesiomorphic pattern of forelimb protraction and retraction (Nyakatura et al. 2010) and only some small scaled morphological changes were deduced to be functionally related to the orientation of the body towards the force of gravity. These include the strong flexor groups of the limbs (Mendel, 1985), a ligamentous connection of the clavicle to the sternum that has been shown to transmit tensile forces (Nyakatura & Fischer, 2010a), and as shown above, the topography of the m. pectoralis superficialis posterior, the topography of the acromial head of m. deltoideus, the relatively small serratus ventralis, as well as the distal insertion of the pectoralis superficialis anterior. All of these characteristics of the shoulder are also found in Bradypus, and thus it remains unclear whether these characteristics are simply homoplasic. The fact that most of the named specializations are less pronounced in Bradypus might be attributed to its postural behavior, which is less dominated by suspension (e.g. Goffart, 1971).
However, there were also anatomical features identified here that might represent different morphological solutions to the new functional demands posed by the adoption of upside-down orientation of the body during locomotion. One example proposed here of convergent evolution is the overall different configuration of the m. pectoralis. Although the m. pectoralis is not as complex in Bradypus as in Choloepus, it may be suited to fulfill the tasks deduced for this muscle complex in Choloepus from the x-ray motion analysis and study of the muscular topography, in spite of being morphologically different. Namely, due to the fibers that the m. pectoralis superficialis exchanges with the m. biceps brachii, it should also be helpful to carry the weight of the thorax at touch down of the forelimb. This also applies for the acromial head of m. deltoideus, which also sends some of its fibers into the m. biceps brachii, but again in a different way as shown for C. didactylus. Significantly, this view implies a conduction of forces generated from proximal muscles to distal limb elements via a ‘muscle chain’ in Bradypus, as has been hypothesized for gibbons and siamangs (Jungers & Stern, 1980), despite differences in general trunk orientation during suspensory locomotion between these apes and sloths. Indeed, in gibbons and siamangs there is a ventral ‘muscle chain’ that connects the m. pectoralis and the m. biceps brachii and a dorsal ‘muscle chain’ that connects the m. latissimus with the m. dorso-epitrochlearis similar to the connections between the same muscles present in tree sloths. In gibbons the ‘muscle chains’ further connect to the relatively strong finger flexors – a feature not evident in either sloth lineage. In an investigation of the muscle activity of the ‘muscle chains’ in gibbons, Jungers & Stern (1980) controverted the hypothesis that forces produced by proximal elements of the chains help to flex distal joints. Instead, the authors identified these chains to be mere by-products of changes in muscle topography of the m. biceps brachii that has an increased mechanical advantage for forearm flexion. Increased mechanical advantages of limb flexor groups have also been found in other suspended quadrupeds (Fujiwara et al. 2011). Possibly driven by similar biomechanical requirements, i.e. a selective pressure towards increased mechanical advantage for powerful flexion, topographic changes of the attachments sites of the m. pectoralis and the m. biceps brachii in Bradypus could have resulted in the now present overlap of attachment sites, thereby creating the impression of a functionally significant connection of these muscles. This argument does not apply to Choloepus, because in two-toed sloths the m. pectoralis superficialis posterior has a common insertion at the forearm with the acromial part of the m. deltoideus and the anterior belly of m. biceps brachii, i.e. here the muscles are not forming a chain but are connected to pull parallel to each other. But even if no forces are conducted by the m. pectoralis superficialis to the forearm in Bradypus, the increased mechanical advantage helps to effectively pull the trunk towards the humerus, helping to support the weight of the body. This is also achieved by the different morphological solution to this functional demand in Choloepus. We suggest this difference to be indicative of convergent evolution of suspensory postures in both modern sloth lineages.
