INTRODUCTION
This Product Profiler introduces health care professionals to Privigen®, Immune Globulin Intravenous (Human), 10% Liquid. Privigen® has been approved by the U.S. Food and Drug Administration (FDA) as replacement therapy for the treatment of primary immunodeficiency disease (PIDD). This includes, but is not limited to, the humoral immunodeficiency in congenital agammaglobulinemia, common variable immunodeficiency (CVID), X-linked agammaglobulinemia (XLA), Wiskott-Aldrich syndrome, and severe combined immunodeficiencies (SCIDs) (Privigen® Prescribing Information 2011). Privigen® is also indicated for the treatment of patients with chronic immune thrombocytopenic purpura (ITP) to raise platelet counts (Privigen® Prescribing Information 2011).
Replacement immunoglobulin therapy is the standard of care for primary humoral immunodeficiency disorders and has also been used for immunomodulatory treatment of autoimmune and inflammatory diseases (Looney 2006, Shehata 2010). Immunoglobulins are isolated from pooled plasma donated by thousands of individuals, which ensures that a broad spectrum of antibodies is contained in the final preparation (Looney 2006). The resulting fractionated blood product provides immunoglobulin G (IgG) antibodies, with minimal IgA and IgM constituents. IgG therapy has been used for the treatment of PIDD since the 1950s (Shehata 2010). Initially, IgG preparations were administered via intramuscular (IM) or subcutaneous (SC) routes. Intravenous immunoglobulin (IVIg) was introduced in the 1970s. Eventually, IM administration of IgG was superceded by IV and SC treatment because of the latter’s improved tolerability (Shehata 2010).
Today, several IVIg products are approved by the FDA for an array of clinical indications: 1) to treat PIDD; 2) to increase platelet counts in patients with ITP to prevent or control bleeding; 3) to prevent bacterial infections in patients with hypogammaglobulinemia or recurrent bacterial infections, or both, associated with B-cell chronic lymphocytic leukemia (CLL); 4) to prevent coronary artery aneurysms in patients with Kawasaki disease (KD); 5) to prevent infections, pneumonitis, and acute graft-versus-host disease (GVHD) after bone marrow transplantation in adults aged ≥20 years; and 6) to reduce the frequency and severity of bacterial infections in children with human immunodeficiency virus (HIV) infection (Orange 2006, Looney 2006).
The following text presents an overview of PIDD and chronic ITP; current treatment options for these disorders; a review of the evidence-based literature supporting the FDA-approved indications for Privigen®; product information pertaining to Privigen®, including clinical trial data and safety information; and considerations for P&T committee decisions regarding this product.
DISEASE OVERVIEW: PRIMARY IMMUNODEFICIENCY
Incidence and Prevalence
PIDD is recognized as an inherited, heterogeneous disorder of the immune system that results in increased rates and severity of infections, immune dysregulation associated with autoimmune diseases, and the development of malignancies (AAAAI 2011, Bonilla 2005, Lindegren 2004). Repeated infections due to PIDDs can lead to severe organ damage, repeated hospitalizations, diminished quality of life (QOL), and reduced life expectancy (AAAAI 2011, Garcia 2010). PIDDs are clinically similar to, but distinct from, secondary immunodeficiencies that may develop in response to viral infections, immunosuppressive therapies, systemic therapy for autoimmune diseases, or chemotherapy for malignancies (Bonilla 2005, Lindegren 2004). At least 50% of all primary immunodeficiency syndromes are primary antibody deficiency disorders (Herriot 2008).
PIDD is more likely to occur in persons aged <20 years, and 70% of cases affect males because of an X-linked recessive pattern of inheritance (Lindegren 2004). It has been estimated that there are currently at least 150 different types of PIDDs, with more than 60 of these involving impaired production of antibodies (Buckley 2009, Orange 2011a). Fewer than 20 PIDDs account for more than 90% of cases (Lindegren 2004).
PIDDs are considered rare, although accurate estimates of incidence or prevalence are unavailable (Boyle 2007, Kumar 2006). Registries established by countries to collect information about PIDDs are believed to underestimate the true prevalence of PIDD for several reasons, among them lack of recognition/diagnosis by clinicians and altered presentation of the disease because of widespread antibiotic use (Kumar 2006, Lindegren 2004).
The incidence and prevalence of the different types of PIDD are widely variable. For example, selective IgA deficiency is recognized as the most common PIDD, with an estimated frequency of 1:223 to 1:1,000 individuals in the US (Kumar 2006, Yel 2010). Higher incidence rates of 1:500 to 1:700 were reported for white individuals of European descent (NPIRC 2011a). CVID affects an estimated 1 in 50,000 persons, and SCID, the most serious primary immune disorder, has an estimated incidence of 1 in 1,000,000 (NPIRC 2011b, 2011c).
Thus, the true incidence and prevalence of PIDD are largely unknown. However, the two most common types are selective IgA deficiency and CVID.
Etiology
Defects in approximately 150 genes are associated with the development of PIDD (Ortutay 2009). Table 1 lists gene mutations that have been identified in key PIDDs (Herriot 2008). These mutations provide researchers with valuable insights into the role of genes in the development and function of immune cells and in immune-related homeostatic mechanisms (Fischer 2004).
TABLE 1.
Gene mutations in key PIDDs
| Disorder | Inheritance | Gene mutations |
|---|---|---|
| Severe reduction in all immunoglobulin isotypes and absent B cells: | ||
| X-linked agammaglobulinemia | XL | Btk |
| Autosomal recessive agammaglobulinemia | AR | μ
chain Igα Igβ λ5 BLNK |
| Severe reduction in at least 2 isotypes with normal or low B cell numbers: | ||
| Common variable immunodeficiency | AR |
ICOS CD19 BAFFR SBDS |
| AD or AR | TACI | |
| Severe reduction in IgG and IgA with normal or increased IgM and normal B cell numbers: | ||
| CD40 ligand deficiency | XL | CD40XL |
| CD40 deficiency | AR | CD40 |
| AID deficiency | AR | AICDA |
| UNG deficiency | AR | UNG |
| Individual isotype or light-chain deficiencies with normal B cell numbers: | ||
| Immunoglobulin heavy-chain deletions | AR | 14q32 deletions |
| K chain deficiency | AR | K constant gene |
| Selective IgA deficiency | AD or AR | TACI |
XL = X-linked; Btk = Bruton tyrosine kinase; AR = autosomal recessive; BLNK = B cell linker protein; ICOS = inducible co-stimulator; BAFFR = B cell activation factor of the TNF family receptor; SBDS = Shwachman-Bodian-Diamond syndrome gene; AD = autosomal dominant; TACI = transmembrane activator and calcium-modulating ligand interactor; AICDA = activation-induced cytidine deaminase; UNG = uracil-DNA glycolase.
Source: Adapted from Herriot 2008.
Pathophysiology
The human body depends on the immune system for protection against invading pathogens (Haynes 2008). There are two basic types of immunity: innate and adaptive (Haynes 2008, Chaplin 2006). The innate immune system, evolved over millions of years, consists of physiologic barriers against invading pathogens (NIAID 2011) along with a variety of host cells bearing gene-encoded receptors that recognize and destroy disease-causing agents (Haynes 2008, Chaplin 2006). The body’s front-line defenses against pathogens include the skin and the respiratory and digestive tracts. Intact skin forms a virtually impenetrable barrier to invaders. Microbes entering the nose often cause the nasal surfaces to secrete protective mucus, and attempts to enter the nose or lungs can trigger a sneeze or cough reflex, which forces microbial invaders out of the respiratory passageways. The stomach contains a strong acid that destroys many pathogens that are swallowed with food. Mucosal surfaces also secrete immunoglobulin A (IgA), a special class of antibody that is often the first type of antibody to encounter an invading microbe. If pathogens are able to bypass the body’s front-line physiologic barriers, they are confronted by a cellular defense system, which is ready to attack without regard for specific antigen markers (NIAID 2011). Key players in this system include NK cells, phagocytes, and complement (a group of proteins that produce inflammation in the presence of infection) (Haynes 2008, NIAID 2011, Blaese 2007).
Unlike the innate immune system, adaptive immunity is characterized by the ability to precisely target invading pathogens (Chaplin 2006). In the adaptive immune response, gene elements are rearranged on the surfaces of B and T lymphocytes to create antigen-binding molecules with high specificity for individual microbial and environmental structures (Haynes 2008, Chaplin 2006). Adaptive immunity consists of both cellular and humoral (antibody) immune functions. In cellular immunity, cytotoxic T lymphocytes recognize and destroy virus-infected or foreign cells (Haynes 2008, Chaplin 2006). In humoral immunity, B cells produce antibodies in response to specific antigens (Haynes 2008). A key feature of the adaptive immune response is the production of long-lived cells that can repeat their effector functions when they encounter an antigen for the second time (Chaplin 2006).
The innate and adaptive immune systems usually work together, with innate responses representing the body’s first line of defense. Adaptive immunity “kicks in” several days later as antigen-specific T and B lymphocytes become activated (Chaplin 2006). Synergy between the innate and adaptive immune systems is crucial for an effective immune response (Chaplin 2006).
Table 2 describes key components of the body’s immune system and their functions.
TABLE 2.
Key Components of the Immune System
| Component | Functions |
|---|---|
| B lymphocytes |
|
| T lymphocytes |
|
| Natural killer cells |
|
| Phagocytes |
|
| Complement system |
|
Sources: Adapted from Blaese 2007, Haynes 2008, Shier 2010.
PIDDs are classified according to the immune mechanisms that are dysfunctional or hypofunctional (Table 3) (Merck Manual 2010). In the adaptive immune system, defective immune functions include humoral (antibody) deficiencies, cellular deficiencies, or a combination of both. In the innate immune system, defects can include phagocyte and complement deficiencies (Merck Manual 2010).
TABLE 3.
Classification of Immune System Component Defects
| Type of component defect | Description | % of PIDD disorders | Common associated disorders |
|---|---|---|---|
| B-cell defects |
|
50–60% |
|
| T-cell defects |
|
5–10% |
|
| Combined B- and T-cell defects |
|
20% |
|
| Natural killer-cell defects |
|
NA | NA |
| Phagocytic-cell defects |
|
10–15% |
|
| Complement defects |
|
≤2% |
|
CVID = common variable immunodeficiency; Ig = immunoglobulin; XLA = X-linked agammaglobulinemia; ZAP-70 = Z-associated protein 70.
Sources: Adapted from Bonilla 2005, Merck Manual 2010.
Clinical Presentation and Evaluation
The hallmark feature of PIDDs is an increased susceptibility to, and severity of, upper and lower respiratory bacterial infections, such as sinusitis, otitis media, bronchitis, or pneumonia, in the absence of other known or suspected contributing factors (e.g., smoking) (Buckley 2009, Herriot 2008, Wood 2007). Patients with PIDDs are at increased risk of multiple or recurrent infections, infections that are refractory to treatment, unusually severe infections, or infections associated with opportunistic pathogens (Blaese 2007, Kumar 2006). Commonly affected sites of persistent or recurrent infections include the gastrointestinal (GI) tract, skin, eyes, skeleton, and central nervous system (Herriot 2008). Table 4 presents estimated infection rates in patients with PIDDs.
TABLE 4.
Estimated Rates of Infection by Site in Patients With PIDDs
| Site of Infection | % (Range) |
|---|---|
| Respiratory/chest (including pneumonia, excluding bronchitis) | 37.0–90.0 |
| Recurrent sinusitis | 19.0–98.0 |
| Gastrointestinal | 6.0–38.0 |
| Cutaneous | 1.0–13.0 |
| Central nervous system/meningitis | 2.0–9.0 |
| Septic arthritis/osteomyelitis | 1.0–7.0 |
| Ophthalmic | 1.4–10.0 |
Source: Adapted from Wood 2009.
While most cases of PIDD are characterized by an increased susceptibility to infections, these patients may develop other clinical disorders, particularly autoimmune diseases, such as autoimmune hemolytic anemia, neutropenia, and thrombocytopenia (Bussone 2009, Wood 2007).
Nonspecific features of PIDD include arthropathy and tissue abnormalities, such as lymphadenopathy, hepatosplenomegaly, and nodular lymphoid hyperplasia (Herriot 2008, Wood 2007). In children, symptoms of antibody deficiency may include failure to thrive, recurrent pyrexia of unknown origin, coping problems at school, poor attendance at school, and recurrent respiratory and GI tract infections (Wood 2007). In addition, individuals with PIDD may demonstrate a poor clinical response to vaccinations, such as immunization against Haemophilus influenzae (Herriot 2008).
The Jeffrey Modell Foundation has published a list of warning signs to help physicians in diagnosing cases of PIDD (Jeffrey Modell Foundation 2009). These signs are described in Table 5.
TABLE 5.
10 Warning Signs of Primary Immunodeficiency
|
Source: Jeffrey Modell Foundation 2009. Reproduced with permission.
Individuals with antibody deficiency may begin to show symptoms of the disorder as early as 7 to 9 months after birth, when maternal antibodies have decreased to below protective levels (Ballow 2002). Most PIDDs (>80%) are diagnosed by the age of 20 years (Kumar 2006, Paul 2002).
A definitive diagnosis of PIDD requires a thorough medical history and physical examination to identify the presence of antibody deficiency and to distinguish between primary and secondary disease (de Vries 2006, Wood 2009). Laboratory evaluations include a complete blood count with differential and platelet count (Paul 2002, Cooper 2003). In addition, an assessment of serum immunoglobulin levels is recommended for patients with a suspected PIDD (Paul 2002, Wood 2009).
Early recognition and referral of patients with suspected PIDD are important since timely initiation of appropriate therapies can prevent or reduce infections as well as prevent the development of systemic complications (Wood 2009). Unfortunately, many patients do not benefit from diagnosis early in life. Among respondents to the third national Immune Deficiency Foundation (IDF) survey of patients with PIDDs, conducted in 2007, only 27% of respondents were diagnosed by the age of 6 years; 51% were not diagnosed until age 30 years or older, and 26% were not diagnosed until age 45 years or older (IDF 2009a).
Effects of PIDD on Health Outcomes and Quality of Life
Despite appropriate therapy, patients with PIDDs are at increased risk of developing organ-specific and systemic complications (Wood 2009). In the 2007 IDF survey, 49% of respondents reported significant, permanent functional impairment prior to their initial diagnosis; 32% indicated permanent loss of lung function, 16% indicated impaired digestive function, and 13% reported permanent loss of hearing (IDF 2009a). Similar results were obtained in a 2008 IDF survey of treatment preferences among PIDD patients, with respondents reporting permanent impairment of their lungs (37%), digestive system (17%), and hearing (13%) (IDF 2009b). In the 2007 survey, delayed diagnoses were associated with higher rates of permanent impairment (IDF 2009a).
The challenges of diagnosing and treating PIDDs can be a significant source of stress and life disruption, with a major impact on the physical and psychologic well-being of patients, caregivers, and family members (Buckley 2009). In the 2007 IDF survey, 26% of respondents described their health status as fair, and 10% indicated poor or very poor health (IDF 2009a). The health-status ratings of patients with PIDDs in the 2007 IDF survey were lower compared with responses from an age-matched US population in the National Health Interview Survey. A health status of good or better was reported by 72.8% of respondents with no permanent functional impairments compared with 26.2% of those with 3 or more impairments (IDF 2009a).
Cost Burden of PIDD
The direct economic impact of the diagnosis, treatment, and long-term care of patients with PIDDs is substantial and can include such factors as the use of antimicrobial agents, hospitalization, and treatment of systemic complications (Simoens 2009).
The cost of care for undiagnosed and untreated individuals with PIDD is likely to be considerably higher than the direct medical costs associated with the care of diagnosed patients. In 2007, physician-experts were surveyed to determine the health care costs of undiagnosed/untreated PIDD versus diagnosed/treated disease (Modell 2007). The findings from this study indicated that the average annual cost of health care for an undiagnosed individual with PIDD was $102,736, compared with $22,696 for a diagnosed patient. The diagnosis of patients with underlying PIDD was estimated to save an average of $79,942 per patient per year (Modell 2007). The National Institutes of Health (NIH) have estimated that there are at least 500,000 cases of undiagnosed PIDD in the US (Modell 2007).
