INTRODUCTION
In a given year, approximately 2.4 million American adults, or about 1.1% of the population 18 years of age and older, have schizophrenia.1,2 Men and women are affected with equal frequency. A severe, chronic brain disorder, schizophrenia is characterized by an onset in early adulthood, a lifelong course, debilitating symptoms, deterioration in functional ability, and lack of social acceptability, making it among the most disabling, and economically catastrophic disorders.2 Symptoms appear in men in their late teens or early 20s, whereas women are generally affected in their 20s or early 30s.3 In the U.S., annual direct and indirect costs of the disorder total more than $62 billion.4
Four symptom clusters have been observed in large groups of patients.5 Positive symptoms (psychosis) are correlated with hospital admission and a first-time diagnosis. Delusions are the most common psychotic symptom and occur in 65% of patients with schizophrenia.6,7 Hallucinations and disorganized thinking occur in approximately 50% of these patients. Auditory hallucinations are the most common sensory disturbance, but visual, tactile, olfactory, and gustatory hallucinations may occur.8 Most patients experience a combination of delusions, hallucinations, and thought disorganization.6
Negative symptoms represent a loss of affective responsiveness, verbal expression, personal motivation, enjoyment, social drive, or attention to the environment.9 All cognitive domains are also affected by the disorder, including attention, language, memory, and executive function.10 The combination of blunted, inappropriate, and odd expressions is the most common affective disturbance in schizophrenia.11 Depressive symptoms are the most common mood disturbance in schizophrenia.12
In 2002, direct annual health care costs were estimated at $22.7 billion for the U.S., $7.6 billion in other direct costs, and $32.4 billion in lost economic productivity and caregiver costs, for a total of $62.7 billion.4 Schizophrenia accounts for 2.5% of all health care expenditures, 25% of hospital beds, and 20% of all Social Security benefit days. 13 Genetic and environmental risk factors contribute to the development of schizophrenia, with alterations in neurotransmitter pathways (e.g., with dopamine) and enlarged sulci and ventricles found on brain imaging.14
CHEMISTRY AND PHARMACOLOGY
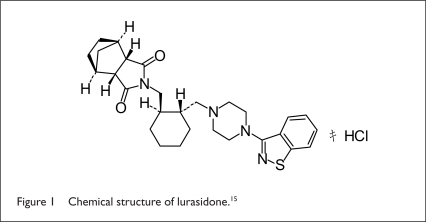
Lurasidone HCl (Latuda, Sunovion), an atypical antipsychotic tablet, belongs to the chemical class of benzoisothiazol derivatives and is indicated for the treatment of patients with schizophrenia. The drug’s chemical name is (3aR,4S,7R,7aS)-2-{(1R,2R)-2-[4-(1,2-benzisothiazol-3-yl piperazin-1-ylmeth yl]cyclohexylmethyl} hexahydro-4,7-methano-2H-isoindole-1, 3-dione HCl. The molecular formula is C28H36N4O2S • HCl, and the molecular weight is 529.14 (Figure 1).
Figure 1.
Chemical structure of lurasidone.15
This white to off-white powder is slightly soluble in water, practically insoluble or insoluble in 0.1 N HCl, slightly soluble in ethanol, sparingly soluble in methanol, practically insoluble or insoluble in toluene, and only slightly soluble in acetone.15
Lurasidone acts as an antagonist at dopamine type-2 (D2) and 5-hydroxytryptamine (5-HT2A) receptors. It also has moderate antagonistic activity at alpha2C- and alpha2A-adrenergic receptors and is a partial agonist at 5-HT1A. receptors.
This medication exhibits minimal or no affinity for histamine type-1 (H1) or muscarinic type-1 (M1) receptors.15
PHARMACOKINETICS AND PHARMACODYNAMICS
Lurasidone works primarily as a result of the activity of the parent drug. Its pharmacokinetic properties are dose-proportional within a total dose of 20 to 160 mg/day. Steady-state concentrations are reached within seven days of initiation of therapy. The elimination half-life of the parent compound is approximately 18 hours.
