Abstract
Maturational differences in brain responsiveness to rewards have been implicated in the increased rates of injury and death in adolescents from behavior-related causes. However, much of this morbidity is related to drug intoxication or other externalizing behaviors, and may be concentrated in a subset of adolescents who are at psychosocial or neurobiological risk. To examine whether individual differences in psychosocial and behavioral symptomatology relate to activation of motivational neurocircuitry, we scanned 26 psychiatrically healthy adolescents using fMRI as they performed a monetary incentive delay task. Overall Problem Density on the Drug Use Screening Inventory (DUSI-OPD) correlated positively with activation of ventral mesofrontal cortex (mFC) during anticipation of responding for rewards (vs responding for no incentive). In addition, DUSI-OPD correlated positively with right ventral striatum recruitment during anticipation of responding to win rewards (vs responding for no incentive or to avoid losses of identical magnitudes). Finally, a psychophysiological interaction (PPI) analysis indicated that increased connectivity between nucleus accumbens and portions of anterior cingulate and mFC as a function of reward prospects also correlated with DUSI-OPD. These findings extend previous reports demonstrating that in adolescents, individual differences in reactivity of motivational neurocircuitry relate to different facets of impulsivity or externalizing behaviors.
Keywords: Reward, Impulsivity, Adolescence, Ventral striatum, Instrumental behavior
1. Introduction
1.1. Neuromaturational differences may account for adolescent vulnerability
Adolescents experience considerable injury due to behavioral causes (U.S. Centers for Disease Control). Hypersensitivity of motivational neurocircuitry to reward-predictive cues or reward deliveries (that is insufficiently checked by immature frontocortical executive control) has been postulated as neurodevelopmental risk factor (Galvan et al., 2006, Somerville et al., 2010, Casey and Jones, 2010). To investigate this possibility, cross-sectional comparisons have compared age-group differences in the responsiveness of mesolimbic incentive neurocircuitry to anticipation and receipt of rewards – specifically in the ventral striatum (VS) and the ventral mesofrontal cortex (mFC), where greater activation of these mesolimbic regions has been interpreted as greater degree of valuation of rewards or of affective reactions to rewards (see below).
1.2. Extant neurodevelopmental difference findings in reward sensitivity are mixed
To study reward processing in humans, functional magnetic resonance imaging (fMRI) paradigms in humans (e.g. Knutson et al., 2001) have been translated from those used in primate electrophysiological studies (Schultz, 2000, Schultz, 2007, Schultz et al., 2003). These experiments show that the VS activates in response to learned, reward-predictive instrumental cues (Knutson et al., 2001, Galvan et al., 2006). In addition, ventral mFC responds to reward deliveries (Knutson et al., 2003), and has activated as a final integrator of the expected or experienced value of several kinds of rewards – such as foods (Hare et al., 2009), odors (Rolls et al., 2010), small-immediate vs larger-delayed rewards (Kable and Glimcher, 2007), and perceived probability of winning money (Knutson and Cooper, 2005). The VS features significant anatomical (Haber and Knutson, 2010, Sesack and Grace, 2010) and functional (Cauda et al., 2011) connectivity with the mFC, in addition to significant connectivity with other limbic frontocortical structures such as insula and perigenual anterior cingulate cortex (ACC), which are frequently recruited by prospective (especially uncertain) rewards.
Extant neuroimaging findings on mesolimbic reward-processing differences between adolescents and other age groups, however, show mixed directionality. An initial comparison between healthy adolescents and young adults who performed a monetary incentive delay (MID) reaction-time task showed that compared to adults, adolescents had reduced activation of right nucleus accumbens (NAcc) by cues to respond for explicit amounts of money (Bjork et al., 2004), with no age difference in recruitment of mFC by reward deliveries. A subsequent study where subjects chose between cartoon images for an unspecified amount of money revealed greater VS recruitment by rewards in adolescents compared to adults and younger children (Galvan et al., 2006).
In gambling tasks, adolescents showed greater VS recruitment compared to younger adults by anticipation of rewards in a simulated slot-machine gambling task (Van Leijenhorst et al., 2010b), and when choosing a risky response option in a roulette-wheel like probability task (Van Leijenhorst et al., 2010a). Similarly, Ernst et al. (2005) reported greater VS sensitivity adolescent to reward deliveries and omissions in the wheel-of-fortune task. In a decision-making learning task with stochastic payoff probabilities for correct choices, adolescents showed an exaggerated VS response to unexpected reward deliveries and omission of expected rewards (i.e. prediction errors), as well as a greater limbic response to reward deliveries in general compared to younger children and adults (Cohen et al., 2010). However, a second developmental comparison using the MID task with a larger sample and improved methodology replicated the modest decrement in right VS recruitment by reward predictive cues in adolescents compared to adults (Bjork et al., 2010b). We suspect these divergent findings resulted from differences in stimuli, learning, or other behavioral contingencies between the tasks used (discussed in Bjork et al. (2010b)), as well as from design emphasis on the anticipatory vs consummatory components of the instrumental behavior (Geier et al., 2010).
