Abstract
In humans, an increasing body of evidence has linked the frequency of cervical ribs to stillbirths, other malformations and early childhood cancers. However, the frequency of cervical ribs in a putatively healthy fetal population is not sufficiently known to assess the actual medical risks of these prenatal findings. We therefore analyzed the presence of skeletal anomalies in a series of 199 electively aborted fetuses, which were whole-mount stained with alizarin red specific for skeletal tissues. Results show that approximately 40% of the fetuses had cervical ribs, even though external congenital abnormalities such as craniofacial and limb defects were absent. A literature overview indicates that the observed frequency of cervical ribs is comparable to results previously obtained for deceased fetuses with no or minor congenital anomalies, and higher than expected for healthy fetuses. This unexpected result can probably in part be explained by a higher detection rate of small cervical ribs when using alizarin red staining instead of radiographs. Additionally, studies in the literature suggest that the size of a cervical rib may indicate the severity of abnormalities, but this possibility requires further research. Anomalies of the axial skeleton are known to be caused by a disturbance of early development, which alters Hox gene expression, but in this study the origin of the stress could not be verified as maternal medical data were not available. The co-occurrence of rudimentary or absent 12th ribs in 23.6% of the cases with cervical ribs indicates that in approximately 8% of the fetuses a homeotic shift occurred over a larger part of the vertebral column. This suggests that the expression of multiple Hox genes may have been affected in these fetuses. Together, the high incidence of cervical ribs and also their co-occurrence with rudimentary or absent 12th ribs suggests that there may have been a disturbance of early development such that the studied fetuses are probably not informative about the general population. Future studies determining the frequency of cervical ribs in a more healthy fetal population are therefore needed to evaluate their potential as an indicator of medical risks.
Keywords: developmental constraint, early organogenesis, evolutionary conservation, homeotic transformation, risk marker
Introduction
For centuries, researchers have been intrigued by the occasional occurrence of variations of the human axial skeleton that alter the typical vertebral formulae (e.g. Topinard, 1877; Bateson, 1894; Davis & King, 1939; see also Fig. 1A–D for details on normal and abnormal vertebral formulae). A case in point is a cervical rib on the seventh cervical vertebra, which due to a homeotic transformation obtains the identity of a thoracic vertebra, and thus reduces the number of cervical vertebrae (Bateson, 1894). The determination of the number of cervical vertebrae forms part of the early anterior–posterior patterning of the paraxial mesoderm, which is mediated by Hox genes (e.g. Kessel & Gruss, 1991; Wellik & Capecchi, 2003; Kmita & Duboule, 2003; Deschamps & van Nes, 2005; Woltering et al., 2009; Mallo et al., 2009). Previously, it has been suggested that cervical ribs observed in human fetuses are ossification centers that fuse with the transverse processes and do not persist into adulthood (O′Rahilly et al., 1990), such that adverse health effects were considered to be limited. However, an increasing number of studies are being published that show that cervical ribs do not disappear later in development (Galis et al., 2006), and that a relationship with pathologies of different origin and severity exists (Bassoe, 1920; Gladstone & Wakeley, 1932; Serck-Hanssen, 1935; Roos, 1976; Naik et al., 1978; Schumacher et al., 1992; Galis & Metz, 2003; Merks et al., 2005; Steigenga et al., 2006; Galis et al., 2006). In humans, cervical ribs are well known to be associated with the thoracic outlet syndrome, in which case the cervical rib may compress the subclavian veins and nerves (e.g. Makhoul & Machleder, 1992; Roos et al., 1999). More seriously, the incidence of cervical ribs in stillborn fetuses has been found positively associated with major congenital abnormalities, and the presence of cervical ribs has also been linked to early childhood cancers (see summary in Table 1; Gladstone & Wakeley, 1932; Schumacher et al., 1992; Merks et al., 2005).
Fig. 1.
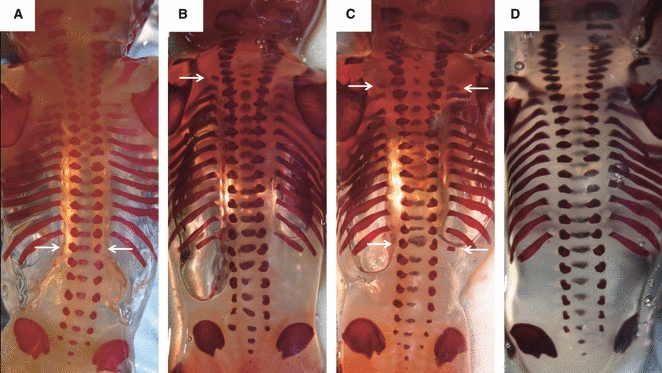
Examples of the observed rib anomalies (indicated by arrows): (A) fetus lacking 12th ribs; (B) fetus with an unilateral cervical rib on the left side; (C) fetus with bilateral cervical ribs, an absent (left) and a rudimentary (right) 12th rib. (D) A fetus with a normal vertebral pattern (seven cervical, 12 thoracic and five lumbar vertebrae) to allow comparison.
