Abstract
Follicular Helper T cells (TFH) are critical for germinal center (GC) formation. The processes that drive their generation and effector potential remain unclear. Here, we define requirements for MHCII antigen presenting cells (APCs) in murine TFH formation by either transiently ablating or restricting antigen presentation to dendritic cells (DCs). We find that cognate interactions with DCs are necessary and sufficient to prime CD4+ T cells towards a CXCR5+ICOS+Bcl6+ TFH intermediate. However, in the absence of additional APCs, these TFH fail to produce IL-21. Furthermore, in vitro priming of naïve T cells by B cells engenders optimal production of IL-21, which induces a GC B cell transcriptional profile. These results support a multi-step model for effector TFH priming and GC initiation, in which DCs are necessary and sufficient to induce a TFH intermediate that requires additional interactions with distinct APCs for full effector function.
INTRODUCTION
Germinal centers (GCs) are transient structures formed during thymus-dependent (TD) immune responses, where expanding B cells undergo affinity maturation, yielding memory B and plasma cells that mediate protective humoral immunity. The exact interactions dictating whether B and helper T cells assume GC status versus alternative fates remain unclear. A key event in GC formation is the Bcl-6 dependent differentiation of Follicular Helper T (TFH) cells (1–2). TFH cells express inducible co-stimulator (ICOS) and CXC chemokine receptor 5 (CXCR5), enabling their migration to the B cell follicle (3–5) and their continue differentiation into GC TFH (6). Moreover, TFH express costimulatory molecules and produce IL-21 that are essential for GC B cell survival and differentiation (7–8).
The sources of MHCII−restricted antigen presentation required for TFH differentiation are controversial. Several lines of evidence suggest that B cells provide unique signal(s) required for complete TFH differentiation. Thus the absence of B cells, the presence of B cells with irrelevant specificity, or an inability of B cells to express ICOSL impairs TFH generation (2, 9–11). Moreover, T cells unable to stably conjugate with B cells display defective TFH differentiation (12). The notion of a unique B cell signal has been challenged by findings that suggest instead that only prolonged, high avidity antigen presentation is needed for GC TFH differentiation that can be mediated by DCs (13) . Indeed, co-stimulation via DCs promotes T cell migration into the B cell follicle (14), suggesting that DC antigen presentation contributes to the TFH program.
We have dissected the steps of TFH differentiation by examining the requirement for MHCII-expressing DCs. We find that cognate interactions with conventional DCs are both necessary and sufficient to prime CD4+ T cells towards a previously unappreciated TFH intermediate that expresses Bcl6, CXCR5 and ICOS. However, DC priming alone is insufficient to generate PD1hi GC TFH and IL-21 production, indicating the need for subsequent, distinct APC interactions for commitment to the TFH program. Furthermore, we show that naïve T cells primed in vitro by B cells express more IL-21 than do those primed by DCs. Moreover, addition of IL-21 to in vitro B cell activation cultures that mimic cognate T cell help induces a GC B transcription profile. Thus, we conclude that TFH differentiation is a multi-step process in which conventional DCs are critical for the initial priming events; however, they are insufficient for imparting complete effector potential.
MATERIALS AND METHODS
Mice & Immunizations
C57BL/6J, OTII and CD11c-DTR mice were purchased from Jackson Laboratories. CD11c/Aβb mice were bred in house (15). 6–14 week old mice were housed under pathogen free conditions, in accordance with the University of Pennsylvania Animal Care and Use Guidelines. CD4+ OTII T cells were enriched by negatively selecting out CD8+, B220+, MHCII+ and FcγRII+ cells using magnetic bead selection (Qiagen) and labeled with CFSE (Invitrogen). OTII were transferred i.v. 1 day prior to i.p. immunization with 50 μg NP15-OVA (Biosearch Technologies) in alum. Some mice were given OVA323–329 peptide (GenWay Biotech). DC depletion and Toxoplasma infection in CD11c DTR mice as described (16).