DISEASE OVERVIEW: CHRONIC IMMUNE THROMBOCYTOPENIC PURPURA
Incidence and Prevalence
Using the Integrated Healthcare Information System data base, which contains demographic and health information for more than 70 million patients in the US, individuals enrolled in participating health plans between 2002 and 2006 were assessed for the incidence and prevalence of chronic ITP, according to appropriate ICD-9 codes (Feudjo-Tepie 2008). The adjusted diagnosed prevalence rate for chronic ITP among adults aged 18 years or older was 23.6 per 100,000 population. This was equivalent to 52,700 adults with chronic ITP, based on 2005 census population estimates. Age- and gender-adjusted prevalence rates were 20.3 per 100,000 persons, or 60,200 cases, in analyses based on the total population, which included individuals aged <18 years. The prevalence of chronic ITP increased with age and was higher for women than for men.
Rates of chronic ITP in children are generally low, with an estimated annual incidence of 0.46 per 100,000 children and an estimated prevalence of 4.6 per 100,000 children (Gernsheimer 2008). Evidence suggests that children aged 8 to 14 years are more likely to develop chronic ITP compared with those aged ≤7 years (Gernsheimer 2008).
Etiology
ITP is an acquired autoimmune disorder caused by inadequate production and increased destruction of platelets (Pruemer 2009, Gernsheimer 2009, Cines 2002). The disease can be acute (lasting ≤6 months) or chronic (>6 months) (Cines 2002). ITP affects all ages and ethnic groups, and women have a 2- to 3-fold greater risk of developing the disease compared with men. The predominance of women is especially apparent in the 30 to 60 age group (Pruemer 2009, PDSA 2011b). Chronic ITP is more common in adults and persists for at least 6 months in the absence of other abnormalities (Gernsheimer 2009). Children are usually diagnosed at a young age (approximately 5 years), and disease onset is characterized by the sudden appearance of petechiae or purpura after an infectious illness (Cines 2002). The condition spontaneously resolves in 80% to 85% of children within 6 months, regardless of the therapeutic intervention (Cines 2002, Gernsheimer 2008). A small proportion (15% to 20%) of children develops chronic ITP that resembles the adult form of the disease (Gernsheimer 2008).
Infectious causes of ITP include human immunodeficiency virus (HIV), hepatitis C virus (HCV), and Helicobacter pylori infection (Stasi 2009). Before HIV infection was treated with highly active antiretroviral therapy (HAART), HIV thrombocytopenia occurred in 5% to 30% of patients infected with HIV-1, with a positive correlation between thrombocytopenia and the progression of immunosuppression due to HIV (Stasi 2009). In several cross-sectional studies, the overall prevalence of HCV was 20% in adult patients with ITP (Stasi 2009). A comparison of the incidence rates of ITP among HCV-infected and uninfected US veterans showed that HCV was associated with an increased risk of ITP, with a hazard ratio of 1.8 regardless of treatment status (Stasi 2009). Evidence has shown that the eradication of H. pylori can improve platelet responses in adults with chronic ITP (Stasi 2009). The overall rate of H. pylori infection was 62.3% across more than two dozen studies of adult ITP patients, conducted mostly in Japan and Italy (Stasi 2009).
Pathophysiology
ITP is an autoimmune disease characterized by the development of platelet autoantigens (Cines 2002, Pruemer 2009). This, in turn, results in the destruction or impaired production of platelets, leading to thrombocytopenia (Pruemer 2009). As platelet membrane proteins become antigenic, the immune system responds by producing autoantibodies directed against glycoprotein complexes on platelet surfaces (Cines 2002, Gernsheimer 2009, Pruemer 2009). These autoantibodies have the ability to attach to circulating platelets, which are then removed from the spleen and liver via the reticuloendothelial system (Gernsheimer 2009).
Cytotoxic T cells also play a role in disrupting the production of new platelets (Gernsheimer 2009, Pruemer 2009). Specifically, CD3+ lymphocytes show higher expression of cytotoxic genes, including tumor necrosis factor, perforin, and granzyme A and B. High numbers of CD56+ CD3− NK cells are detected in patients with treatment-dependent ITP and in those who do not respond to treatment. In addition, a high expression of major histocompatibility complex class II molecules is present in patients with refractory ITP. These findings suggest that NK cells contribute to the pathogenesis of ITP through the destruction of IgG-coated targets (Gernsheimer 2009). Megakaryocyte abnormalities, antibody-induced inhibition of megakaryocyte production and growth, an improper response of the bone marrow to ongoing platelet destruction, and deficiencies in thrombopoietin receptor signaling are all thought to contribute to the pathogenesis of chronic ITP as platelet production is reduced (Pruemer 2009).
Clinical Presentation and Diagnosis
While ITP may be asymptomatic in some patients, the most commonly reported symptom is mucocutaneous bleeding, which manifests as purpura, epistaxis, or menorrhagia, or as oral mucosal, GI, or (in the most severe cases) intracranial hemorrhage (Pruemer 2009). Platelet counts generally remain at one third to one half the normal value of 150 ×109/L but can drop to <10 ×109/L; such dramatic declines in the platelet count may follow viral infection (Gernsheimer 2008).
Chronic ITP may persist for as long as 30 years, but the disease has a generally favorable prognosis for patients who respond to treatment. The risk of bleeding increases with age; a meta-analysis has suggested that age-adjusted bleeding risks are 0.004 per patient-year for individuals aged <40 years, 0.012 per patient-year for patients aged 40 to 60 years, and 0.130 for patients aged >60 years (Gernsheimer 2008).
The diagnosis of ITP is one of exclusion; when all other causes of low platelet counts have been ruled out, then the diagnosis is ITP (PDSA 2011a). Other causes of low platelets include disseminated intravascular coagulation, vitamin deficiency, infection (e.g., HIV and HCV), thrombotic thrombocytopenic purpura, hemolytic-uremic syndrome, systemic lupus erythematosus, IgA deficiency, common variable hypogammaglobulinemia, lymphoproliferative diseases, the use of certain medications, chronic liver disease, and primary bone marrow disease (Cines 2002, Gernsheimer 2008, Pruemer 2009). A history and physical examination are essential, as are a complete blood count and examination of the peripheral blood smear (Pruemer 2009). The physical examination may yield information about the type and severity of bleeding and may identify other symptoms, such as splenomegaly, that could rule out a diagnosis of ITP (Pruemer 2009). The patient’s history should include changes in prescription or nonprescription medications; vaccinations; the use of supplements, vitamins, or herbs; exposure to pesticides, herbicides, or other chemicals; a diagnosis of lymphoma, lupus, HCV, or HIV; insect or animal bites; travel outside the US; a family history of autoimmune diseases; a family or personal history of bruising easily or of a bleeding disorder; and a personal history of numerous colds, flu, or infections (PDSA 2011a).
Effects of Chronic ITP on Health Outcomes and Quality of Life
In 2009, the Platelet Disorder Support Association surveyed 251 members, including patients with acute and chronic ITP (PDSA 2011c). Sixty percent of respondents indicated that ITP had negative effects on their ability to exercise or to engage in sports; 54% reported mood changes; 44% noted that ITP interfered with their ability to travel; and 31% to 36% indicated that the condition affected their ability to take care of children/family members, to engage in social activities, to perform household tasks, and to perform their job responsibilities. Respondents reported fatigue (67%), bruising (63%), anxiety/fear (57%), and bleeding gums (29%). Almost half (44%) indicated that they worried about the future, and 31% were discouraged by the treatment options that were available to them.
An online survey of patients diagnosed with ITP and healthy, age- and gender-matched controls was conducted to determine the impact of ITP on health-related QOL, utilization of health care resources, and workplace productivity (Snyder 2008, Tarantino 2010). A total of 1,002 patients with ITP and 1,031 controls completed the survey, which included a comprehensive health-related QOL assessment. Patients with ITP had significantly lower scores on 7 of 8 health-related QOL domains, which included physical function, bodily pain, and general health, as well as lower scores on the physical and mental summary measures compared with controls (P<.05 for all comparisons) (Snyder 2008). Patients who had been diagnosed with ITP within the past 5 years had significantly (P=.019) lower overall QOL scores compared with patients who had been diagnosed more than 5 years before the survey. Lower platelet counts were consistently associated with worse scores on an ITP-Patient Assessment Questionnaire. This study demonstrated that patients with ITP have significant deficits in their health-related QOL (Snyder 2008).
With respect to health care utilization, a significantly higher proportion of patients with ITP reported visits to their primary care physicians (20%) or to specialists (28%) within the month preceding the survey, whereas only 11% of the healthy controls reported visits to primary care providers or specialists during the same period (P≤.001) (Tarantino 2010). More than half (56%) of the patients with ITP had taken sick leave compared with 30% of the controls (P≤.001), and the ITP patients were more likely to report an inability to perform usual chores (18% and 13% for patients and controls, respectively; P≤.003). Patients with ITP scored significantly worse than the healthy controls on 6 work productivity measures (P≤.05) (Tarantino 2010).
Current Treatment Options
PRIMARY IMMUNODEFICIENCY
Treatment priorities for patients with PIDDs focus on preventing infections, increasing the patient’s life expectancy, and improving QOL (Lindegren 2004). Several therapeutic approaches are available for patients with PIDD, including treatment of infections with antibiotics; immunoglobulin replacement therapy with IgG; and potentially curative treatments, including gene therapy and hematopoietic stem-cell transplantation (HSCT) (Durandy 2005, Lindegren 2004, Notarangelo 2006).
Antibiotics play an essential role in the treatment of infections and also contribute to prophylactic management of infectious diseases, such as Pneumocystis carinii pneumonia (Lindegren 2004). Patients with antibody-deficient PIDDs are at increased risk of infections with encapsulated bacteria, such as Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis. Timely treatment with antibiotics prevents progression of these infections to meningitis, osteomyelitis, consolidated pneumonitis, and mastoiditis (Durandy 2005). However, treatment with antibiotics cannot address all of the types of infections that commonly occur in patients with PIDD. For example, the microorganisms commonly responsible for mycoplasma infections are resistant to most antibiotics, although they are somewhat sensitive to tetracyclines and floxacins (Durandy 205).
IgG replacement therapy in patients with PIDDs is complex, requiring an individualized patient approach for the most appropriate and effective dosage, frequency of infusions, route of administration, and monitoring to ensure optimal patient outcomes (Maarschalk-Ellerbroek 2011, Shehata 2010). Despite these challenges, the efficacy of IgG as replacement or immunomodulatory therapy for PIDDs is well established (Cherin 2010). IgG replacement therapy may be administered via IV infusions or SC injections and is viewed as the primary treatment for PIDDs (Durandy 2005, Lindegren 2004, Orange 2006). Early diagnosis and appropriate management with IgG replacement therapy reduces morbidity and mortality and improves QOL in patients with PIDDs (Shehata 2010). Studies have shown that IgG replacement therapy can decrease infection rates and hospitalizations. Evidence also suggests that IgG replacement therapy may reduce the risk of comorbid chronic illnesses that often affect patients with PIDDs (Shehata 2010).
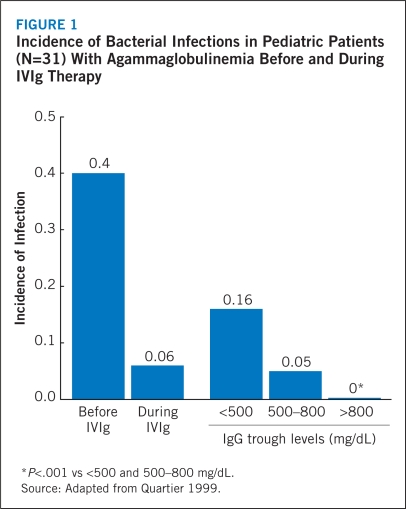
Although no randomized, controlled studies have been conducted, 500 mg/dL has been viewed as a minimum acceptable target for IgG trough serum levels. Increased doses of IgG and subsequent elevations in trough serum levels have been associated with fewer infections and shorter illnesses (Maarschalk-Ellerbroek 2011). It is recommended that IgG trough serum levels range from 600 to 900 mg/dL (Orange 2010). In a study of pediatric patients with agammaglobulinemia, the annual incidence of bacterial infections was lowest when IgG trough serum levels were maintained at >800 mg/dL (Figure 1) (Quartier 1999). A recent meta-analysis of 17 studies involving 676 patients with PIDDs found that IgG trough serum levels increased by 121 mg/dL with each additional 100-mg/kg increase in the IVIg dose (Orange 2010). The incidence of pneumonia progressively declined as IgG trough serum levels increased; pneumonia incidence showed a significant 27% reduction with each 100-mg/dL increase in trough IgG (Orange 2010). These results appear to support the clinical utility of higher IgG trough serum levels (Orange 2010). However, investigators continue to recognize that the IgG replacement dose must be individualized to achieve the best clinical response and long-term outcomes (Lucas 2010, Maarschalk-Ellerbroek 2011). For example, Lucas and colleagues demonstrated that the IgG trough serum level and the dose of replacement therapy required to maintain a minimal infectious burden are unique to each individual patient (Lucas 2010). Protective IgG trough serum levels were achieved with a broad range of replacement doses (0.2–1.2 g/kg/mo), supporting the individualization of doses. Of note, patients with bronchiectasis required twice as much replacement therapy to achieve the same IgG level compared with those free of bronchiectasis. The authors concluded that the goal of IgG replacement therapy should be to improve individualized clinical outcomes rather than to achieve a specific trough serum level.
FIGURE 1.
Incidence of Bacterial Infections in Pediatric Patients (N=31) With Agammaglobulinemia Before and During IVIg Therapy
*P<.001 vs <500 and 500–800 mg/dL.
Source: Adapted from Quartier 1999.
Because there are significant differences in the half-life of IgG among patients with PIDD, the frequency and amount of immunoglobulin therapy may vary from patient to patient. The proper amount may be determined by monitoring clinical response. The dosage should be adjusted over time to achieve the desired IgG trough serum levels and clinical responses. No randomized, controlled trial data are available to determine an optimal trough level in patients receiving immunoglobulin therapy (Privigen® Prescribing Information 2011).
In patients with SCID, allogeneic HSCT has been the definitive treatment for nearly 40 years (Notarangelo 2006). HSCT is also used for the treatment of patients with other types of PIDD. However, patients are at increased risk of life-threatening complications, including GVHD, after undergoing this procedure (Notarangelo 2006). A number of challenges remain before HSCT may be considered standard therapy for patients with PIDDs; these challenges include improving the critical care required by HSCT patients; providing more accurate identification of potential stem-cell donors; developing strategies to prevent or control GVHD; and applying interventions that will improve thymopoiesis (Notarangelo 2006).
CHRONIC IMMUNE THROMBOCYTOPENIC PURPURA
The main treatment goal for both children and adults with ITP is to ensure adequate hemostasis rather than to achieve a “normal” platelet count (Neunert 2011). Children with no bleeding or mild bleeding may require only observation regardless of the platelet count, whereas treatment with a short-term course of corticosteroids may be administered to children with platelet counts below 10 × 109/L or to those experiencing mucosal bleeding; long-term corticosteroid treatment is not recommended in children because of the risk of side effects (Cines 2002, Neunert 2011). First-line treatment of children with ITP may also include a single dose of IVIg (0.8 to 1.0 g/kg) (Cines 2002, Neunert 2011). Second-line treatments for children with ITP who are nonresponders or who have chronic disease include rituximab, high-dose dexamethasone, and splenectomy (Cines 2002, Neunert 2011).
The optimal treatment of adults with chronic ITP is based on the individual patient’s risk of bleeding, on the side effects associated with treatment, and on the patient’s treatment preferences (Neunert 2011). Pharmacologic therapy is recommended for newly diagnosed adults with platelet counts below 30 × 109/L (Neunert 2011). Pharmacologic options for these patients include treatment with IVIg, corticosteroids, or anti-D immunoglobulin (Cines 2002, Neunert 2011, NHLBI 2011). Adults with refractory ITP after first-line corticosteroid therapy are candidates for splenectomy. Thrombopoietin-receptor agonists are recommended for patients who are at increased risk of bleeding, who relapse after splenectomy, or who have contraindications to splenectomy and have failed to respond to at least 1 other therapy (Neunert 2011). Rituximab has also been used (Neunert 2011). Emergency treatments to increase platelet counts in adults with ITP include IVIg, corticosteroids, platelet transfusions, recombinant factor VIIa, antifibrinolytics, and emergent splenectomy (Neunert 2011).