1.3. Differences between adolescents in brain reward-responsiveness may also be critical
Age-dependent differences in reward processing, while important and intriguing in their own right, however, still leave a large amount of variance unexplained. An interesting complimentary question, then, is whether individual differences in brain recruitment by reward-predictive cues also relate to (or even regulate) individual differences in proclivity for risky behaviors or emotional reactivity. Individual differences in reward sensitivity (or its neural instantiation) among adolescents likely has public health implications in that a substantial portion of adolescent death and injury is attributed specifically to behaviors with a high hedonic or psychosocial payoff, such as speeding, violence, or intoxication with alcohol or other drugs, especially in social contexts. With regard to reward anticipation, persons with overly robust activation of motivational neurocircuitry by signals in the environment that a potential reward is available may be more prone to pursuing the reward despite potential risks associated with that reward (where either the generation or the invocation of a mental representation of the potential downside of the behavior is insufficient). With regard to reward consummation or delivery of rewards, individuals with overactive limbic (hedonic) processing or reward deliveries (such as drugs or alcohol) may be at risk for continued or escalating pursuit of that behavior.
Increased reward sensitivity in children at risk has been suggested by laboratory behavior. Subjects diagnosed with externalizing disorders like attention-deficit hyperactivity disorder (ADHD) or conduct disorder (CD) in childhood show increased behavioral (Newman and Wallace, 1993, Fonseca and Yule, 1995, Matthys et al., 1998, Matthys et al., 2004, Lane and Cherek, 2001, Fairchild et al., 2009) and VS (Bjork et al., 2010a) sensitivity to deliveries of rewards or reduced sensitivity to punishments in laboratory tasks. In the real world, persons with these histories also show more reckless driving habits (Barkley et al., 1993, Fischer et al., 2007), and are at increased risk for abuse of drugs or alcohol (Fergusson and Horwood, 1995, Fergusson et al., 2007, Pardini et al., 2007) at follow-up. For example, young adult motorists with childhood histories of ADHD were more likely to suffer traffic accidents, speeding violations, and injuries at follow-up assessment compared to control subjects, where subjects with comorbid CD had the worst motor vehicle outcomes (Barkley et al., 1993).
In essence, it seems likely that among adolescents injured or killed due to behavioral causes, there is an over-representation of individuals with some combination of reward-sensitive or loss-insensitive neurocognitive traits. Notably, we found that among psychiatrically healthy adolescents, high scorers on a brief measure of sensation-seeking (e.g. willingness to bungee jump) showed greater recruitment of the VS by cues to respond to win rewards in another variant of the MID task (Bjork et al., 2008). Similarly, Buckholtz et al. (2010) reported that in healthy young adults, both recruitment of VS by MID reward cues (fMRI) and phasic dopamine responses to rewards in the VS (positron emission tomography) correlated positively with impulsive and antisocial behavior as indexed by the Pychopathic Personality Inventory (PPI) (Lilienfeld and Andrews, 1996).
1.4. Hypotheses
Of interest for this special issue is an exploration of whether individual differences in mesolimbic responses to cues for reward opportunities relate broadly to risk symptomatology (actual psychosocial problems) in adolescents. Would teens with greater levels of risk symptomatology show increased activation of VS at the prospect of a reward, or increased connectivity between the VS and other frontocortical limbic structures when anticipating rewards? We administered the Drug Use Screening Inventory (DUSI) (Tarter, 1990) to 26 adolescents who did not meet DSM-IV criteria for psychiatric disorders and did not report significant drug or alcohol use. The DUSI is a binary endorsement of 149 behavioral and psychosocial symptoms, including potentially rewarding behaviors, such as: “Have you stolen things?”, “Did you do risky or dangerous things a lot?” or “Did you like to play drinking games when you went to parties?” The adolescents then underwent functional magnetic resonance imaging (fMRI) scanning while performing the MID task.
We hypothesized that a tally of psychosocial problems endorsed on the DUSI would correlate positively with VS or mFC responses to reward-predictive cues. Because NAcc activity may reflect the integration of cognitive valuation of prospective incentives (mFC), together with the affective components of incentives (anterior cingulate cortex and insula), we also hypothesized that by extension, DUSI-OPD scores would correlate with the degree of dynamic NAcc-frontocortical connectivity in the presence of an impending reward – as indexed by a psychophysiological interaction (PPI) effect with the NAcc (as the “seed” structure).
