Abstract
Beckwith–Wiedemann syndrome (BWS) is a clinically variable disorder characterized by somatic overgrowth, macroglossia, abdominal wall defects, visceromegaly, and an increased susceptibility to childhood tumors. The disease has been linked to a large cluster of imprinted genes at human chromosome 11p15.5. A subset of BWS patients has been identified with loss-of-function mutations in p57KIP2, a maternally expressed gene encoding a G1 cyclin-dependent kinase inhibitor. Some patients display loss of imprinting of IGF2, a fetal-specific growth factor that is paternally expressed. To understand how the same disease can result from misregulation of two linked, but unrelated, genes, we generated a mouse model for BWS that both harbors a null mutation in p57Kip2 and displays loss of Igf2 imprinting. These mice display many of the characteristics of BWS, including placentomegaly and dysplasia, kidney dysplasia, macroglossia, cleft palate, omphalocele, and polydactyly. Some, but not all, of the phenotypes are shown to be Igf2 dependent. In two affected tissues, the two imprinted genes appear to act in an antagonistic manner, a finding that may help explain how BWS can arise from mutations in either gene.
Keywords: Genomic imprinting, p57Kip2, Igf2, H19, Beckwith–Wiedemann syndrome
Beckwith–Wiedemann syndrome (BWS) is a clinically variable disorder characterized by somatic overgrowth, macroglossia, abdominal wall defects, and visceromegaly (Elias et al. 1998). Children with BWS are also susceptible to a variety of childhood tumors, including Wilms' tumor, hepatoblastomas, and rhabdomyosarcomas. The disease, which affects 1 of every 13,700 live births each year, is genetically heterogeneous with the majority of cases occurring sporadically. Familial cases, which represent ∼15% of BWS patients, have helped link BWS genetically to human 11p15.5, a region containing a large cluster of imprinted genes (Koufos et al. 1989; Ping et al. 1989). That defects in imprinted genes might explain the etiology of the syndrome was first suggested from observations that sporadic cases can be associated with 11p15.5 partial paternal uniparental disomies (UPDs) and trisomies with paternal duplications (Turleau et al. 1984; Henry et al. 1991).
Many of the key defects found in BWS patients could be explained by alterations in the control of cell proliferation, either in the context of organogenesis or tumorigenesis. Thus, along with being imprinted, candidate genes should be tied in some respect to the control of cell proliferation. Two candidate imprinted genes with these properties map to 11p15.5, IGF2, and p57KIP2. These genes have strikingly similar patterns of expression during development in mice and are expressed in all of the tissues affected by BWS (Lee et al. 1990, 1995; Matsuoka et al. 1995).
IGF2 encodes a fetal-specific growth factor that is paternally expressed in both mice and humans (DeChiara et al. 1991; Giannoukakis et al. 1993; Ohlsson et al. 1993). When the expression of IGF2 was examined in BWS patients, it was shown to be elevated as the result of deregulation of its imprinting in some, but not all, cases with normal karyotypes (Weksberg et al. 1993a; Reik et al. 1995). Furthermore, paternal trisomies and UPDs would be expected to increase the expression of IGF2 and could potentially account for the overgrowth observed in these cases of BWS.
Maternally inherited loss-of-function mutations in the p57KIP2 gene have also been identified in ∼5%–10% of sporadic BWS cases examined (Hatada et al. 1996b; O'Keefe et al. 1997) and 30%–50% of familial cases (Lam et al. 1999). p57KIP2encodes a member of the CIP/KIP family of cyclin-dependent kinase inhibitors (CKIs), and is maternally expressed in all mammalian species examined to date (Hatada and Mukai 1995; Lee et al. 1995; Matsuoka et al. 1995, 1996; Hatada et al. 1996a). CKIs of this class inhibit G1/S-phase cyclins, and the absence of p57KIP2 has been shown to affect the ability of cells to exit from the cell cycle. The gene lies ∼800 kb from IGF2 in both mouse and human (Lee et al. 1997; Caspary et al. 1998; Paulsen et al. 1998).
A small number of patients (<1%) have been identified with balanced translocations or inversions 3′ of p57KIP2 in a neighboring imprinted gene, KvLQT1 (Weksberg et al. 1993b; Mannens et al. 1994; Hoovers et al. 1995). This gene encodes a voltage-gated potassium channel that is maternally expressed in humans in all tissues except the heart, but mutations in the gene have been implicated only in the cardiac arrhythmia long QT syndrome, not BWS (Wang et al. 1996; Lee et al. 1997). It has been suggested that the regulation of other genes in the locus, particularly p57KIP2and IGF2, may be disrupted by the translocations. In one such family, IGF2 expression has been shown to be biallelic (Brown et al. 1996). Finally, the methylation imprint and expression of a recently described paternally expressed transcript within an intron of KvLQT1, LIT1, has been shown by two groups to be disrupted in >50% of BWS patients examined (Lee et al. 1999; Mitsuya et al. 1999; Smilinich et al. 1999). These groups suggest that this transcript may mediate the imprinting of other genes in the locus, although they disagree on the likeliest candidate (Lee et al. 1999; Mitsuya et al. 1999; Smilinich et al. 1999).
Several mouse models that shed light on the etiology of BWS have been generated. These studies have confirmed that embryonic growth in mice is very sensitive to the levels of the growth factor IGFII. When a mutated copy of the Igf2 gene was inherited paternally, the offspring were 60% the size of their wild-type littermates (DeChiara et al. 1990). In contrast, in mice in which the cis-acting sequences that control Igf2 imprinting were deleted (H19Δ13), Igf2 was expressed from both parental chromosomes, and the offspring displayed somatic overgrowth and placentomegaly, but none of the other symptoms of BWS (Leighton et al. 1995; Eggenschwiler et al. 1997). This mutation raised the tissue levels of IGFII twofold, but had a less pronounced effect on its circulating levels.
Mutations in the type-2 Igf2 receptor gene (Igf2r), whose product binds IGFII and targets it for lysosomal degradation, also caused elevation of IGFII (Filson et al. 1993; Lau et al. 1994; Wang et al. 1994). Circulating IGFII levels were elevated approximately fourfold, and embryos died late in gestation with many, but not all, of the phenotypes of BWS, such as somatic overgrowth, placentomegaly, heart hypertrophy, omphalocele, and adrenal cysts. In Igf2r and H19Δ13 double mutants (Eggenschwiler et al. 1997), IGFII levels were increased 7- to 11-fold, and the severity of the phenotypes were more pronounced than in either single mutant. In these mice, however, the macroglossia, renal dysplasia, and adrenal cytomegaly commonly found in BWS patients were missing. Overexpression of IGFII has also been achieved in mice carrying Igf2 transgenes (Sun et al. 1997). These animals exhibited overgrowth, polydactyly, and polyhydramnious, all symptoms of BWS patients. Together, these animal models support the hypothesis that BWS results from elevated expression of IGF2.
The impact of loss-of-function mutations in p57Kip2 has also been examined in mice (Yan et al. 1997; Zhang et al. 1997). These mice show abdominal wall defects reminiscent of those seen in Igf2r/H19 double mutants. They also display a unique set of defects such as renal dysplasia and adrenal cytomegaly, defects seen in BWS patients but not observed in mice with elevated IGFII expression. The somatic overgrowth commonly associated with BWS, however, was not observed. In addition, phenotypes not previously associated with BWS, such as lens and gastrointestinal tract abnormalities as well as skeletal defects were present in p57Kip2-null mice.