Another example of a slightly different morphological solution to the same functional demand involves m. teres major and m. dorso-epitrochlearis. We deduced that both muscles help to flex the shoulder joint, and both are well developed in Choloepus and Bradypus. However, in Choloepus the two are of approximately the same size, whereas in Bradypus, the m. teres major is more prominent and the m. dorso-epitrochlearis is smaller. Therefore, although the flexor group is strong in both genera, it is realized in a slightly different way.
Some of the described morphological differences between the two modern lineages of tree sloths were not deduced here to be of significance for the two main functions of the pectoral girdle. Instead, these may be related to the exceptionally high number of cervical vertebrae in Bradypus (Buchholtz & Stepien, 2009), which are used to rotate the head by 180° to maintain a right-side-up orientation of the head during upside-down postures. It is interesting to note that the additional cervical vertebrae in Bradypus are interpreted to be of thoracic origin (Buchholtz & Stepien, 2009; Hautier et al. 2010). Therefore, it seems that not only are the skeletal elements of the pectoral girdle in Bradypus translated caudally, as proposed by Buchholtz & Stepien (2009), but also shoulder muscles that usually attach to the occiput are lost during the process, such as m. levator scapulae and the occipital portion of the m. rhomboideus. These extrinsic shoulder muscles usually play a role in scapula stabilization and protraction during locomotion (Fischer, 1998). Bradypus seems to accomplish this with the cervical portion of m. rhomboideus and the cervical portion of the m. trapezius alone.
Conclusions
The adoption of the suspensory posture and locomotion of C. didactylus had differing effects regarding the two main functional demands of the muscles at the pectoral girdle, supporting the weight of the body and protracting and retracting the forelimbs. The similar kinematic pattern of the pectoral girdle between terrestrial quadrupedal mammals and sloths rendered major evolutionary changes in modes of limb protraction and retraction and related changes in muscular topography unnecessary during the adoption of obligatory quadrupedal suspensory posture. This furthermore reduced the need for new patterns of neural control, and the convergent evolution in the different sloth genera as proposed by phylogenetic analysis appears less improbable.
The peculiar shoulder morphology of C. didactylus is thus likely related to the functional demand of weight bearing at the forelimbs in regard to the inverse orientation of the body to gravitational force. Comparable characteristics are not as clearly present in B. variegatus.
In sum, many characteristics of the muscular topography seem to be related to the inverse orientation of the body in regard to the force of gravity, but only some of these are realized in a morphologically different way in the two species analyzed. The identified different morphological solutions to the new functional demands are indicative of convergent evolution of similar functional capacity in both lineages, confirming the diphyletic origin suggested by phylogenetic studies. The identified similarities may result from the relatively close phylogenetic relationship (compared with other clades within amniotes) and long common phylogenetic history, which may have canalized the possible spectrum of solutions to changed functional demands realized during the evolution of this peculiar locomotor behavior. We suggest that digging adaptations (for example strong limb retractors, long claws) in the common evolutionary history, as proposed for early xenarthrans, had the effect of posing morphological constraints and canalized evolution to convergently realize suspensory postures. In this context it is important to note that the tiny anteater (Cyclopes) is also capable of suspensory locomotion.
Acknowledgments
The authors wish to thank Ilona Schappert of the Zoo Dortmund who kindly provided the sloths Julius and Evita. Dirk Arnold, with whom it was a pleasure to work, provided practical help with the identification of muscles using the criterion of innervation. Sandy Reinhard skillfully prepared Figs 5 and 6. Critical comments from François Pujos and two anonymous reviewers helped to greatly improve an earlier version of the manuscript.