2. Methods
2.1. Subject characterization
Recruitment and testing procedures were approved by the NIAAA IRB, and adolescents participated with written informed assent, with parent or guardian consent. Adolescent subjects (n = 26, 13 males) age 12–17 (mean 14.8) were community-recruited, and deemed medically healthy per medical histories, and physical exam. No subject had a history of chronic psychotropic medication use, and none endorsed significant substance use. No subject met DSM-IV criteria for a psychiatric disorder in either self- or parent-interviews of the Diagnostic Interview for Children and Adolescents (DICA) (Reich, 2000). All but two subjects took part in a recent developmental comparison experiment (Bjork et al., 2010b), where scanning methods, task behavior and brain activation by the MID task contrasts are described in detail. As part of applicant screening, adolescents self-completed the DUSI. Because the most reward-relevant items were distributed across several domains, we related to brain signal the Overall Problem Density (DUSI-OPD) score, which is the total fraction of questionnaire symptoms endorsed.
2.2. Monetary incentive delay task
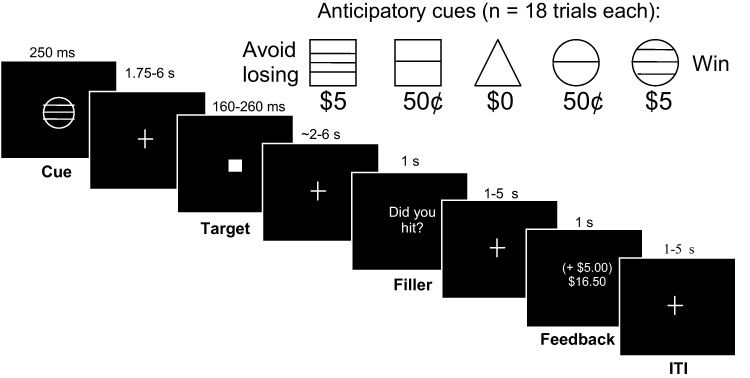
In each MID task trial (Fig. 1), one of the five anticipatory cues was presented. Circle cues (n = 18 trials each) signaled that if the subject responded during the subsequent target presentation, he or she would win money. Square cues (n = 18 trials each) warned that the subject would lose money if he or she did not respond to the subsequent target while it was presented. Triangle cues (n = 18 trials) signaled non-incentive trials, where target hits or misses would not alter winnings, but subjects were instructed to respond anyway. After a variable interval, the target (a white square) was presented. After another variable interval, a filler stimulus, “Did you hit?” was presented, in order to maintain attention to the task until the trial feedback. This was then followed by notification of whether the subject had won or lost money during that trial, along with his or her cumulative earnings. The MID task was administered in three 7-min runs. Prior to scanning, subjects were shown an envelope containing the cash they could win, and were read an instruction script that clarified the anticipatory cues and consequences. Subjects then were administered a practice session, where reaction times were covertly measured to custom-prescribe target durations for the scan task, such that each subject would succeed in roughly two-thirds of trials.
Fig. 1.
Monetary incentive delay (MID) task. Each of the 90 trials began with the presentation of one of the five anticipatory cues. The cue signaled the opportunity to either win money (circle series), avoid losing money (square series), or win/lose no money (triangle) by recording a button press while the following white square target was presented on the screen. After target presentation, the subject then waited a variable delay until notification of whether he or she hit the target, during which a lexical filler stimulus was presented. Intervals between trial stimuli were varied as indicated, and trials were also separated by a 1–5 s variable intertrial interval.
2.3. fMRI acquisition and analysis
Full details are in (Bjork et al., 2010b). In a T2-weighted echoplanar sequence during the MID task, we collected 16 contiguous 5-mm-thick slices with TR = 1 s. These slices were in the saggital plane, and were centered on the intrahemispheric fissure, so as to capture all mesial gray matter and subcortical brain structures, bounded by the lateral extent of the putamen. A T1-weighted MP-RAGE structural scan was then acquired for coregistration of functional data. Blood oxygen-level dependent (BOLD) signal was analyzed as follows: individual brain slice images were time-shifted to simulate simultaneous slice acquisition, then compiled into volumes. Volumes were then warped into Talairach stereotactic space, corrected for head motion, and spatially smoothed to a uniform 8 mm full-width half maximum.