These mouse models have failed to provide a good explanation for the fact that BWS patients with loss of imprinting of IGF2 and those with mutations in p57KIP2 are phenotypically indistinguishable. Unfortunately, it has not been possible to measure the levels of expression of these genes in the relevant tissues at the relevant times in development, and thus a mechanistic connection between them could have gone undetected. Zhang et al. (1997) suggested that IGFII and p57 may act in opposing manners to control cell proliferation during development of human fetuses; that is, that a gain of function of IGF2 would act similarly to a loss of function of p57KIP2. We reasoned that if IGFII and p57 act antagonistically during development, a double mutant in which both BWS-potentiating mutations are present might exhibit phenotypes that are more severe than the sum of those in the single mutants. Such a mouse strain would mimic patients with UPD of 11p15.5, with respect to IGF2 and p57KIP2. To generate such a mouse, we bred a loss-of-function p57Kip2 mutant to an Igf2 loss-of-imprinting mutant (H19Δ13) and screened for meiotic recombination between these tightly linked genes. The double-mutant mice exhibit aspects of BWS that have not been observed in other mouse models, such as macroglossia. In addition, they show an exacerbation of the placental and kidney dysplasias caused by the p57Kip2 mutation alone. Significantly, we observed that reduced levels of Igf2 can overcompensate for these severe placental and kidney dysplasias, leading us to suggest that the two genes act in an antagonistic manner in some tissues in the mouse.
Results
Generating a p57Kip2/H19 double mutant
The phenotypic consequences of mutations in p57Kip2 and H19 are manifested only when the genes are inherited from mothers. Because these genes lie ∼800 kb apart in the mouse, a double-mutant strain could be generated by meiotic recombination between the existing mutations. Toward that end, we crossed heterozygous p57Kip2+/−; H19Δ13−/+ males to C57BL/6 females and screened progeny for animals that were heterozygous for both p57Kip2 and H19Δ13 (p57H19). Among 481 offspring, we identified two p57H19 recombinants, a frequency consistent with the estimate of the genetic distance between the genes (0.5 cM). One recombinant, a male, was fully viable and fertile and was used to establish the line. The other recombinant, a female, failed to give birth to viable p57H19 mice.
Loss of Igf2 imprinting, and the absence of maternal p57Kip2 and H19 expression were confirmed by RNAse protection and RT–PCR assays. The imprinting and expression of Kvlqt1 was unchanged (data not shown).
Perinatal lethality
Maternal inheritance of a null allele of p57Kip2 is lethal, with 10% of offspring dying in utero and the remainder dying within the first 2 weeks after birth (Zhang et al. 1997). We were unable to recover live p57H19 mice at birth, suggesting that the double-mutant phenotype is more severe. Furthermore, parturition invariably occurred at 19 d.p.c., at least 1 day earlier than in p57Kip2 single mutants. At 18.5 d.p.c., p57H19 embryos were present at approximately the expected frequency (Table 1), suggesting that death occurred during or shortly after delivery.
Table 1.
Genetic crosses
| Cross
|
No. of litters
|
Genotype
|
No. of embryos
|
|---|---|---|---|
| p57H19+/− × C57BL/6 | 24 | 170 | |
| p57H19 | 99 | ||
| +/+ | 71 | ||
| C57BL/6 × p57H19+/− | 7 | 55 | |
| p57H19 | 32 | ||
| +/+ | 23 | ||
| p57H19+/− × Igf2+/− | 10 | 75 | |
| p57H19 | 14 | ||
| p57H19; Igf2+/− | 26 | ||
| Igf2+/− | 15 | ||
| +/+ | 20 | ||
| H19Δ13+/− × C57BL/6 | 4 | 26 | |
| H19Δ13+/− | 12 | ||
| +/+ | 14 | ||
| p57Kip2+/− × C57BL/6 | 4 | 30 | |
| p57Kip2+/− | 16 | ||
| +/+ | 14 | ||
| p57Kip2+/− × Igf2+/− | 2 | 22 | |
| p57Kip2+/− | 2 | ||
| Igf2+/− | 7 | ||
| p57Kip2+/−; Igf2+/− | 6 | ||
| +/+ | 7 |
Embryos of the crosses indicated were genotyped between 12 and 18.5 d.p.c. Female parent indicated first.
Prenatal growth
One of the characteristics of BWS is prenatal somatic overgrowth. It had been shown previously that offspring inheriting the H19Δ13 mutation maternally are born 30% bigger than their wild-type littermates (Leighton et al. 1995; Eggenschwiler et al. 1997). p57Kip2 mutants, on the other hand, display no somatic overgrowth at birth (Yan et al. 1997; Zhang et al. 1997). Although p57H19 embryos were indistinguishable in weight from wild-type littermates at 18.5 d.p.c., they were ∼20% larger at 16.5–17.5 d.p.c. (Fig. 1) just as was seen in the single H19Δ13 deletion at the same time (Eggenschwiler et al. 1997). It is conceivable that the gain in growth rate mediated by the loss of Igf2 imprinting is compromised later in gestation by p57Kip2-induced defects.
Figure 1.
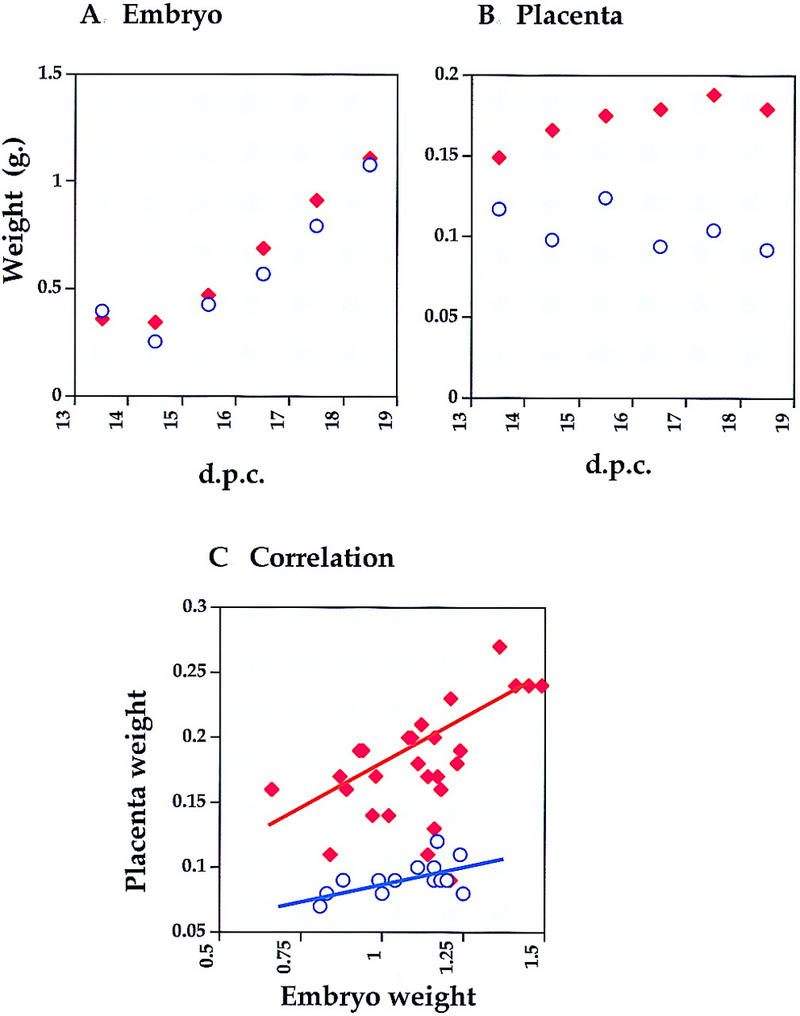
Embryonic growth of p57H19 mutants. p57H19 females were crossed to C57BL/6J males and the wet weights of wild-type (○) and p57H19 (♦) embryos (A) and placentas (B) were determined throughout the last third of gestation. The values are the mean of 9–28 embryos and placentas at each time. (C) Correlation between embryo and placenta weights.