References
- Boczek-Funcke A, Kuhtz-Buschbeck JP, Illert M. Kinematic analysis of the cat shoulder girdle during treadmill locomotion: an x-ray study. Eur J Neurosci. 1996;8:261–272. doi: 10.1111/j.1460-9568.1996.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Brainerd EL, Baier DB, Gatesy SM, et al. X-ray reconstruction of moving morphology (XROMM): precision, accuracy and applications in comparative biomechanics research. J Exp Zool. 2010;313:262–279. doi: 10.1002/jez.589. [DOI] [PubMed] [Google Scholar]
- Buchholtz EA, Stepien CC. Anatomical transformation in mammals: developmental origin of aberrant cervical anatomy in tree sloths. Evol Dev. 2009;11:69–79. doi: 10.1111/j.1525-142X.2008.00303.x. [DOI] [PubMed] [Google Scholar]
- Carrier DR, Deban SM, Fischbein T. Locomotor function of the pectoral girdle ‘muscular sling’ in trotting dogs. J Exp Biol. 2006;209:2224–2237. doi: 10.1242/jeb.02236. [DOI] [PubMed] [Google Scholar]
- Carrier DR, Deban SM, Fischbein T. Locomotor function of the forelimb protractor and retractor muscles of dogs: evidence of strut-like behavior at the shoulder. J Exp Biol. 2008;211:150–162. doi: 10.1242/jeb.010678. [DOI] [PubMed] [Google Scholar]
- Davis DD. The shoulder architecture of bears and other carnivores. Fieldiana Zool. 1949;81:285–305. [Google Scholar]
- Delsuc F, Catzeflis FM, Stanhope MJ, et al. The evolution of armadillos, anteaters, and sloths depicted by nuclear and mitochondrial phylogenies: implications for the status of the enigmatic fossil Eurotamandua. Proc R Soc Lond B. 2001;268:1605–1615. doi: 10.1098/rspb.2001.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton TH., Jr Modifications of the shoulder girdle related to reach and stride in mammals. J Morphol. 1944;75:167–171. [Google Scholar]
- English AWM. An electromyographic analysis of forelimb muscles during overground stepping in the cat. J Exp Biol. 1978;76:105–123. doi: 10.1242/jeb.76.1.105. [DOI] [PubMed] [Google Scholar]
- Evans HE. Miller's Anatomy of the Dog. Philadelphia: WB Sauders; 1993. [Google Scholar]
- Fischer MS. Die Lokomotion von Procavia capensis (Mammalia, Hyracoidea): Zur Evolution des Bewegungssystems bei Säugetieren. Abhandlungen des Naturwissenschaftlichen Vereins in Hamburg. 1998;33:1–188. [Google Scholar]
- Fischer MS, Blickhan R. The tri-segmented limbs of therian mammals: kinematics, dynamics, and self-stabilization – a review. J Exp Zool. 2006;305:935–952. doi: 10.1002/jez.a.333. [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Endo H, Hutchinson JR. Topsy-turvy locomotion: biomechanical specializations of the elbow in suspended quadrupeds reflect inverted gravitational constraints. J Anat. 2011 doi: 10.1111/j.1469-7580.2011.01379.x. doi: 10.1111/j.1469-7580.2011.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatesy SM, Baier DB, Jenkins FA, et al. Scientific rotoscoping: a morphology-based method of 3D-motion analysis and visualization. J Exp Zool. 2010;313:280–298. doi: 10.1002/jez.588. [DOI] [PubMed] [Google Scholar]
- Gaudin TJ. Phylogenetic relationships among sloths (Mammalia, Xenarthra, Tardigrada): the craniodental evidence. Zool J Linn Soc. 2004;140:255–305. [Google Scholar]
- Gaudin TJ, McDonald HG. Morphology-based investigations of the phylogenetic relationships among extant and fossil xenarthrans. In: Vizcaíno SF, Loughry WJ, editors. The Biology of the Xenarthra. Gainesville: University Press of Florida; 2008. pp. 24–36. [Google Scholar]