Table 1.
Overview of the frequency of rib anomalies in different study populations of human fetuses and children
| Frequency cervical ribs (%) | Other rib anomalies (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study population | N | Overall | Bilateral | Unilateral right | Unilateral left | Absent and/or rudimentary 1st rib | Absent and/or rudimentary 12th rib | Lumbar rib | Reference |
| Fetuses | |||||||||
| Healthy unborn* | 367 | 1.4 | 40 | NR | NR | NR | 4.1 | 0.84 | Hershkovitz (2008) |
| Stillborn | 728 | 19.2 | 77.9 | 15.0 | 7.1 | NR | NR | 1.1 | Bagnall et al. (1984) |
| NR† | 136 | 25 | 70.6 | 8.8 | 20.6 | NR | NR | 2.2 | Noback & Robertson (1951) |
| Deceased (no anomalies) | 48 | 31 | NR | NR | NR | 0 | 3.5 | 10.5 | Galis et al. (2006) |
| NR‡ | 177 | 33 | 65 | NR | NR | NR | NR | NR | Meyer (1978) |
| Elective abortions† | 199 | 37.7 | 70.7 | 12 | 17.3 | 1.0 | 12.6 | 1.5 | This study |
| Deceased (single minor anomalies) | 64 | 42 | NR | NR | NR | 0 | 1.5 | 1.5 | Galis et al. (2006) |
| Deceased (multiple minor anomalies) | 44 | 45 | NR | NR | NR | 0 | 4.5 | 7 | Galis et al. (2006) |
| Deceased (single major anomalies) | 56 | 50 | NR | NR | NR | 3.5 | 0 | 0 | Galis et al. (2006) |
| Deceased (multiple major anomalies) | 235 | 63 | NR | NR | NR | 3.5 | 1 | 7.5 | Galis et al. (2006) |
| Stillborn | 715 | 63 | 73 | 16 | 11 | NR | 9.2 | 0.7 | McNally et al. (1990) |
| Children | |||||||||
| < 13 years (hospital database) | 2000 | 0.5 | 89 | NR | NR | NR | NR | NR | Southam & Bythell (1924) |
| < 12 years (healthy siblings of TB patients) | 25949 | 0.5 | NR | NR | NR | NR | NR | NR | Menárguez Carretero & Campo Muñoz (1967) |
| < 13 years (hospital database) | 1000 | 1.2 | 83 | 0 | 17 | NR | NR | NR | Davis & King (1939) |
| ≤ 1 year (deceased myelomeningoceles patients) | 112 | 4.5 | NR | NR | NR | NR | 21.4 | 1.8 | Naik et al. (1978)7 |
| ≤ 14 years (infectious disease patients) | 200 | 4.5 | 78 | 11 | 11 | NR | NR | NR | Schumacher et al. (1992) |
| ≤ 18 years (asthma and infectious disease patients) | 881 | 6.1§ | NR | NR | NR | NR | 5.3 | 0.9 | Merks et al. (2005) |
| ≤ 18 years (tumor patients) | 906 | 8.6§ | NR | NR | NR | NR | 6.6 | 0.9 | Merks et al. (2005) |
| ≤ 21 years (tumor patients) | 1000 | 20.4 | 79 | 10 | 11 | NR | 3.6 | NR | Schumacher et al. (1992) |
All data were collected through radiological examinations unless stated otherwise.
NR, not reported; TB, tuberculosis.
Data collected using 3D ultrasound.
Data collected using alizarin red staining.
Data collected using silver staining.
Combining cases with cervical ribs and transverse apophysemegaly as the latter can be considered a small cervical rib that fused with the vertebral body (Redenbach & Nelems, 1998).