BWS patients often display specific organomegaly, most often affecting the tongue and adrenal glands, and less frequently the liver, kidney, and heart. To distinguish specific organ overgrowth from generalized overgrowth in p57H19 mutant mice, we calculated organ weights at 18.5 d.p.c. as a percentage of total body mass. By this criterion, neither H19Δ13 nor p57Kip2 mutant mice showed specific organ overgrowth (data not shown). In p57H19 offspring, the only organ that displayed significant overgrowth was the tongue, which was 122% of wild type (P < 0.001) (Table 2). If we tabulate those animals whose normalized growth was one standard deviation above the mean of their wild-type littermates, overgrowth of the kidney and heart, as well as overall somatic overgrowth, are observed more frequently in p57H19 mutants than in wild-type littermates (Table 3).
Table 2.
Growth of p57H19 mutant embryos
| Tissue
|
P57H19 (BL/6) n = 20
|
p57H19 (BL/6–129) n = 8
|
Igf2+/− (BL/6–129) n = 10
|
p57H19; Igf2+/− (BL/6–129) n = 19
|
|---|---|---|---|---|
| Embryo | 1.04 | 1.06 | 0.58* | 0.93 |
| Placenta | 1.94* | 1.53* | 0.55* | 1.0 |
| Tongue | 1.22* | 1.11 | 0.86 | 1.00 |
| Heart | 1.03 | 1.17 | 1.03 | 1.16 |
| Kidney | 1.13 | N.D. | N.D. | N.D. |
| Liver | 0.83 | 0.98 | 0.75 | 0.89 |
The wet weights of embryos and internal organs of the genotypes indicated are expressed as a fraction of that of wild type littermates. The p57H19(BL/6) animals were derived from crosses to C57BL/6 males; all others were derived from a p57H19+/− × Igf2+/− cross. (N.D.) Not determined.
P < 0.05.
Table 3.
Summary of phenotypes in BWS animal models
| Phenotypes
|
Percent observance
|
||
|---|---|---|---|
| BWS
|
p57H19
|
+/+
|
|
| Macroglossia | 95 | 60 (12/20) | 11 (1/9) |
| Adrenal defects | |||
| cytomegaly | 94 | 0 | 0 |
| cysts | 69 | 0 | 0 |
| Placentomegaly | 92 | 96 (27/28) | 13 (2/15) |
| Visceromegaly | |||
| hepatomegaly | 85 | 16 (3/19) | 11 (1/9) |
| nephromegaly | 69 | 40 (8/20) | 13 (1/8) |
| cardiomegaly | N.D. | 15 (3/20) | 13 (1/8) |
| Somatic overgrowth | 60 | 39.2 (11/28) | 13 (2/15) |
| Renal dysplasia | 59 | 61.5 (8/13) | 0 |
| Abdominal wall defects | |||
| omphalocele | 60 | 4 (1/23) | 0 |
| umbilical hernia | 32 | 48 (11/23) | 0 |
| Cleft palate | 7.1 | 26 (6/23) | 0 |
| subcutaneous cleft palate | 20 | N.D. | N.D. |
| Cardiac defects | 20 | 39 (5/13) | 0 |
| Polydactyly | 5.5 | 30 (7/23) | 0 |
The frequency of the phenotypes is expressed as a percentage of all individuals examined. The BSW data are adapted from Eggenschwiler et al. (1997). The p57H19 and +/+ data are taken from 18 d.p.c. p57H19 × C57BL/6J litters. (N.D.) Not determined.
Placental defects
The most dramatic overgrowth phenotype observed in p57H19 embryos was placentomegaly, with 18.5-d.p.c. placentas weighing, on average, 190% of those of wild-type littermates (Fig. 1B). On histological analysis, mutant placentas were highly disorganized in the labyrinthine layer, in which both p57Kip2 and Igf2 are expressed. Mutant placentas also displayed fibrin cysts, apoptotic cells, and large accumulations of red blood cells (Fig. 2B). A similar disorganization was seen at 16.5. and 17.5 d.p.c., suggesting that this effect occurs before the normal degeneration of the organ late in gestation. It is unclear whether red blood cells accumulate because the tubule networks through which maternal and fetal blood flow are not established, are breaking down through cell death, or are blocked by surrounding cellular overgrowth. p57Kip2 single-mutant animals showed related morphological placental defects, including reduced vascularization of the labyrinthine zone caused by an overproliferation of trophoblast cells (Fig. 2C). In addition, hyaline membranes, a response to endothelium damage, had been observed and was thought to result in a blockage of the blood supply (Zhang et al. 1998). The p57H19 phenotype that we observed, however, was far more severe.
Figure 2.
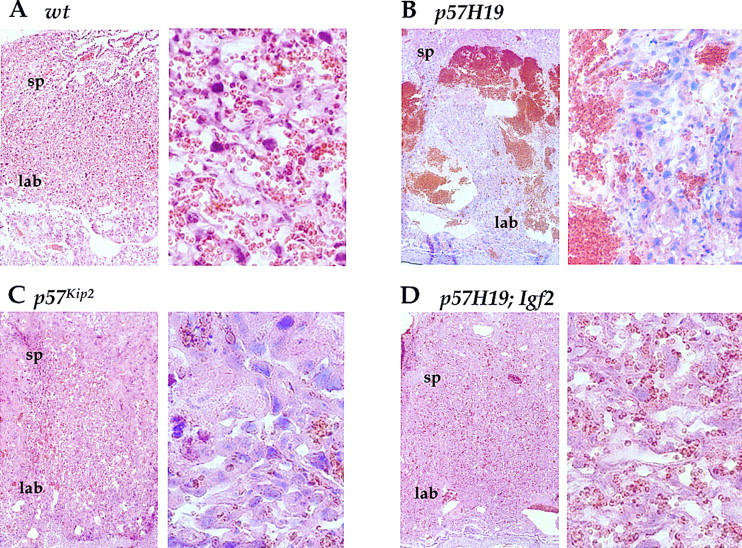
Histological analysis of placental dysplasia in p57H19 mice. Hematoxylin and eosin-stained sections from 18.5-d.p.c placentas generated by crossing p57H19 females to Igf2+/− males (A,B,D) or p57Kip2+/− females to C57BL/6J males (C). The section at right of each group is a magnification of the section at left. (sp) Spongiotrophoblast layer; (lab) labyrinthine layer.