- Goffart M. Function and Form in the Sloth. Oxford: Pergamon Press; 1971. [Google Scholar]
- Goslow GE, Seeherman HJ, Taylor CR, et al. Electrical activity and relative length changes of dog limb muscles as a function of speed and gait. J Exp Biol. 1981;94:15–42. doi: 10.1242/jeb.94.1.15. [DOI] [PubMed] [Google Scholar]
- Grand TI. A mechanical interpretation of terminal branch feeding. J Mammal. 1972;53:198–201. [Google Scholar]
- Greenwood AD, Castresana J, Feldmeier-Fuchs G, et al. A molecular phylogeny of two extinct sloths. Mol Phylogenet Evol. 2001;18:94–103. doi: 10.1006/mpev.2000.0860. [DOI] [PubMed] [Google Scholar]
- Hautier L, Weisbecker V, Sánchez-Villagra MR, et al. Skeletal development in sloths and the evolution of mammalian vertebral patterning. Proc Natl Acad Sci U S A. 2010;107:1890–1898. doi: 10.1073/pnas.1010335107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höss M, Dilling A, Currant A, et al. Molecular phylogeny of the extinct ground sloth Mylodon darwinii. Proc Natl Acad Sci U S A. 1996;93:181–185. doi: 10.1073/pnas.93.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphry GM. The myology of the limbs of the Unau, the Ai, the two-toed anteater, and the Pangolin. J Anat Physiol. 1869;3:2–78. [PMC free article] [PubMed] [Google Scholar]
- Ishida H, Jouffroy FK, Nakano Y. Comparative dynamics of pronograde and upside down horizontal quadrupedalism in the Slow Loris (Nyctecebus coucang) In: Jouffroy FK, Stack MH, Niemitz C, editors. Gravity, Posture and Locomotion in Primates. Florence: Editrice Il Sedicesimo; 1990. pp. 209–220. [Google Scholar]
- Jenkins FA. The movement of the shoulder in claviculate and aclaviculate mammals. J Morphol. 1974;144:71–84. doi: 10.1002/jmor.1051440105. [DOI] [PubMed] [Google Scholar]
- Jenkins FA, Weijs WA. The functional anatomy of the shoulder in the Virginia Opossum (Didelphis virginiana) J Zool (Lond) 1979;188:379–410. [Google Scholar]
- Jenkins FA, Dombrowski PJ, Gordan EP. Analysis of the shoulder in brachiating spider monkeys. Am J Phys Anthropol. 1978;48:65–76. doi: 10.1002/ajpa.1330480110. [DOI] [PubMed] [Google Scholar]
- Jungers WL, Stern JT. Telemetered electromyography of forelimb muscle chains in gibbons (Hylobates lar) Science. 1980;208:617–619. doi: 10.1126/science.7367886. [DOI] [PubMed] [Google Scholar]
- Konstant W, Stern JT, Fleagle JG, et al. Function of subclavius muscle in a nonhuman primate, the spider monkey (Ateles) Folia Primatol. 1982;38:170–182. doi: 10.1159/000156055. [DOI] [PubMed] [Google Scholar]
- Larson SG, Stern JT. The role of propulsive muscles of the shoulder during quadrupedalism in vervet monkeys (Cercopithecus aethiops): implications for neural control of locomotion. J Motor Bahav. 1989;21:457–472. doi: 10.1080/00222895.1989.10735494. [DOI] [PubMed] [Google Scholar]
- Larson SG, Stern JT. Humeral retractor EMG during quadrupedal walking in primates. J Exp Biol. 2007;210:1204–1215. doi: 10.1242/jeb.002337. [DOI] [PubMed] [Google Scholar]
- Lucae JCG. The Muscles and the Skeleton of the Black Lemur and the Sloth (Lemur macaco and Choloepus didactylus) Frankfurt am Main: Mahlau and Waldschmidt Verlag, Senckenbergische Naturforschende Gesellschaft; 1882. [Google Scholar]
- Mackintosh HW. On the myology of the genus Bradypus. Proc R Irish Acad. 1870;1:517–529. [Google Scholar]
- Mackintosh HW. On the muscular anatomy of Choloepus didactylus. Proc R Irish Acad. 1875;2:66–79. [Google Scholar]
