Abstract
Monodisperse dendrimer-entrapped gold nanoparticles (diameter = 3.0 nm) were prepared using G5 poly(amidoamine) (PAMAM) dendrimer functionalized with fluorescein isothiocyanate (FI) and Arg-Gly-Asp (RGD) peptide as template; in vitro targeting efficacy to integrin receptor expressing cells was confirmed by flow cytometry, confocal microscopy, and ICP-MS.
Synthesis of metal nanoparticles has recently gained considerable attention due to the potential for these particles in applications such as catalysis,1–4 optics,5,6 sensing,7–11 drug delivery,12–17 and imaging.18–20 Dendrimer-entrapped metal nanoparticles (DENPs) are a unique class of organic–inorganic hybrid materials where zerovalent metal is entrapped within the interior of a dendrimer.21 The terminal groups of DENPs can be modified with biologically active molecules to create functional nanoparticles. Dendrimer-entrapped gold nanoparticles (Au DENPs) with targeting ligands on their surface have potential for cancer drug targeting and imaging applications. However, the synthesis of multifunctional Au DENPs is a significant challenge as synthetic manipulations of the dendrimer particles often lead to aggregation or precipitation. Despite this, tumor targeted Au DENPs (<5 nm in diameter) remain very attractive for biomedical applications as they are likely to pass through large blood vessels or connective tissue matrices to target cancer cells and their vasculature, while at the same time remaining below the renal filtration threshold allowing excretion through the kidney.21,22
The identification of molecular markers that can differentiate newly formed capillaries from mature counterparts has provided a basis for the delivery of therapeutic agents to tumor neo-vasculature.23–25 The αVβ3 integrin is a specific marker of the neo-vasculature, which is normally found on the luminal surface of endothelial cells during angiogenesis.26 Arg-Gly-Asp (RGD) peptide motifs have been identified by phage display studies as high affinity αVβ3 selective ligands,27 and have been used to deliver doxorubicin23 and proapoptotic peptides28 to tumor vasculature. RGD peptides have also been utilized to target iron oxide nanoparticles to tumor vasculature for imaging applications.29,30 Thus, these molecules appear to be an excellent ligand for targeting and treatment of cancer cells through interaction with αVβ3 integrin that is present on numerous cell types associated with cancer.
We have previously reported that we can couple RGD peptides to generation 5 poly(amidoamine) (PAMAM) dendrimers (G5 PAMAM) to achieve targeting of the dendrimer conjugates to integrin over-expressing cancer cell lines.31,32 In this study we describe the templated synthesis and characterization of monodisperse multifunctional Au-DENPs from a partially acetylated fluorescein isothiocyanate (FI)-labeled G5 PAMAM conjugated with -cRGDyK peptides (G5-Fl-RGD). The specific targeting and internalization of the Au DENPs was demonstrated by flow cytometric analysis and confocal microscopy. In addition, preincubation with excess peptide completely blocked the binding and uptake of either the dendrimer conjugate or gold. This observation suggests that the Au DENPs are stable and specifically taken up by αVβ3 integrin over-expressing cell lines.
Au DENPs were prepared using G5-Fl-RGD as template, which was synthesized as previously reported (see Scheme 1).31 The complexation of aqueous AuCl4− into the dendrimer interior was followed by reduction with NaBH4 in water. Specifically, 74 equiv. of HAuCl4 per dendrimer were mixed with an aqueous solution of G5-Fl-RGD. The solution was vigorously stirred for 30 min to provide enough time for HAuCl4 to be complexed within the dendrimer. Next, a 4-fold molar excess of NaBH4 in water was added to the above solution while stirring to reduce the complexed gold salt to zerovalent Au. This resulted in conversion of the pale yellow solution to the characteristic deep red color of gold nanoparticles (Fig. 1, inset).
Scheme 1.