In p57H19 mutants, there was a direct correlation between the placental weights and disruptions in placental cellular architecture, suggesting that the observed placentomegaly is caused primarily by the increase in red blood cell volume. Interestingly, there was actually a modest positive correlation (R = 0.37) between the disorganization of the placenta, as reflected by its weight, and the size of the embryo (Fig. 1C). Thus, the placental dysmorphologies did not compromise fetal growth.
The placental morphology of BWS patients has been examined in a few cases in which disease was anticipated prenatally or following stillbirths or neonatal death. In these cases, placentas contained large cysts in stem villi. Some of the terminal villi in individual cases were filled with blood, and the trophoblast layer was hyperplastic in some, but not all, cases (McCowan and Becroft 1994). Despite the significantly different architectures of the human and mouse placentas, these phenotypes are similar to those we observe in the p57H19 mice.
Kidney dysplasia
Kidneys in p57H19 mice were underdeveloped in the medullary region, in which p57Kip2 is normally expressed. This region normally consists of stromal mesenchymal cells surrounding a duct collection system. In the mutant mice, fewer collection ducts and dilated renal pelves were observed. Kidneys varied from normal to kidneys in which there was no evident medulla or which contained large cystic-looking regions disrupting the medulla (Fig. 3B). In general, the degree of disorganization in the medulla was significantly more pronounced than had been observed in the p57Kip2 single-mutant strain, in which slightly fewer collection ducts, less mesenchymal tissue, and a reduced medulla as well as more stromal cells were reported (Fig. 3C; Zhang et al. 1997).
Figure 3.
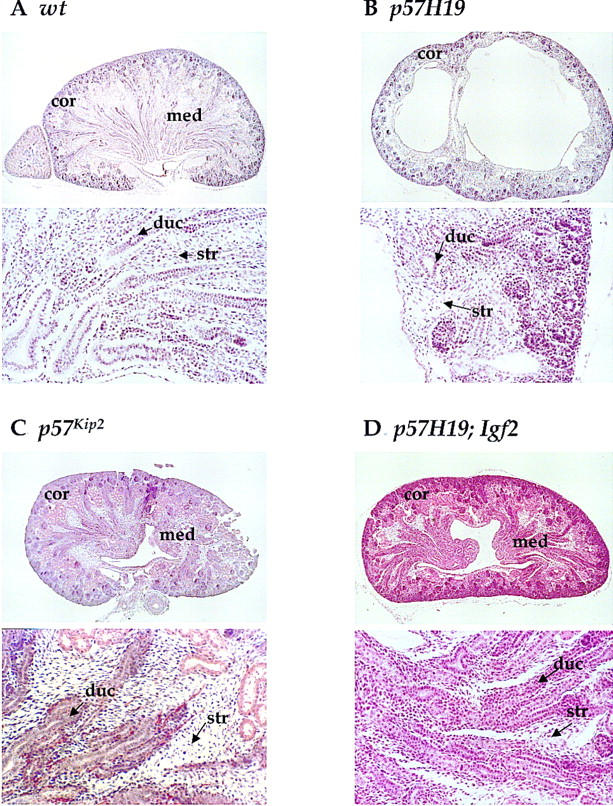
Histological analysis of kidney dysplasia in p57H19 mice. Hematoxylin and eosin-stained sections from 18.5-d.p.c kidneys generated by crossing p57H19 females to Igf2+/− males (A,B,D) or p57Kip2+/− females to C57BL/6J males (C). The bottom section of each panel is a magnification of the top section. (Cor) Cortex; (med) medulla; (duc) collecting duct; (str) stromal cells.
There was a strong correlation between the severity of the placental and kidney phenotypes within individual p57H19 mutant embryos. It is possible that the disorganized placenta, through ineffective nutrient transfer, results in the defects in kidney development. Alternatively, both tissues might independently be sensitized to excess IGFII in the absence of p57Kip2 expression. The variability in the kidney phenotype was observed between animals, not between the two kidneys of a single animal, suggesting that genetic background could play a critical role in its severity.
Renal dysplasia is a common feature of BWS. Specifically, patients with a reduction in the collecting ducts and numerous fluid-filled cysts as well as hypertrophy of the mesenchymal tissue have been reported. In humans, renal dysplasia is thought to result from abnormal metanephric differentiation (Oski et al. 1994). We did not observe hypertrophy in p57H19 kidneys, which may reflect the fact that most BWS kidneys analyzed are from adults, not fetuses. In all other respects, p57H19 dysplastic kidneys appear to mimic most of the aspects of the human disease. In contrast to the kidney dysplasias, we saw no defects in adrenal architecture or size in p57H19 mice. This was surprising in light of the previous finding of adrenomegaly in p57Kip2 mice (Zhang et al. 1997).
Abdominal wall defects
Closure of the ventral abdominal wall in mice occurs around 16.5 d.p.c. and is preceded by the retraction of the midgut into the abdominal cavity. Failure of the intestine to retract results in an umbilical hernia, whereas failure of the abdominal wall to close results in an omphalocele, in which the midgut protrudes from the abdomen. Approximately 60% of BWS patients need surgical correction of omphalocele at birth, and another 32% display umbilical hernias (Table 3). One or the other of these phenotypes was seen in 52% of p57H19 embryos, consistent with the previous report that p57Kip2 mutants display omphalocele as well as body wall muscle dysplasia. Although this condition is not observed in H19Δ13 mutants, it is seen in Igf2r and Igf2r/H19Δ13 double mutants, in which IGFII levels are elevated more than twofold (Filson et al. 1993; Lau et al. 1994; Wang et al. 1994; Eggenschwiler et al. 1997).
Cleft palate and skeletal abnormalities
BWS patients occasionally display cleft palate, a condition that results from the failure of the palatal shelves to elevate, rotate or fuse. Previous studies had detected cleft palate in Igf2r/H19Δ13 as well as in p57Kip2 mutant mice but not in either H19Δ13 or Igf2r mutant mice, implying that either significantly elevated IGFII or reduced p57 could lead to the developmental defect. In p57H19 mutants, the frequency and severity of cleft palate, which was detected in 26% of offspring, was similar to that seen in p57Kip2 single mutants (Table 3), suggesting that the primary cause of cleft palate is loss of p57Kip2 function. It has been proposed that the cleft palate in p57Kip2 mutants results from the failure of p57Kip2 mutant cells to exit the cell cycle and to inappropriately undergo apoptosis (Yan et al. 1997).
Polydactyly has been observed in ∼5% of BWS patients (Table 3). This phenotype has also been observed in mice displaying elevated IGFII, with the frequency and severity increasing with the dosage of IGFII. p57Kip2 single mutants do not display polydactyly, whereas H19Δ13 mice do display postaxial polydactyly 68% of the time on a 129/Sv background (Eggenschwiler et al. 1997). As expected for an Igf2-dependent phenotype, p57H19 double mutants also show postaxial polydactyly in two genetic backgrounds examined (Table 4).
Table 4.