It has been hypothesized that the association between cervical ribs and cancer is caused by a common underlying genetic defect that causes abnormal Hox gene expression (Anbazhagan & Raman, 1997; Galis, 1999). This is based on the fact that Hox genes play an important role in the patterning of the axial skeleton in vertebrates and Hox gene mutants often display abnormalities of the vertebral column, including cervical ribs (reviewed in Wellik, 2009). Also, aberrations in Hox gene expression can affect its role in tumor suppression and oncogenesis (reviewed in Shah & Sukumar, 2010). In addition to this hypothesis, it has been suggested that adverse effects of cervical ribs result from internal selection against deleterious pleiotropic effects associated with developing an atypical number of cervical vertebrae (Galis, 1999). The number of cervical vertebrae is determined during the conserved early organogenesis stage, which is characterized by a high interactivity among organ primordia (Sander, 1983; Raff, 1994). In this situation, mutations causing deleterious pleiotropic effects will be selected against, which may result in increased mortality rates (Galis & Metz, 2001). In agreement, Galis et al. (2006) showed that at least 78% of the fetuses and neonates with a cervical rib die before birth, and 83% before reaching 1 year. In comparison, only 9% of clinically recognized pregnancies of individuals lacking cervical ribs are estimated to die before the age of 1 year.
Hence, current results indicate that fetuses and neonates possessing a cervical rib may face an increased risk of early mortality. A literature overview presented here indeed confirms this pattern in that the frequency of cervical ribs is repeatedly high in deceased fetuses (Table 1). However, this overview also indicates that little information on the frequency of cervical ribs exists for healthy fetuses due to the fact that measurements in uterus are unethical or technically complex (but see Hershkovitz, 2008). This lack of knowledge causes difficulty in relating the presence of a cervical rib to actual risks. For instance, it is possible that the adverse health effects associated with cervical ribs as reported by Galis et al. (2006) were underestimated if deceased fetuses without detectable abnormalities are not representative for the general population. The aim of this study was to quantify the frequency of rib anomalies for human fetuses that can be assumed to more closely resemble the general population. Specifically, we examined a unique collection of fetuses from elective abortions and for which scoring of rib anomalies was possible due to the alizarin red staining technique. Besides rib anomalies, we also inspected fetuses for abnormalities of the craniofacial skeleton and the limbs to obtain reference data on fetus health.
Materials and methods
Study population
Between 1965 and 1975, human fetuses from elective abortions were collected by scientists at the Institute of Dentistry, University of Turku to study craniofacial development. Complete fetuses were preserved by the ‘clearing and single staining’ procedure. In short, the eviscerated specimens were made semi-transparent with potassium hydroxide and treated with alizarin red to stain the skeleton. Thereafter, the fetuses were kept on glycerin. Now, more than 40 years later, the majority of this unique collection is still in suitable condition to allow research. For most fetuses, also information on the gestation age, sex and size is available. However, background information on the mothers has been discarded for ethical reasons.
Scoring rib anomalies
For this study only specimens were selected that had been sufficiently broken down by the potassium hydroxide such that all vertebrae were clearly visible (n = 199). The age of the studied fetuses ranged between 10 and 21 weeks of gestations. First, the vertebra formulae was determined by counting all cervical, thoracic, lumbar, sacral and caudal (if ossified) vertebrae. The vertebra formulae were considered normal when seven cervical, 12 thoracic, five lumbar and four sacral vertebrae were observed. All deviations from the normal vertebral formulae were scored following the approach of Galis et al. (2006), and included cervical ribs, rudimentary 1st ribs, absent or rudimentary 12th ribs and lumbar ribs (see also Fig. 1A–D). The size of cervical ribs was not determined as this was not possible in a non-destructive manner. All fetuses were examined independently by three researchers (FG, LW, JB). In addition, all fetuses were externally evaluated for skeletal abnormalities (including craniofacial and limb defects) by a pediatric pathologist (LW) as we had no knowledge of previous medical examinations.
Statistical analysis
Variation in the frequency of cervical ribs with age, size or between sexes was analyzed with generalized linear mixed models with link logit function and binomial error structure. Sex, age and size (divided into classes: 1 = 10–13 weeks; 2 = 14–16 weeks; 3 = 17–21 weeks) were treated as categorical variables. All analyses were conducted in sas 9.1.
Results
Slightly less than halve (47.2%) of the population had a normal vertebral pattern. Indeed, rib anomalies and especially cervical ribs were common in this Finnish population (Table 1; Fig. 1A–D). By contrast, no abnormalities of the craniofacial skeleton or limbs could be detected in any of the fetuses in the collection.
More specifically, we found that the majority of fetuses had bilateral cervical ribs (Fig. 1C), and that unilateral left ones (Fig. 1B) were slightly more common than unilateral right ones (Table 1). Also, absent or rudimentary 12th ribs occurred frequently (Fig. 1A,C), whereas only a few fetuses with a rudimentary 1st rib or lumbar ribs were observed (Table 1). In some fetuses, a combination of these rib anomalies was observed. When such homeotic transformations occur at multiple vertebral boundaries, they are termed homeotic shifts (Fig. 1C). Of the fetuses having a cervical rib, 23.6% also had absent or rudimentary ribs (i.e. a posterior homeotic shift). Rudimentary 1st ribs only occurred in two cases, and one of these was accompanied by an anterior homeotic shift at the thoraco-lumbar boundary.
