SUMMARY
Type 1 diabetes is an autoimmune disorder leading to loss of pancreatic β-cells and insulin secretion, followed by insulin dependence. Islet and whole pancreas transplantation restore insulin secretion. Pancreas transplantation is often performed together with a kidney transplant in patients with end-stage renal disease. With improved immunosuppression, immunological failures of whole pancreas grafts have become less frequent and are usually categorized as chronic rejection. However, growing evidence indicates that chronic islet autoimmunity may eventually lead to recurrent diabetes, despite immunosuppression to prevent rejection. Thus, islet autoimmunity should be included in the diagnostic work-up of graft failure and ideally should be routinely assessed pretransplant and on follow-up in Type 1 diabetes recipients of pancreas and islet cell transplants. There is a need to develop new treatment regimens that can control autoimmunity, as this may not be effectively suppressed by conventional immunosuppression.
Current status of pancreas & islet transplantation
Type 1 diabetes (T1D) is a chronic autoimmune disorder resulting in the destruction of pancreatic β-cells. This is followed by loss of insulin secretion and lifelong insulin dependence [1–4]. While T1D is traditionally considered T-cell mediated, autoantibodies to islet cell antigens and B lymphocytes also play a role in islet auto-immunity [5–9]. Patients with T1D may develop chronic complications, including diabetic retinopathy, neuropathy and nephropathy, eventually leading to blindness and kidney failure [10]. Both pancreas and islet cell transplantations restore insulin secretion in T1D patients. At present, pancreas transplantation is associated with longer graft survival and function than islet transplantation [11], although it requires invasive surgery compared with the islet infusion procedure and has a higher risk of perioperative morbidity and mortality [12]. Thus, islet transplants are mostly performed in patients with brittle diabetes and severe hypoglycemia, while patients in end-stage renal disease are candidates for simultaneous pancreas–kidney (SPK) transplantation, which accounts for the majority of pancreas transplants. Pancreas transplantation is also a therapeutic option for patients who previously received a kidney allograft. By the end of 2009, over 23,000 patients had received a pancreas transplant in the USA [12]. Most transplants were performed from deceased donors. Most patients (74%) received SPK transplant, while pancreas after kidney (PAK) transplants and pancreas transplants alone (PTA) were performed less frequently (18 and 8%, respectively). As reported by Gruessner for the International Pancreas Transplant Registry, the 1-year patient survival rate (for transplants performed from 1 January 2005 through to 31 December 2009) ranged from 95.5 (SPK transplants) to 97.4% (PTA). The 1-year pancreas graft survival rate has also improved to 78% for PTA and 85% for SPK transplants performed between 2005 and 2009. The half-life of pancreas transplants now extends to approximately 12 years [13,14]. With improved immunosuppression, immunological failures have become less frequent and are usually categorized as chronic rejection [15]. However, patients with T1D receiving a pancreas transplant are exposed to the potential risk of recurrence of the original autoimmune disease in the grafted organ.
Recurrence of T1D in pancreas transplantation
Type 1 diabetes recurrence (T1DR) following pancreas transplantation was initially documented by Sutherland’s group in the 1980s [16–19]. They observed T1DR in living donor recipients of the tail of the pancreas from HLA-identical twins (five cases) and HLA-identical siblings (five cases) in the absence of immunosuppression 4–8 weeks after transplantation. The relative rapid return to hyperglycemia in the absence of pancreatic rejection was consistent with the recurrence of autoimmunity, a conclusion that was supported by the demonstration of insulitis with a mononuclear cell infiltrate and selective β-cell destruction. This seminal observation remains one of the most important pieces of evidence supporting the concept of cellular immunity as a key pathogenic mechanism of T1D in humans.
Sibley et al. subsequently examined tissues obtained by biopsy, pancreatectomy and autopsy from 100 pancreas grafts. Autoimmune diabetes recurrence was not noted in patients receiving immunosuppression [18] and organs from non-HLA-identical siblings or parents. There were no instances (0 out of 12) of recurrence of diabetes in recipients of cadaver donors [19] and no evidence of anti-islet humoral immune responses in these patients [18,17] after measuring islet cell autoantibodies using the islet cell antibodies (ICA) test, which even today remains highly sensitive and predictive [20]. These data supported the belief that immunosuppression effectively prevented recurrence of disease and that this is dependent on HLA matching between the donor and the recipient (i.e., it would only occur in the presence of HLA matching, such as in identical twins or HLA-identical siblings). While HLA matching could theoretically facilitate the emergence of autoimmune responses, it is important to remember that the recipients of the full HLA-matched grafts were not immunosuppressed or mildly immunosuppressed. Thus, a direct comparison of the likelihood of recurrent autoimmunity in HLA-matched and -unmatched donor–recipient pairs cannot be performed.
These concepts were challenged by Bosi et al. who studied 23 pancreas transplant recipients (22 were SPK) and noted the reappearance of ICA in seven patients, with two having persisting ICA titers [21]. Seven of the nine ICA-positive patients experienced graft failure 2–35 months after ICA detection. Since these patients were HLA mismatched, this study provided evidence that the recurrence of ICA may take place in recipients of pancreas transplants regardless of HLA matching. Moreover, these patients were immunosuppressed with azathioprine, cyclosporine and prednisone, with or without induction using anti-lymphocyte globulins.