Effect of reducing IGFII in p57H19 mice
| Phenotype
|
Genotype
|
|
|---|---|---|
|
p57H19
|
p57H19; Igf2
|
|
| Postaxial polydactyly | 50 (4/8) | 0 (0/15) |
| Cleft palate | 12.5 (1/8) | 20 (3/15) |
| Omphalocele | 37.5 (3/8) | 20 (3/15) |
| Umbilical hernia | 25 (2/8) | 27 (4/15) |
The frequency of the phenotypes in 18 d.p.c. fetuses are expressed as a percentage of all individuals examined.
The role of Igf2 in the p57H19 phenotype
The dramatic increase in the severity of kidney and placental dysplasia in p57H19 double mutants occurred in animals overexpressing IGFII. Although the somatic overgrowth observed in H19Δ13 mutant animals was genetically shown to be the consequence of the overproduction of IGFII (Leighton et al. 1995), it is possible that the novel defects observed in p57H19 mice are due to effects of the H19 deletion on genes other than Igf2 or possibly even due to the loss of the H19 mRNA itself. If the new phenotypes are due to IGFII overproduction, a reduction in IGFII levels should ameliorate them. To test this possibility, p57H19 females were crossed to Igf2+/− males with a null allele of Igf2 (p57H19; Igf2) (DeChiara et al. 1990). Previous studies had determined that although the expression of Igf2 mRNA from the maternal chromosome in H19Δ13 mice in mesodermal tissues such as skeletal muscle and heart was essentially equivalent to that from the paternal chromosome, its derepression was less pronounced in endodermal tissues such as liver (Leighton et al. 1995). Furthermore, the circulating levels of IGFII were only modestly elevated (Eggenswiler et al.1997). Thus, overall maternal expression of IGFII in p57H19; Igf2 mutants is lower than that from a wild-type paternal chromosome.
At 18.5 d.p.c., triple-mutant fetuses (p57H19; Igf2) were indistinguishable in size from wild-type, or p57H19 fetuses (Table 2). In contrast, Igf2+/− offspring were 58% the size of wild type, as had been observed previously (DeChiara et al. 1990). Thus, in the absence of p57Kip2, prenatal growth was relatively insensitive to the approximately twofold differences in the levels of IGFII between p57H19 and p57H19; Igf2 mice. The complete absence of IGFII in p57Kip2−/+; Igf2+/− double mutants, on the other hand, results in embryos that are the same size as their Igf2+/− littermates (data not shown).
In contrast to the insensitivity of somatic growth to the reduction in Igf2, the placental overgrowth and dysplasia in p57H19 embryos were almost completely suppressed in triple mutants (Fig. 2D; Table 2). That is, the mean weight of placentas did not differ from that of wild-type littermate placentas, but did differ substantially from that of the p57H19 placentas, which were 53% oversized in this cross. On histological examination, triple-mutant placentas were morphologically normal, lacking even the moderate level of disorganization seen in p57Kip2 single mutants. Thus, the placental dysplasia, which is p57Kip2-dependent, can be suppressed by reducing the levels of IGFII.
The same situation appears to hold for the kidney dysplasia. None of the triple mutants had cystic kidneys, and the only discernible differences observed in a few fetuses were a slightly less-developed medulla and a slight increase in mesenchymal tissue between the renal tubules (Fig. 3D). However, in most animals, the kidneys were less affected than in p57Kip2 animals. Finally, the macroglossia observed in the p57H19 mutant was completely absent in the triple mutant, indicating that overgrowth of the tongue is also Igf2 dependent (Table 2). This is consistent with the finding of macroglossia in Igf2 overexpressing transgenic mice (Sun et al. 1997).
The frequency of abdominal wall defects in p57H19 mice was unaffected by reducing the levels of Igf2 expression in p57H19; Igf2 mice (Table 4). This result is somewhat surprising, as omphalocele was induced in the presence of highly elevated levels of IGFII in Igf2r and Igf2r/H19 mutant mice. Thus, in p57H19 mice, this defect appears to be entirely attributable to p57Kip2 loss of function. Likewise the frequency of cleft palate is not affected by a loss of Igf2 in the triple mutant, even though cleft palate was observed in Igf2r/H19 double mutants. Lastly, the postaxial polydactyly that is observed in both p57H19 and H19 mutants was completely rescued by the reduction in Igf2 expression.
Discussion
This study was prompted by the clinical findings in BWS that mutations in p57KIP2 and overexpression of IGF2 have each been proposed as causes of the disease. Yet, the phenotypes of the mouse models for loss of function of p57Kip2 and loss of imprinting of Igf2 are readily distinguished (Leighton et al. 1995; Yan et al. 1997; Zhang et al. 1997). McLaughlin et al. (1996) have shown that mice containing a UPD of a large portion of the distal end of mouse chromosome 7 die at 9.5 d.p.c., presumably as a consequence of the reduced expression of Mash2, a gene that is required for placental development and is maternally expressed (Guillemot et al. 1994, 1995). By creating a mouse model in which only the two candidate genes are affected, we hoped to gain more precise insight into the ways in which misregulation of these two apparently unrelated genes could lead to the same disease.
The p57H19 mice exhibited a dramatic increase in the severity of several BWS phenotypes such as placental overgrowth and dysplasia and kidney defects. Furthermore, macroglossia, one of the hallmarks of BWS, was seen in these mice. A direct interaction between the p57Kip2 and Igf2 pathways is implied by the ability of reduced levels of Igf2 to compensate for the placental and kidney dysplasias that arise from mutations in p57Kip2. Thus, in the absence of p57Kip2, the disorganized development of those tissues is enhanced in an Igf2-dependent manner. When signaling through Igf2 is reduced, the loss of p57Kip2 is no longer detrimental. Thus, our analysis of p57H19 mice suggests a resolution to the dilemma of how both loss-of-function mutations in p57KIP2 and gain-of-function mutations in IGF2 can lead to BWS. That is, in the mouse, the two genes act in an antagonistic manner in a subset of the tissues in which they are coexpressed.
A pathway that is affected by both genes is that which regulates G1 cell cycle progression. Both IGFII and p57 are involved in regulating the progression of cells through the G1/S phase of the cell cycle, with IGFII promoting the G1/S transition (Zhang et al. 1999a) and p57 inhibiting the G1 cyclin-dependent kinases (CDKs) (Lee et al. 1995; Matsuoka et al. 1995). p57 has not been implicated in regulating embryo size, but is involved in the cell cycle arrest that precedes terminal differentiation of tissues such as skeletal muscle, lens, and placenta (Zhang et al. 1997, 1998, 1999b). IGFII, on the other hand, is a direct regulator of fetal growth, and has been shown to promote progression through the G1 phase of the cell cycle, possibly through its ability to increase the level of the G1 cyclin D1 (Zhang et al. 1999a). The decision to proceed through the G1-to-S phase transition is controlled by the ratio of cyclin/CDK complexes to CKIs, which determines the overall activity of G1-cyclin/CDK complexes. It is possible that the exacerbation of the p57Kip2 phenotype in the presence of excess IGFII and its alleviation when the concentration of the growth factor is reduced reflects cell-type-specific sensitivity to CDK activity in placenta and kidney. Excess IGFII in the absence of p57Kip2 could lead to hyperproliferation, as seen in the placenta, to increased apoptosis, which is observed in both p57Kip2 and p57H19 mutant placentas and kidneys, or to a failure to differentiate, as is suggested by the reduction of medullary cells in the kidney. Increased apoptosis has also been reported in the palatal shelves of p57Kip2 single mutants, and presumably results from alterations in the orderly progression through cell cycle checkpoints (Yan et al. 1997).
