Abstract
In all herpesviruses, the capsid is icosahedral in shape, composed of 162 capsomers, and assembled in the infected cell nucleus. Once a closed capsid has formed, it is packaged with the virus DNA and transported to the cytoplasm where further morphogenetic events take place. Herpesvirus capsid populations are highly uniform in shape, and this property has made them attractive for structural analysis particularly by cryo electron microscopy followed by three-dimensional image reconstruction. Here we describe what is known about herpesvirus capsid structure and assembly with emphasis on herpes simplex virus and on the contribution of structural studies. The overall analysis has demonstrated that herpesvirus capsids are formed by a pathway resembling that established for dsDNA bacteriophage such as P22 and HK97. For example herpes capsid assembly is found to: (1) involve a scaffolding protein not present in the mature virus; (2) proceed through a fragile, spherical procapsid intermediate; and (3) result in incorporation of a portal complex at a unique capsid vertex.
Introduction
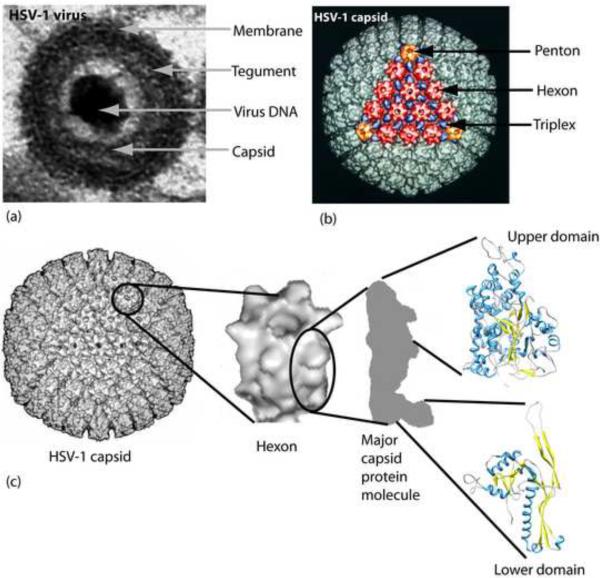
More than 130 characterized viruses are now included in the Herpesvirus family. Most infect vertebrate host animals with a high degree of species specificity and an ability to cause both lytic and latent infections. Humans are the primary host for eight herpesviruses that cause diseases ranging in severity from self-limiting skin infections in the case of herpes simplex virus to mononucleosis, birth defects and cancer in infections by Epstein-Barr virus, human cytomegalovirus and Kaposi's sarcoma-associated herpesvirus, respectively. Herpesviruses all have the same basic structure consisting of four concentric layers, (a) a core composed of the virus dsDNA, (b) a sturdy, icosahedral capsid composed of 162 capsomers that surrounds and protects the DNA, (c) a thick layer of virus-encoded protein called the tegument that lies between the capsid and the envelope membrane, and (d) a membrane derived from the host cell that contains virus-encoded glycoproteins involved in entry and other functions (Fig. 1a).
Figure 1.
Structures of the HSV-1 virion and capsid. (a) Electron micrograph showing a single HSV-1 virion in cross-section. Note the four concentric layers that constitute the virion. The virion diameter is ~210 nm. (b) CryoEM reconstruction of an HSV-1 capsid shown in surface-shaded representation viewed along a face (3-fold axis). One of the capsid faces is illustrated in color with the hexons, pentons and triplexes shown in red, orange and blue, respectively. The capsid diameter is 125 nm. (c) View of the HSV-1 capsid with a single hexon and a single major capsid protein molecule extracted. Also shown are the VP5 upper domain whose structure was determined by X-ray crystallography (1NO7; [17**]), and an example of the bacteriophage capsid protein that docks into the major capsid protein floor domain (2FT1; [47]). Alpha helix, coil and beta structure are indicated in blue, gray and yellow, respectively.