Peak serum concentrations (Cmax) and absorption occur in approximately one to three hours. From 9% to 19% of the administered dose is absorbed. Following administration of a 40-mg dose, the mean apparent volume of distribution is 6,173 (17.2) L. The drug is highly bound (approximately 99%) to serum proteins. According to the manufacturer, patients were instructed to take lurasidone with food (consisting of a minimum of 350 calories) in a clinical trial. The Cmax and area-under-the-curve (AUC) concentration of lurasidone, when consumed with food, were about three times and two times, respectively, the levels observed under fasting conditions.15
Lurasidone is metabolized extensively in the liver mainly via cytochrome P450 (CYP) isoenzyme 3A4. Biotransformation pathways are oxidative N-dealkylation, hydroxylation of the norbornane ring, and S-oxidation. Lurasidone is metabolized into two active metabolites (ID-14283 and ID-14326) and two major non-active metabolites (ID-20219 and ID-20220). The total excretion of radioactivity in urine and feces, combined, was approximately 89%. In clinical trials, 80% was recovered in feces and 9% was recovered in urine. The mean (%CV) apparent clearance was 3,902 (18) mL/minute after a dose of lurasidone 40 mg.15
CLINICAL TRIALS
Four six-week, placebo-controlled studies have shown efficacy in the treatment of schizophrenia among adults whose mean age was 38.8 years (range, 18–72 years of age). To evaluate assay sensitivity, researchers conducted a study that included an active-control arm of patients who received olanzapine (Zyprexa, Eli Lilly). Instruments used to assess psychiatric signs and symptoms in these studies included:
Positive and Negative Syndrome Scale (PANSS). This tool is used to evaluate the effects of drug treatment in schizophrenia (score range, 30–210 points).
Brief Psychiatric Rating Scale (BPRSd) (derived from the PANSS). This multi-item inventory focuses primarily on positive schizophrenia symptoms (score range, 18–126 points).
Clinical Global Impression Severity Scale (CGI–S). This validated clinician-rated scale is used to measure the subject’s current state of illness (score range, 1–7 points).
Primary outcome variables were the change from baseline to week 6 in BPRSd scores in two studies and the change from baseline to week 6 in PANSS total scores in two other studies.15
In study 1 (n = 145), patients received either fixed doses of lurasidone 40 mg or 120 mg or placebo once daily. At six weeks, the 40-mg and 120-mg doses were found to be superior to placebo in improving BPRSd total scores from baseline (mean difference from placebo, −5.6 and −6.7, respectively; P < 0.05).
In study 2 (n = 180), patients received lurasidone 80 mg or placebo once daily. At six weeks, the 80-mg dose was observed to be superior to placebo in improving BPRSd total scores from baseline (mean difference from placebo, −4.7; P < 0.05).
In study 3 (n = 473), patients received fixed doses of lurasidone 40 mg or 120 mg, olanzapine 15 mg (in active controls), or placebo once daily. At six weeks, both doses surpassed placebo in improving PANSS total scores from baseline (mean difference from placebo, −9.7 and −7.5, respectively; P < 0.05).
In study 4 (n = 489), patients received fixed doses of lurasidone 40 mg, 80 mg, and 120 mg or placebo once daily. At six weeks, only the 80-mg dose was superior to placebo (P < 0.05). The mean difference from placebo on the PANSS total score was −2.1, −6.4 and −3.5 for 40 mg, 80 mg, and 120 mg, respectively. A dose of 120 mg/day provided no additional benefit beyond a dose of 40 mg/day.
For all four studies, adverse effects occurring at a rate of 5% or greater, and at least twice the rate of placebo, included somnolence, akathisia, nausea, parkinsonism, and agitation (Table 1).15
Table 1.
Primary Efficacy Endpoints: Results From Four Placebo-Controlled Studies
| Study No. | Primary Endpoint | Least Squares Mean (SE) Difference from Placebo in Change From Baseline | |||
|---|---|---|---|---|---|
| Lurasidone 40 mg/day | Lurasidone 80 mg/day | Lurasidone 120 mg/day | Olanzapine 15 mg/day | ||
| 1 | BPRSd | −5.6* (2.1) | — | −6.7* (2.2) | — |
| 2 | BPRSd | — | −4.7*(1.8) | — | — |
| 3 | PANSS | −9.7* (2.9) | — | −7.5* (3.0) | −12.6* (2.8) |
| 4 | PANSS | −2.1 (2.5) | −6.4* (2.5) | −3.5 (2.5) | |
Adjusted P value, < 0.05 (except for olanzapine).