Other reports provided additional examples of the recurrence of autoantibodies and some evidence for worse graft outcome in autoantibody-positive patients [22–24]. Thus, immunosuppression does not fully prevent the recurrence of humoral autoimmune responses as measured by ICA. An anecdotal SPK transplant patient, reported by Santamaria et al., experienced recurrence of autoimmunity after receiving organs from an HLA-identical sibling, despite immunosuppression with azathioprine and prednisone [25]. However, diabetes recurred 8 years after transplantation. Islet cell antibodies were demonstrated to have been present 5 and 8 years subsequent to the transplantation, and the pathology of the pancreas transplant demonstrated insulitis with selective β-cell loss [25]. The prolonged period of time that preceded recurrence of diabetes in this patient suggests that immunosuppression might have inhibited this process and that immunosuppressed patients may take time to develop recurrence of autoimmunity and diabetes.
Further evidence for T1DR has been reported in recipients of allogeneic cadaver donor SPK transplants, where pancreas transplant biopsies demonstrated the selective loss of β-islet cells [26]. In this report, two SPK transplant recipients were treated with cyclosporine A, steroids and azathioprine. GAD65 and IA2 autoantibodies were expressed in one patient at the time of hyperglycemia and not present in the other. The first patient had a 1A/1DR HLA match with the donor; the second was a six-antigen mismatch. Pancreas transplant pathology demonstrated the selective loss of β-islet cells in both patients and insulitis in one patient (3 months post-hyperglycemia, 29 months following transplantation).
Braghi et al. retrospectively analyzed a cohort of 110 SPK transplant cases and followed 75 of these patients for up to 11.2 years [27]. Pancreas graft survival was not affected by the presence of GAD/IA2 antibodies before the transplant. A total of 59% (n = 44) of the patients remained antibody negative at follow-up, 17% (n = 13) had stable antibody levels, 17% (n = 13) had declining levels and 7% (n = 5) had increasing levels. Of the last five, four lost graft function after 0.7–2.3 years of follow-up. No data were available on the presence of autoreactive T cells in these patients, nor on the lesion in the graft, investigated by means of pancreatic biopsies. Other investigators reported the presence of T1D-associated autoantibodies in SPK transplant recipients [24,28].
Studies of recurrence of T1D at the University of Miami
Most of the aforementioned studies lacked biopsy data to demonstrate insulitis and/or β-cell loss, and none of these studies could examine circulating autoreactive T cells in these patients to a great extent because assays to measure such cells are difficult to perform and were not well established at the time. We have been able to collect such data from studies in our cohort of SPK transplant recipients, as we have identified several patients with T1DR; furthermore, a recent case report of recurrent disease from Japan also acquired biopsy data [29]. Figure 1 illustrates recurrent diabetes, autoimmune responses, biopsy data and treatment effects in one of our patients. All of our SPK transplant patients have T1D and had no detectable C-peptide response to a Sustacal test before transplantation, and they have end-stage renal disease. Pancreas transplants drain into the bladder (exocrine), which allows monitoring of the pancreas transplant exocrine function by measuring urine amylase levels. Our published studies of three such patients demonstrate the cardinal features of T1DR which are [30,31]:
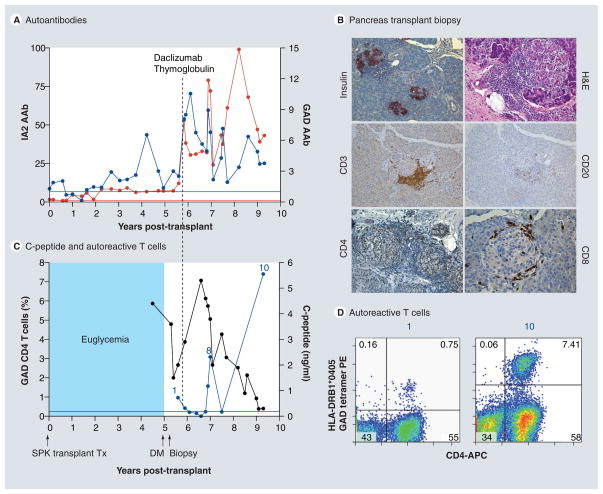
Figure 1. Recurrence of diabetes in a simultaneous pancreas–kidney transplant patient.
The patient was a 41-year-old Caucasian male (HLA A2/A3, B57/B60, DR4 [DRB1*0405]/DR6) who developed Type 1 diabetes 7 years of age. He received a SPK transplant from an HLA A2/A30, B41/B60, DR4/DRX donor at age 32 years. The transplant reversed diabetes, but the patient returned to insulin dependence 5 years later, while kidney and exocrine pancreas allografts had normal function. (A) Autoantibody levels before transplant and at follow-up. The patient had GAD and IA2 autoantibodies before transplantation, which persisted despite immunosuppression and titers increased on follow-up. Color-matched, horizontal lines represent the cut-off level for each autoantibody. Autoantibody levels are expressed as the ratio of the index level of the patient/upper limit of the normal index (cut-off). For all autoantibodies, a value greater than 1 denotes a positive result. (B) Pancreas transplant biopsy stained as labeled, obtained approximately 6 months after the recurrence of hyperglycemia. Insulitis is marked by the presence of T (CD3, CD4) and B (CD20) lymphocytes infiltrating the islets, while insulin staining demonstrates β-cell loss. (C) Serum C-peptide levels and percentage of GAD tetramer-positive T cells in the CD4 T-cell population from the time of hyperglycemia recurrence. C-peptide was still detectable at diagnosis, confirming the function of residual β-cells observed at biopsy. Autoreactive T cells were detected at the time of biopsy, approximately 6 months after the recurrence of hyperglycemia on two samples and again at several time points approximately 1 year after treatment. The horizontal blue line represents the cut-off of the tetramer assay (0.25%). (D) Flow cytometry plots demonstrating GAD-autoreactive CD4 T cells (upper right quadrant, percentage of such cells is indicated). The numbers above the plots match those in (C) to link the data with the time of follow-up. Tetramer staining with irrelevant peptide was <0.1% (not shown).
