Abstract
The formation of salivary glands entails the proliferation of epithelial cells from the stomatodeum into the underlying ectomesenchyme, culminating in a complex network of ducts and acinar bulbs. The extent to which mucins regulate this process is unknown, but they appear to mediate luminal space formation and maturation. Our aim was to examine mucin expression patterns during the morphogenesis of human salivary glands. Mucin expression – MUC1, 2, 3, 4, 5AC, 5B, 6, and 16 – was analyzed in specimens of developing human salivary glands, obtained from fetuses at 4–24 weeks’ gestation, and fully developed salivary glands by immunohistochemistry. Expression patterns were analyzed qualitatively according to the development stage of the salivary glands. Mucins 1, 3, 4, 5B, and 16 were expressed during salivary gland development – being stronger in all ductal segments by the final phases of branching morphogenesis and in mature glands. Acinar cells were negative for most mucins, including MUC1 in mature salivary glands. Mucins 2, 5AC, and 6 were not expressed. Mucins MUC1, 3, 4, 5B, and 16 are expressed in developing human salivary glands and in mature glands, suggesting important roles in the maturation and maintenance of the ductal network.
Keywords: branching morphogenesis, human salivary glands, mucin
Introduction
All salivary glands develop in a similar manner. Formation begins with the proliferation of a solid cord of cells from the epithelium of the stomatodeum into the underlying ectomesenchyme. This cord of cells extends deeply into the ectomesenchyme and branches extensively. Canalization occurs and degradation of the central cells to form the ductal system and the terminal secretory end pieces. The epithelial ingrowths constitute the parenchyma of a salivary gland. The ectomesenchyme differentiates into the connective tissue component of the gland, which supports the glandular parenchyma (Dale, 1994; Klein, 1994; Lourenço et al. 2007, 2008).
At the end of maturation, salivary glands are ready to produce saliva, which occurs in two phases: an acinar phase, during which primary saliva is generated, and a ductal phase of electrolyte resorption, resulting in hypotonic saliva. The final composition of saliva is complex, comprising electrolytes, immunoglobulins, proteins, enzymes, nitrogenous products, and mucins. This amalgam has many functions in maintaining oral health (Guyton & Hall, 2000; Humphrey & Williamson, 2001).
Mucins are the chief glycoprotein components of saliva and other mucous secretions and have many biological actions, such as protection of mucosal surfaces from adverse environmental influences, facilitation of glandular secretion, promotion and modulation of cell adhesion, and regulation of signaling (Braga et al. 1992; Hilkens et al. 1992; Komatsu et al. 2000; Chaturvedi et al. 2008).
In the oral cavity, mucins mediate the protection of dental surfaces against chemical (demineralization and proteolytic degradation) and mechanical (attrition and abrasion) damage. They also prevent caries by limiting bacterial adhesion and controlling oral infection, inhibiting the colonization of pathogenic microbes (Zalewska et al. 2000; Liu et al. 2002; Piras et al. 2010). Mucins regulate the lubrication of the mucosal surface, benefiting physiological functions such as mastication, speech, and swallowing. Further, they prevent mucosal desiccation, based on their capacity to retain water (Nieuw Amerongen et al. 1995; Humphrey & Williamson, 2001; Sonesson et al. 2008).
Mucins are synthesized by epithelial cells and, in some cases, endothelial cells (Hollingsworth & Swanson, 2004; Zhang et al. 2005; Chaturvedi et al. 2008). Nearly 20 mucin (MUC)-encoding genes have been described, some of which have been well characterized (Ho et al. 1993; Gendler & Spicer, 1995). Mucins are membrane-bound (transmembranic) (MUC1, MUC3, MUC4, MUC12, and MUC17), encoded by the 7q22, 3q, and 1q21 loci, or secretory (gel-forming) (MUC2, MUC5AC, MUC5B and MUC6), encoded primarily by the 11p15 locus and restricted to secretory organs and certain cell types (Awaya et al. 2004; Handra-Luca et al. 2005; Chaturvedi et al. 2008). Other products of certain MUC genes do not belong to either class (MUC7, MUC8, MUC9, MUC13, MUC15, and MUC16) (Sasaki et al. 2007).