Several p57Kip2-dependent phenotypes such as cleft palate and omphalocele are neither enhanced by overexpression of Igf2, nor rescued by its reduction. It may be that the insensitivity of cleft palate and omphalocele phenotypes to changes in IGFII levels reflects the fact that these tissues regulate the CDK-to-CKI levels by use of growth factors other than IGFII. On the other hand, both cleft palate and omphalocele are observed in the most severe IGFII gain-of-function mouse model, the Igf2r/H19 double mutant, in which IGFII levels were 7- to 11-fold higher than normal (Eggenschwiler et al. 1997). These animals also displayed the lens abnormalities and skeletal defects that were seen in the p57Kip2 mutants but are not associated with BWS. Thus, it may be that in the presence of p57Kip2, very high levels of IGFII are required to alter the CDK-to-CKI balance.
One of the surprising findings in this study was that the somatic overgrowth that has been shown to be Igf2-dependent in H19Δ13 mice was less pronounced in p57H19 mice. We considered the possibility that the lack of somatic overgrowth was due to the failure of the dysplastic placenta to provide nutrients to the embryo in the later stages of gestation. However, the subset of p57H19 mice in which the placenta was relatively normal were not oversized at birth (data not shown). Furthermore, there was a modest positive correlation between the degree of placental hyperplasia and the size of the embryos in general (Fig. 1C). The fact that p57Kip2 mutants are reduced in size in the presence of a null mutation in Igf2 argues that the p57Kip2 mutation does not completely desensitize the embryo to changes in IGFII concentration. Rather, it may be that the approximately twofold changes in IGFII expression between p57Kip2, p57H19, and p57H19; Igf2 mice do not shift the ratios of CDKs to CKIs sufficiently to effect a change in overall growth rate. Of the Cip/Kip family of CKIs, only p27Kip1 has been directly implicated in overall fetal growth (Deng et al. 1995; Fero et al. 1996; Kiyokawa et al. 1996; Nakayama et al. 1996).
Our results suggest a model in which p57 and IGFII act antagonistically in the control of cell proliferation and development in several tissues affected in BWS patients including the tongue, kidney, and placenta. The affected tissues in the mouse are those in which some rate-limiting step is controlled by the two growth regulators, whereas other tissues, like the liver, presumably utilize other positive or negative growth signals. Although the nature of these other pathways are unknown, IGFI has been shown to function redundantly with IGFII to promote cell growth (Efstratiadis 1998). Likewise, several instances of redundancy between CDK inhibitors have been observed. For example, p21 and p57 are redundant for control of muscle and lung development (Zhang et al. 1999b); p27 and p57 are redundant for control of lens development (Zhang et al. 1998); and p18 and p27 work together to control pituitary gland, spleen, and thymus embryonic growth (Franklin et al. 1998). We propose that BWS phenotypes are observed when the overall balance of regulators is shifted in favor of proliferation by either an increase in IGFII, or a decrease in p57. A shift in the balance of other regulators with differing tissue specificities would presumably produce a phenotype distinct from that of BWS.
The fact that the p57H9 double mutant does not completely recapitulate the BWS phenotype may reflect species-specific differences in the tissues in which the two genes act directly in opposition to one another. Furthermore, because the changes in the cell number in an organ due to a change in the rate of proliferation is an exponential function, the more rounds of cell division a tissue undergoes, the greater will be the effect of a small increase in proliferation rate. Because human organs such as the tongue, kidney, and placenta undergo significantly more cell division, a relatively small change in proliferation rates afforded by p57 loss or increased IGFII may have a more pronounced effect in humans relative to the mouse. It is worth noting that this mouse model would predict that BWS patients with UPD would exhibit more severe defects than patients with different genetic defects. This has not been observed, however, presumably because UPDs are often mosaic.
The variability in the phenotypes in the p57H19 mice is reminiscent of the highly variable phenotypes of BWS patients (Table 3). The mice in this study were not on completely inbred backgrounds, and at least some of the variability we observed could be attributed to genetic modifiers. Another explanation for the variability in the human syndrome is suggested by the curious clinical finding that 10 sets of female identical twins have been reported who are discordant for BWS (Clayton-Smith et al. 1992). Although the high level of discordancy is not understood, it has been suggested that disruptions in epigenetic mechanisms such as X-inactivation and imprinting might explain the occurrence, an explanation that could extend to sporadic cases as well.
In conclusion, the analysis of the defects in p57H19 mice demonstrates that some, but not all, tissues are highly sensitive to the ratio of p57 and IGFII. Perturbations in the levels of either protein may be sufficient to generate the variable range of phenotypes in BWS.
Materials and methods
Generation of the p57H19 double knockout
The mutant mice used in this study have been described previously (DeChiara et al. 1991; Leighton et al. 1995; Zhang et al. 1997). Male p57Kip2+/−/H19Δ13+/− heterozygotes were crossed to C57BL/6J females and the offspring genotyped for the two mutations by Southern blot analysis of tail DNA as described previously (Leighton et al. 1995; Zhang et al. 1997). For genotyping by PCR, the yolk sac or tail DNA was digested overnight at 55°C with 10 μg of proteinase K in buffer containing 50 mm KCl, 10 mm Tris-Cl (pH 8.3), 2 mm MgCl2, 0.45% NP40, and 0.45% Tween 20. The reaction was boiled for 10 min and centrifuged in the microfuge for 10 min. The conditions for H19 and Igf2 PCR reactions have been described previously (Louvi et al. 1997; Caspary et al. 1998). The p57Kip2 mutation was detected by PCR under the following conditions: 94°C for 30 sec; 55°C for 58 sec; 72°C for 60 sec for 35 cycles followed by one cycle of 72°C for 4 min in buffer containing 15 mm MgCl2, 60% sucrose, and 0.15 mm cresol red. The p57Kip2 primers were as follows: forward primer, 5′-GCTGCTAAAGCGCATGCTC-3′ and reverse primer, 5′-AGTTCTCTTGCGCTTG-3′.
Growth analysis
Mice were mated, and noon of the day that the vaginal plug was observed corresponded to 0.5 d.p.c. Embryos and placentas were collected and weighed, and fixed in 4% paraformaldehyde overnight. They were washed successively in saline, 50% ethanol, and 70% ethanol. Organs were dissected from embryos and weighed, with excess liquid removed on absorbent paper.
Histological analysis
Organs, placentas, or 12.5 d.p.c. embryos were embedded in paraffin, and cut in 10-μm sections. The sections were stained with hematoxylin and eosin and examined by a pathologist in a single blind manner.
Acknowledgments
We thank Dr. Argiris Efstratiadis for the Igf2 mutant mice. This work was supported by a grant from the National Institute for General Medical Sciences (NIH) to S.M.T. S.M.T. and S.J.E. are Investigators of the Howard Hughes Medical Institute. P.Z. is supported by a Faculty Research Grant from the Howard Hughes Medical Institute and by a grant from the Colorado Cancer League, Inc.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
Corresponding author.