ROUTES OF ADMINISTRATION
Today, the majority of patients with PIDD receive IgG via IV or SC injection. Each route of administration has both benefits and limitations and must be tailored to the individual patient’s needs and lifestyle (Durandy 2005).
Self-administered SC injections of IgG are more convenient for some patients, are associated with a low rate of systemic AEs, and provide stable IgG trough serum levels. The SC route is particularly useful for patients with venous access problems (Durandy 2005).
Table 6 lists the key features of IVIg. Since IV injections of IgG are administered by health care providers, regular follow-up helps to ensure continuity of care and comprehensive disease management (Durandy 2005).
TABLE 6.
Key Features of IVIg
| Features | Description |
|---|---|
| Pharmacokinetics | High “peak” IgG level immediately after infusion, followed by relatively low level at “trough” just before next dose is due |
| FDA-approved indications | Current indications include use as replacement therapy in patients with primary immunodeficiency syndromes, ITP, CLL, KD, pediatric HIV, and CIDP |
| Systemic side effects | Common |
| Local reactions | Infrequent |
| Administration | Typically administered in outpatient clinic or home setting with nursing support |
| Average length of infusion | 2 to 4 hours |
| Dosing interval | Usually once every 3 or 4 weeks |
| Most common adverse events | Headache, chills, low-grade fever, myalgia, nausea |
| Patient satisfaction | A better option than SCIg for patients who have difficulty with needles and/or self-injection. Preferable in patients who have difficulty with compliance. |
CIDP = chronic inflammatory demyelinating polyradiculoneuropathy; CLL = chronic lymphocytic leukemia; HIV = human immunodeficiency virus; IgG = immunoglobulin G; ITP = immune thrombocytopenic purpura; IVIg = intravenous immunoglobulin; KD = Kawasaki disease; SCIg = subcutaneous immunoglobulin.
Privigen® is FDA-approved for PIDD and chronic ITP.
Sources: Adapted from Blaese 2007, Skoda-Smith 2010, Cherin 2010.
In the 2007 IDF survey of PIDDs in the US, 74% of respondents indicated that they were currently receiving treatment for their disease (IDF 2009a). Of these patients, 58% were receiving IVIg therapy, and 16% were being treated with SC injections. In comparison, in the 2008 IDF treatment survey, 69% and 23% of patients were receiving IV and SC injections, respectively (IDF 2009b).
In patients with PIDDs, standard doses of IVIg range from 300 to 600 mg/kg body weight, administered at 3- to 4-week intervals, although some patients may require more frequent infusions to achieve optimal clinical results (Orange 2011a). For patients with autoimmune diseases, such as chronic ITP, the recommended dose of IVIg is 2 g/kg (Maddur 2009). A typical treatment regimen consists of 5 daily infusions of 400 mg/kg each or a single dose of 2 g/kg, followed by additional infusions of 2 g/kg at intervals of 4 to 6 weeks (Maddur 2009, Negi 2007). The mean half-life of infused IVIg is approximately 25 to 32 days (Maarschalk-Ellerbroek 2011). Patients must be evaluated individually, as the outcomes desired with a specific IgG regimen can differ from patient to patient (Orange 2011a).
Once the decision has been made to use an IVIg product, numerous payer-directed barriers may impede a physician’s efforts to provide optimal therapy in PIDD (Table 7) (Orange 2011b). For example, some payers require trough-level monitoring every 3 months. While trough levels can be helpful in guiding treatment in some patients, frequent monitoring may unnecessarily add to costs. Another potential barrier is frequent requests from payers for a trial of therapy cessation. In a genetically based disorder such as PIDD, the underlying reason for requiring IVIg will not change, and patients will require treatment for the remainder of their lives. A trial of IVIg cessation is not an option in these individuals (Orange 2011b).
TABLE 7.
Potential Payer-directed Barriers to Applying Best Practice in PIDD
|
Source: Orange 2011b. Reproduced with permission.
Guiding principles for the optimal use of IVIg products are listed in Table 8.
TABLE 8.
Eight Guiding Principles for the Use of IVIg Products in PIDD
|
IVIg = intravenous immunoglobulin; SCIg = subcutaneous immunoglobulin.
Source: Orange 2011b. Reproduced with permission.
When choosing the most appropriate IVIg product, physicians should compare the risks and benefits of the available preparations, based on product attributes, as well as the health status of the individual patient. Patients with no known risk factors for serious AEs are likely to respond well to a high-concentration IVIg formulation. However, all patients should be started at the minimum infusion rate and titrated per individual tolerability. Patients known to be at risk of renal failure should not be given IVIg formulations with a high sugar and/or sodium content and high osmolality/osmolarity. Moreover, IVIg products should be administered at the lowest dose and at the slowest possible infusion rate in these patients (Cherin 2010). Similarly, IVIg products with a high sugar and/or sodium content and high osmolality/osmolarity should be used with caution in patients with a history of cardiovascular disease or with an increased risk of thrombotic events. All patients should be carefully monitored during the IV infusion (Cherin 2010).
IVIg therapy is generally well-tolerated, with headache, low-grade fever, flushing, myalgia, backache, and nausea among the most commonly reported treatment-related AEs (Cherin 2010). These events are generally related to the infusion, are of mild severity, and are reversible. The per-infusion rates of mild-to-moderate AEs range from 5% to 15%, although these rates may be higher for the following patients: those with newly diagnosed hypogammaglobulinemia that has never been treated with IVIg; those who are switching from one IVIg product to another; those who have had a dose interruption; and those with a chronic underlying infection, such as bronchitis or sinusitis (Cherin 2010). The presence of aggregates in the IVIg formulation and rapid infusion rates (>0.08 mL/kg/min) are associated with an increased risk of infusion reactions (Cherin 2010). The risk of AEs may be reduced by eliminating aggregates, by administering the product at a slower infusion rate, and by premedicating the patient with corticosteroids, antihistamines, and/or antipyretics (Cherin 2010). Serious AEs have been rarely reported during post-marketing surveillance of IVIg products. These events have included acute renal failure, stroke, myocardial infarction, venous thromboembolism, anaphylaxis, aseptic meningitis, and hemolysis (Cherin 2010).
All IVIg products have a boxed warning regarding the potential for renal failure (FDA 1998). The use of IVIg products, particularly those containing sucrose, has been reported to be associated with a disproportionate occurrence of renal dysfunction, acute renal failure, osmotic nephropathy, and death. Patients at risk of acute renal failure include those with any degree of pre-existing renal insufficiency, diabetes mellitus, advanced age (>65 years), volume depletion, sepsis, or paraproteinemia, or those receiving known nephrotoxic drugs. Privigen® does not contain sucrose or any other sugars in its formulation (Privigen® Prescribing Information 2011).
DIFFERENCES IN INTRAVENOUS IMMUNOGLOBULIN PREPARATIONS
IVIg products are derived from pooled plasma donated by a large number of healthy donors (15,000 to 60,000) to ensure that the products contain a wide spectrum of antibodies (Cherin 2010, Fernandez-Cruz 2009, Orange 2006). All IVIg preparations must adhere to national and international standards to ensure viral safety as well as to achieve optimal therapeutic efficacy and tolerability (Cherin 2010).
The manufacture of IVIg products generally involves the purification of pooled donor plasma using fractionation and chromatography (Cherin 2010). The resulting IVIg products differ in composition, and these variations may affect their tolerability (Cherin 2010). The currently available IVIg products may vary with respect to:
Product formulation. Whether the product is lyophilized or liquid (ready-to-use) has implications for convenience in terms of storage, the time required for reconstitution (lyophilized products), patient administration time, the potential for errors, and transport (Siegel 2005).
Available concentration of IgG. Protein concentrations range from 3% to 12% in the various IVIg products. A product’s concentration affects its administration volume (Siegel 2005).
Type of stabilizer used. Stabilizers include glycine, proline, and sugars (e.g., glucose, maltose, sucrose, or sorbitol) (Cherin 2010). All are used to keep the IgG molecule in its monomeric form. An increased risk of renal dysfunction has been associated with IVIg formulations that contain sucrose (Siegel 2005).
Maximum recommended infusion rate. This determines the amount of time required to complete an infusion and the potential for AEs, and it can have a significant effect on patient convenience and overall experience (Siegel 2005).
Osmolality. This may increase considerably when lyophilized products are reconstituted for administration (Siegel 2005).
Sugar content. This increases the risk of AEs in patients with diabetes. Higher rates of renal dysfunction and acute renal failure have been associated with products that use sucrose as a stabilizer (Siegel 2005).
Sodium content. This may be higher when lyophilized products are reconstituted for administration and may increase certain patients’ risk of renal and cardiac events (Siegel 2005). Sodium content is also an important consideration in patients with fluid restrictions.
pH levels. These are important determinants of product stability. A pH level of approximately 4.5 is associated with greater purity and maximal monomer content. The addition of stabilizers to maintain pH levels may increase the risk of several AEs, including renal toxicity (Siegel 2005). pH is an important consideration in neonates and infants.
IgA content. This increases the risk of anaphylactic shock in patients with antibodies to IgA and a history of hypersensitivity reactions (Siegel 2005).
Volume load. This affects the amount of product that must be given. The administration of large-volume infusions may not be well-tolerated by patients with heart failure, renal dysfunction, hypertension, or vascular disease (Siegel 2005).
Product Information
INDICATIONS AND USAGE
Privigen® is an immune globulin intravenous (human), 10% liquid indicated for the treatment of the following conditions:
Primary humoral immunodeficiency. Privigen® is indicated as replacement therapy for primary humoral immunodeficiency. This includes, but is not limited to, the humoral immune defect in congenital agammaglobulinemia, common variable immunodeficiency (CVID), X-linked agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies (Privigen® Prescribing Information 2011).
Chronic immune thrombocytopenic purpura.Privigen® is indicated for the treatment of patients with chronic immune thrombocytopenic purpura (ITP) to raise platelet counts (Privigen® Prescribing Information 2011).
DESCRIPTION
Privigen® is a ready-to-use, sterile, 10% protein liquid preparation of polyvalent human immunoglobulin G (IgG) for intravenous administration. Privigen® has a purity of at least 98% IgG, consisting primarily of monomers. The balance consists of IgG dimers (≤12%), small amounts of fragments and polymers, and albumin. Privigen® contains ≤25 mcg/mL IgA. The IgG subclass distribution (approximate mean values) is IgG1, 67.8%; IgG2, 28.7%; IgG3, 2.3%; and IgG4, 1.2%. Privigen® has an osmolality of approximately 320 mOsmol/kg (range: 240 to 440) and a pH of 4.8 (range: 4.6 to 5.0) (Privigen® Prescribing Information 2011).
IgG isolated from the pooled plasma of a large number of donors may form idiotype/anti-idiotype antibody dimers. In turn, the presence of IgG dimers in IVIg may be associated with an increased risk of adverse reactions, such as headache, fever, and flushing, during IV infusion of IgG (Berger 2011, Bolli 2010). Dimer levels in IVIg products may be controlled by formulating the product at a low pH and/or by adding small amphiphilic molecules as stabilizers (Berger 2011, Bolli 2010). The hydrophobic groups in these compounds may interact with the hydrophobic domains of IgG molecules. This inhibits the occurrence of hydrophobic interactions and prevents excessive dimerization (Bolli 2010). Amphiphilic molecules are more effective for preventing protein-protein interactions compared with polar compounds or polyols, such as glycerol or sugars (Berger 2011). Masking the hydrophobic regions of the protein with amphiphilic compounds achieves stability of the IgG in the polar-water environment, decreases the likelihood of dimerization, and prevents the formation of aggregates (Berger 2011).
Studies of the effects of proline on IgG dimer and aggregate formation under various conditions (e.g., different IgG concentrations, pH, time, and temperature) have confirmed that proline is an optimal stabilizer. Proline has also been shown to prevent the fragmentation of IgG and the oxidation of proteins; such fragmentation and oxidation have the potential to decrease the activity of specific antibodies during prolonged storage (Berger 2011). Further, proline reduces the formation of idiotype/anti-idiotype dimers in liquid IVIg products (Bolli 2010). The final formulation of Privigen® contains approximately 250 mmol/L of proline (range: 210 to 290 mmol/L) as the sole stabilizer at a pH of 4.8 (Privigen® Prescribing Information 2011, Berger 2011). The formulation of Privigen® with proline at a pH of 4.8 allows the product to remain stable when stored at room temperature (up to 25°C [77°F]) for 36 months (Privigen® Prescribing Information 2011, Berger 2011). Of note, the administered proline is rapidly cleared from the blood circulation after an infusion of Privigen® and does not accumulate in patients with normal proline metabolism (Hagan 2011).
A 3-year study evaluated the stability and activity of Privigen® under long-term storage conditions at 25°C (Cramer 2009). Privigen® maintained good stability under these conditions. The aggregate content remained below the detection limit of 0.1% for up to 9 months in 5 of 7 lots. Increases up to 0.5% were observed by month 36, but this was still well below the specified limit of 2.0%. Fragment levels were below the detection limit of 1.9% for up to 12 months for all 7 lots. These levels increased to 3.9% by month 36. However, this was significantly less than the specification limit of 6.0%. Importantly, the purity of IgG was 98% and the monomer/dimer levels of Privigen® were 96% after 36 months of storage. Initial dimer levels ranged between 3.8% and 4.8%. These levels increased to a range of 4.9% to 7.6% after 36 months of storage, which was below the specification threshold of 12%. The function of the Fc antibody region ranged from 73% to 107% at study initiation and maintained a range of 85% to 104% after 36 months of storage. These results confirm the observation that storage of Privigen® at room temperature for up to 36 months causes minimal degradation, aggregation, or dimerization, and has no detrimental effects on Fc function and on the activity of specific antibodies (Bolli 2010, Berger 2011).
Privigen® does not contain sucrose or other forms of carbohydrate stabilizers. IVIg products that contain sucrose as a stabilizer have been associated with renal dysfunction. Only trace amounts of sodium are present in Privigen®. Privigen® contains no preservatives and does not require refrigeration or reconstitution prior to administration (Privigen® Prescribing Information 2011).
MANUFACTURING PROCESS
Privigen® is prepared from large pools of human plasma by a combination of cold ethanol fractionation, octanoic acid fractionation, and anion exchange chromatography. The IgG proteins are not subjected to heating or to chemical or enzymatic modification. The Fc and Fab functions of the IgG molecule are retained. Fab functions tested include antigen binding capacities, and Fc functions tested include complement activation and Fc-receptor-mediated leukocyte activation (determined with complexed IgG). Privigen® does not activate the complement system or prekallikrein in an unspecific manner (Privigen® Prescribing Information 2011).
All plasma units used in the manufacture of Privigen® have been tested and approved for manufacture using FDA-licensed serologic assays for hepatitis B surface antigen and antibodies to HCV and HIV-1/2 as well as FDA-licensed nucleic acid testing (NAT) for HCV and HIV-1 and have been found to be nonreactive (negative). For HBV, an investigational NAT procedure is used and the plasma units found to be negative; however, the significance of a negative result has not been established. In addition, the plasma has been tested for B19V DNA by NAT. Only plasma that passed virus screening is used for production, and the limit for B19V in the fractionation pool is set not to exceed 104 IU of B19V DNA per mL (Privigen® Prescribing Information 2011).
The manufacturing process for Privigen® includes three steps to reduce the risk of virus transmission. Two of these are dedicated virus clearance steps: pH 4 incubation to inactivate enveloped viruses and virus filtration to remove, by size exclusion, both enveloped and nonenveloped viruses as small as approximately 20 nanometers. In addition, a depth filtration step contributes to the virus reduction capacity. These steps have been independently validated in a series of in vitro experiments for their capacity to inactivate and/or remove both enveloped and nonenveloped viruses (Privigen® Prescribing Information 2011).