Each subject's time series was modeled with canonical hemodynamic responses to anticipatory cues, where responses to targets, trial notification events, and residual head motion were also modeled as regressors of no interest. In addition, auto-correlation of time series data within-voxel was controlled using AFNI module 3dREMLfit. Modeled hemodynamic responses were scaled so that beta weights would be equivalent to percent-BOLD signal-change. We calculated activation by three task contrasts (to correlate with DUSI-OPD): (1) high and low reward cues vs non-incentive cues, (2) high and low loss avoidance cues vs non-incentive cues, and (3) reward-anticipatory cues vs loss-anticipatory cues, to index a motivational bias toward obtaining rewards over avoiding losses of equal magnitude. Trial outcome events (specifically misses) were too few to confidently relate to an external measure.
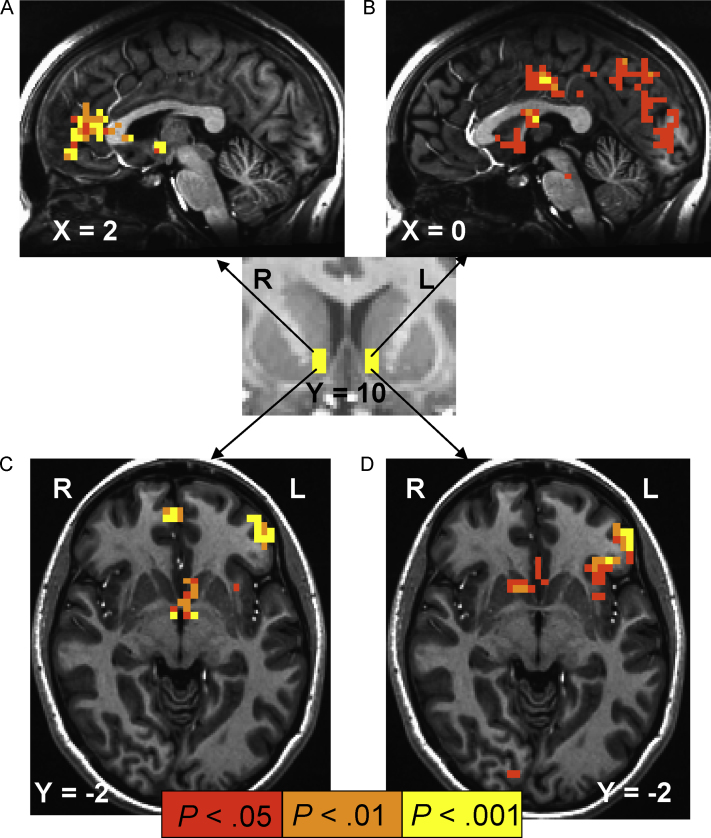
In addition, we performed a psychophysiological interaction (PPI) analysis (Gitelman et al., 2003) to obtain an estimate of the increase in connectivity between the NAcc and other frontocortical structures as a function of presence vs absence of a prospective reward (i.e. circle cues vs triangle cues). In the PPI analysis for individual subjects, the NAcc was anatomically localized as the seed whose time series was deconvolved to obtain the neuronal events, and then to create the regressor of interaction between the NAcc region and the contrast of presence and absence of reward. The groupwise analysis for the interaction was performed initially, by taking the individual interaction effect estimates and their variances to a mixed-effects meta analysis with 3dMEMA (http://afni.nimh.nih.gov/sscc/gangc/MEMA.html) in AFNI. Here, we analyzed frontocortical synchrony of time-series BOLD signal between reward and neutral conditions with that of a “seed” volume-of-interest (placed anatomically) in the bilateral NAcc (Fig. 3, inset). Finally, DUSI-OPD scores were then added to the PPI group analysis as an explanatory variable. In this way, we could determine where in the brain differential time-series activity (as the contrast between reward vs non-reward anticipation) was synchronized with that of NAcc – as a function of individual differences in DUSI-OPD scores.
Fig. 3.
Areas showing a correlation between behavior problem density and reward-dependent connectivity with nucleus accumbens (NAcc). The right and left NAcc were anatomically localized (inset) as the seed masks for a psychophysiological interaction (PPI) analysis. Each of the right and left NAcc time series was deconvolved to obtain the neuronal events, and then to create the regressor of interaction between the NAcc region and the contrast of the presence of prospective reward (cues to win $5 and 50¢) vs absence of reward (cues to win or lose nothing). This interaction effect can be interpreted as either showing structures where the NAcc modulates the effect of reward anticipation relative to non-incentive conditions, or alternatively where the reward stimulus condition (relative to neutral) modulates the connectivity with NAcc. DUSI-OPD scores were entered into the group-wise model as a continuous covariate to determine whether this interaction effect with NAcc was a function of individual differences in risk symptoms. Illuminated voxel clusters survive family-wise error-rate correction to P < .05, and show where right (A and C) and left (B and D) NAcc interaction effects with reward prospects correlated positively with DUSI-OPD scores.