BPRSd = Brief Psychiatric Rating Scale, derived; PANSS = Positive and Negative Syndrome Scale; SE = standard error.
Data from Latuda product information, 2010.15
DRUG INTERACTIONS
Lurasidone should be used with caution when taken with alcohol and other drugs that affect the central nervous system (CNS). Lurasidone is metabolized primarily by CYP3A4, and interactions of this drug with strong and moderate inhibitors or inducers of this isoenzyme have been observed (Table 2); therefore, the drug should not be used with strong inhibitors or inducers of CYP3A4. However, interactions are unlikely to occur with drugs that are inhibitors or inducers of CYP1A1, CYP1A2, CYP2A6, CYP4A11, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP2E1 enzymes. Dose adjustments are not needed when lurasidone is taken with digoxin (Lanoxin, GlaxoSmithKline), midazolam (Versed, Roche), or oral contraceptives (estrogen/progesterone).15
Table 2.
Effect of Coadministered Drugs on Exposure to Lurasidone in Healthy Subjects and in Patients with Schizophrenia
| Effect on Pharmacokinetics | |||||
|---|---|---|---|---|---|
| Coadministered Drug | Dose Schedule | Lurasidone | Cmax | AUC | Recommendation |
| Ketoconazole (strong CYP3A4 inhibitor) | 400 mg/day for 5 days | 10 mg, single dose | 6.9 times that of lurasidone alone | 9 times that of lurasidone alone | Should not be coadministered with lurasidone |
| Diltiazem (moderate CYP3A4 inhibitor) | 240 mg/day for 5 days | 20 mg, single dose | 2.1 times that of lurasidone alone | 2.2 times that of lurasidone alone | Lurasidone dose should not exceed 40 mg/day if coadministered |
| Rifampin (strong CYP3A4 inducer) | 600 mg/day for 8 days | 40 mg, single dose | 1/7 of lurasidone alone | 1/5 of lurasidone alone | Should not be coadministered with lurasidone |
| Lithium | 600 mg twice daily for 8 days | 120 mg/day for 8 days | 0.9 times that of lurasidone alone | 1.1 times that of lurasidone alone | No dose adjustment required |
AUC = area-under the curve concentration; Cmax = peak concentration; CYP = cytochrome P450.
Data from Latuda product information, 2010.15
DOSAGE AND ADMINISTRATION
For adults with schizophrenia, the recommended initial dose is lurasidone 40 mg orally daily with food. Initial dose titration is not required. The maximum recommended daily dose is 80 mg. In patients with moderate and severe renal impairment (creatinine clearance [CrCl], 10–50 mL/minute) and for those with moderate and severe hepatic impairment (Child–Pugh class B and C), the lurasidone dose should not exceed 40 mg/day. No dosage adjustments are necessary in the geriatric population.
WARNINGS
A boxed warning is included in the prescribing information for lurasidone to reflect an increased mortality rate in elderly patients with dementia-related psychosis. In an analysis of 17 placebo-controlled trials (modal duration, 10 weeks) in patients taking atypical antipsychotic drugs, the risk of death in these patients was between 1.6 times and 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week randomized controlled trial, mortality rates were 4.5% for treated patients and 2.6% for the placebo group. Most deaths appeared to be either cardiovascular (heart failure or sudden death) or infectious (pneumonia). As with similar atypical antipsychotic medications, lurasidone is not approved for the treatment of dementia-related psychosis.15
ADVERSE REACTIONS
During clinical trials, the most common adverse reactions noted in patients receiving lurasidone were somnolence, akathisia, nausea, parkinsonism, and agitation. Serious adverse effects included agranulocytosis, seizure, suicidal thoughts, and syncope.