The expression of each MUC gene is specific to an organ, tissue, or cell type (Nieuw Amerongen et al. 1995; Alos et al. 2005). Some mucins are expressed in most epithelial and glandular tissues, such as in pancreas (MUC1), breast (MUC1), the gastrointestinal (MUCs3, 4, 5AC, 5B, 6, 17) and respiratory tracts, tracheobronchial mucosa (MUC 2, 4, 5AC, 5B, 6), gallbladder (MUC3), and salivary glands (1, 2, 5AC, 5B, 6, 7, 16) (Ho et al. 1993; Alos et al. 2005; Chaturvedi et al. 2008, Piras et al. 2010). MUC5B is also expressed in the endocervix, respiratory tract, gallbladder, and colon (Audie et al. 1993; Porchet et al. 1995; van Klinken et al. 1998).
The expression patterns of mucins in fully developed salivary glands and salivary gland neoplasms have been described recently (Liu et al. 2002; Alos et al. 2005; Handra-Luca et al. 2005; Piras et al. 2010). But such patterns in salivary gland tissue have not been determined, and mucin expression during human salivary gland morphogenesis has not been reported. The purpose of this study is to determine the distribution of mucins during human salivary gland development and its relationship with gland morphology and maturation.
Materials and methods
Tissue preparation
Fragments from the oral cavity of 20 postmortem human fetuses (from natural miscarriages) were used. The fetuses were at 4–24 weeks’ gestation and were obtained from the Medical School of the University of São Paulo, with permission from the ethical committee of the institution.
The specimens were collected from the buccal mucosa, tongue, mandible, and hard palate. Specimens of fully developed salivary glands from oral mucosa biopsies were retrieved from the archives of the Laboratory of Dermatopathology of the same institution and used as controls. Histologically, they were normal and exhibited no inflammation or neoplasms.
All specimens were fixed in 10% buffered formalin for 24 h and embedded in paraffin. They were then processed histologically, serial-sectioned, and stained with hematoxylin and eosin to determine the presence of salivary glands and study their morphology. Specimens that contained developing minor salivary glands were selected for the immunohistochemical analysis (80 specimens). The expression of MUC1, 2, 3, 4, 5AC, 5B, 6, and 16 was examined in all developing salivary gland samples and controls.
Immunohistochemistry
Serial sections of 4 μm of the developing salivary gland samples were deparaffinized, rehydrated, and subjected to antigen retrieval. The antigen retrieval methods and primary antibody clones, sources, and names are listed in Table 1. The sections were incubated in 3% aqueous hydrogen peroxide for 15 min to quench endogenous peroxidase activity and with Protein Block Serum-Free (DakoCytomation, Carpinteria, CA, USA) for 20 min at room temperature to prevent nonspecific binding of subsequent reagents.
Table 1.
Antibody sources, clones, and working protocols
| Primary serum | Source | Clone | Titer | Antigen retrieval |
|---|---|---|---|---|
| MUC1 | Novocastra, Benton Lane, Newcastle, UK | Ma695 | 1 : 2000 | Citrate buffer/pH 6.0 |
| MUC2 | Novocastra | Ccp58 | 1 : 2000 | Citrate buffer/pH 6.0 |
| MUC3 | Neomarkers, Fremont, CA, USA | 1143/B7 | 1 : 700 | EDTA/TRIS,pH 9.0 |
| MUC4 | Zymed, San Francisco, CA, USA | 1G8 | 1 : 800 | EDTA/pH 8.0 |
| MUC5AC | Novocastra, Benton Lane, Newcastle, UK | CLH2 | 1 : 1000 | Citrate buffer/pH 6.0 |
| MUC5B | LifeSpan, Seattle, WA, USA | Muc5b | 1 : 100 | Citrate buffer/pH 6.0 |
| MUC6 | Novocastra | CLH5 | 1 : 2000 | Citrate buffer/pH 6.0 |
| MUC16 | Abcam, Washington, DC, USA | X75 | 1 : 100 | EDTA/TRIS pH 9.0 |
The samples were incubated with primary antibody for 2 h at room temperature. The antigen-antibody complexes were visualized using the Advance system (DakoCytomation) and developed with 3′ 3-diaminobenzidine tetrachloride (DAB) (DakoCytomation) for 5 min. The sections were then counterstained with Mayer's hematoxylin, dehydrated, and mounted with glass coverslips and xylene-based mounting media. All immunohistochemical reactions and analyses were performed in duplicate.
The slides were examined on a conventional optical microscope that was equipped with a digital camera for photographic registration.
As negative controls, the specimens were incubated with nonimmune serum. Positive controls were included as per the manufacturer's recommendations.