Voxelwise correlations between DUSI-OPD scores and either contrast activations or the PPI interaction were performed using AFNI plug-in 3dMEMA. Correlations are reported in the VS uncorrected as the a priori structure of interest, due to its consistent recruitment by the MID task (Knutson et al., 2001, Bjork et al., 2008, Bjork et al., 2010b). Across the remainder of scan coverage, voxelwise correlations between DUSI-OPD and either simple task contrast activations or the PPI effect are reported only where clusters survived family-wise error-rate correction (AFNI plug-in 3dClustSim) to a corrected P < .05.
3. Results
3.1. Correlations between DUSI-OPD scores and contrast-based activations
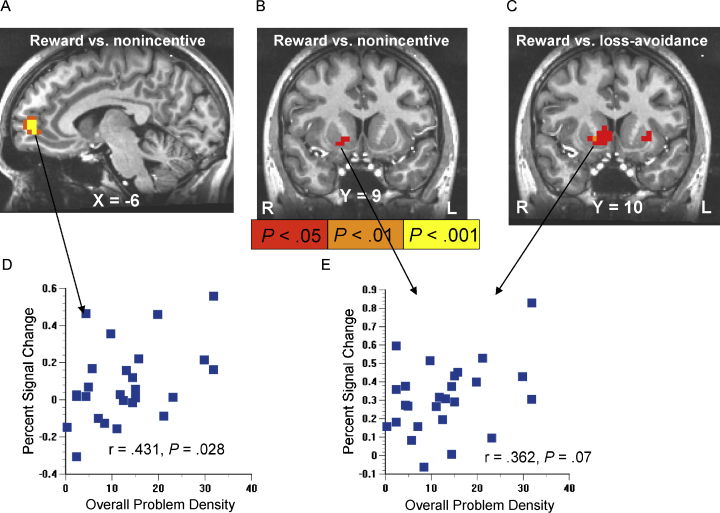
DUSI-OPD scores ranged from 0 to 31.5 (mean 12.5) and did not correlate with age or differ by sex. DUSI-OPD correlated positively with activation by the reward-vs-nonincentive anticipation contrast in anteroventral mFC (Brodmann area 10; Fig. 2A). In voxels in the right putamen (lateral VS), DUSI-OPD scores correlated with activation by the reward-vs-nonincentive anticipation contrast (Fig. 2B), and also with activation by the reward-vs-loss-avoidance anticipation contrast (Fig. 2C). There were no significant voxelwise correlations between DUSI-OPD and anticipation of potential losses vs nonincentive.
Fig. 2.
Correlations between behavior problem density and activation by reward anticipation. The underlay is the structural image from a representative subject, with the Talairach coordinate of the image plane indicated. Illuminated voxels illustrate where activation by reward anticipation in the MID correlated with Drug Use Screening Inventory-total Problem Density (DUSI-OPD) scores. Correlations are shown after family-wise error rate correction in the anterior mesofrontal cortex (A), and uncorrected in the anterior ventral striatum (VS) as the a priori structure of interest (B and C). Peak modeled signal change elicited by the high-reward cues themselves correlated with DUSI-OPD in the mFC (D), with a trend toward a correlation in right VS (E).
We then qualitatively compared which anticipatory cue types (singly) drove these illuminated correlations between contrast-based activations and DUSI-OPD. First, peak post-cue modeled signal change was averaged by trial type, and extracted from a mask placed at the center of the mFC correlation cluster. This indicated that the mFC activation correlation with DUSI-OPD resulted specifically from individual differences in signal change elicited by both the high (r = .431, P = .028; Fig. 2D) and low (r = .412, P = .037)1 reward cues, but not by individual differences in signal change elicited by the non-incentive cues. Similarly, we extracted peak post-cue signal change from the right ventral putamen by averaging across a two-voxel mask, where these two voxels were a shared-edge conjunction of the correlation maxima voxels of the DUSI-OPD by reward vs nonincentive contrast, and the DUSI-OPD by reward vs loss-avoidance contrast. There was a trend for individual differences in activation by high reward cues to correlate with DUSI-OPD (r = .362, P = .07; Fig. 2E). In contrast, DUSI-OPD scores did not significantly correlate with ventral putamen signal change by the other four cue types.