Flow cytometry
Antibodies were purchased from Biolegend, eBioscience, BD Pharmingen or Invitrogen. DAPI or AQUA™ (Invitrogen) was used to identify live cells. FoxP3 fixation and permeabilization kit was used for intracellular Bcl6 staining (eBioscience). Cells were acquired or sorted on an LSR II cytometer or FacsAria II, respectively (BD Biosciences). Data was analyzed using FlowJo software (TreeStar).
Immunohistochemistry
Spleens immersed in O.C.T. (Tissue Tek) were flash frozen using 2-methylbutane / liquid nitrogen. 8 μm sections were fixed with cold acetone and stained with PNA and antibodies to IgD and CD90.1. Sections were imaged with a Zeiss LSM510META NLO laser scanning confocal system.
In vitro stimulations
OTII cells were polarized towards TFH lineage as published (9) with CD11chi or CD23+ cells at a ratio of 1 APC:10 T cells and harvested on day 4. B cell stimulations were performed as described (17) with anti-CD40 (10 ng/ml; BD Biosciences) and IL-21 (100ng/ml; R&D).
QPCR
QPCR was conducted as previously described (17). 18S or GAPDH were used as housekeeping gene for T and B cells, respectively.
ELISPOT
Splenocytes were incubated on 10 ug/ml of NP33-BSA (Biosearch Technologies) coated plates and developed with biotin-anti-mouse IgM or -IgG1 (Southern Biotech) followed by ExtrAvidin-Alkaline Phosphatase and NBT/BCIP substrate (Sigma). Spots were enumerated on CTL-ImmunoSpot (Cellular Technologies).
RESULTS AND DISCUSSION
Dendritic Cells are necessary for TFollicular Helper formation
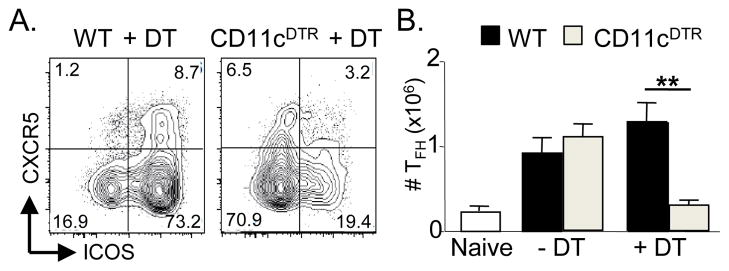
Using a loss of function approach, we determined whether DC-T cell interactions are necessary for initiation of TFH cells priming in vivo. We transiently ablated DCs by Diptheria toxin (DT) in CD11c-DTR mice (18), and infected the mice with Toxoplasma gondii as described (16). TFH cells were present in untreated mice at day 7 post-infection, but were significantly reduced in DT-treated CD11c-DTR animals (Fig. 1A). Normal DC numbers were required for optimal generation of CD4+CXCR5+ cells (WT 1.71 ± 0.15; CD11c-DTR 0.67 ± 0.13; p<0.01) as well as induction of ICOS (Fig 1B). To verify that these results were not limited to toxoplasmosis, we transferred 105 CD90.1+ ovalbumin(OVA)-specific OTII CD4 T cells into CD90.2+ WT or CD11c-DTR recipient mice followed by DT treatment and i.p. immunization with NP-OVA/alum. Again, there was a profound decrease in the number of OTII TFH generated (data not shown). The few CD4+CXCR5+ T cells formed after DC depletion could potentially migrate into the follicle and initiate GC via other co-stimulatory molecules, including CD40L. We conclude that DCs are necessary to generate TFH cells, as expected from their role in priming other TH lineages (19).
Figure 1.