DM: Diabetes; H&E: Hematoxylin and eosin; PE: Phycoerythrin; SPK: Simultaneous pancreas–kidney; Tx: Treatment.
Reproduced with permission from [29] © American Diabetes Association (2010).
Hyperglycemia without rejection and no functional changes of the exocrine pancreas (urine amylase) or kidney (serum creatinine) grafts, with selective loss of insulin secretion;
Biopsies demonstrating insulitis and/or β-cell loss;
The persistence or reappearance of autoanti-bodies, prior to diabetes recurrence;
The presence of circulating autoreactive T cells around the time of diabetes recurrence and on further follow-up, which in vitro predominantly produced proinflammatory cytokines (e.g., IFN-γ);
In vivo evidence that the SPK transplant patients’ autoreactive CD4 T cells specific for the islet autoantigen GAD65 can specifically mediate β-cell destruction in HLA-mismatched islet grafts when T cells and islets are co-transplanted under the kidney capsule of immunodeficient mice;
The presence of autoreactive T cells in the circulation of several patients, both CD4 and CD8 T cells, correlated with disease activity and progression. In patients who received additional immunosuppression in an attempt to salvage the residual β-cell mass demonstrated at biopsy, autoreactive T cells were no longer detected after treatment but reappeared on later follow-up. Their return was followed by a further and complete loss of C-peptide.
Thus, with frequent sampling, it was possible to detect changes in autoantibodies and circulating autoreactive T cells. Fluctuations of autoreactive T cells reflected the effects of immunosuppressive therapy and disease progression, which were assessed by metabolic evaluation and pancreas transplant biopsy [30]. Another key component of our work is that we can study autoreactive T cells in the pancreas transplant and pancreas transplant lymph nodes (obtained through open biopsy based on clinical need). We demonstrated proinsulin-reactive CD4 T cells in the peripheral blood, the pancreas transplant and pancreas transplant lymph nodes of a SPK transplant patient; in another [30], we demonstrated GAD-reactive CD4 T cells against the same epitope in both pancreas transplant lymph nodes and the circulation. In this patient, the cells in the circulation and pancreas transplant lymph nodes had the same T-cell receptor (identical V-β and CDR3). Thus, T cells reacting against the same autoantigens were present in the circulation and in the target organ, which links these T cells with the disease process and shows that tetramer assays can detect disease-relevant autoreactive T cells. The return and further persistence of T-cell responses against the same autoantigen specificity with similar/identical T-cell receptor restriction provided indirect evidence for the persistence of a T-cell memory phenotype, which was associated with recurrent diabetes in these patients [30,31].
Memory cells & autoimmunity in islet cell transplantation & spontaneous T1D
Studies in islet cell transplant recipients also show that autoimmunity negatively affects graft outcome [32,33] and that some immunosuppression regimens may actually favor the emergence of autoreactive memory T cells. Lymphopenia has been demonstrated to stimulate homeostatic proliferation, resulting in proliferation of memory cell populations, which are enriched in autoreactive T cells [34–36]. The homeostatic expansion of memory cells has been linked with islet autoimmunity. Specifically, in patients receiving islet transplants, the lymphopenia induced by immunosuppression is followed by increased production of IL-7, IL-15 and in vivo proliferation of CD45RO+ T cells, which are highly enriched in autoreactive T cells. GAD-reactive CD8 T cells (detected using the HLA-A*0201–GAD65114–122 pentamer) were enriched up to 34-fold in the patients studied and expressed markers of memory cells (CD45R0) [37]. These findings implicate the involvement of memory T cells with recurrent islet autoimmunity in islet transplant recipients, for which there is histological evidence from two patients [38,39]. HLA-A*0201-restricted CD8 T cells with cytotoxic function reacting with a preproinsulin epitope were also linked with spontaneous diabetes development and recurrent autoimmunity in islet graft recipients [40–43]. The presence of autoreactive CD8 T cells pretransplant was recently correlated with graft failure in islet transplant recipients treated with ATG, tacrolimus and mycophenolate mofetil therapy [32,44].
Literature data also show strong associations of memory autoreactive T cells when patients originally develop T1D. Indeed, memory T cells [45–51] and B cells [52–54] have been linked to a variety of autoimmune conditions, including in models of T1D [55,56], and not exclusively under conditions of lymphopenia [55,57]. A partial explanation for this phenomenon may be that normal stimuli for the generation and survival of memory cells also include self-antigens [36]. Recent studies show that the memory cell compartment of T1D patients is enriched in autoreactive T cells. A study by Viglietta et al. [58] provided initial evidence for an association of memory cells with T1D. These investigators discovered that GAD65-reactive T cells from T1D patients no longer required a second costimulatory signal for clonal expansion, as these cell were strikingly less dependent on CD28/B7-1 costimulation for proliferation than equivalent cells from healthy controls. These findings were consistent with previous in vivo activation and differentiation into memory cells of these GAD65-reactive T cells. Monti et al. examined islet-specific autoreactive T cells in patients and healthy subjects [59]. While such responses were detected in both patients and controls when examining in vitro, stimulated responses in unselected peripheral blood mononuclear cells or in unselected CD4 T-cell populations, CD45RO+ memory autoreactive T cells were only detected in T1D patients and not in controls. The same was true for GAD65 CD8 autoreactive T cells, which were directly assessed ex vivo following purification with an HLA-A*0201 pentamer that detects T cells reacting against the GAD65114-122 peptide. In another study, Waid et al. identified a T-cell subset defined by CD4lo and CD40 expression (TCD40) that is significantly expanded in the peripheral blood of T1D patients but not in healthy subjects or patients with Type 2 diabetes [60]. Most TCD40 cells in T1D patients carried a memory phenotype and previous reports have associated CD40 function with the generation of CD8 memory T cells [61].