3.2. Psychophysiological Interaction (PPI) of reward vs nonreward conditions and NAcc
The core PPI group-wise analysis indicated several regions that demonstrated increased connectivity with the NAcc as a seed region interacting with the presence vs absence of reward – including posterior mFC, anterior mFC, orbitofrontal cortex, mesial and later occipital cortex, as well as midbrain and VS voxels contralateral to the respective right and left NAcc seeds (Supplemental Fig. 1). Inclusion of DUSI-OPD scores as a continuous covariate revealed that DUSI-OPD scores correlated positively with the magnitude of the reward-dependent PPI effects of right NAcc on mFC (Fig. 3A), and left NAcc on posterior cingulate and mesial occipital cortices (Fig. 3B). DUSI-OPD scores also correlated positively with the effect of reward-dependent signal change of each NAcc seed on the contralateral VS, and on the left insula extending into left orbitofrontal cortex (Fig. 3C and D). Put simply, reward-anticipatory signal change in the aforementioned structures was significantly accounted for by the differential effects of reward vs nonreward prospects on NAcc activation, where this variance capture itself correlated positively with DUSI-OPD scores.
4. Discussion
4.1. Main findings
In healthy adolescents, an omnibus tally of psychosocial problems correlated positively with recruitment of the VS by cues to respond for rewards, and this symptomatology also correlated with an approximation of right NAcc connectivity with frontocortical structures – as a function of the presence (vs absence) of prospective rewards. The positive directionality of this relationship is similar to what we found between a personality measure of sensation-seeking and reward-anticipation VS recruitment in healthy adolescents (Bjork et al., 2008). This directionality was also evident in other recent reports. In healthy young adults, individual differences in impulsive and antisocial behavior also correlated positively with mesolimbic (especially VS) recruitment by reward-predictive cues in the MID task (Buckholtz et al., 2010), and in a perception paradigm, individual differences in impulsivity correlated positively with attentional capture by reward-linked stimuli (Anderson et al., 2011). In a variant of the MID task with more numerous outcome events to model, adolescents who met DSM-IV criteria for a disruptive behavior disorder (but no substance use disorders) showed elevated VS responsiveness to reward deliveries compared to matched controls (Bjork et al., 2010a).
We note that this positive directionality may differ from experiments that use other incentive paradigms, such as choice tasks where rewards are paired with potential penalties. Findings may also differ depending on whether adolescents with actual substance use disorders (SUD) were tested (Crowley et al., 2010). The recent Crowley paper reported that highly externalizing adolescents with SUD showed blunted responses to rewards. It may be that impulsive or externalizing traits relate to motivational processing differently once an adolescent begins chronic exposure to alcohol and other drugs that may damage mesolimbic neurocircuitry to progressively decrease incentive-related positive affect (Koob and Le Moal, 2008). Notably, self-reported impulsivity correlated negatively with MID task reward anticipation in alcoholics, but not in controls (Beck et al., 2009).
Individual differences in symptomatology also correlated with reward cue-elicited activation of the mFC (as a contrast with cues for non-reward), and also correlated with a measure of connectivity increase between right NAcc and this same region of mFC as a function of prospective reward. We note that this correlated mFC region spatially overlaps foci where BOLD activation has tracked valuation of several rewards – such as foods (Hare et al., 2009), odors (Rolls et al., 2010), and small-immediate vs larger-delayed rewards (Kable and Glimcher, 2007). Moreover, our PPI analyses of the NAcc seed revealed fronto-striatal connectivity consistent with structural connectivity between NAcc and mFC and insula as described in anatomical (Haber and Knutson, 2010, Sesack and Grace, 2010) and functional (Cauda et al., 2011) studies of connectivity in human and non-human primates. The right-lateralization (right NAcc seed connectivity > left NAcc seed connectivity) of striatal connectivity is consistent with findings that anticipatory BOLD signal change in right (but not left) NAcc correlates with self-reported positive affect elicited by reward-predictive cues (e.g. Knutson et al., 2001, Bjork et al., 2004). These findings collectively suggest that psychosocial symptomatology relates both to increased valuation of prospective rewards, as well as to increased mobilization of attentional and motor effector circuitry to prepare for and execute the instrumental responses (instantiated in VS, specifically the ventral putamen).
4.2. Study limitations
This MID task variant was intended to isolate hemodynamic responses to anticipatory cues, which were presented on average every 16 s. This slower pace resulted in an insufficient number of trial outcome events (especially misses) for analysis, since actual hits and misses bifurcated the five trial types. Second, this sample was comprised of controls, only six of whom were at risk for substance abuse problems by virtue of a DUSI-OPD score ≥ 20 (Tarter, personal communication). From a public health standpoint, adolescents with clinically significant neurocognitive or affective symptomatology are at greater risk of addiction, injury, and death. It may be that a more severe sample would show greater effects. Third, it cannot be assumed that psychometric correlations with mesolimbic recruitment by abstract secondary rewards like money will generalize to recruitment by potential primary rewards like sex, food, alcohol or other psychotropic drugs.