DCs are necessary for initiating TFH priming. WT and CD11cDTR mice were treated with DT and infected with T. gondii as described (16). (A) Numbers of TFH (CD4+CXCR5+ICOS+) were calculated at day 7 p.i. Data are pooled from two experiments (n>8 per group) (B) Representative FACS plots of CXCR5 and ICOS expression on CD4+ T cells in WT or CD11cDTR treated with DT. ** denotes statistical significance of p<0.01 in a 2-tailed t test at α=0.05
Dendritic cells are sufficient for the generation of a Bcl6+ TFH intermediate
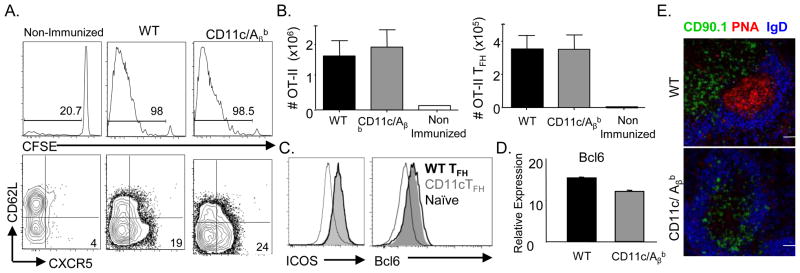
Using a gain of function approach, we asked if cognate DC interactions are sufficient to generate TFH cells. We used the transgenic CD11c/Aβb mouse model, in which MHCII antigen presentation is limited to conventional CD11chi DCs (15). As CD11c/Aβb mice lack endogenous CD4 T cells, we transferred CFSE labeled 106 OTII cells. Splenic OTII cells proliferated similarly in both WT and CD11c recipients following i.p. immunization with NP-OVA/alum (Fig. 2A, 2B). Further, the numbers of OTII derived CD62LloCXCR5+ TFH generated in WT (WT-TFH) or CD11c/Aβb mice (CD11c-TFH) were not significantly different, and both subsets displayed equivalent expression of ICOS (Fig 2B, 2C). Moreover, transfer of 105 OTII cells yielded similar results (Supp. Fig. 1A). Furthermore, OTII cells migrated into the follicle in both mice strains (Fig. 2E). Since Bcl6 is necessary and sufficient for differentiation of CXCR5+ TFH cells (1–2), we asked if antigen presentation restricted to DCs effectively drove Bcl6 induction. Compared to naïve OTII T cells, WT-TFH or CD11c-TFH expressed higher but equivalent levels of Bcl6 message (Fig. 2D) and protein (Fig. 2C). Thus, cognate interactions with DCs are sufficient to skew naïve CD4+ T cells towards Bcl6+ TFH.
Figure 2.
Cognate interactions by dendritic cells are sufficient for generation of TFH cells. 106 CFSE labeled CD90.1+ OTII T cells were transferred into either WT or CD11c/Aβbmice and mice were immunized with NP-OVA/alum. (A) Representative FACS gating strategy for identification of splenic OTII T cells (CD19− TCRβ+ CD4+ CD90.1+). OTII TFH cells were identified as CD62L− CXCR5+ cells. CFSE dilution of transferred OT-II cells was assessed (middle panel). (B) Total number of splenic OTII and OTII TFH present in immunized WT or CD11c/Aβb recipients at day 8 p.i. or in non-immunized WT mice at d8 post-transfer (n=4–5 mice, representative of >5 experiments). (C) Representative histogram overlay of ICOS and Bcl6 staining intensity on OTII TFH generated in WT or CD11c/Aβb mice and naïve endogenous CD4+ T cells (CD19− TCRβ+ CD4+CD62L+) present in WT mice (D) Bcl-6 mRNA in FACS sorted OT-II TFH generated in WT or CD11c/Aβ mice at d7 p.i.. relative to naive OTII cells (representative of 3 experiments) (E) Confocal micrographs of splenic sections to identify CD90.1+ OTII along with PNA+ GCs and IgD+ naïve B cells in the follicle. Scale bar denotes 50 μm.