The viral removal, inactivation, and nanofiltration processes involved in the manufacture of Privigen® result in ≥16 log-fold reductions for HIV and West Nile virus; ≥12 log-fold reductions for large DNA viruses, such as herpes; ≥7.9 and ≥9.4 log-fold reductions for model viruses corresponding to hepatitis A and B, respectively; and ≥7.8 log-fold reductions for a model of B19V. In addition, the process used to manufacture Privigen® decreases prions that contribute to transmissible spongiform encephalopathies by at least 14.8 log-fold (Berger 2011).
CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism of action of IVIg is complex and is not completely understood. It is thought to involve modulation of the expression and function of Fc receptors; interference with the activation of complement and the cytokine network; effects on the activation, differentiation, and effector functions of T cells and B cells; and other significant processes (Kazatchkine 2001).
Two widely studied and accepted mechanisms of IVIg action are supplementation of essential antibodies and immunomodulatory effects (Simon 2003, Kazatchkine 2001). In supplementation, IVIg therapy is used to provide protective antibodies to patients with PIDDs by delivering immune antibodies against common pathogens (Simon 2003). With immunomodulatory effects, a number of events take place (Kazatchkine 2001), including:
Blockade of Fc receptors on macrophages and effector cells
Attenuation of complement-mediated inflammatory damage
Neutralization of circulating autoantibodies by anti-idiotypes
Selective down-regulation of antibody production
Regulation of apoptosis
Mechanism of action in primary immunodeficiency diseases. Privigen® is a replacement therapy for PI, and supplies a broad spectrum of opsonic and neutralizing IgG antibodies against bacterial, viral, parasitic, and mycoplasma agents and their toxins (Privigen® Prescribing Information 2011). As recommended by the FDA and by the Plasma Protein Therapeutics Association, Privigen® is manufactured from the pooled plasma of thousands of blood donors and therefore includes a broad variety of antibody specificities against common pathogens to which the donor population has been exposed (Cramer 2009, Orange 2006). Moreover, the antibodies in Privigen® are structurally and functionally intact, and their effector functions are fully operative (Cramer 2009). The mechanism of action of Privigen® in PI has not been fully elucidated (Privigen® Prescribing Information 2011).
Mechanism of action in immune thrombocytopenic purpura. Studies have suggested that IVIg mediates short-term increases in platelet counts as a result of reticuloendothelial system (RES) blockade, which may occur via two independent mechanisms: 1) IVIg contains IgG dimers and multimers that can bind to Fc receptors and block platelet clearance and prolong platelet survival, and 2) IVIg contains IgG molecules that bind to host antigens, form immune complexes, and compete with antibody-sensitized platelets for Fc receptors in the RES, resulting in prolonged platelet survival (Lazarus 1998). Other mechanisms may also contribute to the inhibition of thrombocytopenia. For example, IVIg has been shown to have effects on the cellular immune response itself. Specifically, IVIg-induced changes in cellular immunity may modulate B- and T-cell functions, leading to the suppression of autoantibody production (Lazarus 1998). The mechanism of action of high doses of immunoglobulins in the treatment of chronic ITP has not been fully elucidated (Privigen® Prescribing Information 2011).
Drug Metabolism and Pharmacokinetics
Treatment of primary humoral immunodeficiency. In the pivotal clinical study of Privigen®, which assessed the product’s efficacy and safety in 80 subjects with PI, serum concentrations of total IgG and IgG subclasses were measured in 25 subjects (ages 13 to 69 years) following the 7th infusion for 3 subjects on a 3-week dosing interval and following the 5th infusion for 22 subjects on a 4-week dosing interval. The doses of Privigen® used in these subjects ranged from 200.0 mg/kg to 714.3 mg/kg. After the infusion, blood samples were taken until Day 21 and Day 28 for the 3-week and 4-week dosing intervals, respectively. Table 9 summarizes the pharmacokinetic parameters of Privigen®, based on serum concentrations of total IgG (Privigen® Prescribing Information 2011).
TABLE 9.
Pharmacokinetic Parameters of Privigen® in Subjects With PI
| 3-Week Dosing Interval (n=3) | 4-Week Dosing Interval (n=22) | |||
|---|---|---|---|---|
| Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | |
| Cmax (peak, mg/dL) | 2,550 (400) | 2,340 (2,290–3,010) | 2,260 (530) | 2,340 (1,040–3,460) |
| Cmin (trough, mg/dL) | 1,230 (230) | 1,200 (1,020–1,470) | 1,000 (200) | 1,000 (580–1,360) |
| t1/2 (days) | 27.6 (5.9) | 27.8 (21.6–33.4) | 45.4 (18.5) | 37.3 (20.6–96.6) |
| AUC0–t (day × mg/dL)* | 32,820 (6,260) | 29,860 (28,580–40,010) | 36,390 (5,950) | 36,670 (19,680–44,340) |
| AUC0–∞ (day × mg/dL)* | 79,315 (20,170) | 78,748 (59,435–99,762) | 104,627 (33,581) | 98,521 (64,803–178,600) |
| Clearance (mL/day/kg) | 1.3 (0.1) | 1.3 (1.1–1.4) | 1.3 (0.3) | 1.3 (0.9–2.1) |
| Mean residence time (days)* | 38.6 (8.1) | 39.5 (30.1–46.2) | 65.2 (24.7) | 59.0 (33.2–129.6) |
| Volume of distribution at steady state (mL/kg)* | 50 (13) | 44 (40–65) | 84 (35) | 87 (40–207) |
Cmax = maximum (peak) serum concentration; Cmin = minimum (trough) serum concentration; t½ = elimination half-life; AUC0-t = area under the curve from 0 hour to last sampling time; AUC0-∞ = area under the curve from 0 hour to infinite time.
Calculated by log-linear trapezoidal rule.
The median half-life of Privigen® was 36.6 days for the 25 subjects in the pharmacokinetic subgroup (Privigen® Prescribing Information 2011).
Although no systematic study was conducted to evaluate the effect of gender and age on the pharmacokinetics of Privigen®, based on a small sample size (11 males and 14 females) it appears that clearance of Privigen® is comparable in males (1.27 ± 0.35 mL/day/kg) and females (1.34 ± 0.22 mL/day/kg). In 6 subjects between 13 and 15 years of age, the clearance of Privigen® (1.35 ± 0.44 mL/day/kg) was comparable with that observed in 19 adult subjects 19 years of age or older (1.29 ± 0.22 mL/day/kg) (Privigen® Prescribing Information 2011).
The IgG subclass levels observed in the pharmacokinetic study were consistent with a physiologic distribution pattern (mean trough values): IgG1, 564.91 mg/dL; IgG2, 394.15 mg/dL; IgG3, 30.16 mg/dL; and IgG4, 10.88 mg/dL (Privigen® Prescribing Information 2011).
Treatment of chronic immune thrombocytopenic purpura. Pharmacokinetic studies with Privigen® were not performed in subjects with chronic ITP (Privigen® Prescribing Information 2011).
DOSAGE AND ADMINISTRATION
Preparation and Handling
Privigen® is a clear or slightly opalescent, colorless to pale yellow solution. Inspect parenteral drug products visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if the solution is cloudy or turbid, or if it contains particulate matter (Privigen® Prescribing Information 2011).
Do not shake.
Do not freeze. Do not use if Privigen® has been frozen.
Privigen® should be at room temperature (up to 25°C [77°F]) at the time of administration.
Do not use Privigen® beyond the expiration date on the product label.
The Privigen® vial is for single-use only. Promptly use any vial that has been entered. Privigen® contains no preservative. Discard partially used vials or unused product in accordance with local requirements.
Infuse Privigen® using a separate infusion line. Prior to use, the infusion line may be flushed with Dextrose Injection, USP (D5W) or 0.9% Sodium Chloride for Injection, USP.
Do not mix Privigen® with other IVIg products or other IV medications. Privigen® may be diluted with Dextrose Injection, USP (D5W).
An infusion pump may be used to control the rate of administration.
If large doses of Privigen® are to be administered, several vials may be pooled using aseptic technique. Begin infusion within 8 hours of pooling.
Dosage
Treatment of primary humoral immunodeficiency. Because there are significant differences in the half-life of IgG among patients with PI, the frequency and amount of immunoglobulin therapy may vary from patient to patient. The proper amount can be determined by monitoring clinical response (Privigen® Prescribing Information 2011).
The recommended dosage of Privigen® for patients with PI is 200 to 800 mg/kg (2 to 8 mL/kg) administered every 3 to 4 weeks. If a patient misses a dose, the missed dose should be administered as soon as possible, and then scheduled treatments should be resumed every 3 or 4 weeks, as applicable (Privigen® Prescribing Information 2011).
The dosage should be adjusted over time to achieve the desired trough serum levels and clinical responses. No randomized controlled data are available to determine an optimal trough level in patients receiving immune globulin therapy (Privigen® Prescribing Information 2011).
Treatment of chronic immune thrombocytopenic purpura. The recommended dosage of Privigen® for patients with chronic ITP is 1 g/kg (10 mL/kg) administered daily for 2 consecutive days, resulting in a total dose of 2 g/kg. The high-dose regimen (2 g/kg divided over 2 days) is not recommended for individuals with expanded fluid volumes or where fluid volume may be a concern (Privigen® Prescribing Information 2011).
Administration
Privigen® is for intravenous administration only. Monitor the patient’s vital signs throughout the infusion. Slow or stop the infusion if adverse reactions occur. If symptoms subside promptly, the infusion may be resumed at a lower rate that is comfortable for the patient (Privigen® Prescribing Information 2011).
Ensure that patients with pre-existing renal insufficiency are not volume depleted. For patients judged to be at risk of renal dysfunction or thrombotic events, administer Privigen® at the minimum infusion rate practicable, and discontinue Privigen® administration if renal function deteriorates. Table 10 provides the recommended infusion rates for Privigen® (Privigen® Prescribing Information 2011).
TABLE 10.
Recommended Infusion Rates for Privigen®
| Condition | Initial Infusion Rate | Maintenance Infusion Rate (If Tolerated) |
|---|---|---|
| Primary immunodeficiency disease (PIDD) | 0.5 mg/kg/min (0.005 mL/kg/min) | Increase to 8 mg/kg/min (0.08 mL/kg/min) |
| Immune thrombocytopenic purpura (ITP) | 0.5 mg/kg/min (0.005 mL/kg/min) | Increase to 4 mg/kg/min (0.04 mL/kg/min) |
Source: Adapted from Privigen® Prescribing Information 2011.
The following patients may be at risk of developing inflammatory reactions on rapid infusion of Privigen® (>4 mg/kg/min [0.04 mL/kg/min]): 1) those who have never received Privigen® or another IgG product or have not received it within the past 8 weeks, and 2) those who are switching from another IgG product. These patients should be started at a slow rate of infusion (e.g., 0.5 mg/kg/min [0.005 mL/kg/min] or less) and gradually advanced to the maximum rate, as tolerated (Privigen® Prescribing Information 2011).
How Supplied
Privigen® is supplied in a single-use, tamper-evident vial containing the labeled amount of functionally active IgG. The components used in the packaging for Privigen® are latex-free (Privigen® Prescribing Information 2011). The following presentations of Privigen® are available:
| NDC Number | Fill Size (mL) | Grams Protein |
|---|---|---|
| 44206-436-05 | 50 | 5 |
| 44206-437-10 | 100 | 10 |
| 44206-438-20 | 200 | 20 |
Each vial has an integral suspension band and a label with two peel-off strips showing the product name, lot number, and expiration date (Privigen® Prescribing Information 2011).
Storage and Handling
When stored at room temperature (up to 25°C [77°F]), Privigen® is stable for up to 36 months, as indicated by the expiration date printed on the outer carton and on the vial label (Privigen® Prescribing Information 2011).
Keep Privigen® in its original carton to protect it from light.
Do not freeze.
Clinical Trials
TREATMENT OF PRIMARY IMMUNODEFICIENCY
Safety and Efficacy of Privigen®, a Novel 10% Liquid Immunoglobulin Preparation for Intravenous Use, in Patients With Primary Immunodeficiencies
Methods. A phase III, open-label, single-arm, multicenter trial evaluated the efficacy and safety of Privigen® in 80 patients with PIDD (59 with CVID and 21 with XLA) (Stein 2009). The patients’ median age was 25 years (range: 3–69 years). All patients received Privigen® at 3- to 4-week intervals for 12 months. Doses ranged from 200 to 800 mg/kg.
The study’s primary end point was the number of acute serious bacterial infections (aSBIs), including pneumonia, bacteremia/septicemia, osteomyelitis/septic arthritis, bacterial meningitis, and visceral abscess. Secondary end points included the occurrence of any infection, the number of days missed from work or school due to illness, the number of days hospitalized, and the number of days on which antibiotics were taken.
Results. During the 12-month study period, an aSBI occurred in 6 patients (7.5%), including 3 patients with pneumonia (3.8%) and 1 patient each with septic arthritis, osteomyelitis, and iatrogenic visceral abscess (following bowel perforation during surgery) (1.3% each), corresponding to an annual rate of 0.08 for the intention-to-treat population (N=80). Sixty-six patients (82.5%) experienced 255 episodes of any type of infection, including aSBIs, for an annual infection rate of 3.55 per patient. The majority of infections were mild or moderate; 16 infections (6.3%) experienced by 10 patients were considered to be severe. The most common infection was sinusitis. Table 11 lists the aSBIs and other infections that occurred during this study.
TABLE 11.
aSBIs and All Other Infections Occurring in >5% of PIDD Patients Treated With Privigen® for 12 Months
| Infections | ITT Population [N=80] No. (%) |
|---|---|
| aSBIs* | 6 (7.5) |
| Pneumonia | 3 (3.8) |
| Septic arthritis | 1 (1.3) |
| Osteomyelitis | 1 (1.3) |
| Visceral abscess | 1 (1.3) |
| All other infections | 66 (82.5) |
| Sinusitis | 25 (31.3) |
| Nasopharyngitis | 18 (22.5) |
| URTI | 15 (18.8) |
| Bronchitis | 11 (13.8) |
| Rhinitis | 11 (13.8) |
| Influenza | 10 (12.5) |
| Gastroenteritis | 7 (8.8) |
| Conjunctivitis | 6 (7.5) |
| Ear infection | 6 (7.5) |
| Urinary tract infection | 6 (7.5) |
Blood cultures were negative in 3 of the 6 patients with aSBIs and were not performed in 2 patients. In the patient with osteomyelitis, vancomycin-resistant enterococci and yeast-like fungi were found. aSBIs = acute serious bacterial infections; ITT = intention-to-treat; URTI = upper respiratory tract infection.
Source: Stein 2009. Reproduced with kind permission from Springer+Business Media: J Clin Immunol. Safety and efficacy of Privigen®, a 10% liquid immunoglobulin preparation for intravenous use, in patients with primary immunodeficiencies. 2009;29(1):137–144. Stein MR, Nelson RP, Church JA, et al. Table II.
Fifty-three patients (66.3%) missed work, school, or daycare or were unable to perform normal activities because of illness, for an annual average rate of 7.94 days per patient. Fifteen patients were hospitalized for a total of 166 days, which corresponded to an annual rate of 2.31 days of hospitalization per patient. The annual rate of antibiotic use was 87.4 days. Mean IgG trough serum levels ranged from 8.84 to 10.27 g/L for all infusions.
At least one AE occurred in 78 patients (97.5%) during the study period. Headache was the most common AE, affecting 67.5% of patients. Most AEs (60%) were mild in severity and not related to study treatment (84%). Sixteen patients (20%) experienced 38 serious AEs. Only 1 patient had serious AEs that were considered to be related to the study drug (i.e., hypersensitivity, chills, fatigue, dizziness, and body temperature increased).
Conclusions. In this pivotal phase III study, Privigen® provided stable IgG trough serum levels, a low annual aSBI rate, and a low proportion of infusions with temporally associated AEs in patients requiring regular immunoglobulin replacement therapy.
Tolerability of a New 10% Liquid Immunoglobulin for Intravenous Use, Privigen®, at Different Infusion Rates
Methods. Patients who completed the pivotal phase III trial of Privigen®, described above, were enrolled in a phase III, open-label, single-arm, prospective, multicenter extension study for further treatment for up to 124 weeks at stable doses (up to 875 mg/kg) (Sleasman 2010). Forty-five patients with PIDD (34 with CVID and 11 with XLA) participated in the study. The patients’ median age was 19 years (range: 4–66 years).