Herpesvirus capsids are highly uniform, robust and symmetric in structure making them attractive for analysis by the methods of structural biology. Cryo electron microscopy followed by three-dimensional image reconstruction (cryoEM) has proved to be well-suited to capsid studies, and structures have now been determined for the capsids of human and several animal herpesviruses. The nature of the capsid structure has suggested testable hypotheses about how it is assembled, and revealing studies have now been pursued with a variety of in vivo and in vitro systems. Here we give an account of herpes capsid structure followed by a description of what has been learned about the mechanism of its assembly. Emphasis is on herpes simplex virus and on the results of structural analysis, an approach that continues to yield important advances.
Capsid structure
Initial studies of herpes simplex virus (HSV-1) capsid structure were carried out with capsids isolated from the infected cell nucleus. Three types of capsids, A, B and C capsids, were isolated and all were found to have the same shell structure. The three differ in the content of the capsid cavity with only C capsids containing the virus DNA and able to mature into infectious virus. A and B capsids are considered to be developmental dead ends [1,2].
CryoEM analysis has been carried out with all three capsid types [3–5]. The resolution has continued to improve in successive reconstructions with 8.5Å the current standard [6**]. In the future, enhanced resolution is expected as advances are made in cryoEM and in methods of sample preparation. Reconstructions have demonstrated that the three capsid types are icosahedral in shape with a diameter of 125 nm. They resemble the capsids of all herpesviruses in that their major structural components are 162 capsomers (150 hexons and 12 pentons) that lie on a T=16 icasahedral lattice (Fig. 1b) Capsomers are connected by way of the capsid floor layer and by 320 triplexes blue in Fig. 1b; [7]). The triplexes are smaller than capsomers, compact in shape, quite uniform in size and located on the surface of the capsid floor (Fig. 1b). Each triplex lies at the local three-fold position created by a group of three capsomers.
One of the twelve pentons, the portal, differs in structure from the other eleven. It is about the same size as a penton and cylindrical in shape with twelve-fold rotational symmetry rather than the five-fold symmetry found in other pentons (Fig. 2a; [8–10]). The portal has an axial channel through which the virus DNA enters and exits the capsid.
Figure 2.
Structures of the HSV-1 portal (a) and CCSC (b). In (a), the structure of the HSV-1 portal is shown beside the portals of phages T7 and P22 [8, 48–49]. Note that the three are similar in size and structure suggesting their function is the same. All are cylindrical in shape with 12-fold rotational symmetry and an axial channel through which virus DNA enters and exits the capsid. In (b) the CCSC is highlighted in green and shown in the full capsid (left) and at higher magnification in a single vertex (right). Note that five CCSC rods project radially outward from each capsid vertex. CCSC graphic courtesy of James Conway (University of Pittsburgh; [25]).
C capsids and capsids in the intact virion have an additional structural feature called the C capsid specific component (CCSC) not found in HSV-1 A or B capsids [11–12*]. CCSCs are rod-shaped with five rods located near each capsid vertex (Fig. 2b; green color). Each rod extends radially outward from the penton connecting it with two adjoining triplexes. The CCSC is considered to support its neighboring penton against the pressure created by the packaged DNA mass. It also provides an interface with the tegument as a prominent tegument protein, UL36, is found to bind the CCSC [13*].
Capsid proteins; VP5
The protein content of HSV-1 C capsids is shown in Table 1. All the hexons and eleven of the twelve pentons are found to be composed of the major capsid protein (MCP; VP5); hexons and pentons contain six and five molecules, respectively. At their distal ends, the hexons also contain six molecules of VP26 arranged in a ring and attached one to each VP5 molecule [14–16]. The outline of VP5 molecules in the hexons and pentons is well described in cryoEM reconstructions (Fig. 1c). In both, VP5 molecules are found to be arranged to create a roughly cylindrical structure (12 nm in diameter × 15 nm long) with an axial channel running through the center. The channel is thought to function as an exit site for the scaffolding protein as DNA enters the capsid (see below). A constriction is found near the center of the axial channel suggesting the channel may be closed at some point in the virus life cycle. High resolution cryoEM reconstructions of the capsid show the location of alpha helices in the VP5 structure [6**].