Cognate DC-T interactions cannot induce GC status in T cells
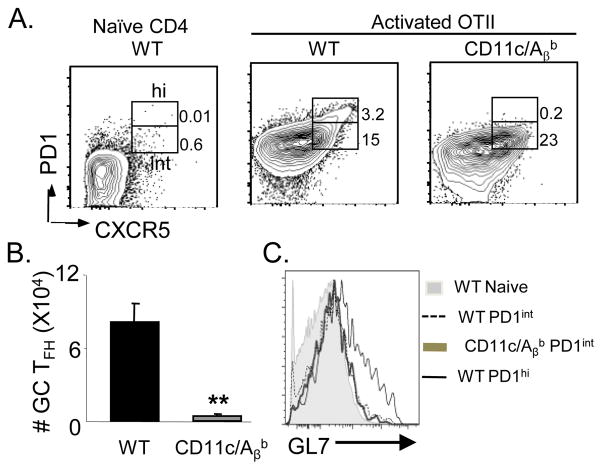
MHCII expression by B cells is necessary for antibody class switching (15, 20) and presumably for GC formation. Consistent with this view, GC B cells were absent both phenotypically (Supp. Fig. 2A, 2B) and histologically GCs (Fig. 2E) in immunized CD11c/Aβb mice. Further, CD11c/Aβb mice generated few NP-specific IgG1 ASCs, despite an intact IgM ASC response (Supp. Fig 2C), as reported before (15, 20). PD1 expression is thought be an indicator of chronic antigen exposure in the GC, as correlative evidence links the presence of GC B cells with PD1 expression on TFH cells (4). Thus, we examined the expression of PD1 on TFH cells. Amongst the CXCR5+ cells, a small subset of the WT-TFH cells expressed high levels of PD1 as well as GL7, which were absent within the CD11c-TFH cells (Fig. 3A, B) consistent with GC TFH phenotype (Fig. 3C, (6)). Moreover, the transfer of 107 polyclonal WT CD4+ T cells did not alter the observed phenotype (data not shown), indicating that the absence of endogenous helper T cells does not impact the evolution of this response in the CD11c/Aβb mice. Since TFH down regulate P-selectin glycoprotein ligand 1 (PSGL1) (11) and upregulate and B- and T-lymphocyte attenuator (BTLA) (6), thus we examined their expression on CD11c-TFH and WT-TFH. Compared to naïve WT T cells, both CD11c-TFH and WT-TFH had significantly upregulated BTLA and down regulated PSGL1 (data now shown). However, CD11c-TFH expressed more PSGL1 than WT-TFH (data not shown) (11) while BTLA expression was comparable. Collectively, we conclude that not only do GC B cells require TFH cells for their induction, GC TFH also rely on interactions with other APCs, including activated B cells, to complete their phenotype.
Figure 3.
DC restricted antigen presentation is insufficient for the generation of GC TFH 106 OTII T cells were transferred into WT and CD11c/Aβb mice and mice were immunized with NP-OVA/alum. (A) Representative FACS gating strategy to identify PD1+CXCR5+ within activated OTII TFH (CD19−TCRβ+CD4+CD90.1+CD62L−) in immunized WT or CD11c/Aβb on d7 p.i. as well as naïve WT CD4+ T cells as a staining control. The PD1+ cells were parsed into PD1hi (GC TFH) and PD1int populations. (B) The total number of OTII GC TFH in WT or CD11c/Aβb mice on d7 p.i. (n=4–5) (C) Histogram overlay of GL7 staining on various subsets gated in (A) (n=4–5 mice). Results are representative of 3 experiments. ** denotes statistical significance of p<0.01 in a 2-tailed t test at α=0.05.
IL-21 expression is profoundly reduced in TFH cells primed by DCs alone
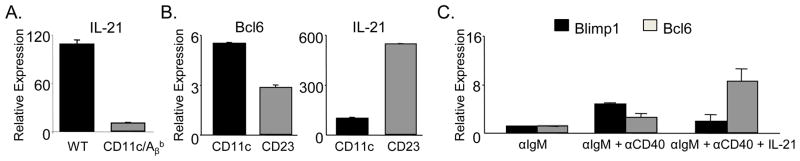
IL-21 is a key TFH effector cytokine that mediates differentiation of GC B cells (6–8). Strikingly, CD11c-TFH primed solely by DCs expressed minimal IL-21 compared to WT-TFH (Fig. 4A). To determine if B cells were intrinsically more adept at inducing IL-21 producing TFH, we polarized naïve OTII T cells in vitro towards the TFH lineage with splenic CD11chi DCs or CD23+ B cells as APCs as described (9). While both B cells and DCs induced expression of Bcl6, B cells induced greater IL-21 transcript in polarized TFH cells (Fig. 4B). Together, these data suggest that B cells provide qualitatively different signal(s) than DCs, perhaps via expression of co-stimulatory molecules that facilitate optimal IL-21 production by TFH cells.
Figure 4.