Finally, we note that the DUSI is primarily a psychosocial screening instrument, and is not organized around specific neuropsychological constructs, such as “hot” (emotion-laden) impulsivity or “cold” (purely cognitive) impulsivity. For example, item content pertaining to theft is mixed in the same domain as psychosis. However, we think it noteworthy that limbic responses to potential rewards would correlate with such a broad metric of psychosocial and behavioral symptoms.2 This may have resulted from a non-specific greater emotional reactivity in high DUSI-OPD scorers. This finding also reflects the strong correlations found in epidemiological surveys between internalizing symptomatology, externalizing symptomatology, work or school problems, substance use or abuse, negative peer relations, and unstable or aversive familial environments – all of which are indexed in DUSI domains.
4.3. Summary and future directions
We found that individual differences in a tally of psychosocial and behavioral problems correlated positively with recruitment of mesolimbic incentive neurocircuitry by cues for rewards, and this tally also correlated with NAcc-mediated signal change in cortical structures as a function of reward opportunity. While correlation should not imply causality per se, the data nonetheless raise the possibility that increased engagement in problematic behaviors in some adolescents may partly result from mesolimbic sensitivity to reward-predictive cues. Considering the findings of maturational differences in the behavioral (Figner et al., 2009) and neurophysiological (Galvan et al., 2006, Bjork et al., 2010b, Casey and Jones, 2010) manifestations of incentive processing, as well maturational differences in brain recruitment by behavior-control (Bjork et al., 2007, Eshel et al., 2007), it is tempting to speculate that increased mesolimbic sensitivity as a trait may interact with the general immaturity of the adolescent brain – to partly explain behavior-related injury or death in “at-risk” adolescents.
Future research could explore how incentive processing in adolescence relates to hot impulsivity as measured by a psychometric instrument (validated for adolescents) that focuses on propensity for impulsive acts committed under highly emotional conditions, such as to terminate an aversive mood state. Crucial too would be longitudinal studies exploring whether individual differences in mesolimbic responsiveness to potential or delivered rewards in early adolescence portends adverse psychosocial outcomes in later adolescence or young adulthood.
Acknowledgement
This research was funded by NIAAA intramural research funding.
Footnotes
Correlation values may be exaggerated due to extraction of signal change data from voxels defined by the correlation between the questionnaire measure and task contrast activation (c.f. Vul et al., 2009). We note, however, that these masks/voxels were localized at coordinates typically activated by MID task rewards.
The individual DUSI domain that assessed the most severe externalizing symptoms like physical fighting, vandalism, and theft was the “psychiatric disorder” domain. The correlations reported in this paper were also substantially evident if the psychiatric disorder domain scores were substituted for the overall problem density, since these two measures were highly correlated (r = .92).
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.dcn.2011.07.005.
Appendix A. Supplementary data
References
- Anderson B.A., Laurent P.A., Yantis S. Value-driven attentional capture. Proc. Natl. Acad. Sci. U.S.A. 2011;108:10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R.A., Guevremont D.C., Anastopoulos A.D., DuPaul G.J., Shelton T.L. Driving-related risks and outcomes of attention deficit hyperactivity disorder in adolescents and young adults: a 3- to 5-year follow-up survey. Pediatrics. 1993;92:212–218. [PubMed] [Google Scholar]
- Beck A., Schlagenhauf F., Wustenberg T., Hein J., Kienast T., Kahnt T., Schmack K., Hagele C., Knutson B., Heinz A., Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol. Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Chen G., Smith A.R., Hommer D.W. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J. Child Psychol. Psychiatry. 2010;51:827–837. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G.W., Caggiano D.M., Bennett S.M., Hommer D.W. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Hommer D.W. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Danube C.L., Hommer D.W. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J. Neurosci. 2007;27:4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz J.W., Treadway M.T., Cowan R.L., Woodward N.D., Benning S.D., Li R., Ansari M.S., Baldwin R.M., Schwartzman A.N., Shelby E.S., Smith C.E., Cole D., Kessler R.M., Zald D.H. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat. Neurosci. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. quiz 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F., Cavanna A.E., D’Agata F., Sacco K., Duca S., Geminiani G.C. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J. Cogn. Neurosci. 2011 doi: 10.1162/jocn.2011.21624. http://www.ncbi.nlm.nih.gov/pubmed/21265603. [DOI] [PubMed] [Google Scholar]