The purpose of this study was to evaluate further the safety and efficacy of Privigen® in patients with PIDD and to assess the tolerability of Privigen® at a higher maximum infusion rate (12 mg/kg/min). The study’s primary tolerability end point was the frequency of temporally associated AEs, which were defined as any AE that occurred during an infusion or within 72 hours after completion of an infusion, regardless of assessment of causal relation to the study drug. AEs were continuously monitored at each study visit and with patient diaries.
All of the patients received infusions of Privigen® at 3- to 4-week intervals. Treatment lasted for up to 124 weeks, with all doses of study medication remaining at the same level (not exceeding 875 mg/kg) unless a dose adjustment was required for medical reasons. At the investigators’ discretion (based on the patients’ tolerability), 23 patients were selected to receive a high maximum infusion rate (HIR; 12 mg/kg/min), and the remaining 22 patients received a low maximum infusion rate (LIR; 8 mg/kg/min).
Results. Privigen® was well tolerated at the high infusion rate without any compromise in tolerability. The percentage of infusions with temporally associated AEs was significantly lower in the HIR group compared with the LIR group (8% vs 21%, respectively; P=.0089). Moreover, the most frequently associated AEs were less common in patients receiving HIR than in those receiving LIR (Table 12). Overall, headache was the most common temporally associated AE, occurring in 56 (8%) of 688 infusions.
TABLE 12.
Most Frequent (≥1% of Infusions in Any Group) Temporally Associated Adverse Events in PIDD Patients Treated With Privigen® for Up to 124 Weeks
| Adverse Event | Number (%*) of Temporally Associated AEs | |
|---|---|---|
| Low Max. Infusion Rate 8 mg/kg/min 423 Infusions | High Max. Infusion Rate 12 mg/kg/min 265 Infusions | |
| Headache | 54 (12.8) | 2 (0.8) |
| Pain† | 23 (5.4) | 4 (1.5) |
| Nausea | 8 (1.9) | 2 (0.8) |
| Chills | 7 (1.7) | 0 (0) |
| Vomiting | 4 (0.9) | 0 (0) |
| Pyrexia | 9 (2.1) | 1 (0.4) |
Percentage of infusions associated with each AE (i.e., number of AEs divided by number of infusions).
Includes the following AE preferred terms: pain, abdominal pain, abdominal pain lower, abdominal pain upper, back pain, chest pain, infusion site pain, injection site pain, neck pain, pain in extremity, urinary tract pain, pharyngolaryngeal pain, and ear pain.
Source: Adapted from Sleasman 2010.
Conclusions. The results of this study demonstrate that patients can tolerate Privigen® at high infusion rates without compromising tolerability.
Efficacy and Safety of Privigen® in Children and Adolescents With Primary Immunodeficiency
Methods. A phase III, prospective, open-label, multi-center, single-arm study was conducted to evaluate the efficacy and safety of Privigen® in children and adolescents with CVID or XLA (Church 2009). The study population consisted of 31 pediatric patients who had participated in the pivotal phase III trial of Privigen®, described above. This population was divided into children (aged 3 to 11 years; n = 19) and adolescents (aged 12 to 15 years; n = 12). The study’s primary end point was the annual rate of aSBIs (e.g., pneumonia, bacteremia, and septicemia) per patient. Secondary end points included the annual rate of any infections per patient, the number of days missed from school or daycare, the number of days unable to perform usual activities, the number of days hospitalized, and the use of antibiotics.
All of the patients received Privigen® at doses of 200 to 800 mg/kg body weight at 3- to 4-week intervals for 12 months. The Privigen® infusions were increased to a maximum of 4 mg/kg/min for the first 3 infusions. From the fourth infusion onward, the infusion rate could be increased to a maximum of 8 mg/kg/min, if well tolerated.
Results. The annual rates of aSBIs per patient were 0.12 for children and 0.10 for adolescents. The corresponding annual rates of all infections (including aSBIs) per patient were 4.63 and 2.42, respectively. URTIs were the most common infections, occurring in 32% of all patients. The annual rates of days missed from school or daycare per patient were 11.5 in children and 4.8 in adolescents. Table 13 summarizes the study’s primary and secondary efficacy results.
TABLE 13.
Annual Rates of Efficacy Parameters in Pediatric Patients With PIDD Treated With Privigen® for 12 Months
| End Point | Children (3–11 Years) [n=19] | Adolescents (12–15 Years) [n=12] | Total [n=31] |
|---|---|---|---|
| Total number of study days | 6,152 | 3,776 | 9,928 |
| Annual rate of aSBIs per patient | 0.12 | 0.10 | 0.11 |
| No. of patients with aSBI | 2 | 1 | 3 |
| No. of aSBIs | 2 | 1 | 3 |
| Annual rate of all infections per patient | 4.63 | 2.42 | 3.79 |
| No. of patients with infections | 17 | 11 | 28 |
| No. of infections | 78 | 25 | 103 |
| Annual rate of days missed from school/daycare per patient | 11.51 | 4.83 | 8.97 |
| No. of patients who missed | 16 | 7 | 23 |
| No. of days missed | 194 | 50 | 244 |
| Annual rate of days hospitalized per patient | 0.53 | 0 | 0.33 |
| No. of patients hospitalized | 5 | 0 | 5 |
| No. of days hospitalized | 9 | 0 | 9 |
| Annual rate of days with antibiotics per patient | 47.88 | 56.64 | 51.21 |
| No. of patients treated with antibiotics | 16 | 9 | 25 |
| No. of days on antibiotics | 807 | 586 | 1,393 |
Source: Adapted from Church 2009.
Fourteen children (74%) and 7 adolescents (58%) experienced temporally associated AEs (occurring during or within 72 hours after the end of an infusion) on at least 1 occasion. The most common temporally associated AEs were headache, fatigue, chills, and vomiting. Most of these AEs were mild or moderate in severity. The most common AEs related to Privigen® infusion were headache, vomiting, fatigue, chills, nausea, and back pain. Fourteen serious AEs occurred in 6 children, and 1 serious AE occurred in 1 adolescent. Five of the 14 serious AEs in children occurred in a single patient and were considered related to Privigen®. These events included hypersensitivity, chills, fatigue, dizziness, and increased body temperature.
Conclusions. This study demonstrated that Privigen® is effective and safe in children and adolescents with PIDD.
TREATMENT OF CHRONIC IMMUNE THROMBOCYTOPENIC PURPURA
Efficacy and Safety of Privigen®, a Novel Liquid Intravenous Immunoglobulin Formulation, in Adolescent and Adult Patients With Chronic Immune Thrombocytopenic Purpura
Methods. A phase III, prospective, open-label study was conducted to evaluate the efficacy and safety of Privigen® in 57 adolescent and adult patients with chronic ITP (Robak 2009). Patients were included in the study if they had a platelet count of ≤20 × 109/L at screening, no other known cause for thrombocytopenia, and a platelet count of ≤150 × 109/L over 6 months or a response to previous treatment with a subsequent decrease in platelet count even if the duration of chronic ITP was <6 months. The patients’ mean age was 38 years (range: 15–69 years). Fifty-two patients (91.2%) had received previous treatment for ITP.
The administration of concomitant medications, including other immunoglobulins, intravenous steroids, immunosuppressant agents, blood products, and medications that could affect the blood-clotting response, was not permitted, although oral steroids were allowed if the dose had been stable for at least 15 days prior to screening and for the duration of the study period.
Patients were treated with Privigen® 1 g/kg body weight on 2 consecutive days, for a total dose of 2 g/kg. The initial infusion rate was 0.3 mL/kg/h (0.5 mg/kg/min), which could be increased at 30-minute intervals (first, to 0.6 mL/kg/h, then to 1.2 mL/kg/h, and then to the maximum rate of 2.4 mL/kg/h). Platelet counts and bleeding status were evaluated at screening for study eligibility and on study days 1, 2, 4, 6, 8, 15, 22, 29, 57, and 85. Laboratory samples for blood chemistry, hematology, and urinalysis were obtained at screening and on days 1, 2, 4, 8, and 29.
The study’s primary efficacy end point was the response rate, with response defined as an increase in the platelet count to ≥50 × 109/L within 7 days after the first infusion of Privigen®. Secondary efficacy end points included the regression of hemorrhage, increases in platelet counts at different time points, time to and duration of the platelet response, and the maximum platelet level. The frequency and severity of AEs and changes in laboratory parameters were recorded to assess safety.
Results. Fifty-seven patients (the intention-to-treat [ITT] population) received two infusions of Privigen®, with almost all of the patients receiving the planned total dose of 2 g/kg body weight. The infusion rate was increased to the specified maximum of 2.4 mL/kg/h in 104 (91.2%) of the 114 infusions.
Forty-six patients (80.7%) achieved the primary efficacy end point of a platelet response ≥50 × 109/L within 7 days after the first administration of Privigen® (P<.0001) (Table 14). Forty-three percent of the patients showed a response after only 1 infusion. The median time to a platelet response was 2.5 days after the start of Privigen® therapy. The median duration of responses was 15.4 days (range: 1 to >82 days).
TABLE 14.
Platelet Response Rate After 2 Infusions of Privigen® in Adolescent and Adult Patients With Chronic ITP
| Population | ITT Cohort, N | Response Rate*, N (%) |
|---|---|---|
| Total | 57 | 46 (80.7) |
| Gender subgroups | ||
| Female | 34 | 24 (70.6) |
| Male | 23 | 22 (95.7) |
| Corticosteroid use | ||
| No | 39 | 31 (79.5) |
| Yes | 18 | 15 (83.3) |
| Blood group ABO | ||
| A | 22 | 18 (81.8) |
| AB | 1 | 1 (100) |
| B | 9 | 7 (77.8) |
| O | 23 | 19 (82.6) |
| Blood group Rhesus | ||
| Positive | 45 | 38 (84.4) |
| Negative | 10 | 7 (70.0) |
| Unknown | 2 | 1 (50.0) |
Response defined as platelet count ≥50 × 109/L within 7 days of first Privigen® infusion.
ITT = intention-to-treat.
Source: Adapted from Robak 2009.
Platelet counts increased rapidly after Privigen® therapy (Figure 2). At 1 day after the first infusion, the mean platelet count had increased from 13 × 109/L to 52 × 109/L. The mean peak platelet count (227 × 109/L ) was achieved on Day 8. Thereafter, platelet counts decreased but still remained above baseline levels for the duration of the study.
FIGURE 2.
Platelet Counts After Privigen® Infusion on Days 1 and 2 in Adolescent and Adult Patients With Chronic ITP
Data are presented as mean ± standard error of the mean.
Source: Robak 2009. Reproduced with permission.
Bleeding status also improved rapidly. Three days after the first infusion of Privigen®, the proportion of patients experiencing bleeding events had decreased from 70.2% to 41.8%, with a further decrease to 21.8% by the eighth day after infusion. Hemorrhage regression rates were 86.1% for skin (31 of 36 patients), 100% for the oral cavity (11 of 11 patients), and 77.8% for the genitourinary tract (7 of 9 patients).
A total of 52 patients (91.2%) experienced at least 1 AE, with the majority of AEs being classified as either mild or moderate in severity. Headache was the most common AE, occurring in 66.7% of patients. Table 15 summarizes the most common AEs in this study.
TABLE 15.
Most Frequent (>10% of Patients) Adverse Events in Adolescents and Adults With Chronic ITP Treated With Privigen®
| Event | Number of Patients (%) | ||
|---|---|---|---|
| With Premedication (n=32) | Without Premedication (n=25) | Total (n=57) | |
| Headache | 16 (50.0) | 22 (88.0) | 38 (66.7) |
| Increased temperature* | 11 (34.4) | 9 (36.0) | 20 (35.1) |
| Epistaxis | 6 (18.8) | 5 (20.0) | 11 (19.3) |
| Contusion† | 4 (12.5) | 6 (24.0) | 10 (17.5) |
| Petechiae‡ | 3 (9.4) | 4 (16.0) | 7 (12.3) |
| Anemia | 2 (6.3) | 4 (16.0) | 6 (10.5) |
| Nausea | 2 (6.3) | 4 (16.0) | 6 (10.5) |
| Skin hemorrhage | 3 (9.4) | 3 (12.0) | 6 (10.5) |
| Vomiting | 2 (6.3) | 4 (16.0) | 6 (10.5) |
Includes pyrexia, body temperature increased, and hyperthermia.
Includes adverse events reported as contusions or as bruises.
Includes skin petechiae and oral petechiae.
Source: Robak 2009. Reproduced with permission.
After the implementation of a protocol amendment that permitted premedication, 32 patients (56.1%) were pre-treated with acetaminophen/antihistamine. Headache occurred in 50% of pretreated patients compared with 88% of those who were not pretreated. Moreover, mild-to-moderate headaches were experienced by 40.6% of pre-treated patients versus 80.0% of non-pretreated patients.
Conclusions. In this phase III study, Privigen® rapidly reduced the signs and symptoms of disease in patients with chronic ITP, as evidenced by platelet-response end points and bleeding event rates. Privigen® was generally well tolerated in both adolescents and adults.
Safety
BOXED WARNING: ACUTE RENAL DYSFUNCTION/FAILURE.
The use of intravenous immunoglobulin (IVIg) products, particularly those containing sucrose, has been reported to be associated with renal dysfunction, acute renal failure, osmotic nephropathy, and death. Patients at risk of acute renal failure include those with any degree of pre-existing renal insufficiency, diabetes mellitus, advanced age (above 65 years of age), volume depletion, sepsis, paraproteinemia, or receiving known nephrotoxic drugs. Privigen® does not contain sucrose.
For patients at risk of renal dysfunction or failure, administer Privigen® at the minimum infusion rate practicable.
CONTRAINDICATIONS
Privigen® is contraindicated in patients who have a history of anaphylactic or severe systemic reaction to the administration of human immune globulin.
Privigen® is contraindicated in patients with hyperprolinemia because it contains the stabilizer L-proline.
Privigen® is contraindicated in IgA-deficient patients with antibodies to IgA and a history of hypersensitivity.
WARNINGS AND PRECAUTIONS
Hypersensitivity
Severe hypersensitivity reactions may occur. In case of hypersensitivity, discontinue the Privigen® infusion immediately and institute appropriate treatment. Medications such as epinephrine should be available for immediate treatment of acute hypersensitivity reactions.
Privigen® contains trace amounts of IgA (≤25 mcg/mL). Individuals with IgA deficiency can develop anti-IgA antibodies and anaphylactic reactions (including anaphylaxis and shock) after administration of blood components containing IgA. Patients with known antibodies to IgA may have a greater risk of developing potentially severe hypersensitivity and anaphylactic reactions with the administration of Privigen®. Privigen® is contraindicated in patients with antibodies against IgA and a history of hypersensitivity.
Renal Dysfunction/Failure
Acute renal dysfunction/failure, osmotic nephropathy, and death may occur with the use of IVIg products, including Privigen®. Ensure that patients are not volume depleted and assess renal function, including measurement of blood urea nitrogen (BUN) and serum creatinine, before the initial infusion of Privigen® and at appropriate intervals thereafter.
Periodic monitoring of renal function and urine output is particularly important in patients judged to be at increased risk of developing acute renal failure. If renal function deteriorates, consider discontinuing Privigen®. For patients judged to be at risk of developing renal dysfunction because of pre-existing renal insufficiency, or predisposition to acute renal failure (such as those with diabetes mellitus or hypovolemia, those who are overweight, those who use concomitant nephrotoxic medicinal products, or those who are over 65 years of age), administer Privigen® at the minimum infusion rate practicable.
Hyperproteinemia, Increased Serum Viscosity, and Hyponatremia
Hyperproteinemia, increased serum viscosity, and hyponatremia may occur after treatment with IVIg products, including Privigen®. The hyponatremia is likely to be a pseudohyponatremia, as demonstrated by a decreased calculated serum osmolality or elevated osmolar gap. It is critical to distinguish true hyponatremia from pseudohyponatremia, as treatment aimed at decreasing serum-free water in patients with pseudohyponatremia may lead to volume depletion, a further increase in serum viscosity, and a possible predisposition to thromboembolic events.