Thus, memory autoreactive CD4 and CD8 T cells appear to be a major obstacle to the treatment of islet autoimmunity in both spontaneous disease and its recurrence after transplantation. As noted, our clinical experience in attempting to treat recurrent diabetes shows that additional T- and B-cell-directed immunosuppression does not afford lasting protection once autoimmunity has been reactivated. Therefore, conventional immunosuppression may control rejection but not always control recurrent autoimmunity [30]. A possible explanation is that, as suggested by our data and the aforementioned literature, memory autoreactive T cells play a role in T1DR and, once activated, these cells cannot be controlled by conventional anti-rejection regimens.
T1DR is an underappreciated condition
In our cohort, recurrent diabetes explains 50% of the immunological failures observed, the other 50% being caused by chronic rejection. Thus, chronic islet autoimmunity is as frequent as chronic rejection and represents a major cause of immunological failure. In our cohort, approximately 5% of our patients may develop T1DR while another 5% may develop chronic rejection. Similar rates were described in earlier studies [19,27]. With more than 23,000 pancreas transplants performed in the USA (and more worldwide) a condition that compromises graft outcome with similar frequency to chronic rejection is of clear clinical significance. The loss of insulin secretion compromises blood glucose control, negatively impacts quality of life and may jeopardize the survival of the kidney graft given the deleterious effects of hyperglycemia on the kidney. Thus, there is a compelling case that further studies of islet autoimmunity in pancreas transplant recipients are needed to address key questions about this previously underappreciated condition. While at present islet autoimmunity is not routinely assessed in pancreas transplant recipients, it should be possible for most transplant centers to at least monitor autoantibodies, given that these assays are widely available even through clinical laboratories.
Conclusion
Overall, there is mounting evidence that diabetes-associated autoantibodies may persist or reappear in SPK transplant recipients, suggesting the possibility of recurrent autoimmunity despite immunosuppression and regardless of HLA matching. The prevalence of this phenomenon, which is so far mostly assessed through the presence of autoantibodies, may be higher than previously estimated. Underestimates could result from the frequent occurrence of rejection in earlier studies, which until the early 1990s curtailed the long-term graft survival rates and prevented studies with a long follow-up period. A critical consideration is that recurrent autoimmunity may take many years to develop in immunosuppressed patients [25] while it develops quickly in HLA-matched recipients of living related HLA-matched donors who are not immunosuppressed [16,25]. Similarly, we lack long-term follow-up data and robust studies in recipients of islet cell transplants [24,27,62–65]. Pancreas and islet transplantation in T1D recipients pose a special challenge regarding the need to address the underlying autoimmunity. This problem has been largely underestimated. Future studies will enable improvements in diagnosis, prediction and therapy of diabetes recurrence in pancreas transplant recipients. The lessons learned will be highly relevant to spontaneous disease and may ultimately contribute to curing T1D.
Future perspective
We identify two major obstacles to advancing progress in the field: the lack of extensively validated laboratory markers of T1DR to aid in the diagnosis and prediction of this condition, and a satisfactory knowledge of the phenotypes and function of T cells involved in recurrent disease that will guide new and more directed therapeutic efforts. Our ongoing studies are focused on specifically addressing these critical problems.
For the past few years we have been investigating whether islet-specific autoimmune responses are risk factors for T1DR in SPK transplant recipients, independent of alloimmunity. We were aided in this endeavor by access to serial, banked samples from more than 200 SPK transplant recipients, from whom we are generating extensive data by testing for GAD and IA2 autoantibodies. Combined with many years of past and ongoing follow-up and outcomes data, data/samples are proving quite powerful in assessing T1DR risk. Our unpublished data from ongoing analysis of over 200 SPK transplant patients show that GAD and IA2 autoantibodies also mark islet autoimmunity in transplant recipients. For each patient, we test a pretransplant sample and multiple samples on follow-up, with the number of samples tested varying according to the length of follow-up, sample availability (this is an infrequent limitation) and in relation to relevant events during the clinical follow-up. A key finding, which confirms and extends earlier studies [27,66], is that autoantibody conversion, as opposed to persistence or negativity, is associated with a high risk of developing T1DR. It therefore appears that it is not just the mere presence of autoantibodies but rather the reactivation of the humoral autoimmune response on follow-up that marks recurrent autoimmunity and perhaps follows some as yet unknown triggering event. In this context, we believe that autoantibody testing will become the mainstay in T1DR diagnosis and prediction [67–69]. Advances in prediction through expanded autoantibody panels that include additional autoantibodies and novel biomarkers will facilitate the design of additional trials.
Since our pancreas transplants are performed with bladder drainage, urine amylase levels are of critical importance for the clinical diagnosis of T1DR, allowing differentiation from transplant rejection and for validating autoantibodies as risk factors for T1DR. Most transplant centers perform pancreas transplants with enteric drainage and cannot easily differentiate exocrine and endocrine function. As we progress with our studies, our cohort will be exceptionally well suited to address the next set of critical questions and to validate biomarkers of diabetes recurrence. Findings from this cohort can then be applied to clinical practice, and help in diagnosing T1DR and assessing its prevalence at multiple centers. Better predictive tools are within reach and are required for prevention trials to block autoimmunity at earlier stages in transplanted patients.