Results
The specimens were minor human salivary glands at various stages of development, comprising the initial, pseudoglandular, canalicular, and terminal buds (Fig. 1), according to the stages of salivary gland morphogenesis proposed by Tucker (2007). The results concerning salivary gland morphology and morphogenetic stage are shown in Table 2.
Fig. 1.
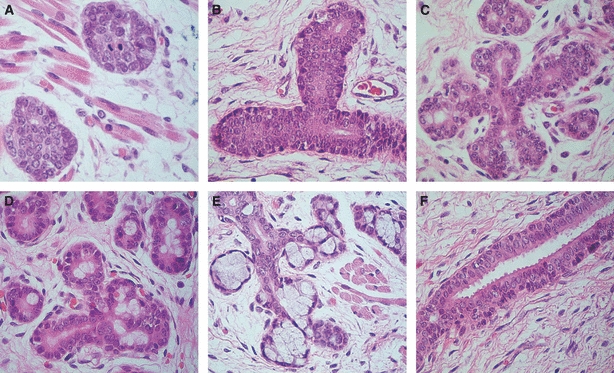
Morphological phases of human salivary gland development. (A) Initial bud: solid nests of epithelial cells surrounded by a loose mesenchyme (original magnification ×400). (B) Pseudoglandular stage: solid cords of epithelial cells with central vestigial luminal spaces (original magnification ×400). (C) Canalicular stage: proliferation of the glandular structure, forming a complex network of canalized structures (original magnification ×400). (D) Terminal bud – initial stage: presence of terminal bulbs composed of cells with large and clear cytoplasm (original magnification ×400). (E) Terminal bud – advanced stage: presence of well established acinar lobules (original magnification ×400). (F) Well developed excretory duct composed of multiple layers of epithelial cells. Note the secretion of eosinophilic material (mucous material) on the luminal pole of luminal cells (original magnification ×400).
Table 2.
Semiquantitative expression of mucins during human salivary gland morphogenesis and in fully developed salivary glands
| MUC1 | MUC2 | MUC3 | MUC4 | MUC5AC | MUC5B | MUC6 | MUC16 | |
|---|---|---|---|---|---|---|---|---|
| Developing human salivary gland | ||||||||
| (80 sp) | ||||||||
| Initial bud (6 sp) | ||||||||
| Epithelial cells | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stroma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudoglandular (24 sp) | ||||||||
| Luminal space | +++ | 0 | 0 | 0 | 0 | 0 | 0 | + |
| Ductal cells | + | 0 | + | + | 0 | + | 0 | 0 |
| Canalicalicular (40 sp) | ||||||||
| Luminal space | +++ | 0 | 0 | + | 0 | 0 | 0 | ++ |
| Ductal cells | + | 0 | ++ | 0 | 0 | ++ | 0 | 0 |
| Terminal bud (10 sp) | ||||||||
| Acinar cells | 0 | 0 | 0 | 0 | 0 | 0 | 0 | +++ |
| Ductal cells | +++ | 0 | ++ | + | 0 | +++ | 0 | 0 |
| Luminal space | +++ | 0 | 0 | ++ | 0 | 0 | 0 | ++ |
| Adult salivary glands | ||||||||
| (6 sp) | ||||||||
| Acinar cells | +++ | 0 | + | + | 0 | 0 | 0 | +++ |
| Ductal cells | 0 | 0 | + | +++ | 0 | +++ | 0 | 0 |
| Luminal space | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blood vessels | 0 | 0 | +++ | +++ | 0 | ++ | 0 | 0 |
+, weak expression; ++, moderate expression; +++, strong expression; 0, negative; sp, specimens.
Immunohistochemistry
MUC 1, 3, 4, 5B, and 16 was expressed in various phases of human salivary gland development and in mature human salivary glands. Mucins MUC 2, 5AC, and 6 were not expressed in any specimen.
At the initial bud stage, when salivary glands begin to develop, only MUC1 was detected around a few clusters of cells (Fig. 2A1,A2), and MUC5B and 16 were expressed weakly in some cells (Fig. 3A1,B1). At this stage, MUC3 and MUC4 were not present (Fig. 2A3,A4).
Fig. 2.