Thrombotic Events
Thrombotic events may occur following treatment with IVIg products, including Privigen®. Patients at risk include those with a history of atherosclerosis, multiple cardiovascular risk factors, advanced age, impaired cardiac output, coagulation disorders, prolonged periods of immobilization, and/or known/suspected hyperviscosity.
Because of the potentially increased risk of thrombosis, consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients judged to be at risk of developing thrombotic events, administer Privigen® at the minimum rate of infusion practicable.
Aseptic Meningitis Syndrome (AMS)
AMS may occur infrequently following treatment with Privigen® and other human immune globulin products. Discontinuation of treatment has resulted in remission of AMS within several days without sequelae. AMS usually begins within several hours to 2 days after IVIg treatment.
AMS is characterized by the following signs and symptoms: severe headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, nausea, and vomiting. Cerebrospinal fluid (CSF) studies are frequently positive, with pleocytosis up to several thousand cells per cubic millimeter, predominantly from the granulocytic series, and with elevated protein levels up to several hundred mg/dL, but negative culture results. Conduct a thorough neurologic examination of patients exhibiting such signs and symptoms, including CSF studies, to rule out other causes of meningitis.
AMS may occur more frequently in association with high doses (2 g/kg) and/or rapid infusion of IVIg.
Hemolysis
Privigen® may contain blood group antibodies that can act as hemolysins and induce in vivo coating of red blood cells (RBCs) with immunoglobulin, causing a positive direct antiglobulin test (DAT) (Coombs’ test) result and hemolysis. Delayed hemolytic anemia can develop subsequent to Privigen® therapy because of enhanced RBC sequestration, and acute hemolysis, consistent with intravascular hemolysis, has been reported.
Hemolysis, possibly intravascular, occurred in 2 subjects treated with Privigen® in the ITP study. These cases resolved uneventfully. Six other subjects experienced hemolysis in the ITP study, as documented from clinical laboratory data.
Monitor patients for clinical signs and symptoms of hemolysis. If these are present after a Privigen® infusion, perform appropriate confirmatory laboratory testing. If transfusion is indicated for patients who develop hemolysis with clinically compromising anemia after receiving IVIg, perform adequate cross-matching to avoid exacerbating ongoing hemolysis.
Transfusion-related Acute Lung Injury (TRALI)
Noncardiogenic pulmonary edema may occur in patients following treatment with IVIg products, including Privigen®. TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever. Symptoms typically appear within 1 to 6 hours after treatment.
Monitor patients for pulmonary adverse reactions. If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil antibodies and anti-human leukocyte antigen (HLA) antibodies in both the product and the patient’s serum.
TRALI may be managed using oxygen therapy with adequate ventilatory support.
Volume Overload
The high-dose regimen (1 g/kg/day for 2 days) used to treat patients with chronic ITP is not recommended for individuals with expanded fluid volumes or where fluid volume may be of concern.
Transmissible Infectious Agents
Because Privigen® is made from human blood, it may carry a risk of transmitting infectious agents (e.g., viruses and, theoretically, the Creutzfeldt-Jakob disease agent). The risk of infectious agent transmission has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by including virus inactivation/removal steps in the manufacturing process for Privigen®. Healthcare professionals should report any infection thought to be possibly transmitted by Privigen® to CSL Behring Pharmacovigilance at 1-866-915-6958.
Interference With Laboratory Tests
Various passively transferred antibodies in immunoglobulin preparations may lead to misinterpretation of the results of serologic testing.
ADVERSE REACTIONS
The most serious adverse reaction observed in clinical study subjects receiving Privigen® for PI was hypersensitivity in 1 subject. The most common adverse reactions observed in >5% of clinical study subjects with PI were headache, pain, nausea, fatigue, chills, vomiting, joint swelling/effusion, pyrexia, and urticaria.
The most serious adverse reactions observed in clinical study subjects receiving Privigen® for chronic ITP were aseptic meningitis syndrome in 1 subject and hemolysis in 2 subjects. Six other subjects in the ITP study experienced hemolysis as documented from clinical laboratory data. The most common adverse reactions observed in >5% of clinical study subjects with chronic ITP were headache, pyrexia/hyperthermia, positive DAT, anemia, vomiting, nausea, hyperthermia, bilirubin conjugated increased, bilirubin unconjugated increased, hyperbilirubinemia, and blood lactate dehydrogenase increased.
Clinical Trials Experience
Because different clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Treatment of Primary Humoral Immunodeficiency. In a prospective, open-label, single-arm, multicenter clinical study (pivotal study), 80 subjects with PIDD (with a diagnosis of XLA or CVID) received Privigen® intravenously every 3 or 4 weeks for up to 12 months. All subjects had been on regular IVIg replacement therapy for at least 6 months before participating in the study. Subjects ranged in age from 3 to 69 years; 46 (57.5%) were male and 34 (42.5%) were female.
The safety analysis included all 80 subjects, 16 (20%) on the 3-week schedule and 64 (80%) on the 4-week schedule. The median dose of Privigen® administered was 428.3 mg/kg (3-week schedule) or 440.6 mg/kg (4-week schedule) and ranged from 200 to 888 mg/kg. A total of 1,038 infusions of Privigen® were administered, 272 in the 3-week schedule and 766 in the 4-week schedule.
Routine premedication was not allowed. However, subjects who experienced 2 consecutive infusion-related adverse events (AEs) that were likely to be prevented by premedication were permitted to receive antipyretics, antihistamines, nonsteroidal anti-inflammatory drugs (NSAIDs), or antiemetic agents. During the study, 8 subjects (10%) received premedication prior to 51 (4.9%) of the 1,038 infusions administered.
Temporally associated AEs are those occurring during or within 72 hours after the end of an infusion, irrespective of causality. In this study, the upper bound of the 1-sided 97.5% confidence interval for the proportion of Privigen® infusions temporally associated with 1 or more AEs was 23.8% (actual proportion: 20.8%). The total number of temporally associated AEs was 397 (a rate of 0.38 AEs per infusion), reflecting that some subjects experienced more than 1 AE during the observation period.
Table 16 lists the temporally associated AEs that occurred in >5% of subjects, irrespective of causality.
TABLE 16.
Adverse Events Occurring in >5% of Subjects With Primary Immunodeficiency During a Privigen® Infusion or Within 72 Hours After the End of an Infusion, Irrespective of Causality (PIDD Pivotal Study)
| Adverse Event* | Subjects (%) [N=80] | Infusions (%) [N=1,038] |
|---|---|---|
| Headache | 35 (43.8) | 82 (7.9) |
| Pain | 20 (25.0) | 44 (4.2) |
| Fatigue | 13 (16.3) | 27 (2.6) |
| Nausea | 10 (12.5) | 19 (1.8) |
| Chills | 9 (11.3) | 15 (1.4) |
| Vomiting | 7 (8.8) | 13 (1.3) |
| Pyrexia | 6 (7.5) | 10 (1.0) |
| Cough | 5 (6.3) | 5 (0.5) |
| Diarrhea | 5 (6.3) | 5 (0.5) |
| Stomach discomfort | 5 (6.3) | 5 (0.5) |
Excluding infections.
Source: Adapted from Privigen® Prescribing Information 2011.
Of the 397 temporally associated AEs reported for the 80 subjects with PIDD, the investigators judged 192 to be at least possibly related to the infusion of Privigen® (including 5 serious, severe AEs, described below). Of these, 91 were mild, 81 were moderate, 19 were severe, and 1 was of unknown severity.
Table 17 lists the adverse reactions (AEs at least possibly related to the infusion of Privigen®) that occurred in >5% of subjects with PIDD, irrespective of time of occurrence.
TABLE 17.
Adverse Reactions Occurring in >5% of Subjects With Primary Immunodeficiency Treated With Privigen®, Irrespective of Time of Occurrence (PIDD Pivotal Study)
| Adverse Reaction | Subjects (%) [N=80] | Infusions (%) [N=1,038] |
|---|---|---|
| Headache | 24 (30.0) | 62 (6.0) |
| Pain, all types* | 12 (15.0)† | 26 (2.5) |
| Nausea | 10 (12.5) | 18 (1.7) |
| Fatigue | 9 (11.3) | 16 (1.5) |
| Chills | 9 (11.3) | 15 (1.4) |
| Vomiting | 6 (7.5) | 11 (1.1) |
Includes abdominal pain lower, abdominal tenderness, arthralgia, back pain, chest pain, infusion-site pain, injection-site pain, neck pain, pain, pain in extremity, and pharyngolaryngeal pain.
Some subjects experienced more than one type of pain.
Source: Adapted from Privigen® Prescribing Information 2011.
Sixteen subjects (20%) experienced 41 serious AEs. Five of these AEs (hypersensitivity, chills, fatigue, dizziness, and increased body temperature, all severe) were related to Privigen®, occurred in 1 subject, and resulted in the subject’s withdrawal from the study. Two other subjects withdrew from the study because of AEs related to Privigen® treatment (chills and headache in 1 subject; vomiting in the other).
Seventy-seven of the 80 subjects enrolled in this study had a negative direct antiglobulin test (DAT) at baseline. Of these 77 subjects, 36 (46.8%) developed a positive DAT at some time during the study. However, no subjects showed evidence of hemolytic anemia.
During this study, no subjects tested positive for infection due to HIV, HBV, HCV, or B19V.
An extension of the pivotal study was conducted in 55 adult and pediatric subjects with PIDD to collect additional efficacy, safety, and tolerability data. This study included 45 subjects from the pivotal study who were receiving Privigen® and 10 new subjects who were receiving another IVIg product prior to enrolling in the extension study. Subjects ranged in age from 4 to 81 years; 26 (47.3%) were male and 29 (52.7%) were female.
Subjects were treated with Privigen® at median doses ranging from 286 to 832 mg/kg per infusion over a treatment period ranging from 1 to 27 months. Twelve (21.8%) subjects were on a 3-week treatment schedule, with the number of infusions per subject ranging from 4 to 38 (median: 8 infusions); 43 (78.2%) subjects were on a 4-week schedule, with the number of infusions ranging from 1 to 31 (median: 15 infusions). A total of 771 infusions were administered in this study.
In this study, subjects who continued from the pivotal study were permitted to receive infusions of Privigen® at a rate up to 12 mg/kg/min (as opposed to the maximum of 8 mg/kg/min allowed in the pivotal study) at the discretion of the investigator, based on individual tolerability. Twenty-three (51%) of the 45 subjects from the pivotal study (41.8% of the 55 subjects in the extension study) received 265 (38.4%) infusions at a maximum rate greater than the recommended rate of 8 mg/kg/min. The median of the maximum infusion rate in this subset was 12 mg/kg/min. However, because the study was not designed to compare infusion rates, no definitive conclusions regarding tolerability could be drawn for infusion rates higher than the recommended rate of 8 mg/kg/min.
In this study, the proportion of infusions temporally associated with 1 or more AEs that occurred during a Privigen® infusion or within 72 hours after the end of an infusion was 15%. The total number of temporally associated AEs, irrespective of causality, was 206 (a rate of 0.27 AEs per infusion), reflecting that some subjects experienced more than 1 AE during the observation period. Table 18 lists the temporally associated AEs that occurred in >5% of subjects, irrespective of causality.
TABLE 18.
Adverse Events Occurring in >5% of Subjects With Primary Immunodeficiency During a Privigen® Infusion or Within 72 Hours After the End of an Infusion, Irrespective of Causality (PIDD Extension Study)
| Adverse Event* | Subjects (%) [N=55] | Infusions (%) [N=771] |
|---|---|---|
| Headache | 18 (32.7) | 56 (7.3) |
| Pain, all types† | 14 (25.5)‡ | 31 (4.0) |
| Abdominal pain§ | 3 (5.5) | 4 (0.5) |
| Chest pain | 3 (5.5) | 4 (0.5) |
| Pharyngolaryngeal pain | 3 (5.5) | 4 (0.5) |
| Nausea | 6 (10.9) | 10 (1.3) |
| Pyrexia | 4 (7.3) | 9 (1.2) |
| Chills | 3 (5.5) | 7 (0.9) |
| Influenza-like illness | 3 (5.5) | 4 (0.5) |
Note: The AE rates in this study cannot be compared directly with the rates in other IVIg studies, including the original pivotal study of Privigen®, because 1) the extension study used an enriched population, and 2) the selective use of higher infusion rates at the investigators’ discretion in a subset of subjects may have introduced bias.
Excluding infections.
Includes abdominal pain, abdominal pain upper, arthralgia, back pain, chest pain, fibromyalgia, injection-site pain, myalgia, pain, pain in extremity, painful respiration, pharyngolaryngeal pain, and toothache.
Also includes abdominal pain, upper.
Some subjects experienced more than one type of pain.
Source: Adapted from Privigen® Prescribing Information 2011.
Of the 206 temporally associated AEs reported for the 55 subjects with PIDD, the investigators judged 125 to be at least possibly related to the infusion of Privigen®. Of these, 76 were mild, 40 were moderate, and 9 were severe. Table 19 lists the adverse reactions that occurred in >5% of subjects, irrespective of time of occurrence.
TABLE 19.
Adverse Events Occurring in >5% of Subjects With Primary Immunodeficiency Treated With Privigen®, Irrespective of Time of Occurrence (PIDD Extension Study)
| Adverse Reaction | Subjects (%) [N=55] | Infusions (%) [N=771] |
|---|---|---|
| Headache | 16 (29.1) | 53 (6.9) |
| Pain, all types* | 11 (20.0)† | 26 (3.4) |
| Abdominal pain‡ | 4 (7.3) | 6 (0.8) |
| Chest pain | 3 (5.5) | 4 (0.5) |
| Chills | 3 (5.5) | 7 (0.9) |
| Fatigue | 3 (5.5) | 5 (0.6) |
| Joint swelling/effusion | 3 (5.5) | 7 (0.9) |
| Pyrexia | 3 (5.5) | 10 (1.3) |
| Urticaria | 3 (5.5) | 4 (0.5) |
Includes abdominal pain, abdominal pain lower, abdominal pain upper, arthralgia, back pain, chest pain, injection-site pain, musculoskeletal pain, myalgia, pain, and painful respiration.
Some subjects experienced more than one type of pain.
Includes abdominal pain, lower and abdominal pain, upper.
Source: Adapted from Privigen® Prescribing Information 2011.
Eleven (20%) subjects experienced 17 serious AEs, none of which was considered to be related to Privigen®. Three subjects experienced AEs that were considered to be at least possibly related to Privigen®: dyspnea and pancytopenia in 1 subject; a transient ischemic attack 16 days after the infusion in 1 subject; and mild urticaria in 1 subject, resulting in the subject’s withdrawal from the study.
Treatment of Chronic Immune Thrombocytopenic Purpura. In a prospective, open-label, single-arm, multi-center clinical study, 57 subjects with chronic ITP and a platelet count of ≤20 × 109/L received a total 2 g/kg dose of Privigen® administered as 1 g/kg infusions daily for 2 consecutive days. Subjects ranged in age from 15 to 69 years; 23 (40.4%) were male and 34 (59.6%) were female.
Concomitant medications affecting platelets or other treatments for ITP were not allowed. Thirty-two (56.1%) subjects received premedication with acetaminophen and/or an antihistamine.
Table 20 lists the temporally associated AEs that occurred in >5% of subjects with chronic ITP during a Privigen® infusion or within 72 hours after the end of a treatment cycle (two consecutive infusions) with Privigen®, irrespective of causality.
TABLE 20.
Adverse Events Occurring in >5% of Subjects During a Privigen® Infusion or Within 72 Hours After the End of a Treatment Cycle*, Irrespective of Causality (Chronic ITP Study)
| Adverse Event | Subjects (%) [N=57] | Infusions (%) [N=114] |
|---|---|---|
| Headache | 37 (64.9) | 41 (36.0) |
| Pyrexia/hyperthermia | 21 (36.8) | 22 (19.3) |
| Nausea | 6 (10.5) | 6 (5.3) |
| Epistaxis | 6 (10.5) | 6 (5.3) |
| Vomiting | 6 (10.5) | 6 (5.3) |
| Blood, unconjugated bilirubin increased | 6 (10.5) | 6 (5.3) |
| Blood, conjugated bilirubin increased | 5 (8.8) | 5 (4.4) |
| Blood, total bilirubin increased | 4 (7.0) | 4 (3.5) |
| Hematocrit decreased | 3 (5.3) | 3 (2.6) |
Treatment cycle = 2 consecutive daily infusions.