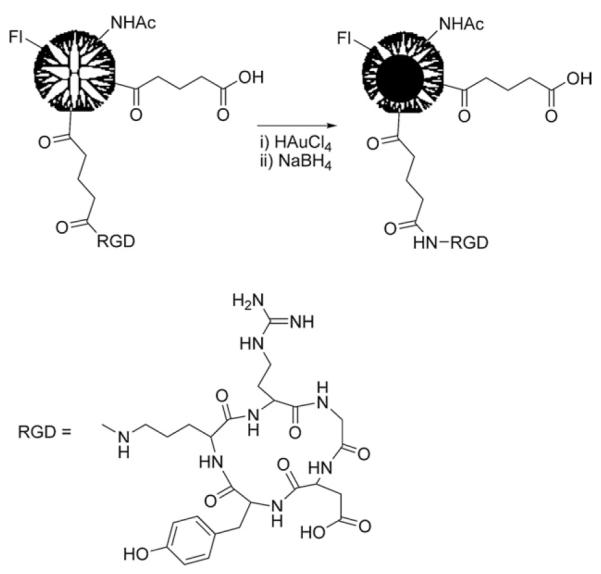
Synthesis of {(Au0)74-G5-FI-RGD} DENPs.
Fig. 1.
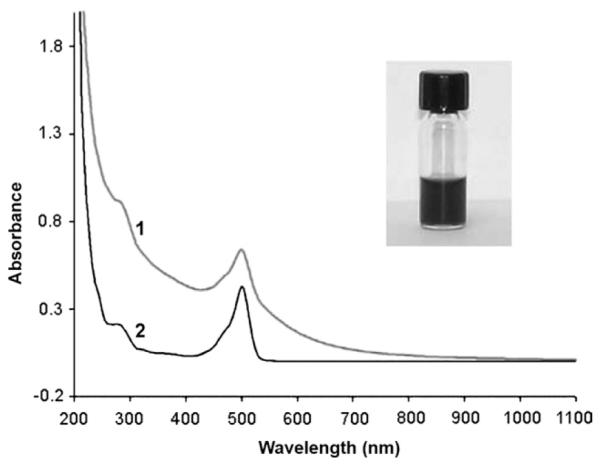
UV-vis absorption spectra of (1) {(Au0)74-G5-FI-RGD} DENPs and (2) G5-FI-RGD conjugate. Inset shows the Au DENP solution in water (0.5 mg mL−1).
The Au DENPs show characteristic absorption peaks at 500 and 280 nm for FI and RGD peptide, respectively (Fig. 1). An overlap of the surface plasmon resonance band of Au DENPs (510 nm) with the absorption of FI is also observed. A shoulder resulting from light scattering induced by nanoparticles (900–550 nm) is also observed. The functionalized Au-DENPs are stable in both water solution and cell culture medium, and no precipitation of the solution was observed even after storage for 2 months (a photomicrograph of the aqueous solution of the Au DENPs is shown in the inset of Fig. 1).
Transmission electron microscopy (TEM) shows that the synthesized Au DENPs are relatively monodisperse with a mean diameter of 3.0 nm (Fig. 2b). It is noteworthy that these gold particles are larger than those reported in literature (Fig. 2a).33,34 This is possibly due to the different reaction conditions employed to prepare the nanoparticles. For instance, Kim et al. used partially quaternized dendrimers to prepare entrapped Au nanoparticles with varying molar ratios of Au atom and dendrimer.33 The positive charge of the used partially quaternized dendrimers led to formation of smaller Au particles possibly by reducing the particle aggregation. The synthesized Au-DENPs are highly polycrystalline as shown by both the high-resolution TEM images and selected area electron diffraction (SAED) patterns. In Fig. 2c, typical crystal lattices for both single crystals and twin crystals of Au are observed. In order to confirm the composition of the synthesized Au-DENPs, energy dispersive spectroscopy (EDS) was performed on Au DENP samples, which clearly indicates the existence of Au elements (Fig. 2d).
Fig. 2.