Expression of MUC1, 3, and 4 in developing human salivary glands. (A1–A4) Initial bud: (A1,A2) Minimal expression of MUC1 in the epithelial cords (arrows). (A3,A4) No MUC3 or 4 at this stage of glandular development. Original magnification: ×400. (B1–B3) Pseudoglandular stage: (B1) MUC1 in the luminal space of canalizing structures. (B2) Mucin MUC3 – diffuse expression in the cytoplasm of epithelial cells. Expression of this mucin was also observed in blood vessel walls (arrows). (B3) Weak expression of MUC4 mainly in the luminal space of the ductal system and in blood vessel walls (arrows). Original magnification: ×400 (B1), ×600 (B2), and ×250 (B3). (C1–C3) Canalicular stage: (C1) Strong expression of MUC1 in the luminal spaces of the ductal system. (C2) Weak expression of MUC3 in ductal cell cytoplasm, mainly in the basal pole. (C3) Weak expression of MUC4 in the luminal pole of ducts and in blood vessel walls (arrows). Original magnification: ×150 (C1), ×600 (C2), and ×400 (C3). (D1–D4) Terminal bud: (D1,D2) Strong expression of MUC1 in the luminal space of the ductal system and in the basal pole of excretory duct cell cytoplasm. (D3) Expression of MUC3 in the cytoplasm of ductal cells and in some demilune structures. (D4) Presence of MUC4 in blood vessel walls (arrows) and in the luminal space of excretory ducts. Original magnification: ×600 (D1), ×150 (D2), ×400 (D3), ×400 (D4).
Fig. 3.

Expression of MUC5B and 16 in developing human salivary glands. (A1,B1) Pseudoglandular stage: (A1) No MUC5B at this stage of glandular development. (B1) MUC 16 in several ductal lumens (arrow). Original magnification: ×400. (A2,B2) Canalicular stage: (A2) MUC5B in the luminal space of canalized ducts. (B2) MUC16 in the luminal spaces of well canalized and differentiated glandular structures. Acinar lobules – positive for MUC16 in the central spaces. Original magnification ×400. (A3,B3) Terminal bud: (A3) Strong expression of MUC5B in the luminal spaces of the ductal system. (B3) MUC16 in the central space of acinar lobules. Luminal borders of ducts are negative. Original magnification: ×400 (A3) and ×600 (B3).
At the pseudoglandular stage, MUC1 was observed as brown deposits in the rudimentary luminal space (Fig. 2B1). In this phase, MUC3 staining was faint in the vessel walls at the initial phase of canalization but formed small cytoplasmic deposits in the glandular cells of future ductal structures as this phase of glandular morphogenesis progressed (Fig. 2B2). MUC4 expression was robust in the luminal pole of luminal cells (Fig. 2B3). MUC5B and 16 were poorly expressed in the luminal space in all specimens (Fig. 3A1,B1).
MUC1, 3, and 4 were present in the canalicular stage of salivary gland morphogenesis. MUC1 appeared in the luminal space of all glandular ductal systems, and MUC4 was expressed only in the luminal space of well-developed excretory ducts, but not to a greater extent than MUC1 (Fig. 2C1,C3). MUC4 was also observed in blood vessel walls (Fig. 2C3– arrow), similar to MUC5B (Fig. 3A3). MUC3 was expressed in the cytoplasm of ductal cells (Fig. 2C2). MUC5B and 16 were not expressed at this stage of morphogenesis.
During the terminal bud stage, MUC1 maintained its strong expression along the luminal pole of luminal cells throughout the entire ductal system (Fig. 2D1,D2). Further, MUC1 was expressed in the cytoplasm of basal cells of larger excretory ducts in certain areas (Fig. 2D1). MUC3 was present in the cytoplasm of many glandular cells – in all ductal portions (intercalated, interlobular and excretory extralobular ducts), and in the demilune structures around acinar lobules (Fig. 2D3). MUC4 maintained its positivity only in the luminal pole of luminal cells of larger excretory ducts (Fig. 2D4). MUC3 and MUC4 were also expressed in the luminal border of blood vessel walls (Fig. 2D4– arrow). All these three mucins were absent in well-developed acinar structures, and MUC5B and 16 were highly expressed in mucous cells (Fig. 3A2,B2). In minor mucous salivary glands, MUC5B was not expressed, and MUC16 was present only at the luminal pole of ductal cells and as deposits in the luminal space (Fig. 3A3,B3).