Source: Adapted from Privigen® Prescribing Information 2011.
Table 21 lists the adverse reactions that occurred in >5% of subjects, irrespective of the time of occurrence.
TABLE 21.
Adverse Reactions Occurring in >5% of Subjects Treated With Privigen®, Irrespective of Time of Occurrence (Chronic ITP Study)
| Adverse Reaction | Subjects (%) [N=57] | Infusions (%) [N=114] |
|---|---|---|
| Headache | 37 (64.9) | 52 (45.6) |
| Pyrexia/hyperthermia | 19 (33.3) | 21 (18.4) |
| Positive DAT | 6 (10.5) | 7 (6.1) |
| Anemia | 6 (10.5) | 6 (5.3) |
| Vomiting | 5 (8.8) | 6 (5.3) |
| Nausea | 5 (8.8) | 7 (6.1) |
| Bilirubin conjugated, increased | 5 (8.8) | 5 (4.4) |
| Bilirubin unconjugated, increased | 5 (8.8) | 5 (4.4) |
| Hyperbilirubinemia | 3 (5.3) | 3 (2.6) |
| Blood lactate dehydrogenase increased | 3 (5.3) | 3 (2.6) |
| Hematocrit decreased | 3 (5.3) | 3 (2.6) |
DAT = direct antiglobulin test (Coombs’ test).
Source: Adapted from Privigen® Prescribing Information 2011.
Of the 149 non-serious AEs related to Privigen®, 103 were mild, 37 were moderate, and 9 were severe. Three subjects experienced 3 serious AEs, one of which (aseptic meningitis) was related to the infusion of Privigen®. One subject withdrew from the study because of gingival bleeding that was not related to Privigen®.
Eight subjects, all of whom had a positive DAT, experienced transient drug-related hemolytic reactions, which were associated with elevated bilirubin, elevated lactate dehydrogenase, and a decrease in the hemoglobin level within 2 days after the infusion of Privigen®. Two of the 8 subjects were clinically anemic but did not require clinical intervention; these cases resolved uneventfully. Four other subjects with active bleeding were reported to have developed anemia without evidence of hemolysis.
In this study, there was a decrease in hemoglobin after the first Privigen® infusion (median decrease of 1.2 g/dL by Day 8), followed by a return to near baseline by Day 29.
Fifty-six of the 57 subjects in this study had a negative DAT at baseline. Of these 56 subjects, 12 (21.4%) developed a positive DAT during the 29-day study period.
Postmarketing Experience
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions or to establish a causal relationship to product exposure.
The following adverse reactions have been indentified and reported during the post-approval use of IVIg products:
Infusion reactions: Hypersensitivity (e.g., anaphylaxis), headache, diarrhea, tachycardia, fever, fatigue, dizziness, malaise, chills, flushing, urticaria or other skin reactions, wheezing or other chest discomfort, nausea, vomiting, rigors, back pain, myalgia, arthralgia, changes in blood pressure
Renal: Acute renal dysfunction/failure, osmotic nephropathy
Respiratory: Apnea, acute respiratory distress syndrome, TRALI, cyanosis, hypoxemia, pulmonary edema, dyspnea, bronchospasm
Cardiovascular: Cardiac arrest, thromboembolism, vascular collapse, hypotension
Neurologic: Coma, loss of consciousness, seizures, tremor, AMS
Integumentary: Stevens-Johnson syndrome, epidermolysis, erythema multiforme, bullous dermatitis
Hematologic: Pancytopenia, leukopenia, hemolysis, positive DAT (Coombs’ test)
Musculoskeletal: Back pain
Gastrointestinal: Hepatic dysfunction, abdominal pain
General/Body as a Whole: Pyrexia, rigors
P&T Committee Considerations
Primary immunodeficiency syndromes comprise a heterogeneous group of genetic disorders linked by a common inability to produce effective immune responses to pathogens (Wood 2007). Replacement IgG therapy is the standard of care for primary humoral immunodeficiencies and is also used for its immunomodulatory mechanisms in autoimmune and inflammatory diseases (Looney 2006, Shehata 2010). IVIg therapy has also been used to treat other conditions, including neuroimmune disorders, infectious and infection-related diseases, dermatologic diseases, and neurocognitive disorders (Orange 2006, Looney 2006).
Early diagnosis and appropriate management with IgG replacement therapy is associated with reduced morbidity and mortality in patients with PIDDs (Shehata 2010). Studies have shown that IVIg therapy decreases both infection rates and hospitalizations in patients with PIDDs and achieves adequate hemostasis in patients with chronic ITP (Shehata 2010, Robak 2009). Evidence also suggests that IgG therapy may reduce the risk of chronic illnesses and complications that often affect patients with PIDDs (Shehata 2010). IVIg replacement therapy is therefore an important treatment option for patients with PIDD or chronic ITP.
Health-related Quality of Life
Despite appropriate therapy, patients with PIDDs are at increased risk of organ-specific and systemic complications (Wood 2009). Importantly, the risk of significant permanent functional impairments is high among patients with PIDDs, with 49% of respondents to the 2007 IDF survey indicating pulmonary, GI, and auditory impairments (IDF 2009a). Similar results were reported in the 2008 IDF survey, in which PIDD patients had permanent impairment of the lungs, digestive system, and hearing (IDF 2009b). Delayed diagnosis was associated with higher rates of permanent impairment, and a positive correlation existed between the time from symptom onset and the number of permanent functional impairments reported by patients, especially for neurologic, mobility, digestive, and visual impairments (IDF 2009a).
Patients with ITP have also reported that their disease significantly compromises their QOL. In an online survey conducted by the Platelet Disorders Support Association, between 30% and 60% of respondents indicated that ITP moderately, strongly, or completely interfered with their ability to exercise or engage in sports, negatively affected their emotional well-being and mood, compromised their ability to travel, interfered with work performance, and undermined their ability to manage daily life responsibilities and activities (PDSA 2011c).
Cost Burden
The cost of care for undiagnosed and untreated individuals with PIDDs is considerably higher than the direct medical costs associated with the care of patients with PIDD. A survey of 118 physician-experts in the management of PIDD found that the average annual cost of health care for undiagnosed patients was $102,736, compared with $22,696 for each diagnosed patient (Modell 2007). The National Institutes of Health estimate that there are at least 500,000 cases of undiagnosed PIDD in the US (Modell 2007).
Treatment Considerations
IgG replacement therapy requires individualized decisions regarding the most appropriate and effective product, dosage, frequency of infusions, route of administration, and monitoring to ensure optimal patient outcomes (Maarschalk-Ellerbroek 2011, Shehata 2010). A product’s sodium, sugar, and IgA contents, osmolarity and osmolality, pH, volume load, and infusion rate may affect tolerability (Siegel 2005). Some IVIg products are more appropriate than others for a given patient, depending on the patient’s health status, as shown in Table 22 (Siegel 2005). In addition, each patient should be treated at the dose and IgG serum trough level that keep them infection- and complication-free versus treating all patients to a target serum trough level (Maarschalk-Ellerbroek 2011, Shehata 2010, Lucas 2010).
TABLE 22.
Features of IVIg Products That Are Considerations for Patients With Risk Factors
| Risk Factor | Sodium Content | Sugar Content | Osmolality/Osmolarity | pH | IgA | Volume Load |
|---|---|---|---|---|---|---|
| Renal dysfunction | X | X | X | X | ||
| Heart disease | X | X | X | |||
| Diabetes mellitus, prediabetes | X | |||||
| Elderly | X | X | X | X | ||
| Neonatal, pediatric | X | X | X | X | ||
| Thromboembolic risk | X | X | X | |||
| IgA deficiency | X |
Source: Adapted from Siegel 2005.
Convenience of administration for both patients and health care providers is also an important factor to be considered when selecting the most appropriate IVIg replacement product (Siegel 2005). Health care providers must be aware of the clinically relevant differences between liquid and lyophilized products, including storage, transport, and reconstitution requirements (Siegel 2005). Patients appreciate the fact that low AE rates and faster infusion times decrease the impact of treatment on their lives and well-being (Siegel 2005).
In the 2008 IDF survey, the majority of PIDD patients treated with IVIg replacement therapy reported high levels of satisfaction with the efficacy of their treatment, with 91% describing their immunodeficiency as completely, well, or adequately controlled (IDF 2009b). Further, 78% of patients were very satisfied with the IVIg product that they were currently receiving. The results of this survey confirmed that regular administration of IgG replacement therapy significantly improves various aspects of a patient’s health, including health status, limitations on activities, and QOL. The respondents perceived IgG replacement therapy as life-saving. Notably, 17% of patients reported difficulties in receiving IVIg therapy during the previous 2 to 3 years, including delayed infusions, reimbursement problems, lack of product availability, and increased intervals between therapies.
Privigen®: An Effective and Well-tolerated Formulary Option
Privigen® (Immune Globulin Intravenous [Human], 10% Liquid) is approved for replacement therapy in patients with PIDD and for the treatment of patients with chronic ITP to raise platelet counts (Privigen® Prescribing Information 2011).
In the pivotal phase III study of Privigen® in patients with PIDD, the product provided adequate IgG trough serum levels, a low annual aSBI rate (0.08 for the intention-to-treat cohort [N=80]), and a low percentage of infusions with temporally associated AEs, thus demonstrating the product’s efficacy and safety in PIDD (Stein 2009). Results from an extension study further supported the long-term tolerability of Privigen® in patients with PIDD (Sleasman 2010).
Privigen® was also shown to be effective in sustaining a low incidence of infections, including aSBIs, and showed a good tolerability profile in children and adolescents with PIDD (Church 2009). The annual rate of aSBIs was 0.12 per patient in children (aged 3 to 11 years) and 0.10 per patient in adolescents (aged 12 to 15 years). The corresponding annual rates of all infections (including aSBIs) per patient were 4.63 and 2.42, respectively. The most common temporally associated AEs were headache, fatigue, chills, and vomiting.
Further, Privigen® has been established as an effective and well-tolerated treatment in adolescents and adults with chronic ITP. In a pivotal phase III trial, the primary efficacy end point (an increase in the platelet count to ≥50 × 109/L within 7 days after the first Privigen® infusion) was achieved by 80.7% of patients (P<.001) (Robak 2009). Three days after the first infusion of Privigen®, the proportion of patients with bleeding events decreased from 70.2% to 41.8%, and to 21.8% by Day 8. Hemorrhage regression rates were 86.1% for skin, 100% for the oral cavity, and 77.8% for the genitourinary tract. The most common AE was headache (66.7% of patients).
Privigen® is prepared from large pools of human plasma by a combination of cold ethanol fractionation, octanoic acid fractionation, and anion exchange chromatography. The IgG proteins are not subjected to heating or to chemical or enzymatic modification. Only plasma that passed virus screening is used for production (Privigen® Prescribing Information 2011). A unique feature of Privigen® is the use of proline (a nonessential amino acid) as a stabilizer. Proline has favorable stabilizing activity compared with that of glycine and other amino acids and polyols. Proline has also been shown to prevent fragmentation and oxidation of IgG (Berger 2011). Further, proline prevents the formation of idiotype/anti-idiotype dimers in liquid IVIg products (Bolli 2010). The final formulation of Privigen® contains approximately 250 mmol/L proline as the sole stabilizer at pH 4.8 (Privigen® Prescribing Information 2011, Berger 2011). Privigen® is also low in sodium and has an osmolality level that is compatible with physiologic parameters (approximately 320 mOsmol/kg) (Privigen® Prescribing Information 2011). The unique formulation of Privigen® provides clinicians with an effective treatment option for patients with PIDD or chronic ITP. The product’s formulation eliminates the need for reconstitution, remains stable for up to 36 months at room temperature, and allows high infusion rates, as tolerated (Privigen® Prescribing Information 2011, Sleasman 2010).
Conclusion
IVIg replacement therapy has been established as a safe and effective way to supplement patients’ immune systems to:
prevent infection
decrease the risk of bleeding events
decrease the risk of systemic and end-organ complications
improve patients’ lives
reduce the use of health care resources
Similar to the inherent differences that exist among patients, all IVIg products are not equivalent, and they are not interchangeable. The evaluation of Privigen® for inclusion in a formulary requires that P&T committee members consider the product’s attributes and features (including efficacy and safety) as well as its potential benefits for patients. Numerous clinical trials, including studies that evaluated higher infusion rates, have demonstrated that Privigen® is effective and well tolerated in both adult and pediatric patients. The vast majority of AEs associated with the use of Privigen® are mild or moderate in severity and may be managed by premedication and/or by slowing the infusion rate.
GLOSSARY OF ABBREVIATIONS
- AE
adverse event
- AMS
aseptic meningitis syndrome
- aSBI
acute serious bacterial infection
- B19V
B19 virus
- CSF
cerebrospinal fluid
- CVID
common variable immunodeficiency
- DAT
direct antiglobulin test (Coombs’ test)
- DNA
deoxyribonucleic acid
- FDA
Food and Drug Administration
- GI
gastrointestinal
- GVHD
graft-versus-host disease
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HSCT
hematopoietic stem-cell transplantation
- IDF
Immune Deficiency Foundation
- Ig
immunoglobulin
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IM
intramuscular
- ITP
immune thrombocytopenic purpura
- IV
intravenous
- IVIg
intravenous immunoglobulin
- NAT
nucleic acid testing
- NK
natural killer (cell)
- PIDD
primary immunodeficiency disease
- RES
reticuloendothelial system
- SC
subcutaneous
- SCID
severe combined immunodeficiency
- TRALI
transfusion-related acute lung injury
- XLA
X-linked agammaglobulinemia
REFERENCES
- AAAAI (American Academy of Allergy, Asthma, and Immunology) 8 guiding principles for the safe, effective, and appropriate use of IVIG for PI. 2011. Available at: http://www.aaaai.org/members/resources/initiatives/ivig_toolkit/ivig_coverage.pdf. Accessed Mar. 15, 2011.
- Ballow M. Primary immunodeficiency disorders: antibody deficiency. J Allergy Clin Immunol. 2002;109(4):581–591. doi: 10.1067/mai.2002.122466. [DOI] [PubMed] [Google Scholar]
- Berger M. L-proline-stabilized human IgG: Privigen® 10% for intravenous use and Hizentra® 20% for subcutaneous use. Immunotherapy. 2011;3(2):163–176. doi: 10.2217/imt.10.108. [DOI] [PubMed] [Google Scholar]
- Blaese RM, Winkelstein JA, editors. Patient and Family Handbook for Primary Immunodeficiency Diseases. 4th ed. Towson, MD: Immune Deficiency Foundation; 2007. Available at: http://www.primaryimmune.org/publications/book_pats/book_pats.htm. Accessed Mar. 26, 2011. [Google Scholar]
- Bolli R, Wood K, Bartschi M, Hofferer L, Lerch P. L-proline reduces IgG dimer content and enhances the stability of intravenous immunoglobulin (IVIG) solutions. Biologicals. 2010;38(1):150–157. doi: 10.1016/j.biologicals.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Bonilla FA, Bernstein IL, Khan DA, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94(5 Suppl 1):S1–S63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- Boyle JM, Buckley RH. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. J Clin Immunol. 2007;27(5):497–502. doi: 10.1007/s10875-007-9103-1. [DOI] [PubMed] [Google Scholar]
- Buckley RH, editor. Diagnostic and Clinical Care Guidelines for Immunodeficiency Diseases. 2nd ed. Towson, MD: Immune Deficiency Foundation; 2009. Available at: http://www.primaryimmune.org/publications/book_diag/IDF_Diagnostic_and_Clinical_Care_Guidelines-Final.pdf. Accessed Mar. 21, 2011. [Google Scholar]
- Bussone G, Mouthon L. Autoimmune manifestations in primary immune deficiencies. Autoimmun Rev. 2009;8(4):332–336. doi: 10.1016/j.autrev.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Chaplin DD. Overview of the human immune response. J Allergy Clin Immunol. 2006;117:S430–S435. doi: 10.1016/j.jaci.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Cherin P, Cabane J. Relevant criteria for selecting an intravenous immunoglobulin preparation for clinical use. Biodrugs. 2010;24(4):211–223. doi: 10.2165/11537660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Church JA, Borte M, Taki H, et al. Efficacy and safety of Privigen in children and adolescents with primary immunodeficiency. Pediatr Asthma Allergy Immunol. 2009;22(2):53–61. [Google Scholar]
- Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346(13):995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Pommering TL, Koranyi K. Primary immunodeficiencies. Am Fam Physician. 2003;68(10):2001–2011. [PubMed] [Google Scholar]
- Cramer M, Frei R, Sebald A, Mazzoletti P, Maeder W. Stability over 36 months of a new liquid 10% polyclonal immunoglobulin (IgPro10, Privigen®) stabilized with L-proline. Vox Sang. 2009;96(3):219–225. doi: 10.1111/j.1423-0410.2008.01143.x. [DOI] [PubMed] [Google Scholar]
- de Vries E. Patient-centered screening for primary immunodeficiency: a multi-stage diagnostic protocol for non-immunologists. Clin Exp Immunol. 2006;145(2):204–214. doi: 10.1111/j.1365-2249.2006.03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durandy A, Wahn V, Petteway, Gelfand EW. Immunoglobulin replacement therapy in primary antibody deficiency diseases—maximizing success. Int Arch Allergy Immunol. 2005;136(3):217–219. doi: 10.1159/000083948. [DOI] [PubMed] [Google Scholar]
- FDA (Food and Drug Administration) Dear doctor letter––important drug warning: immune globulin intravenous (human) Nov 13, 1998. Available at: http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ucm105914.htm. Accessed May 3, 2011.