Consideration should be given to coordinated efforts to collect pancreas transplant biopsies from patients with suspected T1DR when the opportunity presents or there is clinical indication. In our experience, a biopsy demonstrating insulitis but the presence of surviving β-cells around the time of T1DR diagnosis has been critical in the decision to proceed with additional immunosuppression or, in the case of one patient with complete β-cell loss, with re-transplantation [30]. The study of pancreas transplant biopsy specimens will lead to improved understanding of the pathology of T1DR. It will also allow further investigations of the pancreas remodeling phenomena involving the conversion of ductal cells into insulin-producing cells, which we have observed in several of our SPK transplant recipients with T1DR [70].
The assessment of autoreactive CD4 and CD8 T cells against multiple autoantigen specificities, both pre- and post-transplant, will be critical to study recurrent autoimmunity. Our ongoing studies are also addressing the limited knowledge regarding autoreactive T cells in SPK transplant patients [30,31]. We are investigating phenotypic and functional changes in autoreactive T cells in relation to autoantibody conversion. Demonstrating a link between autoantibody conversion and functional/phenotypic changes in persisting memory autoreactive T cells would provide a strong rationale for therapeutic interventions that specifically target memory cells and could allow us to identify novel therapeutic targets based on the functional assessments. During the last few years, there has been tremendous development and increased availability of multiple platforms to study autoreactive T cells. While our studies have so far been based on class II tetramers to detect CD4 autoreactive T cells after in vitro antigenic stimulation, novel tetramer reagents and other assay platforms allow autoreactive T cells to be measured, both CD4 and CD8 T cells, against a variety of islet autoantigens and multiple epitopes [71]. Moreover, some T-cell assays measure autoreactive T cells directly ex vivo, some simultaneously with multiple autoantigens using MHC multimers [71], thus allowing for more extensive evaluation of the autoimmune response and more meaningful phenotypic studies.
Based on the above, the best way forward is probably the organization of coordinated, multicenter research efforts that bring together expertise, technology and facilitate patient enrollment to most effectively identify recipients with T1DR or islet autoimmunity. This would allow clinical trials to test the safety and efficacy of new interventions at the onset of T1DR, and studies could be conducted in autoantibody converters to monitor auto-reactive T cells and identify additional predictive markers. There are a number of therapeutic regimens that could be tested by clinical trials; for example, based on the suspected role of memory cells, antimemory cell drugs could be a reasonable approach. There are other agents that have been and are being used in clinical trials for the prevention and treatment of new-onset T1D that could be used in transplant patients. In addition to immunosuppressive drugs, there are agents that are believed to have immunomodulatory functions and approaches, based on the administration of autoantigens [72,73]. Much consideration has recently been given to the concept of using combination therapies to contain autoimmunity in newly diagnosed patients, when effects of multiple agents are believed to be synergistic [74,75], and this may help to target immunosuppression, immunomodulation, antigen specificity and regeneration of β-cells. As discussed, pancreas transplant biopsies could be very valuable for understanding the effects of therapies and evaluating possible regeneration [76–78]. Thus, cross-fertilization between the immunology of T1D and transplantation fields is likely to play a key role in the design of future trials in transplant patients with recurrent autoimmunity and will be relevant to the treatment of spontaneous disease.
Practice Points.
Immunological loss of a pancreas or islet graft in a patient with Type 1 diabetes may be caused by rejection, but also autoimmunity.
Ideally, transplant recipients should be monitored for islet cell autoantibodies to identify those who acquire autoantibodies on follow-up; these patients are more likely to develop Type 1 diabetes recurrence within a few years.
It is important to be aware of ongoing research studies that could enroll patients with autoantibody conversions of suspected Type 1 diabetes recurrence and conduct further investigations with tools not easily available in clinical practice.
Conventional immunosuppression may suppress alloimmunity and autoimmunity in many patients, but may not control islet autoimmunity once this has been triggered on follow-up.
Given the limited knowledge and therapeutic choices, further research is clearly needed, including pilot trials to test therapies that might interfere with recurrent autoimmunity. Thus, new patients with suspected recurrent diabetes could be referred to centers that are active in this area for further evaluation and possible inclusion in future trials.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
Studies from the author’s center reviewed in this article are supported by grants from the NIH (5RO1-DK-070011 and AI-50864), the American Diabetes Association (1-09-RA-413, 1-05-RA-105), the Juvenile Diabetes Research Foundation (JDRF 1-2005-257) and the Diabetes Research Institute Foundation, Hollywood, FL, USA. These studies were approved by the University of Miami Institutional Review Board (Protocol # 20053039). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