In fully developed salivary glands, MUC1 was detected in the cytoplasm of acinar cells and in the luminal space border of the ducts (Fig. 4A). MUC3 and MUC4 were expressed in ductal cells, acinar lobules, and blood vessels (Fig. 4B,C1 and C2). MUC3 staining was faint in the cytoplasm of ductal cells, whereas MUC4 was intense in several layers of cells that constitute the excretory ducts (Fig. 4C2). MUC5B was detected in the luminal border of excretory ducts, and MUC16 was observed in acinar cells and the luminal border of excretory ducts (Fig. 4D1 and D2, respectively).
Fig. 4.

Expression of MUC1, 3, 4, and 16 in fully developed human salivary glands. (A) Expression of MUC1 in the cytoplasm of acinar cells and in the luminal portions of ducts. Original magnification: ×400. (B) Presence of MUC3 in the cytoplasm of ductal cells and in myoepithelial cells. Original magnification: ×400. (C) MUC4 expressed in the luminal portion of interlobular ducts (C1) and in blood vessel walls (C1 arrows). Marked expression of MUC4 in excretory ducts (C2). Original magnification: ×400. (D) Expression of MUC16 in acinar cells (D1) and luminal border of excretory ducts (D2). Original magnification: ×400.
Discussion
This study demonstrates the expression of a panel of mucins throughout human salivary gland development. Each mucin has been suggested to have disparate properties, e.g. an epidermal growth factor-like domain, a transmembrane region, and a capacity to form gels, conferring diverse functions (Sasaki et al. 2007). This model is supported by our results.
Five of the eight mucins that we examined were positive throughout human salivary gland morphogenesis: MUC1, MUC3, MUC4, MUC5B, and MUC16. Further, the expression of each of these mucins varied, depending on glandular developmental phase. MUC2, 5AC, and 6 were not detected in our specimens; according to Alos et al. (2005), they are negative or only focally expressed in certain fully developed salivary glands, confirming our findings.
MUC1 and MUC4 are the best-characterized membrane-associated mucins in salivary glands and are classified structurally as monomers. MUC3 is also considered a membrane-bound mucin (Liu et al. 2002). Whereas the functions of gel-forming mucins are believed to be primarily protective, the functions of membrane-associated mucins are poorly understood, although recent evidence suggests that they mediate signal transduction cascades, a function that is believed to be linked to the presence of EGF-like motifs in MUC3A, MUC3B, MUC4, MUC12, and MUC17. Such domains might modulate epithelial growth, which explains in part the expression of MUC3 and 4 during salivary gland morphogenesis.
Although the mechanisms by which these mucins function in human salivary glands are unknown, Liu et al. (2002) demonstrated that MUC1 and MUC4 in fully developed parotid and submandibular glands and membrane-associated mucins might regulate the signaling pathways that govern their normal and physiological functions.
Despite this significant evidence on mucin function in fully developed glands, there is very little information available on mucin expression in human salivary gland development. Nevertheless, MUC1 is an important glycoprotein that might facilitate the flow of secretions through the ductal system (Liu et al. 2002). We detected MUC1 deposits at the bud phase, immediately before morphological evidence of epithelial cord proliferation and canalization. Braga et al. (1992) observed that MUC1 lines the luminal border of epithelia that fold and branch, even before the completion of organ maturation; this pattern is also observed in salivary glands. MUC1 deposits increased gradually with subsequent luminal opening during salivary gland morphogenesis, and its expression was greater in pseudoglandular and canalicular phases, primarily in the luminal pole of epithelial cells.
These results are consistent with those of other groups. Alos et al. (2005) noted MUC1 primarily in the apical pole of normal salivary glands ductal cells, as observed by other investigators in various tubular organs (Braga et al. 1992), strengthening a model in which MUC1 expression is induced concomitantly with epithelial differentiation in individual organs.
High MUC1 expression decreases cell–cell interactions on the luminal surface of ducts, which likely facilitates lumen formation and maintenance by reducing adhesive associations in the apical domain (Lightenberg et al. 1992). Moreover, the structure and biochemical properties of MUC1 might help prevent interactions between adhesive molecules, favoring the maintenance of luminal spaces. The maintenance of the luminal space might also be aided by the expression of other mucins; this hypothesis is supported by our results, in which MUC4 is expressed in the late stages of salivary gland morphogenesis, when ductal lumina are already well formed, especially on the luminal surface of excretory ducts.