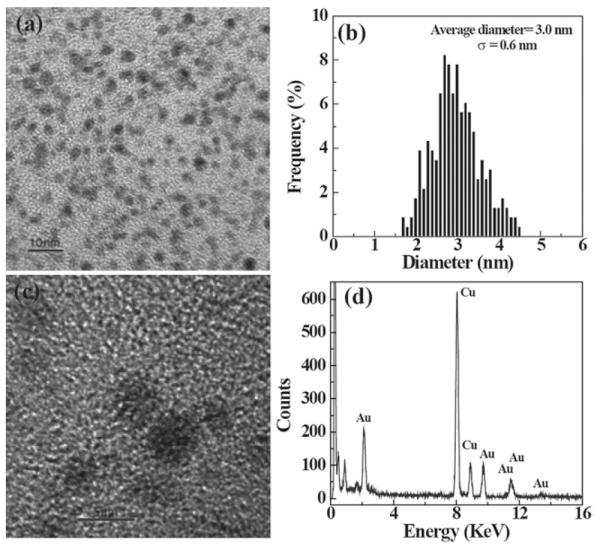
(a) A typical TEM image of {(Au0)74-G5-FI-RGD}; (b) size distribution histogram of {(Au0)74-G5-FI-RGD}; (c) high-resolution TEM image of {(Au0)74-G5-FI-RGD}; and (d) EDS spectrum of {(Au0)74-G5-FI-RGD}.
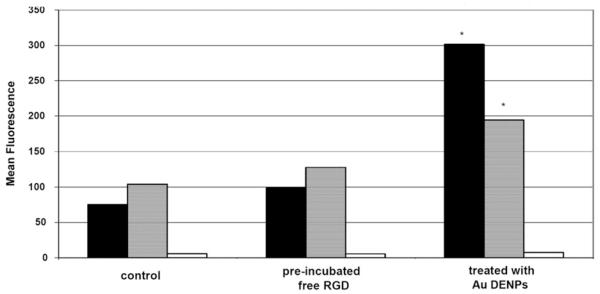
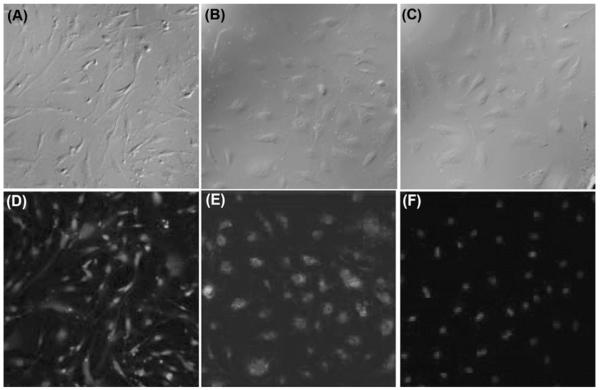
To examine specific binding of Au DENPs to integrin over-expressing human dermal microvessel endothelial cells (HDMEC) and human vascular endothelial cells (HUVEC), the cells were incubated with Au DENPs and examined via flow cytometry (Fig. 3). The Au DENPs were shown to bind the integrin-expressing cell lines in a dose-dependent fashion, whereas it failed to bind to a control cell line (L1210) known to lack the integrin receptor at all concentrations tested.31 Saturation binding for this conjugate was obtained with both integrin expressing cell lines (Fig. 4). The Au DENP conjugate was also shown to internalize in both integrin expressing cell lines by confocal microscopic analysis (Fig. 5) and is primarily localized in the cytosolic compartment. Quantitative analysis of gold in Au-DENP treated cells by inductively coupled plasma mass spectroscopy (ICP-MS) further confirmed the specific internalization of gold in the cells (Table 1). Maximum gold uptake was observed in HDMEC cells whereas control cell lines did not have any gold content. The uptake of gold was inhibited by preincubation with RGD peptide alone, together clearly indicating that the Au-DENPs specifically target the integrin receptor expressing cell lines.
Fig. 3.
Binding of {(Au0)74-G5-FI-RGD} DENPs to HUVEC (■) and HDMEC ( ) cells examined with and without pre-incubation with free RGD peptide. L1210 cells were used as a control (□), which is known to lack the αVβ3 integrin (p < 0.01).
) cells examined with and without pre-incubation with free RGD peptide. L1210 cells were used as a control (□), which is known to lack the αVβ3 integrin (p < 0.01).
Fig. 4.