The frequency of cervical ribs did not vary with gestation age (df = 10, χ2 = 8.02, P = 0.63). Furthermore, the frequency of cervical ribs did not vary with the size of fetus (df = 8, χ2 = 10.59, P = 0.23), nor did it differ between males and females (df = 1, χ2 = 0.6, P = 0.44).
Discussion
Unexpected high frequency of cervical ribs
We detected a high frequency of cervical ribs and other rib anomalies in this Finnish population, despite the fact that we studied fetuses from elective – and not therapeutic – abortions, and no craniofacial abnormalities and limb defects were observed. In fact, because approximately 40% cervical ribs were found, the frequency in this population is 20 times higher than previously reported in healthy fetuses (Hershkovitz, 2008). Our result is thus more comparable to that found in deceased fetuses with no or minor anomalies (Table 1). This is true even when recognizing that fetuses from our study population were on average younger and some of them may have become subject to natural miscarriages. Several reasons may be put forward to explain this unexpected finding, including the type of study material and the medical condition of the mothers.
The majority of previous studies are based on radiographs for determining cervical ribs (see Table 1), whereas data for this study were collected using single stained fetuses. Differences in detection rate between these methods can be expected. Indeed, it is known that radiological recognition of prenatal ossification centers of cervical ribs is possible from approximately 14 weeks of gestation (McNally et al., 1990), while they are observable with alizarin red staining from approximately 10 weeks of gestation (Noback & Robertson, 1951). Furthermore, a considerable number of ossification centers observed in this study were particularly small (F. Galis, personal observation), and as such it cannot be excluded that such anomalies or variations remained undetected on radiographs even when taking precautions for age effects. It is possible that the smallest ossification centers may not be associated with as adverse health effects as has been found for those that can be detected on radiographs (Galis et al., 2006; Furtado et al., in press). The observation that stillbirths were more frequent and cervical ribs were larger in mouse mutants homozygous for a Hoxa-5 mutation than in heterozygotes (Jeannotte et al., 1993) indeed suggests that the size of a cervical rib is associated with the severity of medical problems (see also Tabariès et al., 2007). This possibility is supported by other studies that found cervical ribs to be larger in homozygous compared with heterozygous Hoxb-8 (Charité et al., 1995) and bmi-1 mutants (Alkema et al., 1995); however, formal testing remains to be done. If it is correct that cervical rib size indicates the severity of medical problems, this may help explaining why we observed many but small cervical ribs in spite of the absence of major external or skeletal abnormalities.
It is also possible that the health status of the mothers negatively affected the early development of the fetuses with cervical ribs. Unfortunately, we could not empirically examine this possibility as all medical data of the mothers of our study population had been discarded for ethical reasons. In general, it can be expected that women carrying an unwanted child are less concerned about its health; however, it is difficult to speculate about possible effects. Information on whether an unknown stress disturbed early development of the fetuses may also be obtained indirectly from data on fluctuating asymmetry. Indeed, fluctuating asymmetry is determined as random deviations from perfect symmetry of bilateral symmetric traits (i.e. here the absolute difference in size between left and right limb bones) and is considered a proxy of developmental disturbance due to genetic or environmental stress (e.g. Polak, 2003; Van Dongen, 2006). Unpublished data collected for the same set of fetuses show an augmented level of limb asymmetry when compared with deceased fetuses with no or minor congenital anomalies from a population from the Netherlands (Van Dongen et al., 2009). In fact, the level of fluctuating asymmetry in our Finnish population was similar to Dutch fetuses with major congenital abnormalities and fetuses with both major and chromosomal abnormalities (S. Delen, J. Bots, K. Heikinheimo, F. Galis, S. Van Dongen, unpublished data; Van Dongen et al., 2009). Although populations may differ in various aspects, this finding may indicate that development was disturbed in our Finnish study population, but the exact causes of the stress remain unknown.