MUC4 is expressed in many normal epithelial tissues during development and in adults (Gendler & Spicer, 1995; Van Klinken et al. 1995; Zhang et al. 2006). During development, MUC4 levels rise progressively with gestational age, and in certain sections of the digestive tube, its expression correlates with the stage of differentiation (Zhang et al. 2006). This fact was observed in our results – MUC4 expression increased as gland maturation occurred and was more significant in the squamous epithelium of excretory ducts. Other groups have documented expression of MUC4 in excretory, striated, and intercalated ducts and moderate expression in acinar structures of the parotid submandibular gland and other minor salivary glands (Liu et al. 2002; Alos et al. 2005).
The expression of MUC1 and MUC4 in the late stages of salivary gland morphogenesis might be indicative of their other physiological functions in the fully developed gland, such as facilitation of salivary flow secretion through the duct system and interactions with other molecules on oral epithelial surfaces for further association with the gel-forming mucin MUC5B, which has protective functions as part of MG1 (Liu et al. 1998). This latter function has been ascribed to MUC4 in the breast milk of humans and other mammals (Zhang et al. 2005; Ruvoen-Clouet et al. 2006; Chaturvedi et al. 2008).
Moreover, MUC1 and MUC4 are modulators of cell signaling, whereby MUC1 acts as a docking protein for signaling pathways and MUC4 regulates the receptor tyrosine kinase ErbB2 (Carraway et al. 2002; Zhang et al. 2006). These MUCs might participate in such mechanisms during salivary gland development, a hypothesis that should be examined in future studies.
The membrane-bound mucin MUC3 was detected in the pseudoglandular/canalicular phases of salivary gland morphogenesis in the cytoplasm of cells that compose the glandular ductal system. The cysteine (Cys)-rich domain of MUC3 and its epidermal growth factor-like domains govern epithelial growth and function in epithelial restitution. Therefore, MUC3 expression in salivary gland morphogenesis might be crucial for the growth and determination of the final shape of the ductal system and can be developed as a novel therapeutic agent in inflammatory glandular diseases, as proposed by Ho et al. (2006) for intestinal wound healing.
MUC5B is the chief mucin in human saliva, originally termed MG1. It is a high-molecular-weight oligomeric glycoprotein that is the principal secretory product of mucous acini of the submandibular, sublingual, and palatal glands (Piras et al. 2010). Notably, MUC5B was expressed in blood vessel walls. In an excellent review on the structure and biosynthesis of salivary gland mucins, Zalewska et al. reported that six subdomains of the C-terminal region of MUC5B have conserved sequences that are similar to those in von Willebrand factor, which is expressed in the subendothelial area of most blood vessels (Sadler, 1998; Zalewska et al. 2000). Thus, the MUC5B expression in blood vessels walls might have been due to cross-reaction with its vWF homolog. We are testing this hypothesis.
In contrast to MUC5B, there are no data on the expression of MUC16 in human salivary glands. This protein is primarily expressed in human nasal mucosa, corneal epithelial cells, and ovarian cancers (Davies et al. 2007; Woo et al. 2010).
Whereas MUC1, 3, and 4 levels were highest at the initial stages of the developmental human salivary glands that we analyzed, MUC5B and 16 were more important during the final stages of their development, when mucus production begins. According to Alos et al. (2005) and Piras et al. (2010), MUC5B is robustly expressed in the cytoplasm of mucous acini of all normal submandibular and minor salivary glands.
An understanding of the expression of certain mucins in the early phases of glandular morphogenesis might allow us to comprehend the reported expression of mucins in salivary gland neoplasms. Hamada et al. (2004) have shown that MUC1 expression in pleomorphic adenomas is associated with tumor recurrence. Other groups have linked MUC1 expression with a poor prognosis in mucoepidermoid carcinomas (Hilkens et al. 1992; Alos et al. 2005). It is possible that MUC1 regulates cell migration and invasion during embryogenesis and organ formation and with regard to the invasiveness of neoplastic cells. This issue, however, merits further investigation using experimental approaches to evaluate MUC1 as a prognostic marker of salivary gland neoplasms.
Author contributions
Tathyane Harumi N. Teshima contributed to the study design, acquisition of data, data analysis, interpretation, and manuscript drafting. Renata Fraga Ianez, Claudia M. Coutinho-Camillo, and Marcilei E. Buim contributed to the study design, acquisition of data, data analysis, and interpretation. Fernando A. Soares and Silvia V. Lourenço contributed to the study concept and design, data interpretation, critical revision of the manuscript, and final approval of the materials and manuscript.