E-MAIL stilghman@molbio.princeton.edu; FAX (609) 258-3345.
References
- Brown KW, Villar AJ, Bickmore W, Clayton-Smith J, Catchpole D, Maher ER, Reik W. Imprinting mutation in the Beckwith-Wiedemann syndrome leads to biallelic IGF2 expression through an H19-independent pathway. Hum Mol Genet. 1996;5:2027–2032. doi: 10.1093/hmg/5.12.2027. [DOI] [PubMed] [Google Scholar]
- Caspary T, Cleary MA, Baker CC, Guan X-J, Tilghman SM. Multiple mechanisms regulate imprinting of the mouse distal chromosome 7 gene cluster. Mol Cell Biol. 1998;18:3466–3474. doi: 10.1128/mcb.18.6.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton-Smith J, Read AP, Donnai D. Monozygotic twinning and Wiedemann-Beckwith syndrome. Am J Med Genet. 1992;42:633–637. doi: 10.1002/ajmg.1320420440. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A. Genetics of mouse growth. Int J Dev Biol. 1998;42:955–976. [PubMed] [Google Scholar]
- Eggenschwiler J, Ludwig T, Fisher P, Leighton PA, Tilghman SM, Efstratiadis A. Mouse mutant embryos overexpressing IGF-II exhibit phenotypic features of the Beckwith-Wiedemann and Simpson-Golabi-Behmel syndromes. Genes & Dev. 1997;11:3128–3142. doi: 10.1101/gad.11.23.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias ER, DeBaun MR, Feinberg AP. Beckwith-Wiedemann Syndrome. In: Jameson JL, editor. Principles of molecular medicine. Totowa, NJ: Humana Press; 1998. pp. 1047–1052. [Google Scholar]
- Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- Filson A, Louvi A, Efstradiatis A, Roberston EJ. Rescue of the T-associated maternal effect in mice carrying null mutations in Igf-2 and Igf2r, two reciprocally imprinted genes. Development. 1993;118:731–736. doi: 10.1242/dev.118.3.731. [DOI] [PubMed] [Google Scholar]
- Franklin DS, Godfrey VL, Lee H, Kovalev GI, Schoonhoven R, Chen-Kiang S, Su L, Xiong Y. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes & Dev. 1998;12:2899–2911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoukakis N, Deal C, Paquette J, Goodyer CG, Polychronakos C. Parental genomic imprinting of the human gene. Nat Genet. 1993;4:98–101. doi: 10.1038/ng0593-98. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL. Essential role of Mash-2 in extraembryonic development. Nature. 1994;371:333–336. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Caspary T, Tilghman SM, Copeland NG, Gilbert DJ, Jenkins NA, Anderson DJ, Joyner AL, Rossant J, Nagy A. Genomic imprinting of Mash-2, a mouse gene required for trophoblast development. Nat Genet. 1995;9:235–241. doi: 10.1038/ng0395-235. [DOI] [PubMed] [Google Scholar]
- Hatada I, Mukai T. Genomic imprinting of p57/KIP2, a cyclin-dependent kinase inhibitor, in mouse. Nat Genet. 1995;11:204–206. doi: 10.1038/ng1095-204. [DOI] [PubMed] [Google Scholar]
- Hatada I, Inazawa J, Abe T, Nakyama M, Kaneko Y, Jinno Y, Niikawa N, Ohashi H, Fukushima Y, Iida K, Yutani C, Takahashi S, Chiba Y, Ohishi S, Mukai T. Genomic imprinting of human p57KIP2 and its reduced expression in Wilms' tumors. Hum Mol Genet. 1996a;6:783–788. doi: 10.1093/hmg/5.6.783. [DOI] [PubMed] [Google Scholar]
- Hatada I, Ohashi H, Fukushima Y, Kanetko Y, Inoue M, Komoto Y, Okada A, Ohishi S, Nabetani A, Morisaki H, et al. An imprinted gene p57KIP2 is mutated in Beckwith-Wiedemann syndrome. Nat Genet. 1996b;14:171–173. doi: 10.1038/ng1096-171. [DOI] [PubMed] [Google Scholar]
- Henry I, Bonalti-Pellie C, Chehensse V, Beldjord C, Schwartz C, Utermann G, Junien C. Uniparental paternal disomy in a genetic cancer-predisposing syndrome. Nature. 1991;351:665–667. doi: 10.1038/351665a0. [DOI] [PubMed] [Google Scholar]
- Hoovers JM, Kalikin LM, Johnson LA, Alders M, Redeker B, Law DJ, Bliek J, Steenman M, Benedict M, Wiegant J. Multiple genetic loci within 11p15 defined by Beckwith-Wiedemann syndrome rearrangement breakpoints and subchromosomal transferable fragments. Proc Natl Acad Sci. 1995;92:12456–12460. doi: 10.1073/pnas.92.26.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Koufos A, Grundy P, Morgan K, Aleck KA, Hadro T, Lampkin BC, Kalbakji A, Cavenee W. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989;44:711–719. [PMC free article] [PubMed] [Google Scholar]
- Lam WW, Hatada I, Ohishi S, Mukai T, Joyce JA, Cole TR, Donnai D, Reik W, Schofield PN, Maher ER. Analysis of germline CDKN1C (p57KIP2) mutations in familial and sporadic Beckwith-Wiedemann syndrome (BWS) provides a novel genotype/phenotype correlation. J Med Genet. 1999;7:518–523. [PMC free article] [PubMed] [Google Scholar]
- Lau MMH, Stewart CEH, Liu Z, Bhatt H, Rotwein P, Stewart CL. Loss of the imprinted Igf2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes & Dev. 1994;8:2953–2963. doi: 10.1101/gad.8.24.2953. [DOI] [PubMed] [Google Scholar]
- Lee JE, Pintar J, Efstratiadis A. Pattern of the insulin-like growth factor II gene expression during early mouse embryogenesis. Development. 1990;110:151–159. doi: 10.1242/dev.110.1.151. [DOI] [PubMed] [Google Scholar]
- Lee MH, Reynisdottir I, Massagué J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes & Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- Lee MP, Hu R, Johnson LA, Feinberg AP. Human KVLQT1 gene shows tissue-specific imprinting and encompasses Beckwith-Wiedemann syndrome chromosomal rearrangements. Nat Genet. 1997;15:181–185. doi: 10.1038/ng0297-181. [DOI] [PubMed] [Google Scholar]
- Lee MP, DeBaun MR, Mitsuya K, Galonek HL, Brandenburg S, Oshimura M, Feinberg AP. Loss of imprinting of a paternally expressed transcript, with antisense orientation to KVLQT1, occurs frequently in Beckwith-Wiedemann syndrome and is independent of insulin-like growth factor II imprinting. Proc Natl Acad Sci. 1999;96:5203–5208. doi: 10.1073/pnas.96.9.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton PA, Ingram RS, Eggenschwiler J, Efstratiadis A, Tilghman SM. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature. 1995;375:34–39. doi: 10.1038/375034a0. [DOI] [PubMed] [Google Scholar]
- Louvi A, Accili D, Efstratiadis A. Growth promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Dev Biol. 1997;189:33–48. doi: 10.1006/dbio.1997.8666. [DOI] [PubMed] [Google Scholar]