High frequency of homeotic shifts of the cervico-thoracic and thoraco-lumbar boundary
Our finding that the number of cervical ribs in our study population is elevated with respect to the general population is strongly supported by the high co-occurrence of cervical ribs with homeotic transformations at the thoraco-lumbar boundary. Indeed, 23.6% of the fetuses with cervical ribs also had absent or rudimentary 12th ribs, pointing to a posterior homeotic shift of the seventh cervical vertebrae and all thoracic vertebrae. Similar results, namely 26.4% posterior homeotic shifts, were found in deceased fetuses and infants with a variety of minor and major congenital abnormalities (Galis et al., 2006). Such a vertebral pattern with homeotic shifts of larger part of the vertebral column corresponds to the vertebral pattern of mice with mutations in genes upstream of Hox that affect several Hox genes simultaneously (e.g. Alkema et al., 1995; van der Lugt et al., 1996; Ikeya & Takada, 2001; van den Akker et al., 2002).
Disparity in the frequency of cervical ribs between fetuses and adults
Multiple studies, including this one, have indicated that the frequency of cervical ribs is much higher in fetuses than in adult humans (e.g. Chernoff & Rogers, 2004; Galis et al., 2006). Indeed, the overview in Table 1 shows that most studies in deceased fetuses report frequencies in the range of 19–63% (but see Hershkovitz, 2008). Much lower frequencies, in the range of 0–3%, have been found in adults (summarized in Galis et al., 2006; Brewin et al., 2009).
To explain the significant disparity between fetal and adult incidences, it has been hypothesized that cervical ribs may disappear by fusing with the vertebral body during postnatal growth (Chernoff & Rogers, 2004). Chernoff & Rogers (2004) subsequently suggested that the intermediate frequencies of cervical ribs in children (see summary Table 1) may be indicative of such an age-related decrease. These ideas were also influenced by results of toxicological studies in rodents that followed the fate of supernumerary lumbar ribs after birth. These studies suggested that there are two types of supernumerary ribs that may occur at the thoraco-lumbar boundary, i.e. ‘rudimentary’ and ‘extra’ ribs (Chernoff et al., 1991; Foulon et al., 2000). Rudimentary ribs are defined as < 50% of the 13th rib length and have been suggested to disappear after birth, whereas extra ribs are more than 50% of the 13th rib length and not transient (Chernoff et al., 1991; Foulon et al., 2000). However, these rudimentary ribs do not actually disappear, but fuse with the transverse process, which remains visible from its changed anatomy (Wickramaratne, 1988). Furthermore, Wéry et al. (2005) provided evidence against the transient nature of supernumerary ribs by showing that these are caused by a posterior shift of the expression of Hoxa-10 and Hoxd-9 while using the same toxicological treatment as Foulon et al. (2000). In other words, rudimentary and extra ribs both reflect the abnormal specification of developing somites, but vary in completeness of the homeotic transformation. Wéry et al. (2005) emphasized that full penetrance of a phenotype (i.e. a complete homeotic transformation) requires altered expression of several homologous or paralogous Hox genes (see also Kessel & Gruss, 1991; Kmita & Duboule, 2003; Deschamps & van Nes, 2005).
Cervical ribs in humans also occur in various sizes such that homeotic transformations are often incomplete (Fischel, 1906; Roos, 1976; Merks et al., 2005; Varela-Lasheras et al., 2011). Instead of fusing with the sternum such as true thoracic ribs, cervical ribs often fuse with the adjacent rib. But when very short, they fuse to the transverse process, leading to an ‘enlarged transverse processes’ or ‘transverse apophysemegaly’ (Roos, 1976; Redenbach & Nelems, 1998; Merks et al., 2005; Varela-Lasheras et al., 2011; see also fig. 5 in Fischel, 1906). Thus, as argued above, all variations have to be considered homeotic transformations, which is further corroborated by the fact that even small cervical ribs that can still be detected on radiographs are significantly associated with childhood cancers (Merks et al., 2005) and the thoracic outlet syndrome (Roos, 1976). In addition, Galis et al. (2006) showed that age has no effect on the incidence of cervical ribs in fetuses and infants, which again invalidates the hypothesis of cervical ribs disappearing later in development. Therefore, the only finding that can currently explain the disparity between fetal and adult incidences of cervical ribs is the early mortality of individuals with a cervical rib due to negative pleiotropic effects (Galis et al., 2006).
Conclusion
The high frequency of cervical ribs and other rib anomalies detected in this population of electively aborted fetuses appears to reflect a disturbance of early development, but the origin and intensity of the stress are uncertain. An alternative explanation includes the sensitivity of the assay. Nevertheless, the high frequency of homeotic shifts of a larger part of the vertebral column strongly suggests that the studied fetuses are probably not informative about the frequency of cervical ribs in the general population. Therefore, information on the general population currently remains solely available from one study in which cervical ribs were determined for healthy fetuses using 3D ultrasound (Hershkovitz, 2008). It should be noted though that in spite of the advantage of obtaining information on fetuses in uterus, it may be more difficult to observe tiny skeletal abnormalities on 3D ultrasound images, but progress in imaging techniques is expected to take care of this problem. Future studies determining the frequency of cervical ribs in healthy fetuses are thus clearly needed to allow evaluating their potential as an indicator of medical risks. Also, it will be valuable to investigate whether the size of a cervical rib is indicative of the severity of medical problems.