Table 1.
Protein composition of the HSV-1 C capsid
| Gene | Protein | Amino Acids | Copies/capsid | Location in capsid |
|---|---|---|---|---|
| UL19 | VP5 | 1374 | 955 | Hexons; pentons |
| UL38 | VP19C | 465 | 320 | Triplexes |
| UL18 | VP23 | 318 | 640 | Triplexes |
| UL35 | VP26 | 112 | 900 | Tip of hexon |
| UL6 | pUL6 | 676 | 12 | Unique vertex |
| UL17 | pUL17 | 703 | 60 | Near vertices |
| UL25 | pUL25 | 546 | 60 | Near vertices |
Further information about VP5 structure was obtained by X-ray crystallography. When purified VP5 (1374 aa) was digested with trypsin in solution, a specific fragment corresponding to amino acids 451–1054 was found to be protected from proteolysis [17**]. After purification, this fragment was found to crystallize, and its structure was determined by X-ray crystallography at 2.9Å resolution [17**]. Examination of the structure demonstrated that it fits uniquely into the upper domain of the VP5 volume as defined by cryoEM reconstruction of HSV-1 capsids (Fig. 1c). The fragment, now called the VP5 upper domain (VP5ud), is observed to consist mostly of short regions of α-helix connected by short coils. Two regions of β-structure are found near the fragment base.
The VP5ud fold is unique in that it is not found in the coat protein of any other virus or indeed in any other protein [17**]. The fold is expected to be found, however, in the major capsid proteins of other herpesviruses. Resistance of the VP5ud to trypsin digestion suggests it is a structurally robust region perhaps serving as a nucleation site for folding of the overall molecule [17**]. Determination of the VP5ud structure was a most significant advance in herpes virology as it was the first high resolution structure of any herpes major capsid protein.
Information about the VP5 floor layer came from a most un-expected source. Investigators using X-ray crystallography to study the capsids of dsDNA bacteriophage such as HK97 and T4 noticed that the capsid protein fit reasonably well into the volume of the VP5 floor layer as defined by cryoEM (Fig. 1c; [18*]. It is reasonable to speculate that this structural conservation may underlie the similar mechanisms of capsid formation observed in herpesviruses and dsDNA bacteriophage (see below). The structural conservation also supports the idea that there may be an evolutionary relationship linking herpesviruses and dsDNA bacteriophage [19].
Capsid proteins; triplex proteins
All the triplexes are composed of one VP19C and two VP23 molecules yielding a particle molecular weight of ~119 kDa [7, 20]. Six distinct triplex types, Ta-Tf, can be distinguished in cryoEM reconstructions. The six differ slightly in structure and in the identity of their neighboring capsomers (Fig. 1b; [21**]). The triplexes are required for capsid assembly where they function to hold capsomers together as the structure is formed. High resolution structure analysis of the triplexes has frustrated the best efforts of crystallographers. When insect cells are infected with recombinant baculoviruses (rBV) encoding VP19C and VP23, triplexes form readily and can be purified. Crystals suitable for analysis have not been obtained, however, despite many attempts. It is speculated that the conformational heterogeneity of the triplexes may affect crystal formation.
Capsid proteins: the portal
The portal is composed of twelve copies of the portal protein, pUL6 [22**]. The UL6 protein is required for HSV-1 replication, and homologs are encoded in the genomes of all herpesviruses. Structural analysis of the HSV-1 portal has been carried out with purified portals [8] and with portals in situ in the capsid [9–10]. The two structures are in agreement. They show that the portal is cylindrical in shape with an outside diameter of 16.5 nm, a length of 9 nm and an axial channel ~3 nm in diameter (Fig. 2a). The channel is slightly wider in diameter at the end facing outside the capsid. The structure of the HSV-1 portal is found to be recognizably similar to the portals of dsDNA bacteriophage (Fig. 2a). Like the HSV-1 portal, the dsDNA phage structures are also located at a single capsid vertex, 12-fold symmetric in shape and involved in transport of DNA into and out of the capsid.