- Mannens M, Hoovers JM, Redeker E, Verjaal M, Feinberg AP, Little P, Boavida M, Coad N, Steenman M, Bliek J, et al. Parental imprinting of human chromosome region 11p15.3-pter involved in the Beckwith-Wiedemann syndrome and various human neoplasia. Eur J Hum Genet. 1994;2:3–23. doi: 10.1159/000472337. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Edwards MC, Bai C, Parker S, Zhang P, Baldini A, Harper JW, Elledge SJ. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes & Dev. 1995;9:650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Thompson JS, Edwards MC, Barletta JM, Grundy P, Kalikin LM, Harper JW, Elledge SJ, Feinberg AP. Imprinting of the gene encoding a human cyclin-dependent kinase inhibitor, p57KIP2, on chromosome 11p15. Proc Nat Acad Sci. 1996;93:3026–3030. doi: 10.1073/pnas.93.7.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan LME, Becroft DMO. Beckwith-Wiedemann Syndrome, placental abnormalities, and gestational proteinuric hypertension. Obstet Gynecol. 1994;83:813–817. [PubMed] [Google Scholar]
- McLaughlin KJ, Szabo P, Haegel H, Mann JR. Mouse embryos with paternal duplication of an imprinted chromosome 7 region die at midgestation and lack placental spongiotrophoblast. Development. 1996;122:265–270. doi: 10.1242/dev.122.1.265. [DOI] [PubMed] [Google Scholar]
- Mitsuya K, Meguro M, Lee MP, Katoh M, Schulz TC, Kugoh H, Yoshida MA, Niikawa N, Feinberg AP, Oshimura M. LIT1, an imprinted antisense RNA in the human KvLQT1 locus identified by screening for differentially expressed transcripts using monochromosomal hybrids. Hum Mol Genet. 1999;8:1209–1217. doi: 10.1093/hmg/8.7.1209. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Ohlsson R, Nystrom A, Pfeifer-Ohlsson S, Tohonen V, Hedborg F, Schofield P, Flam F, Ekstrom TJ. IGF2 is parentally imprinted during human embryogenesis and in the Beckwith-Wiedemann syndrome. Nat Genet. 1993;4:94–97. doi: 10.1038/ng0593-94. [DOI] [PubMed] [Google Scholar]
- O'Keefe D, Dao D, Zhao L, Sanderson R, Warburton D, Weiss L, Anyane-Yeboa K, Tycko B. Coding mutations in p57KIP2 are present in some cases of Beckwith-Wiedemann syndrome but are rare or absent in Wilms tumors. Am J Hum Genet. 1997;61:295–303. doi: 10.1086/514854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oski FA, DeAngelis CA, Feigin RD, McMillan JA, Warshaw JB. Principles and practice of pediatrics. Philadelphia, PA: J.B. Lippincott Company; 1994. [Google Scholar]
- Paulsen M, Davies KR, Bowden LM, Villar AJ, Franck O, Fuermann M, Dean WL, Moore TF, Rodrigues N, Davies KE, et al. Syntenic organization of the mouse distal chromosome 7 imprinting cluster and the Beckwith-Wiedemann syndrome region in chromosome 11p15.5. Hum Mol Genet. 1998;7:1149–1159. doi: 10.1093/hmg/7.7.1149. [DOI] [PubMed] [Google Scholar]
- Ping AJ, Reeve AE, Law DJ, Young MR, Boehnke M, Feinberg AP. Genetic Linkage of Beckwith-Wiedemann syndrome to 11p15. Am J Hum Genet. 1989;44:720–723. [PMC free article] [PubMed] [Google Scholar]
- Reik W, Brown KW, Schneid H, LeBouc Y, Bickmore W, Maher ER. Imprinting mutations in the Beckwith-Wiedemann syndrome suggested by an altered imprinting pattern in the IGF2-H19 domain. Hum Mol Genet. 1995;4:2379–2385. doi: 10.1093/hmg/4.12.2379. [DOI] [PubMed] [Google Scholar]
- Smilinich NJ, Day CD, Fitzpatrick GV, Caldwell GM, Lossie AC, Cooper PR, Smallwood AC, Joyce JA, Schofield PN, Reik W, et al. A maternally methylated CpG island in KvLQT1 is associated with an antisense paternal transcript and loss of imprinting in Beckwith-Wiedemann syndrome. Proc Natl Acad Sci. 1999;96:8064–8069. doi: 10.1073/pnas.96.14.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F-L, Dean W, Kelsey G, Allen ND, Reik W. Transactivation of Igf2 in a mouse model of Beckwith-Wiedemann syndrome. Nature. 1997;389:809–815. doi: 10.1038/39797. [DOI] [PubMed] [Google Scholar]
- Turleau C, deGrouchy J, Chjavin-Colin F, Martelli H, Voyer M, Charlas R. Trisomy 11p15 and Beckwith-Wiedemann syndrome: A report of two cases. Hum Genet. 1984;67:219–221. doi: 10.1007/BF00273006. [DOI] [PubMed] [Google Scholar]
- Wang Z-Q, Fung MR, Barlow DP, Wagner EF. Regulation of embryonic growth and lysosomal targeting by the imprinted Igf2r/Mpr gene. Nature. 1994;372:464–467. doi: 10.1038/372464a0. [DOI] [PubMed] [Google Scholar]
- Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- Weksberg R, Shen DR, Fei YL, Song QL, Squire J. Disruption of insulin-like growth factor 2 imprinting in Beckwith-Wiedemann syndrome. Nat Genet. 1993a;5:143–150. doi: 10.1038/ng1093-143. [DOI] [PubMed] [Google Scholar]
- Weksberg R, Teshima I, Williams BR, Greenberg CR, Pueschel SM, Chernos JE, Fowlow SB, Hoyme E, Anderson IJ, Whiteman DA. Molecular characterization of cytogenetic alterations associated with the Beckwith-Wiedemann syndrome (BWS) phenotype refines the localization and suggests the gene for BWS is imprinted. Hum Mol Genet. 1993b;2:549–556. doi: 10.1093/hmg/2.5.549. [DOI] [PubMed] [Google Scholar]
- Yan Y, Frisén J, Lee M-H, Massagué J, Barbacid M. Ablation of the CDK inhibitor p57KIP2 results in increased apoptosis and delayed differentiation during mouse development. Genes & Dev. 1997;11:973–983. doi: 10.1101/gad.11.8.973. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liégeois NJ, Wong C, Finegold M, Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA, Elledge SJ. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann sydrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ. Cooperation between the Cdk inhibitors p27KIP1 and p57KIP2 in the control of tissue growth and development. Genes & Dev. 1998;12:3162–3167. doi: 10.1101/gad.12.20.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kim M, Choi YH, Goemans B, Yeung C, Hu Z, Zhan S, Seth P, Helman LJ. Diminished G1 checkpoint after gamma-irradiation and altered cell cycle regulation by insulin-like growth factor II overexpression. J Biol Chem. 1999a;274:13118–13126. doi: 10.1074/jbc.274.19.13118. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wong C, Liu D, Finegold M, Harper JW, Elledge SJ. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes & Dev. 1999b;13:213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]