Acknowledgments
We thank two anonymous referees for their constructive comments. This research was financially supported by Grant for a Short Scientific Mission for the COST 23B Action (Oral Facial Development and Regeneration) of the European Science Foundation to FG and by the Fund for Scientific Research (FWO)-Flanders (postdoctoral grant to JB and research program G.0027.07 to SVD). Ethical approval (approval number 648/32/300/05) to study the human collection of embryos and fetuses owned by the University of Turku was obtained from the National Supervisory Authority for Welfare and Health of Finland (VALVIRA).
Author contributions
Designed research: FG, LW, KH, SVD; collected data: FG, LW, JB, SD; performed pathological examinations: LW; analyzed data: JB; wrote paper: JB, FG; revised paper: JB, FG.
References
- van den Akker E, Forlani S, Chawengsaksophak K, et al. Cdx1 and Cdx2 have overlapping functions in anteriorposterior patterning and posterior axis elongation. Development. 2002;129:2181–2193. doi: 10.1242/dev.129.9.2181. [DOI] [PubMed] [Google Scholar]
- Alkema MJ, Vanderlugt NMT, Bobeldijk RC, et al. Transformation of the axial skeleton due to overexpression of bmi-1 in transgenic mice. Nature. 1995;374:724–727. doi: 10.1038/374724a0. [DOI] [PubMed] [Google Scholar]
- Anbazhagan R, Raman V. Homeobox genes: molecular link between congenital anomalies and cancer. Eur J Cancer. 1997;33:635–637. doi: 10.1016/s0959-8049(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Bagnall KM, Harris PF, Jones PRM. A radiographic study of variations of the human spine. Anat Rec. 1984;208:265–270. doi: 10.1002/ar.1092080213. [DOI] [PubMed] [Google Scholar]
- Bassoe P. The coincidence of cervical ribs and syringomyelia. Arch Neurol Psychiat. 1920;4:542–545. [Google Scholar]
- Bateson W. Materials for the Study of Variation: Treated with Special Regard to Discontinuity in the Origin of Species. London: Macmillan; 1894. [Google Scholar]
- Brewin J, Hill M, Ellis H. The prevalence of cervical ribs in a London population. Clin Anat. 2009;22:331–336. doi: 10.1002/ca.20774. [DOI] [PubMed] [Google Scholar]
- Charité J, De Graaf W, Deschamps J. Specifications of multiple vertebral identities by ectopically expressed Hoxb-8. Dev Dyn. 1995;204:13–21. doi: 10.1002/aja.1002040103. [DOI] [PubMed] [Google Scholar]
- Chernoff N, Rogers JM. Supernumerary ribs in developmental toxicity bioassays and in human populations: incidence and biological significance. J Toxicol Env Health B Crit Rev. 2004;7:437–449. doi: 10.1080/10937400490512447. [DOI] [PubMed] [Google Scholar]
- Chernoff N, Rogers JM, Turner CI, et al. Significance of supernumerary ribs in rodent developmental toxicity studies: postnatal persistence in rats and mice. Fundam Appl Toxicol. 1991;17:448–453. doi: 10.1016/0272-0590(91)90196-b. [DOI] [PubMed] [Google Scholar]
- Davis DB, King JC. Cervical rib in early life. Am J Dis Child. 1939;56:744–755. [Google Scholar]
- Deschamps J, van Nes J. Developmental regulation of Hox genes during axial morphogenesis in the mouse. Development. 2005;132:2931–2942. doi: 10.1242/dev.01897. [DOI] [PubMed] [Google Scholar]
- Fischel A. Untersuchungen über die Wirbelsäule und den Brustkorb des Menschen. Anat Hefte. 1906;31:462–588. [Google Scholar]
- Foulon O, Jaussely C, Repetto M, et al. Postnatal evolution of supernumerary ribs after a single administration of sodium silicylate. J Appl Toxicol. 2000;20:205–209. doi: 10.1002/(sici)1099-1263(200005/06)20:3<205::aid-jat635>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Furtado F, Thaker H, Erickson L, et al. Cervical ribs are more prevalent in stillborn foetuses than in liveborn infants and are strongly associated with fetal aneuploidy. Pediat Devel Pathol. doi: 10.2350/11-01-0974-OA.1. (in press) in press. [DOI] [PubMed] [Google Scholar]