References
- Alos L, Lujan B, Castillo M, et al. Expression of membrane-bound mucins (MUC-1 and MUC-4) and secreted mucins (MUC-2, MUC-5AC, MUC-5B, MUC-6 and MUC7) in mucoepidermoid carcinomas of salivary glands. Am J Surg Pathol. 2005;29:806–813. doi: 10.1097/01.pas.0000155856.84553.c9. [DOI] [PubMed] [Google Scholar]
- Audie JP, Janin A, Porchet N, et al. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993;41:1479–1485. doi: 10.1177/41.10.8245407. [DOI] [PubMed] [Google Scholar]
- Awaya H, Takeshima Y, Yamasaki M, et al. Expression of MUC-1, MC-2, MUC-5AC, and MUC-6 in atypical adenomatous hyperplasia, bronchioloalveolar carcinoma, adenocarcinoma with mixed subtypes, and mucinous bronchioloalveolar carcinoma of the lung. Am J Clin Pathol. 2004;121:644–653. doi: 10.1309/U4WG-E9EB-FJN6-CM8R. [DOI] [PubMed] [Google Scholar]
- Braga VMM, Pemberton LF, Duhig T, et al. Spatial and temporal expression of an epithelial mucin, Muc-1, during mouse development. Development. 1992;115:427–437. doi: 10.1242/dev.115.2.427. [DOI] [PubMed] [Google Scholar]
- Carraway LK, Perez A, Idris N, et al. MUC4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog Nucleic Acids Res Mol Biol. 2002;171:149–185. doi: 10.1016/s0079-6603(02)71043-x. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Singh AP, Batra SK. Structure, evolution and biology of the MUC4 mucin. FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AC. Salivary gland. In: Ten Cate AR, editor. Oral Histology. 4th edn. St. Louis: Mosby; 1994. pp. 312–333. [Google Scholar]
- Davies J, Kirkham S, Svitacheva N, et al. MUC16 is produced in tracheal surface epithelium and submucosal glands and is present in secretions from normal human airway and cultured bronchial epithelial cells. Int J Biochem Cell Biol. 2007;39:1943–1954. doi: 10.1016/j.biocel.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Hall JE. Textbook of Medical Physiology. 10th edn. Philadelphia: WB Saunders Company; 2000. pp. 738–753. [Google Scholar]
- Hamada T, Matsukita S, Goto M, et al. Mucin expression in pleomorphic adenoma of salivary gland: a potential role for MUC1 as a marker to predict recurrence. J Clin Pathol. 2004;57:813–821. doi: 10.1136/jcp.2003.014043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handra-Luca A, Lamas G, Jacques-Charles B, et al. MUC 1, MUC 2, MUC 4 and MUC 5AC expression in salivary gland mucoepidermoid carcinoma – diagnostic and prognostic implications. Am J Surg Pathol. 2005;29:881–889. doi: 10.1097/01.pas.0000159103.95360.e8. [DOI] [PubMed] [Google Scholar]
- Hilkens J, Ligtenberg JL, Vos HL, et al. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem Sci. 1992;17:359–363. doi: 10.1016/0968-0004(92)90315-z. [DOI] [PubMed] [Google Scholar]
- Ho SB, Niehans GA, Lyftogt C, et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641–651. [PubMed] [Google Scholar]
- Ho SB, Dvorak LA, Moor RE, et al. Cysteine-rich domains of MUC3 intestinal mucin promote cell migration, inhibit apoptosis, and accelerate wound healing. Gastroenterology. 2006;131:1501–1517. doi: 10.1053/j.gastro.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Humphrey SP, Williamson RT. A review of saliva: normal composition, flow and function. J Prosthet Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- Klein RM. Development, structure and function of salivary glands. In: Avery JK, editor. Oral Development and Histology. 2nd edn. New York: Thieme; 1994. pp. 352–379. [Google Scholar]
- van Klinken BJ, Dekker J, van Gool SA, et al. MUC5B is the prominent mucin in human gallbladder and is also expressed in a subset of colonic goblet cells. Am J Physiol. 1998;274:G871–G878. doi: 10.1152/ajpgi.1998.274.5.G871. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Tatum L, Altman NH, et al. Potentiation of metastasis by cell surface sialomucin complex (MUC4), a multifunctional anti-adhesive glycoprotein. Int J Cancer. 2000;87:480–486. doi: 10.1002/1097-0215(20000815)87:4<480::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Lightenberg MJL, Kruijshaar L, Buijs F, et al. Cell associated episialin is a complex containing two proteins derived from a common precursor. J Biol Chem. 1992;267:6171–6177. [PubMed] [Google Scholar]