Reduced expression of IL-21 in TFH cells primed in CD11c/Aβb mice. (A) Expression of IL-21 transcript in FACS sorted OT-II TFH generated in WT or CD11c/Aβb mice at d7 p.i. normalized to naïve OTII cells. Results are representative of 3 experiments. (B) Relative expression of Bcl6 and IL-21 in OTII cells polarized towards TFH lineage using splenic CD11c+ or CD23+ cells as APCs compared to Th0 condition. Results are representative of 2 experiments. (C) QPCR analysis of Bcl6 and Blimp1 on CD23+ cells stimulated with F(ab)2 fragments of αIgM alone or in combination with αCD40 with or without IL-21 for 3 days. Results pooled from 3 experiments.
Consistent with a previous study (9), in vitro primed TFH expressed little to no IL-17 transcript (Supp. Fig. 1E), further confirming their TFH character. Moreover, the superior ability of DCs to induce IL-17 when compared to B cells was recapitulated in vivo, as OTII TFH and OTII Teff primed solely via conventional DCs in CD11c/Aβb mice expressed more IL-17 compared to their WT counterparts (Supp. Fig. 1F). Together, these data highlight the critical role played by non-DC APCs, presumably B cells, in enforcing progression to TFH status while inhibiting other lineage potential.
Sustained antigen presentation on conventional DCs is insufficient to rescue GC TFH or production of IL-21
Recent findings suggest that sustained antigen presentation restores the formation of PD1hiCXCR5+ TFH in the absence of B cells (13). We performed a similar experiment in the CD11c/Aβb mice. We transferred 105 OTII cells, immunized with NP-OVA the next day and then administered 10 μg of OVA323–339 peptide i.v. on day 3 p.i.. Consistent with Deenick et al. (2010), we observed greater expansion of OTII cells in the CD11c/Aβb mice given exogenous peptide (Supplemental Fig. 1A) on day 7 p.i.. However, neither PD1hi GC TFH nor IL-21 production were observed in CD11c/Aβb mice that received extra peptide (Supp. Fig. 1B–D). Whether these differences arise because of irradiation chimeras used by Deenick et. al. (2010) or due to the lack of other MHCII+ APCs in the CD11c/Aβb mice is unclear. Thus, sustained antigen presentation by conventional DCs is insufficient to induce GC TFH cells and a second, distinct, antigen presenting cell population is required.
In conjunction with BCR and CD40 ligation, IL-21 induces a GC B transcription profile
Recent studies suggest that IL-21R ligation yields maximal Bcl6 expression in B cells, thus fostering GC formation in vivo (7–8). However, in the presence of anti-CD40 in vitro, IL-21 induces B Lymphocyte-induced maturation protein 1 (Blimp1) (21) skewing B cells towards a plasma cell fate. To simulate antigen-driven, T-dependent activation of Follicular B cells in vitro, we ligated both the BCR and CD40 with anti-IgM and anti-CD40 antibodies respectively, then assayed for the induction of Blimp1 and Bcl6 transcripts. Compared to B cells stimulated with only anti-IgM or anti-IgM plus anti-CD40, IL-21 markedly augmented the transcriptional profile associated with adoption the GC fate. Both AID (data not shown) and Bcl6 were substantially upregulated, whereas Blimp1 transcripts, which were elevated following BCR and CD40 ligation alone, were lowered in the presence of IL-21 (Fig 4C). We conclude that concurrent stimulation of BCR, CD40 and IL-21R potently induces Bcl6 expression, which in turn represses Blimp1 (22). This reciprocal interaction is key to skew the B cells towards a GC fate and away from the short-lived plasma cell fate, which can also inhibit TFH differentiation (23).
Collectively, our data suggest that complete differentiation of TFH is most effectively accomplished via successive, cognate interactions with distinct APCs. Herein, we demonstrate that DCs are necessary and sufficient to initiate the TFH program, arguing against the notion that B cells are critical for priming (2, 9–11). Priming solely via DCs in the CD11c/Aβb system leads to accumulation and persistence of a novel intermediate or pre-TFH, which express CXCR5, ICOS and Bcl6, but lacks IL-21. Thus, optimal induction of IL-21 as well as further differentiation towards GC TFH requires cognate interactions with other APCs, including activated B cells. Thus, we propose that as poised CXCR5+ICOS+TFH relocate from the DC-rich T cell zone to B cell-rich follicles, their interactions with activated B cells via costimulatory ligands including PDL2, ICOSL increases the likelihood of adopting a TFH cytokine profile, including IL-21 production (24–25). Whether B cells provide qualitatively distinct signal(s) to TFH for optimal effector cytokine production remains unclear.