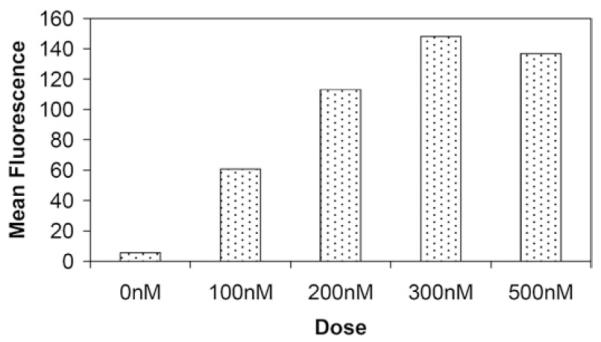
Dose-dependent binding of {(Au0)74-G5-FI-RGD} DENPs to HDMEC cells.
Fig. 5.
Confocal microscopic images (20x) of both HUVEC (A, D) and HDMEC (B, E) cells that were specifically bound with {(Au0)74-G5-FI-RGD} DENPs. In addition, images reveal that Au DENP binding was eliminated when cells were pre-incubated with free RGD peptide (C, F) prior to treatment.
Table 1.
ICP-MS analysis of HDMEC cells treated with {(Au0)74-G5-FI-RGD} DENPs
| Sample | Au concentration (ppm) |
Au concentration (nM) |
|---|---|---|
| Control | 0.0009 | 0.004 |
| HDMEC + RGD | 1.47 ± 0.07 | 0.07 |
| HDMEC | 4.56 ± 0.23 | 23.1 |
In summary, we present here a simple approach for the synthesis of nearly uniform, multifunctional Au DENPs. These nanoparticles specifically target integrin receptor expressing tumor cells. Further studies will focus on determining the targeting and therapeutic efficacy of these nanoparticles in vivo. The approach of using a multifunctional dendrimer as a template to synthesize gold nanoparticles could be a useful general strategy for creating multifunctional materials for a range of biomedical applications.
Footnotes
Electronic supplementary information (ESI) available: Experimental details of synthesis, characterization of nanoparticles and cell targeting and binding assays. See DOI: 10.1039/b810885d
Notes and references
- 1.Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK. Acc. Chem. Res. 2001;34:181–190. doi: 10.1021/ar000110a. [DOI] [PubMed] [Google Scholar]
- 2.Li D, Cui Y, Wang K, He Q, Yan X, Li J. Adv. Funct. Mater. 2007;17:3134–3140. [Google Scholar]
- 3.Li D, He Q, Cui Y, Li J. Chem. Mater. 2007;19:412–417. [Google Scholar]
- 4.Lu Y, Mei Y, Drechsler M, Ballauff M. Angew. Chem., Int. Ed. 2006;45:813–816. doi: 10.1002/anie.200502731. [DOI] [PubMed] [Google Scholar]
- 5.Olkhov RV, Shaw AM. Biosens. Bioelectron. 2008;23:1298–1302. doi: 10.1016/j.bios.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Zheng J, Zhang C, Dickson RM. Phys. Rev. Lett. 2004;93:077402. doi: 10.1103/PhysRevLett.93.077402. [DOI] [PubMed] [Google Scholar]
- 7.Drechsler U, Erdogan B, Rotello VM. Chem.–Eur. J. 2004;10:5570–5579. doi: 10.1002/chem.200306076. [DOI] [PubMed] [Google Scholar]
- 8.Han MS, Lytton-Jean AKR, Oh BK, J. H, Mirkin CA. Angew. Chem., Int. Ed. 2006;45:1807–1810. doi: 10.1002/anie.200504277. [DOI] [PubMed] [Google Scholar]
- 9.Lee JS, Han MS, Mirkin CA. Angew. Chem., Int. Ed. 2007;46:4093–4096. doi: 10.1002/anie.200700269. [DOI] [PubMed] [Google Scholar]
- 10.Li D, He Q, Zhu H, Tao C, Li J. J. Nanosci. Nanotechnol. 2007;7:3089–3094. doi: 10.1166/jnn.2007.637. [DOI] [PubMed] [Google Scholar]
- 11.You CC, Miranda OR, Gider B, Ghosh PS, Kim IB, Erdogan B, Krovi SA, Bunz UHF, Rotello VM. Nat. Nanotechnol. 2007;2:318–323. doi: 10.1038/nnano.2007.99. [DOI] [PubMed] [Google Scholar]
- 12.Gibson JD, Khanal BP, Zubarev ER. J. Am. Chem. Soc. 2007;129:11653–11661. doi: 10.1021/ja075181k. [DOI] [PubMed] [Google Scholar]
- 13.Han G, Ghosh P, Rotello VM. Nanomedicine. 2007;2:113–123. doi: 10.2217/17435889.2.1.113. [DOI] [PubMed] [Google Scholar]