- Meincke F. Morphologische Untersuchungen über die Myologie an den Extremitäten bei Bradypus tridactylus. Morph Jahrb. 1911;2:311–358. [Google Scholar]
- Mendel FC. Adaptations for suspensory behavior in the limbs of two-toed sloths. In: Montgomery GG, editor. The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas. Washington, DC: Smithsonian Institution Press; 1985. pp. 151–162. [Google Scholar]
- Miller RA. Functional adaptations in the forelimb of the sloths. J Mammal. 1935;16:38–51. [Google Scholar]
- Nyakatura JA, Fischer MS. Three-dimensional kinematic analysis of the pectoral girdle during upside down locomotion of two-toed sloths (Choloepus didactylus, Linné 1758) Front Zool. 2010a;7:1–16. doi: 10.1186/1742-9994-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyakatura JA, Fischer MS. Functional morphology and three-dimensional kinematics of the thoraco-lumbar region of the spine of the two-toed sloth. J Exp Biol. 2010b;213:4278–4290. doi: 10.1242/jeb.047647. [DOI] [PubMed] [Google Scholar]
- Nyakatura JA, Petrovitch A, Fischer MS. Limb kinematics during locomotion in the two-toed sloth (Choloepus didactylus, Xenarthra) and its implications for the evolution of the sloth locomotor apparatus. Zoology. 2010;113:221–234. doi: 10.1016/j.zool.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Poinar H, Kuch M, McDonald HG, et al. Nuclear gene sequences from a late pleistocene sloth coprolite. Curr Biol. 2003;13:1150–1152. doi: 10.1016/s0960-9822(03)00450-0. [DOI] [PubMed] [Google Scholar]
- Pujos F, DeIuliis G, Argot C, et al. A peculiar climbing Megalonychidae from the pleistocene of Peru and its implication for sloth history. Zool J Linn Soc. 2007;149:179–235. [Google Scholar]
- Schmidt M, Voges D, Fischer MS. Shoulder movements during quadrupedal locomotion in arboreal primates. Z Morphol Anthrop. 2002;83:235–242. [PubMed] [Google Scholar]
- Schmitt D, Larson SG, Stern JT. Serratus ventralis function in vervet monkeys (Cercopithecus aethiops): are primate quadrupeds unique? J Zool Lond. 1994;232:215–230. [Google Scholar]
- Stern JT, Wells JP, Jungers WL, et al. An electromyographic study of the pectoralis major in the Atelines and Hylobates, with special reference to the evolution of a pars clavicularis. Am J Phys Anthropol. 1980;52:13–25. doi: 10.1002/ajpa.1330520104. [DOI] [PubMed] [Google Scholar]
- Sudhaus W. Die Notwendigkeit morphologischer Analysis zur Rekonstruktion der Stammesgeschichte. Species Phylogeny Evol. 2006;1:17–32. [Google Scholar]
- Tokuriki M. Electromyographic and joint-mechanical studies in quadrupedal locomotion. I. Walk. Jpn J Vet Sci. 1973a;35:433–446. doi: 10.1292/jvms1939.35.433. [DOI] [PubMed] [Google Scholar]
- Tokuriki M. Electromyographic and joint-mechanical studies in quadrupedal locomotion. II. Trot. Jpn J Vet Sci. 1973b;35:525–533. doi: 10.1292/jvms1939.35.525. [DOI] [PubMed] [Google Scholar]
- Tokuriki M. Electromyographic and joint-mechanical studies in quadrupedal locomotion. III. Gallop. Jpn J Vet Sci. 1974;36:121–132. doi: 10.1292/jvms1939.36.121. [DOI] [PubMed] [Google Scholar]
- Wetzel RM. The identification and distribution of recent Xenarthra ( = Edentata) In: Montgomery GG, editor. The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas. Washington, DC: Smithsonian Institution Press; 1985. pp. 5–22. [Google Scholar]
- Windle BCA, Parsons FG. On the myology of the Edentata. Proc Zool Soc Lond. 1899;1899:990–1017. [Google Scholar]