- Galis F. Why do almost all mammals have seven cervical vertebrae? Developmental constraints, Hox genes, and cancer. J Exp Zool B. 1999;285:19–26. [PubMed] [Google Scholar]
- Galis F, Metz JAJ. Testing the vulnerability of the phylotypic stage. J Exp Zool B. 2001;291:195–204. doi: 10.1002/jez.1069. [DOI] [PubMed] [Google Scholar]
- Galis F, Metz JAJ. Anti-cancer selection as a source of developmental and evolutionary constraints. Bioessays. 2003;25:1035–1039. doi: 10.1002/bies.10366. [DOI] [PubMed] [Google Scholar]
- Galis F, Van Dooren TJM, Feuth JD, et al. Extreme selection in humans against homeotic transformations of cervical vertebrae. Evolution. 2006;60:2643–2654. [PubMed] [Google Scholar]
- Gladstone RJ, Wakeley CPG. Cervical ribs and rudimentary first thoracic ribs considered from the clinical and etiological standpoints. J Anat. 1932;66:334–337. [PMC free article] [PubMed] [Google Scholar]
- Hershkovitz R. Prenatal diagnosis of isolated abnormal number of ribs. Ultrasound Obstet Gynecol. 2008;32:506–509. doi: 10.1002/uog.5296. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Takada S. Wnt-3a is required for somite specification along the anteriorposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech Dev. 2001;103:27–33. doi: 10.1016/s0925-4773(01)00338-0. [DOI] [PubMed] [Google Scholar]
- Jeannotte L, Lemieux M, Charron J, et al. Specification of the axial identity in the mouse: role of the Hoxa-5 (Hox1.3) gene. Genes Dev. 1993;7:2085–2096. doi: 10.1101/gad.7.11.2085. [DOI] [PubMed] [Google Scholar]
- Kessel M, Gruss P. Homeotic transformation of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Kmita M, Duboule D. Organizing axes in time and space; 25 years of collinear tinkering. Science. 2003;301:331–333. doi: 10.1126/science.1085753. [DOI] [PubMed] [Google Scholar]
- van der Lugt NMT, Alkema M, Berns A, et al. The Polycomb-group homolog Bmi-1 is a regulator of murine Hox gene expression. Mech Dev. 1996;58:153–164. doi: 10.1016/s0925-4773(96)00570-9. [DOI] [PubMed] [Google Scholar]
- Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16:534–545. [PubMed] [Google Scholar]
- Mallo M, Vinagre T, Carapuco M. The road to the vertebral formula. Int J Dev Biol. 2009;53:1469–1481. doi: 10.1387/ijdb.072276mm. [DOI] [PubMed] [Google Scholar]
- McNally E, Sandin B, Wilkens RA. The ossification of the costal element of the seventh cervical vertebra with particular reference to cervical ribs. J Anat. 1990;170:125–129. [PMC free article] [PubMed] [Google Scholar]
- Menárguez Carretero L, Campo Muñoz M. Estudio radiologico y tipos morfologicos de costillas cervicales en el sexo femenino. Enferm Torax. 1967;16:285–308. [Google Scholar]
- Merks JHM, Smets AM, Van Rijn RR, et al. Prevalence of RIB anomalies in normal Caucasian children and childhood cancer patients. Eur J Med Genet. 2005;48:113–129. doi: 10.1016/j.ejmg.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Meyer DB. Appearance of cervical ribs during early human fetal development. Anat Rec. 1978;2:481. [Google Scholar]
- Naik DR, Lendon RG, Barson AJ. A radiological study of vertebral and rib malformations in children with myelomeningocele. Clin Radiol. 1978;29:427–430. doi: 10.1016/s0009-9260(78)80105-6. [DOI] [PubMed] [Google Scholar]
- Noback CR, Robertson GG. Sequences of appearance of ossification centers in the human skeleton during the first five prenatal months. Am J Anat. 1951;89:1–28. doi: 10.1002/aja.1000890102. [DOI] [PubMed] [Google Scholar]
- O′Rahilly R, Muller F, Meyer DB. The human vertebral column at the end of the embryonic period proper. 3. The thoracico-lumbar region. J Anat. 1990;168:81–93. [PMC free article] [PubMed] [Google Scholar]
- Polak M. Developmental Instability: Causes and Consequences. Oxford: Oxford University Press; 2003. [Google Scholar]
- Raff RA. Developmental mechanisms in the evolution of animal form: origins and evolvability of body plans. In: Bengston S, editor. Early Life of Earth. New York: Columbia University Press; 1994. pp. 489–500. [Google Scholar]