•• of considerable interest
- 1.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood Type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23(10):1516–1526. doi: 10.2337/diacare.23.10.1516. [DOI] [PubMed] [Google Scholar]
- 3.Soltesz G, Patterson CC, Dahlquist G. Worldwide childhood Type 1 diabetes incidence – what can we learn from epidemiology? Pediatr Diabetes. 2007;8(Suppl 6):6–14. doi: 10.1111/j.1399-5448.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 4.DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23(8):857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 5.Reijonen H, Daniels TL, Lernmark A, Nepom GT. GAD65-specific autoantibodies enhance the presentation of an immunodominant T-cell epitope from GAD65. Diabetes. 2000;49(10):1621–1626. doi: 10.2337/diabetes.49.10.1621. [DOI] [PubMed] [Google Scholar]
- 6.Hampe CS, Hammerle LP, Bekris L, et al. Recognition of glutamic acid decarboxylase (GAD) by autoantibodies from different GAD antibody-positive phenotypes. J Clin Endocrinol Metab. 2000;85(12):4671–4679. doi: 10.1210/jcem.85.12.7070. [DOI] [PubMed] [Google Scholar]
- 7.Serreze DV, Silveira PA. The role of B lymphocytes as key antigen-presenting cells in the development of T cell-mediated autoimmune Type 1 diabetes. Curr Dir Autoimmun. 2003;6:212–227. doi: 10.1159/000066863. [DOI] [PubMed] [Google Scholar]
- 8.Miao D, Yu L, Eisenbarth GS. Role of autoantibodies in Type 1 diabetes. Front Biosci. 2007;12:1889–1898. doi: 10.2741/2195. [DOI] [PubMed] [Google Scholar]
- 9.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of β-cell function. N Engl J Med. 2009;361(22):2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi N. The burden of diabetes and its complications: trends and implications for intervention. Diabetes Res Clin Pract. 2007;76(Suppl 1):S3–S12. doi: 10.1016/j.diabres.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Mineo D, Pileggi A, Alejandro R, Ricordi C. Point: steady progress and current challenges in clinical islet transplantation. Diabetes Care. 2009;32(8):1563–1569. doi: 10.2337/dc09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruessner AC, Sutherland DE, Gruessner RW. Pancreas transplantation in the United States: a review. Curr Opin Organ Transplant. 2010;15(1):93–101. doi: 10.1097/MOT.0b013e32833552d2. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland DE, Gruessner AC. Long-term results after pancreas transplantation. Transplant Proc. 2007;39(7):2323–2325. doi: 10.1016/j.transproceed.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Gruessner AC, Sutherland DE. Pancreas transplant outcomes for United States (US) and non-US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of June 2004. Clin Transplant. 2005;19(4):433–455. doi: 10.1111/j.1399-0012.2005.00378.x. [DOI] [PubMed] [Google Scholar]
- 15.White SA, Shaw JA, Sutherland DE. Pancreas transplantation. Lancet. 2009;373(9677):1808–1817. doi: 10.1016/S0140-6736(09)60609-7. [DOI] [PubMed] [Google Scholar]
- 16••.Sutherland DE, Sibley R, Xu XZ, et al. Twin-to-twin pancreas transplantation: reversal and reenactment of the pathogenesis of Type I diabetes. Trans Assoc Am Physicians. 1984;97:80–87. Seminal paper describing the recurrence of Type 1 diabetes in HLA-identical, non or minimally immunosuppressed pancreas transplant recipients. [PubMed] [Google Scholar]
- 17.Sibley RK, Sutherland DE, Goetz F, Michael AF. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985;53(2):132–144. [PubMed] [Google Scholar]
- 18.Sutherland DE, Goetz FC, Sibley RK. Recurrence of disease in pancreas transplants. Diabetes. 1989;38(Suppl 1):85–87. doi: 10.2337/diab.38.1.s85. [DOI] [PubMed] [Google Scholar]
- 19.Sibley RK, Sutherland DE. Pancreas transplantation. An immunohistologic and histopathologic examination of 100 grafts. Am J Pathol. 1987;128(1):151–170. [PMC free article] [PubMed] [Google Scholar]
- 20.Bottazzo GF, Florin-Christensen A, Donniach D. Islet cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2:1279–1283. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 21.Bosi E, Bottazzo GF, Secchi A, et al. Islet cell autoimmunity in Type I diabetic patients after HLA-mismatched pancreas transplantation. Diabetes. 1989;38(Suppl 1):82–84. doi: 10.2337/diab.38.1.s82. [DOI] [PubMed] [Google Scholar]
- 22.Esmatjes E, Rodriguez-Villar C, Ricart MJ, et al. Recurrence of immunological markers for Type 1 (insulin-dependent) diabetes mellitus in immunosuppressed patients after pancreas transplantation. Transplantation. 1998;66(1):128–131. doi: 10.1097/00007890-199807150-00022. [DOI] [PubMed] [Google Scholar]
- 23.Petruzzo P, Andreelli F, McGregor B, et al. Evidence of recurrent Type I diabetes following HLA-mismatched pancreas transplantation. Diabetes Metab. 2000;26(3):215–218. [PubMed] [Google Scholar]
- 24.Da Silva M, Thivolet C, Lefrancois N, et al. Combined analysis of autoantibodies against β-cells for prediction of pancreas allograft failure. Transplant Proc. 2000;32(8):2773. doi: 10.1016/s0041-1345(00)01877-7. [DOI] [PubMed] [Google Scholar]
- 25.Santamaria P, Nakhleh RE, Sutherland DE, Barbosa JJ. Characterization of T lymphocytes infiltrating human pancreas allograft affected by isletitis and recurrent diabetes. Diabetes. 1992;41(1):53–61. doi: 10.2337/diab.41.1.53. [DOI] [PubMed] [Google Scholar]
- 26.Tyden G, Reinholt FP, Sundkvist G, Bolinder J. Recurrence of autoimmune diabetes mellitus in recipients of cadaveric pancreatic grafts. N Engl J Med. 1996;335(12):860–863. doi: 10.1056/NEJM199609193351205. [DOI] [PubMed] [Google Scholar]