- Liu B, Offner GD, Nunes DP, et al. MUC4 is a major component of salivary mucin MG1 secreted by the human submandibular gland. Biochem Biophys Res Commun. 1998;250:757–761. doi: 10.1006/bbrc.1998.9390. [DOI] [PubMed] [Google Scholar]
- Liu B, Lague JR, Nunes DP, et al. Expression of membrane-associated mucins MUC1 and MUC4 in major human salivary glands. J Histochem Cytochem. 2002;50:811–820. doi: 10.1177/002215540205000607. [DOI] [PubMed] [Google Scholar]
- Lourenço SV, Coutinho-Camillo CM, Buim MEC, et al. Human salivary gland branching morphogenesis: morphological localization of claudins and its parallel relation with developmental stages revealed by expression of cytoskeleton and secretion markers. Histochem Cell Biol. 2007;128:361–369. doi: 10.1007/s00418-007-0322-6. [DOI] [PubMed] [Google Scholar]
- Lourenço SV, Uyekita SH, Lima DMC, et al. Developing human minor salivary glands: morphological parallel relation between the expression of TGF-beta isoforms and cytoskeletal markers of glandular maturation. Virchows Arch. 2008;452:427–434. doi: 10.1007/s00428-007-0552-y. [DOI] [PubMed] [Google Scholar]
- Nieuw Amerongen AV, Bolscher JGM, Veerman ECI. Salivary mucins: protective functions in relation to their diversity. Glycobiology. 1995;5:733–740. doi: 10.1093/glycob/5.8.733. [DOI] [PubMed] [Google Scholar]
- Piras M, Hand AR, Tore G, et al. Ultrastructural localization of salivary mucins MUC5B and MUC7 in human labial glands. Eur J Oral Sci. 2010;118:14–18. doi: 10.1111/j.1600-0722.2009.00700.x. [DOI] [PubMed] [Google Scholar]
- Porchet N, Pigny P, Buisine MP, et al. Human mucin genes: genomic organization and expression of MUC4, MUC5AC and MUC5B. Biochem Soc Trans. 1995;23:800–805. doi: 10.1042/bst0230800. [DOI] [PubMed] [Google Scholar]
- Ruvoen-Clouet N, Mas E, Marionneau S, et al. Bile-salt-stimulated lipase and mucins from milk of ‘secretor’ mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands. Biochem J. 2006;393:626–634. doi: 10.1042/BJ20050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler JE. Biochemistry and genetics of Von Willebrand Factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Ikeda H, Nakanuma Y. Expression profiles of MUC mucins and trefoil factor family (TFF) peptides in the intrahepatic biliary system: physiological distribution and pathological significance. Prog Histochem Cytochem. 2007;42:61–110. doi: 10.1016/j.proghi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Sonesson M, Wickström C, Kinnby B, et al. Mucins MUC5B and MUC7 in minor salivary gland secretion of children and adults. Arch Oral Biol. 2008;53:523–527. doi: 10.1016/j.archoralbio.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Tucker AS. Salivary gland development. Semin Cell Dev Biol. 2007;18:237–244. doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Van Klinken BJW, Decker J, Buller HA, et al. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol. 1995;269:G613–G627. doi: 10.1152/ajpgi.1995.269.5.G613. [DOI] [PubMed] [Google Scholar]
- Woo H, Bae CH, Song S, et al. Expression of membrane-bound mucins in human nasal mucosa. Different patterns for MUC4 and MUC16. Arch Otolaryngol Head Neck Surg. 2010;136:603–609. doi: 10.1001/archoto.2010.71. [DOI] [PubMed] [Google Scholar]
- Zalewska A, Zwierz K, Zólkowski K, et al. Structure and biosynthesis of human salivary mucins. Acta Biochim Pol. 2000;47:1067–1079. [PubMed] [Google Scholar]
- Zhang J, Perez A, Yasin M, et al. Presence of MUC4 in human milk and at the luminal surfaces of blood vessels. J Cell Physiol. 2005;204:166–177. doi: 10.1002/jcp.20277. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yasin M, Carraway CAC, et al. MUC4 expression and localization in gastrointestinal tract and skin of human embryos. Tissue Cell. 2006;38:271–275. doi: 10.1016/j.tice.2006.06.004. [DOI] [PubMed] [Google Scholar]