Since CD4+ T cells require continued interactions with APCs for their expansion and effector functions (26), the assumption of alternate effector fates likely reflects the receipt of chronologically ordered signals during the early primary response. This suggests a step-wise model for GC TFH formation. T cells primed by DCs differentiate into a novel pre-TFH subset. These cells can then migrate towards the T-B border, where cognate interactions with antigen primed B cells induce optimal IL-21 production, thus acquiring effector potential characteristic of TFH subset while inhibiting other fates, such as Th17. IL-21, in turn, reinforces a GC transcriptional program in the activated B cells. Thus, fully-differentiated TFH are only generated when a humoral response is appropriate and antigen-specific T cell help is very specifically directed towards antigen-experienced B cells.
Supplementary Material
Acknowledgments
This work was supported by Merit Award BX000435 from the VA to TML, RO1 AI 42334 to CAH and R01 AI 073939 to MPC. RG is supported by NIH Training grant T32 AI-055428.
Abbreviations used in the paper
- DC
Dendritic Cells
- PSGL1
P selectin glycoprotein ligand 1
- BTLA
B- and T-lymphocyte attenuator
- PD1
Programmed Death Ligand 1
Footnotes
Please address issues regarding the manuscript to Terri M. Laufer.
References
- 1.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang Yh, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston RJ, Poholek AC, Ditoro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Förster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes NM, Allen CDC, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 5.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, Crotty S. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) J Immunol. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. The Journal of experimental medicine. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang Y-h, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, Crotty S, Craft J. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular Helper T Cell Differentiation Requires Continuous Antigen Presentation that Is Independent of Unique B Cell Signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fillatreau S, Gray D. T cell accumulation in B cell follicles is regulated by dendritic cells and is independent of B cell activation. J Exp Med. 2003;197:195–206. doi: 10.1084/jem.20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemos MP, Fan L, Lo D, Laufer TM. CD8alpha+ and CD11b+ dendritic cell-restricted MHC class II controls Th1 CD4+ T cell immunity. J Immunol. 2003;171:5077–5084. doi: 10.4049/jimmunol.171.10.5077. [DOI] [PubMed] [Google Scholar]
- 16.Tait ED, Jordan KA, Dupont CD, Harris TH, Gregg B, Wilson EH, Pepper M, Dzierszinski F, Roos DS, Hunter CA. Virulence of Toxoplasma gondii is associated with distinct dendritic cell responses and reduced numbers of activated CD8+ T cells. J Immunol. 2010;185:1502–1512. doi: 10.4049/jimmunol.0903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowley JE, Stadanlick JE, Cambier JC, Cancro MP. FcgammaRIIB signals inhibit BLyS signaling and BCR-mediated BLyS receptor up-regulation. Blood. 2009;113:1464–1473. doi: 10.1182/blood-2008-02-138651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung S, Unutmaz D, Wong P, Sano GI, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 20.Williams GS, Oxenius A, Hengartner H, Benoist C, Mathis D. CD4+ T cell responses in mice lacking MHC class II molecules specifically on B cells. Eur J Immunol. 1998;28:3763–3772. doi: 10.1002/(SICI)1521-4141(199811)28:11<3763::AID-IMMU3763>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, Carotta S, Donovan CE, Goldman ML, Tailor P, Ozato K, Levy DE, Nutt SL, Calame K, Leonard WJ. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, Staudt LM. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 23.Pelletier N, McHeyzer-Williams LJ, Wong KA, Urich E, Fazilleau N, McHeyzer-Williams MG. Plasma cells negatively regulate the follicular helper T cell program. Nat Immunol. 2010;11:1110–1118. doi: 10.1038/ni.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. The Journal of experimental medicine. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.