- 27.Braghi S, Bonifacio E, Secchi A, Di Carlo V, Pozza G, Bosi E. Modulation of humoral islet autoimmunity by pancreas allotransplantation influences allograft outcome in patients with Type 1 diabetes. Diabetes. 2000;49(2):218–224. doi: 10.2337/diabetes.49.2.218. [DOI] [PubMed] [Google Scholar]
- 28.Thivolet C, Abou-Amara S, Martin X, et al. Serological markers of recurrent β cell destruction in diabetic patients undergoing pancreatic transplantation. Transplantation. 2000;69(1):99–103. doi: 10.1097/00007890-200001150-00018. [DOI] [PubMed] [Google Scholar]
- 29.Ishida-Oku M, Iwase M, Sugitani A, et al. A case of recurrent Type 1 diabetes mellitus with insulitis of transplanted pancreas in simultaneous pancreas–kidney transplantation from cardiac death donor. Diabetologia. 2010;53(2):341–345. doi: 10.1007/s00125-009-1593-3. [DOI] [PubMed] [Google Scholar]
- 30.Vendrame F, Pileggi A, Laughlin E, et al. Recurrence of Type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010;59(4):947–957. doi: 10.2337/db09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laughlin E, Burke G, Pugliese A, Falk B, Nepom G. Recurrence of autoreactive antigen-specific CD4+ T cells in autoimmune diabetes after pancreas transplantation. Clin Immunol. 2008;128(1):23–30. doi: 10.1016/j.clim.2008.03.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huurman VA, Hilbrands R, Pinkse GG, et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS ONE. 2008;3(6):E2435. doi: 10.1371/journal.pone.0002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 34.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117(2):265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 35.Calzascia T, Pellegrini M, Lin A, et al. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc Natl Acad Sci USA. 2008;105(8):2999–3004. doi: 10.1073/pnas.0712135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 37••.Monti P, Scirpoli M, Maffi P, et al. Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J Clin Invest. 2008;118(5):1806–1814. doi: 10.1172/JCI35197. Demonstrates the expansion of memory autoreactive T cells in immunosuppressed islet cell transplant recipients with Type 1 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stegall MD, Lafferty KJ, Kam I, Gill RG. Evidence of recurrent autoimmunity in human allogeneic islet transplantation. Transplantation. 1996;61(8):1272–1274. doi: 10.1097/00007890-199604270-00027. [DOI] [PubMed] [Google Scholar]
- 39.The Worcester Human Islet Transplantation Group. Autoimmunity after islet-cell allotransplantation. N Engl J Med. 2006;355(13):1397–1399. doi: 10.1056/NEJMc061530. [DOI] [PubMed] [Google Scholar]
- 40.Pinkse GG, Boitard C, Tree TI, Peakman M, Roep BO. HLA class I epitope discovery in Type 1 diabetes: independent and reproducible identification of proinsulin epitopes of CD8 T cells – report of the IDS T Cell Workshop Committee. Ann NY Acad Sci. 2006;1079:19–23. doi: 10.1196/annals.1375.003. [DOI] [PubMed] [Google Scholar]
- 41.Pinkse GG, Tysma OH, Bergen CA, et al. Autoreactive CD8 T cells associated with β cell destruction in Type 1 diabetes. Proc Natl Acad Sci USA. 2005;102(51):18425–18430. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skowera A, Ellis RJ, Varela-Calvino R, et al. CTLs are targeted to kill β cells in patients with Type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118(10):3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roep BO. Islet autoreactive CD8 T-cells in Type 1 diabetes: licensed to kill? Diabetes. 2008;57(5):1156. doi: 10.2337/db08-0264. [DOI] [PubMed] [Google Scholar]
- 44.Hilbrands R, Huurman VA, Gillard P, et al. Differences in baseline lymphocyte counts and autoreactivity are associated with differences in outcome of islet cell transplantation in Type 1 diabetic patients. Diabetes. 2009;58(10):2267–2276. doi: 10.2337/db09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawakami N, Odoardi F, Ziemssen T, et al. Autoimmune CD4+ T cell memory: lifelong persistence of encephalitogenic T cell clones in healthy immune repertoires. J Immunol. 2005;175(1):69–81. doi: 10.4049/jimmunol.175.1.69. [DOI] [PubMed] [Google Scholar]
- 46.Beeton C, Chandy KG. Potassium channels, memory T cells, and multiple sclerosis. Neuroscientist. 2005;11(6):550–562. doi: 10.1177/1073858405278016. [DOI] [PubMed] [Google Scholar]
- 47.Fujii R, Kanai T, Nemoto Y, et al. FTY720 suppresses CD4+CD44highCD62L− effector memory T cell-mediated colitis. Am J Physiol Gastrointest Liver Physiol. 2006;291(2):G267–G274. doi: 10.1152/ajpgi.00496.2005. [DOI] [PubMed] [Google Scholar]
- 48.Langer S, Langnickel D, Enghard P, et al. The systemic and SmD183–119-autoantigen-specific cytokine memory of Th cells in SLE patients. Rheumatology. 2007;46(2):238–245. doi: 10.1093/rheumatology/kel180. [DOI] [PubMed] [Google Scholar]
- 49.Lamprecht P, Csernok E, Gross WL. Effector memory T cells as driving force of granuloma formation and autoimmunity in Wegener’s granulomatosis. J Intern Med. 2006;260(3):187–191. doi: 10.1111/j.1365-2796.2006.01698.x. [DOI] [PubMed] [Google Scholar]
- 50.Le SC, Mennechet S, Taylor N, Hernandez J. Memory-like CD8+ and CD4+ T cells cooperate to break peripheral tolerance under lymphopenic conditions. Proc Natl Acad Sci USA. 2008;105(49):19414–19419. doi: 10.1073/pnas.0807743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sattler A, Wagner U, Rossol M, et al. Cytokine-induced human IFN-γ-secreting effector-memory Th cells in chronic autoimmune inflammation. Blood. 2009;113(9):1948–1956. doi: 10.1182/blood-2008-02-139147. [DOI] [PubMed] [Google Scholar]