- Cohen J.R., Asarnow R.F., Sabb F.W., Bilder R.M., Bookheimer S.Y., Knowlton B.J., Poldrack R.A. A unique adolescent response to reward prediction errors. Nat. Neurosci. 2010;13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T.J., Dalwani M.S., Mikulich-Gilbertson S.K., Du Y.P., Lejuez C.W., Raymond K.M., Banich M.T. Risky decisions and their consequences: neural processing by boys with Antisocial Substance Disorder. PLoS One. 2010;5:e12835. doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E., Blair J., Pine D.S. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Eshel N., Nelson E.E., Blair R.J., Pine D.S., Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G., van Goozen S.H., Stollery S.J., Aitken M.R., Savage J., Moore S.C., Goodyer I.M. Decision making and executive function in male adolescents with early-onset or adolescence-onset conduct disorder and control subjects. Biol. Psychiatry. 2009;66:162–168. doi: 10.1016/j.biopsych.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson D.M., Horwood L.J. Early disruptive behavior, IQ, and later school achievement and delinquent behavior. J. Abnorm. Child Psychol. 1995;23:183–199. doi: 10.1007/BF01447088. [DOI] [PubMed] [Google Scholar]
- Fergusson D.M., Horwood L.J., Ridder E.M. Conduct and attentional problems in childhood and adolescence and later substance use, abuse and dependence: results of a 25-year longitudinal study. Drug Alcohol. Depend. 2007;88(Suppl. 1):S14–S26. doi: 10.1016/j.drugalcdep.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Figner B., Mackinlay R.J., Wilkening F., Weber E.U. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. J. Exp. Psychol. Learn. Mem. Cogn. 2009;35:709–730. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Fischer M., Barkley R.A., Smallish L., Fletcher K. Hyperactive children as young adults: driving abilities, safe driving behavior, and adverse driving outcomes. Accid. Anal. Prev. 2007;39:94–105. doi: 10.1016/j.aap.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Fonseca A.C., Yule W. Personality and antisocial behavior in children and adolescents: an enquiry into Eysenck's and Gray's theories. J. Abnorm. Child Psychol. 1995;23:767–781. doi: 10.1007/BF01447476. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C.F., Terwilliger R., Teslovich T., Velanova K., Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb. Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman D.R., Penny W.D., Ashburner J., Friston K.J. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Camerer C.F., Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Cooper J.C. Functional magnetic resonance imaging of reward prediction. Curr. Opin. Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Bennett S.M., Adams C.M., Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Le Moal M. Addiction and the Brain Antireward System. Annu. Rev. Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Lane S.D., Cherek D.R. Risk taking by adolescents with maladaptive behavior histories. Exp. Clin. Psychopharmacol. 2001;9:74–82. doi: 10.1037/1064-1297.9.1.74. [DOI] [PubMed] [Google Scholar]
- Lilienfeld S.O., Andrews B.P. Development and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal populations. J. Pers. Assess. 1996;66:488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Matthys W., van Goozen S.H., de Vries H., Cohen-Kettenis P.T., van Engeland H. The dominance of behavioural activation over behavioural inhibition in conduct disordered boys with or without attention deficit hyperactivity disorder. J. Child Psychol. Psychiatry. 1998;39:643–651. [PubMed] [Google Scholar]
- Matthys W., van Goozen S.H., Snoek H., van Engeland H. Response perseveration and sensitivity to reward and punishment in boys with oppositional defiant disorder. Eur. Child Adolesc. Psychiatry. 2004;13:362–364. doi: 10.1007/s00787-004-0395-x. [DOI] [PubMed] [Google Scholar]
- Newman J.P., Wallace J.F. Diverse pathways to deficient self-regulation: implications for disinhibitory psychopathology in children. Clin. Psychol. Rev. 1993;13:699–720. [Google Scholar]
- Pardini D., White H.R., Stouthamer-Loeber M. Early adolescent psychopathology as a predictor of alcohol use disorders by young adulthood. Drug. Alcohol Depend. 2007;88(Suppl. 1):S38–S49. doi: 10.1016/j.drugalcdep.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W. Diagnostic Interview for Children and Adolescents (DICA) J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Grabenhorst F., Parris B.A. Neural systems underlying decisions about affective odors. J. Cogn. Neurosci. 2010;22:1069–1082. doi: 10.1162/jocn.2009.21231. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat. Rev. Neurosci. 2000;1:199. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W., Tremblay L., Hollerman J.R. Changes in behavior-related neuronal activity in the striatum during learning. Trends Neurosci. 2003;26:321–328. doi: 10.1016/S0166-2236(03)00122-X. [DOI] [PubMed] [Google Scholar]
- Sesack S.R., Grace A.A. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Casey B.J. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter R.E. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am. J. Drug Alcohol Abuse. 1990;16:1–46. doi: 10.3109/00952999009001570. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L., Moor B.G., Op de Macks Z.A., Rombouts S.A., Westenberg P.M., Crone E.A. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L., Zanolie K., Van Meel C.S., Westenberg P.M., Rombouts S.A., Crone E.A. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb. Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Vul E. Puzzingly High Correlations in fMRI studies of Emotion, Personality, and Social Cognitition. Perspect. Psychol. Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.