Other reactions observed during the premarketing evaluation of lurasidone included anemia, leukopenia, neutropenia, tachycardia, first-degree atrioventricular block, angina pectoris, bradycardia, vertigo, and blurred vision.15 Also noted were abdominal pain, diarrhea, dysphagia, gastritis, sudden death, elevated creatine phosphatase levels, decreased appetite, rhabdomyolysis, tardive dyskinesia, cerebrovascular accident, dysarthria, syncope, and neuroleptic malignant syndrome. Patients also experienced abnormal dreams, panic attacks, sleep disorder, dysuria, renal failure, amenorrhea, dysmenorrhea, breast enlargement and breast pain, galactorrhea, erectile dysfunction, rash, pruritus, hyperprolactinemia, angioedema, hypertension, and orthostatic hypotension.15
CONTRAINDICATIONS AND PRECAUTIONS
Lurasidone HCl is contraindicated in patients using strong CYP3A4 inhibitors and inducers and for those with a hypersensitivity to lurasidone HCl or any components in the formulation. The drug should be used with caution in elderly patients with dementia-related psychosis because of an increased risk of death.
The white blood cell (WBC) count should be monitored periodically, because agranulocytosis, leukopenia, and neutropenia have been reported during clinical trials. Additional precautions include cardiovascular or cerebrovascular disease or conditions that predispose patients to hypotension; cerebrovascular adverse events, including fatalities, that have been reported in association with antipsychotic agents in elderly patients with dementia. Dysregulation of body temperature; development of diabetes mellitus or risk factors for diabetes mellitus, owing to a potential for an increased risk of severe hyperglycemia; an increased risk of tardive dyskinesia, especially in elderly women; and aspiration pneumonia, resulting from potential esophageal dysmotility and aspiration caused by the drug, have also been documented.15
MONITORING REQUIREMENTS
During the first few months of therapy, the manufacturer recommends obtaining a complete blood count with differential in patients with a pre-existing low WBC count or a history of drug-induced leukopenia or neutropenia. Patients should be monitored regularly if they have diabetes mellitus or worsening of glucose control. In patients with risk factors for diabetes, such as obesity and a family history, fasting blood glucose testing should be performed at baseline and periodically thereafter. Orthostatic vital signs should be monitored in patients taking lurasidone, especially if they are susceptible to hypotension. Monitoring of body weight is recommended, and patients at a high risk for suicide should be closely monitored during therapy.
PREGNANCY AND BREAST-FEEDING
The FDA has classified lurasidone as a Pregnancy Category B agent, although no adequate or well-controlled studies of the drug’s use have been conducted during pregnancy. Animal studies have shown no evidence of teratogenicity, but exposure to antipsychotic medications during the third trimester has been associated with extrapyramidal and withdrawal symptoms in neonates. The manufacturer recommends that lurasidone be used during pregnancy only if the maternal benefit justifies the fetal risk.
No lactation studies of lurasidone have been performed in humans, and it is not clear whether the drug is excreted in human milk. It is known that lurasidone is excreted in the milk of lactating rats; therefore, the manufacturer recommends that mothers not breast-feed while they are taking this medication. Lurasidone is recommended for nursing mothers only if the potential maternal benefit outweighs the risk to the nursing infant.15
P&T COMMITTEE CONSIDERATIONS
Lurasidone (Latuda) is a novel atypical antipsychotic agent that was approved in October 2010 by the FDA for the treatment of schizophrenia; it is also being studied for the treatment of bipolar disorder. Efficacy data for lurasidone have been evaluated for doses of 20 mg to 120 mg daily; the 20-mg/day dose has been found to be less effective than higher doses in improving schizophrenia symptoms. Based on clinical trial results, the 40-mg/day dose is considered optimal in the outpatient setting, whereas the 80-mg/day dose is more effective in the inpatient setting.
Nausea, vomiting, akathisia, dizziness, and sedation are the most common adverse events associated with lurasidone. Although the newer atypical antipsychotic agents can cause metabolic disturbances and metabolic syndrome, studies of lurasidone have shown a minimal risk for the development of the syndrome, no significant increase in the corrected QT (QTc) interval, and only slight elevations in prolactin levels.
In addition to lurasidone’s affinity for the dopamine D2 and 5-HT2A receptors, the drug has the highest affinity for the 5-HT7 receptor. Experimental data support the theory that antagonism of 5-HT7 can improve cognition, memory, and mood symptoms. Lurasidone has shown a much greater affinity for 5-HT1A and alpha2-adrenergic receptors, which have been implicated in improving cognition and in alleviating negative mood and related symptoms, such as depression and anxiety.