- 52.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26(2):205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duddy M, Niino M, Adatia F, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178(10):6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 54.Nicholas MW, Dooley MA, Hogan SL, et al. A novel subset of memory B cells is enriched in autoreactivity and correlates with adverse outcomes in SLE. Clin Immunol. 2008;126(2):189–201. doi: 10.1016/j.clim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117(2):265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 56.Filippi CM, Juedes AE, Oldham JE, et al. Transforming growth factor-β suppresses the activation of CD8+ T-cells when naive but promotes their survival and function once antigen experienced: a two-faced impact on autoimmunity. Diabetes. 2008;57(10):2684–2692. doi: 10.2337/db08-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khoruts A, Fraser JM. A causal link between lymphopenia and autoimmunity. Immunol Lett. 2005;98(1):23–31. doi: 10.1016/j.imlet.2004.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viglietta V, Kent SC, Orban T, Hafler DA. GAD65-reactive T cells are activated in patients with autoimmune Type 1a diabetes. J Clin Invest. 2002;109(7):895–903. doi: 10.1172/JCI14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monti P, Scirpoli M, Rigamonti A, et al. Evidence for in vivo primed and expanded autoreactive T cells as a specific feature of patients with Type 1 diabetes. J Immunol. 2007;179(9):5785–5792. doi: 10.4049/jimmunol.179.9.5785. [DOI] [PubMed] [Google Scholar]
- 60.Waid DM, Wagner RJ, Putnam A, et al. A unique T cell subset described as CD4loCD40+ T cells (TCD40) in human Type 1 diabetes. Clin Immunol. 2007;124(2):138–148. doi: 10.1016/j.clim.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297(5589):2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 62.Jaeger C, Brendel MD, Hering BJ, Eckhard M, Bretzel RG. Progressive islet graft failure occurs significantly earlier in autoantibody-positive than in autoantibody-negative IDDM recipients of intrahepatic islet allografts. Diabetes. 1997;46(11):1907–1910. doi: 10.2337/diab.46.11.1907. [DOI] [PubMed] [Google Scholar]
- 63.Bosi E, Braghi S, Maffi P, et al. Autoantibody response to islet transplantation in Type 1 diabetes. Diabetes. 2001;50(11):2464–2471. doi: 10.2337/diabetes.50.11.2464. [DOI] [PubMed] [Google Scholar]
- 64.Vantyghem M, Fajardy I, Pigny P, et al. Kinetics of diabetes-associated autoantibodies after sequential intraportal islet allograft associated with kidney transplantation in Type 1 diabetes. Diabetes Metab. 2003;29(6):595–601. doi: 10.1016/s1262-3636(07)70074-5. [DOI] [PubMed] [Google Scholar]
- 65.Jaeger C, Brendel MD, Eckhard M, Bretzel RG. Islet autoantibodies as potential markers for disease recurrence in clinical islet transplantation. Exp Clin Endocrinol Diabetes. 2000;108(5):328–333. doi: 10.1055/s-2000-8125. [DOI] [PubMed] [Google Scholar]
- 66.Sundkvist G, Tyden G, Karlsson FA, Bolinder J. Islet autoimmunity before and after pancreas transplantation in patients with Type 1 diabetes mellitus [letter] Diabetologia. 1998;41(12):1532–1533. doi: 10.1007/s001250051102. [DOI] [PubMed] [Google Scholar]
- 67.Verge CF, Gianani R, Kawasaki E, et al. Prediction of Type 1 diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–933. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 68.Krischer JP, Cuthbertson DD, Yu L, et al. Screening strategies for the identification of multiple antibody-positive relatives of individuals with Type 1 diabetes. J Clin Endocrinol Metab. 2003;88(1):103–108. doi: 10.1210/jc.2002-020760. [DOI] [PubMed] [Google Scholar]
- 69.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human Type 1 diabetes. Proc Natl Acad Sci USA. 2007;104(43):17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin-Pagola A, Sisino G, Allende G, et al. Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with Type 1 diabetes and recurrence of autoimmunity. Diabetologia. 2008;51(10):1803–1813. doi: 10.1007/s00125-008-1105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Velthuis JH, Unger WW, Abreu JR, et al. Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell-associated epitopes using combinatorial MHC-multimers. Diabetes. 2010;59(7):1721–1730. doi: 10.2337/db09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Staeva-Vieira T, Peakman M, von Herrath M. Translational mini-review series on Type 1 diabetes: immune-based therapeutic approaches for Type 1 diabetes. Clin Exp Immunol. 2007;148(1):17–31. doi: 10.1111/j.1365-2249.2007.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skyler JS. Immunomodulation for Type 1 diabetes mellitus. Int J Clin Pract. 2010;166:59–63. doi: 10.1111/j.1742-1241.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 74.Matthews JB, Staeva TP, Bernstein PL, Peakman M, von Herrath M. Developing combination immunotherapies for Type 1 diabetes: recommendations from the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin Exp Immunol. 2010;160(2):176–184. doi: 10.1111/j.1365-2249.2010.04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haller MJ, Atkinson MA, Schatz DA. Efforts to prevent and halt autoimmune β cell destruction. Endocrinol Metab Clin North Am. 2010;39(3):527–539. doi: 10.1016/j.ecl.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. β-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59(10):2340–2348. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atkinson MA, Gianani R. The pancreas in human Type 1 diabetes: providing new answers to age-old questions. Curr Opin Endocrinol Diabetes Obes. 2009;16(4):279–285. doi: 10.1097/MED.0b013e32832e06ba. [DOI] [PubMed] [Google Scholar]
- 78.Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of β cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab. 2007;3(11):758–768. doi: 10.1038/ncpendmet0647. [DOI] [PubMed] [Google Scholar]