- Fernandez-Cruz E, Alecsandru D, Ramon SS. Mechanisms of action of immune globulin. Clin Exp Immunol. 2009;157(Suppl 1):1–2. doi: 10.1111/j.1365-2249.2009.03955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feudjo-Tepie MA, Robinson NJ, Bennett D. Prevalence of diagnosed chronic immune thrombocytopenic purpura in the US: analysis of a large US claim database: a rebuttal [letter] J Thromb Haemost. 2008;6:711–712. doi: 10.1111/j.1538-7836.2008.02911.x. [DOI] [PubMed] [Google Scholar]
- Fischer A. Human primary immunodeficiency diseases: a perspective. Nature Immunol. 2004;5(1):23–30. doi: 10.1038/ni1023. [DOI] [PubMed] [Google Scholar]
- Fischer A, Hacein-Bey-Abina S, Cavazzana-Calvo M. Gene therapy for primary immunodeficiencies. Hematol Oncol Clin North Am. 2011;25(1):89–100. doi: 10.1016/j.hoc.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Garcia JM, Gamboa P, de la Calle A, Hernandez MD, Cabellero MT. Diagnosis and management of immunodeficiencies in adults by allergologists. J Invest Allergol Clin Immunol. 2010;20(3):185–194. [PubMed] [Google Scholar]
- Gernsheimer T. Epidemiology and pathophysiology of immune thrombocytopenic purpura. Eur J Haematol Suppl. 2008;69:3–8. doi: 10.1111/j.1600-0609.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- Gernsheimer T. Chronic idiopathic thrombocytopenic purpura: mechanisms of pathogenesis. Oncologist. 2009;14(1):12–21. doi: 10.1634/theoncologist.2008-0132. [DOI] [PubMed] [Google Scholar]
- Hagan JB, Robak T, Church JA, Rojavin M, Wasserman RL. Rapid clearance of L-proline after intravenous and subcutaneous infusion with L-proline-stabilized immunoglobulin replacement products. Poster presented at the Second Annual Meeting of the Clinical Immunology Society; May 19–22, 2011; Chicago, Illinois. [Google Scholar]
- Haynes BF, Soderberg KA, Fauci AS. Introduction to the immune system. In: Fauci AS, Braunwald E, Kasper DL, et al., editors. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Companies; 2008. pp. 2019–2045. [Google Scholar]
- Herriot R, Sewell WA. Antibody deficiency. J Clin Pathol. 2008;61(9):994–1000. doi: 10.1136/jcp.2007.051177. [DOI] [PubMed] [Google Scholar]
- IDF (Immune Deficiency Foundation) Primary Immunodeficiency Diseases in America: 2007. The Third National Survey of Patients. 2009a. Prepared by Abt SRBI, Inc., May 1, 2009. Available at: http://www.primary-immune.org/publications/surveys/National_Patient_Survey_Report%282007%29.pdf. Accessed Mar. 15, 2011.
- IDF (Immune Deficiency Foundation) Treatment Experiences and Preferences Among Patients With Primary Immunodeficiency Diseases. National Survey of Patients: 2008. 2009b. Prepared by Abt SRBI, Inc.; May 6, 2009. Available at: http://www.primaryimmune.org/publications/surveys/2008_treatment_report_06_08_09.pdf. Accessed Mar. 15, 2011.
- Jeffrey Modell Foundation Ten warning signs of primary immunodeficiency. 2009. Available at: http://www.info4pi.org/aboutPI/pdf/General10WarningSignsFINAL.pdf. Accessed Mar. 15, 2011.
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747–755. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- Kumar A, Teuber SS, Gershwin ME. Current perspectives on primary immunodeficiency diseases. Clin Dev Immunol. 2006;13(2–4):223–259. doi: 10.1080/17402520600800705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus AH, Freedman J, Semple JW. Intravenous immunoglobulin and anti-D in idiopathic thrombocytopenic purpura (ITP): mechanisms of action. Transfus Sci. 1998;19(3):289–294. doi: 10.1016/s0955-3886(98)00043-5. [DOI] [PubMed] [Google Scholar]
- Lindegren ML, Kobrynski L, Rasmussen SA, et al. Applying public health strategies to primary immunodeficiency diseases: a potential approach to genetic disorders. MMWR Recomm Rep. 2004;53(RR-1):1–29. [PubMed] [Google Scholar]
- Looney RJ, Huggins J. Use of intravenous immunoglobulin (IVIG) Best Pract Res Clin Haematol. 2006;19(1):3–25. doi: 10.1016/j.beha.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125(6):1354–1360. doi: 10.1016/j.jaci.2010.02.040. [DOI] [PubMed] [Google Scholar]
- Maarschalk-Ellerbroek LJ, Hoepelman IM, Ellerbroek PM. Immunoglobulin treatment in primary antibody deficiency. Int J Antimicrob Agents. 2011 doi: 10.1016/jijantimicag201011027. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Maddur MS, Lacroix-Desmazes S, Bayry J, Kaveri SV. Intravenous polyclonal immunoglobulin in autoimmune diseases: clinical indications and mechanisms of action. Drug Discov Today. 2009;6(1):5–11. [Google Scholar]
- Merck Manual Online Library for Patients and Caregivers, Professional Edition. 2010 Available at: http://www.merck.com/mmpe/sec13/ch164/ch164a.html. Accessed Mar. 31, 2010. [Google Scholar]
- Negi VS, Elluru S, Siberil S, et al. Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007;27(3):233–245. doi: 10.1007/s10875-007-9088-9. [DOI] [PubMed] [Google Scholar]
- Modell V. The impact of physician education and public awareness on early diagnosis of primary immunodeficiencies: Robert A. Good Immunology Symposium. Immunol Res. 2007;38:43–47. doi: 10.1007/s12026-007-0048-5. [DOI] [PubMed] [Google Scholar]
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. doi: 10.1182/blood-2010-08-302984. Prepublished online, February 16, 2011; [DOI] [PubMed] [Google Scholar]
- NHLBI (National Heart Lung and Blood Institute) How is idiopathic thrombocytopenic purpura treated? Available at: http://www.nhlbi.nih.gov/health/dci/Diseases/Itp/ITP_Treatments.html. Accessed Mar. 15, 2011.
- NIAID (National Institute of Allergy and Infectious Diseases) Immune system: mounting an immune response. 2011. Available at: http://www.niaid.nih.gov/TOPICS/IMMUNESYSTEM/Pages/response.aspx. Accessed May 2, 2011.
- Notarangelo LD, Forino C, Mazzolari E. Stem cell transplantation in primary immunodeficiencies. Curr Opin Allergy Clin Immunol. 2006;6(6):443–448. doi: 10.1097/01.all.0000246616.47708.2f. [DOI] [PubMed] [Google Scholar]
- NPIRC (National Primary Immunodeficiency Resource Center) Primary immunodeficiency diseases: selective IgA deficiency. 2011a. Available at: http://www.info4pi.org/aboutPI/index.cfm?section=aboutPI&content=syndromes&area=2&CFID=40309203&CFTOKEN=52038630. Accessed Mar. 15, 2011.
- NPIRC (National Primary Immunodeficiency Resource Center) Primary immunodeficiency diseases: common variable immunodeficiency. 2011b. Available at: http://www.info4pi.org/aboutPI/index.cfm?section=aboutPI&content=syndromes&area=4&CFID=40309203&CFTOKEN=52038630. Accessed Mar. 15, 2011.
- NPIRC (National Primary Immunodeficiency Resource Center) Primary immunodeficiency diseases: severe combined immunodeficiency. 2011c. Available at: http://www.info4pi.org/aboutPI/index.cfm?section=aboutPI&content=syndromes&area=6&CFID=40309203&CFTOKEN=52038630. Accessed Mar. 15, 2011.
- Orange JS. Clinical update in immunoglobulin therapy for primary immunodeficiency diseases. Clinical Focus on Primary Immunodeficiencies. 2011a Mar;(14) Available at: http://www.primaryimmune.org/publications/clinic_focus/cf_mar11.pdf. Accessed Mar. 15, 2011. [Google Scholar]
- Orange JS. Immunoglobulin therapy: standardizing approaches and best practices in rare diseases in the managed care setting. J Manag Care Med. 2011b;14(2):26–33. [Google Scholar]
- Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta-analysis of clinical studies. Clin Immunol. 2010;137(1):21–30. doi: 10.1016/j.clim.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma, and Immunology. J Allergy Clin Immunol. 2006;117(4 Suppl):S525–S553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Ortutay C, Vihinen M. Identification of candidate disease genes by integrating gene ontologies and protein-interaction networks: case study of primary immunodeficiencies. Nucleic Acids Res. 2009;37:622–628. doi: 10.1093/nar/gkn982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul ME. Diagnosis of immunodeficiency: clinical clues and diagnostic tests. Curr Allergy Asthma Rep. 2002;2(5):349–355. doi: 10.1007/s11882-002-0066-2. [DOI] [PubMed] [Google Scholar]
- PDSA (Platelet Disorder Support Association) Diagnosing ITP—Important Information for Patients. 2011a Available at: http://www.pdsa.org/about-itp/diagnosis-questions.html. Accessed Mar. 16, 2011. [Google Scholar]
- PDSA (Platelet Disorder Support Association) ITP in Adults. 2011b. Available at: http://www.pdsa.org/about-itp/in-adults.html. Accessed Mar. 16, 2011.
- PDSA (Platelet Disorder Support Association) ITP Patient Questionnaire Key Findings. 2011c. Available at: http://www.pdsa.org/images/stories/pdf/FINAL_ITP_Patient_Questionnaire_One-Pager.pdf. Accessed Mar. 24, 2011.
- Kankakee, IL: CSL Behring LLC; Feb, 2011. Privigen®, Immune Globulin Intravenous (Human), 10% Liquid [prescribing information] Available at: http://www.privigen.com/pdf/Privigen_PI.pdf. Accessed May 3, 2011. [Google Scholar]
- Pruemer J. Epidemiology, pathophysiology, and initial management of chronic immune thrombocytopenic purpura. Am J Health Syst Pharm. 2009;66(2 Suppl 2):S4–S10. doi: 10.2146/ajhp080490. [DOI] [PubMed] [Google Scholar]
- Quartier P, Debré M, De Blic J, et al. Early and prolonged intravenous and subcutaneous immunoglobulin replacement therapy in childhood agammaglobulinemia: a retrospective survey of 31 patients. J Pediatr. 1999;134:589–596. doi: 10.1016/s0022-3476(99)70246-5. [DOI] [PubMed] [Google Scholar]
- Robak T, Salama A, Kovaleva L, et al. Efficacy and safety of Privigen®, a novel liquid intravenous immunoglobulin formulation, in adolescent and adult patients with chronic immune thrombocytopenic purpura. Hematology. 2009;14(4):227–236. doi: 10.1179/102453309X439773. Available at: www.maney.co.uk/journals/hem and at www.ingentaconnect.com/content/maney/hem. Accessed May 3, 2011. [DOI] [PubMed] [Google Scholar]
- Shehata N, Palda V, Bowen T, et al. The use of immunoglobulin therapy for patients with primary immune deficiency: an evidence-based practice guideline. Transfus Med Rev. 2010;24(Suppl 1):S7–S27. doi: 10.1016/j.tmrv.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Shier D, Butler J, Lewis R, editors. Hole’s Human Anatomy & Physiology. 12th ed. New York, NY: McGraw Hill; 2010. pp. 616–650. [Google Scholar]
- Siegel J. All immunoglobulins are not equivalent. Pharmacotherapy. 2005;25(11 Pt 2):78S–84S. doi: 10.1592/phco.2005.25.11part2.78S. [DOI] [PubMed] [Google Scholar]
- Simoens S. Pharmacoeconomics of immunoglobulin in primary immunodeficiency. Expert Rev Pharmacoeconomics Outcomes Res. 2009;9(4):375–386. doi: 10.1586/erp.09.37. [DOI] [PubMed] [Google Scholar]
- Simon HU, Späth PJ. IVIG––mechanisms of action. Allergy. 2003;58:543–552. doi: 10.1034/j.1398-9995.2003.00239.x. [DOI] [PubMed] [Google Scholar]
- Skoda-Smith S, Torgerson TR, Ochs HD. Subcutaneous immunoglobulin replacement therapy in the treatment of patients with primary immunodeficiency disease. Ther Clin Risk Manag. 2010;6:1–10. doi: 10.1057/rm.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleasman JW, Duff CM, Dunaway T, Rojavin MA, Stein MR. Tolerability of a new 10% liquid immunoglobulin for intravenous use, Privigen®, at different infusion rates. J Clin Immunol. 2010;30(3):442–448. doi: 10.1007/s10875-010-9373-x. [DOI] [PubMed] [Google Scholar]
- Snyder CF, Mathias SD, Cella D, Isitt JJ, Wu AW, Young J. Health-related quality of life of immune thrombocytopenic purpura patients: results from a web-based survey. Curr Med Res Opin. 2008;24(10):2767–2776. doi: 10.1185/03007990802377461. [DOI] [PubMed] [Google Scholar]
- Stasi R, Willis F, Shannon MS, Gordon-Smith EC. Infectious causes of chronic immune thrombocytopenia. Hematol Oncol Clin North Am. 2009;23(6):1275–1297. doi: 10.1016/j.hoc.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Stein MR, Nelson RP, Church JA, et al. Safety and efficacy of Privigen®, a novel 10% liquid immunoglobulin preparation for intravenous use, in patients with primary immunodeficiencies. J Clin Immunol. 2009;29(1):137–144. doi: 10.1007/s10875-008-9231-2. [DOI] [PubMed] [Google Scholar]
- Tarantino MD, Mathias SD, Snyder CF, Isitt JJ, Gernsheimer T, Young Impact of ITP on physician visits and workplace productivity. Curr Med Res Opin. 2010;26(2):319–328. doi: 10.1185/03007990903451298. [DOI] [PubMed] [Google Scholar]
- Wood P, Stanworth S, Burton J. Recognition, clinical diagnosis, and management of patients with primary antibody deficiencies: a systematic review. Clin Exp Immunol. 2007;149(3):410–423. doi: 10.1111/j.1365-2249.2007.03432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. Primary antibody deficiencies: recognition, clinical diagnosis, and referral of patients. Clin Med. 2009;9(6):595–599. doi: 10.7861/clinmedicine.9-6-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30(1):10–16. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]