- Redenbach DM, Nelems B. A comparative study of structures of comprising the thoracic outlet in 250 human cadavers and 72 surgical cases of thoracic outlet syndrome. Eur J Cardio-Thorac Surg. 1998;13:353–360. doi: 10.1016/s1010-7940(98)00037-2. [DOI] [PubMed] [Google Scholar]
- Roos DB. Congenital anomalies associated with thoracic outlet syndrome – anatomy, symptoms, diagnosis and treatment. Am J Surg. 1976;132:771–778. doi: 10.1016/0002-9610(76)90456-6. [DOI] [PubMed] [Google Scholar]
- Roos DB, Annest SJ, Brantigan CO. Historical and anatomic perspectives on thoracic outlet syndrome. Chest Surg Clin N Am. 1999;9:713–723. [Google Scholar]
- Sander K. The evolution of patterning mechanisms: gleanings from insect embryogenesis and spermatogenesis. In: Goodwin BC, Holder N, Wylie CC, editors. Development and Evolution. Cambridge: Cambridge University Press; 1983. pp. 137–159. [Google Scholar]
- Schumacher R, Mai A, Gutjahr P. Association of rib anomalies and malignancy in childhood. Eur J Pediatr. 1992;151:432–434. doi: 10.1007/BF01959357. [DOI] [PubMed] [Google Scholar]
- Serck-Hanssen T. Cervical ribs combined with other anomalies of the vertebral column as a family condition. Acta Chir Scandinav. 1935;76:551. [Google Scholar]
- Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer. 2010;10:361–371. doi: 10.1038/nrc2826. [DOI] [PubMed] [Google Scholar]
- Southam AH, Bythell WJS. Cervical ribs in children. Br Med J. 1924;88:844–845. doi: 10.1136/bmj.2.3332.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigenga M, Ruinard S, de Koning J, et al. Evolutionary conserved structures as indicators of medical risks. Increased incidence of cervical ribs after ovarian hyper stimulation in mice. J Anim Biol. 2006;56:63–68. [Google Scholar]
- Tabariès S, Lemieux M, Aubin J, et al. Comparative analysis of Hoxa5 allelic series. Genesis. 2007;45:218–228. doi: 10.1002/dvg.20292. [DOI] [PubMed] [Google Scholar]
- Topinard P. Des anomalies de nombre de la colonne vertébrale chez l′homme. Rev Anthropol. 1877;6:575–659. [Google Scholar]
- Van Dongen S. Fluctuating asymmetry and developmental instability in evolutionary biology: past, present and future. J Evol Biol. 2006;19:1727–1743. doi: 10.1111/j.1420-9101.2006.01175.x. [DOI] [PubMed] [Google Scholar]
- Van Dongen S, Wijnaendts LCD, ten Broek CMA, et al. Fluctuating asymmetry does not consistently reflect severe developmental disorders in human fetuses. Evolution. 2009;63:832–1844. doi: 10.1111/j.1558-5646.2009.00675.x. [DOI] [PubMed] [Google Scholar]
- Varela-Lasheras I, Bakker A, van der Mije S, et al. Braking evolutionary and pleiotropic constraints in mammals: on sloths, manatees and homeotic mutations. EvoDevo. 2011;2 doi: 10.1186/2041-9139-2-11. (11), doi: 10.1186/2041-9139-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellik DM. Hox genes and vertebral pattern. In: Pourquie O, editor. Current Topics in Developmental Biology. Burlington: Academic Press; 2009. pp. 257–278. [DOI] [PubMed] [Google Scholar]
- Wellik DM, Capecchi MR. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. 2003;301:363–367. doi: 10.1126/science.1085672. [DOI] [PubMed] [Google Scholar]
- Wéry N, Foulon O, Blacker A, et al. Vertebral malformations induced by sodium salicylate correlate with shifts in expression domains of Hox genes. Reprod Toxicol. 2005;20:39–45. doi: 10.1016/j.reprotox.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Wickramaratne GAS. The post-natal fate of supernumerary ribs in rat teratogenicity studies. J Appl Toxicol. 1988;8:91–94. doi: 10.1002/jat.2550080205. [DOI] [PubMed] [Google Scholar]
- Woltering JM, Vonk FJ, Muller H, et al. Axial patterning in snakes and ceacilians: evidence for an alternative interpretation of the Hox code. Dev Biol. 2009;332:82–89. doi: 10.1016/j.ydbio.2009.04.031. [DOI] [PubMed] [Google Scholar]