Clinicians might consider lurasidone when making a formulary decision because of its favorable metabolic profile, low incidence of extrapyramidal symptoms, low risk of QTc prolongation, and a minimal potential risk of nausea, vomiting, sedation, and dizziness. The receptor-binding profile may provide lurasidone with a unique place in the treatment of schizophrenia and bipolar disorder, although longer-term studies are needed to further explore these ideas.
CONCLUSION
Schizophrenia is a major public health problem and a burden on families and the community. It tends to be a lifelong disorder requiring multimodal treatment at all stages of illness. With the cause of the illness still unknown, current treatments focus on eliminating symptoms with antipsychotic medications and various psychosocial treatments. With long-term use, the older, typical antipsychotic drugs have often led to tardive dyskinesia. The newer second-generation (atypical) antipsychotic drugs have recently been blamed for serious side effects such as agranulocytosis and metabolic syndrome.
The efficacy of lurasidone, an atypical antipsychotic agent, was established in four six-week controlled studies of adults with schizophrenia. The agent’s effectiveness beyond six weeks has not been clarified in controlled studies. Therefore, the use of this product for an extended length of time should be periodically reevaluated in each patient.
Footnotes
Disclosure: The author reports no financial or commercial relationships in regard to this article.
REFERENCES
- 1.U.S. Census Bureau, Population, Division Estimates by Demographic Characteristics. Jun 9, 2005. Available at www.census.gov/popest/national/asrh/nc-est2004/nc-est2004-02.xls. Accessed May 25, 2011.
- 2.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, D.C: American Psychiatric Association; 1994. p. 285. [Google Scholar]
- 3.Robins LN, Regier DA, editors. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. New York: The Free Press; 1991. [Google Scholar]
- 4.Wu EQ, Birnbaum HG, Shi L. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry. 2005;66:1122–1129. doi: 10.4088/jcp.v66n0906. [DOI] [PubMed] [Google Scholar]
- 5.Lindenmayer JP, Bernstein-Hyman R, Grochowski S. Five-factor model of schizophrenia: Initial validation. J Nerv Ment Dis. 1994;182:631. doi: 10.1097/00005053-199411000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Breier A, Berg PH. The psychosis of schizophrenia: Prevalence, response to typical antipsychotics, and prediction of outcome. Biol Psychiatry. 1999;46:361. doi: 10.1016/s0006-3223(99)00040-2. [DOI] [PubMed] [Google Scholar]
- 7.Applebaum PS, Robbins PC, Roth LH. Dimensional approach to delusions: Comparison across types and diagnoses. Am J Psychiatry. 1999;156:1938. doi: 10.1176/ajp.156.12.1938. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura T, Okazaki Y, Fujinawa A, et al. Dimensions of schizophrenic positive symptoms: An exploratory factor analysis investigation. Eur Arch Psychiatry Clin Neurosci. 1998;248:130. doi: 10.1007/s004060050029. [DOI] [PubMed] [Google Scholar]
- 9.Andreasen NC. Negative symptoms in schizophrenia: Definition and reliability. Arch Gen Psychiatry. 1982;39:784. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 10.Saykin AJ, Gur RC, Gur RE, et al. Neuropsychological function in schizophrenia: Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48:618. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- 11.Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: A methodological review. Clin Psychol Rev. 2002;22:789. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch SR, Jolley A, Barnes T. Are depressive symptoms part of the schizophrenic syndrome? In: DeLisi L, editor. Depression in Schizophrenia. Washington, D.C: American Psychiatric Press; 1990. p. 25. [Google Scholar]
- 13.Meltzer HY. Outcome in schizophrenia: Beyond symptom reduction. J Clin Psychiatry. 1999;60(Suppl 3):3. [PubMed] [Google Scholar]
- 14.Seeman P. Atypical antipsychotics: Mechanism of action. Can J Psychiatry. 2002;47:27. [PubMed] [Google Scholar]
- 15.Latuda (lurasidone), product information. Fort Lee, N.J.: Sunovion; 2010. [Google Scholar]