- 14.Han G, Ghosh P, Rotello VM. Adv. Exp. Med. Biol. 2007;620:48–56. doi: 10.1007/978-0-387-76713-0_4. [DOI] [PubMed] [Google Scholar]
- 15.Li D, He Q, Cui Y, Wang K, Zhang X, Li J. Chem.–Eur. J. 2007;13:2224–2229. doi: 10.1002/chem.200600839. [DOI] [PubMed] [Google Scholar]
- 16.Saha B, Bhattacharya J, Mukherjee A, Ghosh AK, Santra CR, Dasgupta AK, Karmakar P. Nanoscale Res. Lett. 2007;2:614–622. [Google Scholar]
- 17.Seferos DS, Giljohann DA, Rosi NL, Mirkin CA. ChemBioChem. 2007;8:1230–1232. doi: 10.1002/cbic.200700262. [DOI] [PubMed] [Google Scholar]
- 18.Copland JA, Eghtedari M, Popov VL, Kotov N, Mamedova N, Motamedi M, Oraevsky AA. Mol. Imaging Biol. 2004;6:341–349. doi: 10.1016/j.mibio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.El-Sayed I, Huang X, Macheret F, Humstoe JO, Kramer R, El-Sayed M. Technol. Cancer Res. Treat. 2007;6:403–412. doi: 10.1177/153303460700600505. [DOI] [PubMed] [Google Scholar]
- 20.Rosi NL, Mirkin CA. Chem. Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 21.Shi X, Wang S, Meshinchi S, Van Antwerp M, Bi X, Lee I, Baker JR., Jr. Small. 2007;3:1245–1252. doi: 10.1002/smll.200700054. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharya R, Patra CR, Earl A, Wang SF, Katarya A, Lu LC, Kizhakkedathu JN, Yaszemski MJ, Greipp PR, Mukhopadhyay D, Mukherjee P. Nanomedicine. 2007;3:224–238. [Google Scholar]
- 23.Arap W, Pasqualini R, Ruoslahti E. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 24.Baillie CT, Winslet MC, Bradley NJ. Br. J. Cancer. 1995;72:257–267. doi: 10.1038/bjc.1995.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruoslahti E. Nat. Rev. Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 26.Cleaver O, Melton DA. Nat. Med. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- 27.Pasqualini R, Koivunen E, Ruoslahti E. Nat. Biotechnol. 1997;15:542–546. doi: 10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- 28.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Del Rio G, Krajewski S, Lombardo CR, Rao R, Ruoslahti E, Bredesen DE, Pasqualini R. Nat. Med. 1999;5:1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 29.Uchida M, Flenniken ML, Allen M, Willits DA, Crowley BE, Brumfield S, Willis AF, Jackiw L, Jutila M, Young MJ, Douglas T. J. Am. Chem. Soc. 2006;128:16626–16633. doi: 10.1021/ja0655690. [DOI] [PubMed] [Google Scholar]
- 30.Zhang CF, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, Zentgraf H, Bock M, Eisenhut M, Semmler W, Kiessling F. Cancer Res. 2007;67:1555–1562. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- 31.Hill E, Shukla R, Park SS, Baker JR., Jr. Bioconjugate Chem. 2007;18:1756–1762. doi: 10.1021/bc0700234. [DOI] [PubMed] [Google Scholar]
- 32.Shukla R, Thomas TP, Peters J, Kotlyar A, Myc A, Baker JR., Jr. Chem. Commun. 2005:5739–5741. doi: 10.1039/b507350b. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y-G, Oh S-K, Crooks RM. Chem. Mater. 2004;16:167–172. [Google Scholar]
- 34.Shi X, Wang S, Sun H, Baker JR., Jr. Soft Matter. 2007;3:71–74. doi: 10.1039/b612972b. [DOI] [PubMed] [Google Scholar]