Capsid proteins; the CCSC
Each CCSC molecule is composed of one molecule of UL17 protein and one of UL25 (green rods in Fig. 2b; [11–12*, 23]). The two proteins are linearly arranged in the structure with the pUL17 molecule closest to the neighboring penton and pUL25 more distal [11]. CCSC molecules are attached to the capsid in the infected cell nucleus as DNA packaging is completed; pUL17 attachment is required for binding of pUL25 [24]. Binding of pUL25 is found to require amino acids 1–50 [25]. X-ray crystallographic analysis has been done with a fragment (aa 134–580) of the 580 aa pUL25 molecule [26]. The structure is found to consist of a compact core from which four loops project outward.
Capsid assembly
In HSV-1-infected cells, capsid formation takes place in the nucleus in large, spherical structures called inclusion bodies (~5–10/cell; [27]). Assembly-competent inclusion bodies form within 4 hours after initiation of an infection and persist throughout the infection increasing somewhat in number. In electron micrographs, inclusion bodies can be seen to have progeny DNA-containing capsids at their outer edges supporting the view that they are involved in capsid formation. Inclusion bodies stain darkly in electron micrographs, however, so images provide little information about the mechanism of capsid formation. Assembly-competent inclusion bodies have not been isolated from infected cells.
The capsid assembly pathway has been more productively studied with systems based on a panel of recombinant baculoviruses (rBV) encoding HSV-1 capsid proteins [28**–29**]. A uniform population of morphologically normal capsids is formed when insect cells are co-infected with rBV encoding the major capsid, triplex and scaffolding proteins. The panel of rBV is also central for in vitro systems of capsid formation involving: (a) extracts of rBV-infected insect cells [30]; and (b) capsid proteins purified from infected rBV extracts [31**]. Important information about capsid assembly has also been obtained with the use of HSV-1 mutants containing temperature-sensitive lesions in the viral maturational protease (ts1201 and tsProtA; [32–33]). At the non-permissive temperature, capsid formation with these mutants is arrested at the procapsid stage. When cells are transferred to the permissive temperature the ts lesion is reversed and capsid formation proceeds to completion.
Capsid assembly pathway
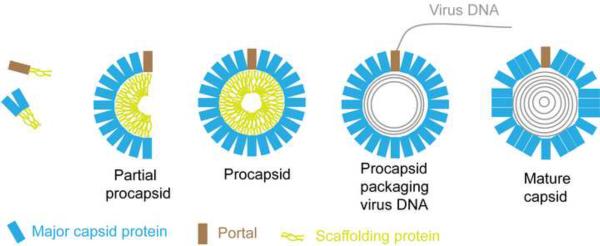
Fig. 3 shows the pathway of capsid assembly deduced from studies with the experimental systems described above. The basic assembly unit is a complex of the major capsid and scaffolding proteins. These associate with each other to form angular segments of the spherical procapsid with binding promoted by scaffold-scaffold interactions and by the triplexes which link VP5 molecules. The angular segments, called partial procapsids, enlarge progressively in size to create the closed, spherical procapsid [34*]. The procapsid has the same diameter as the mature capsid (125 nm), and the same T=16 icosahedral symmetry, but differs in that it is spherical rather than polyhedral in shape (Fig. 4a–4c). Like the major capsid protein, the portal is thought to be incorporated into the nascent procapsid by way of a complex with the scaffolding protein [35*–38]. Incorporation of the portal takes place during initiation or other early stage of procapsid formation [39**].
Figure 3.
Drawing illustrating the pathway of herpesvirus capsid formation. The basic assembly units are small complexes of the major capsid and scaffolding proteins. In the presence of the triplexes (not illustrated), complexes of the major capsid-scaffolding proteins assemble to form angular segments of the closed, spherical procapsid (partial procapsids). A complex of the portal and scaffolding protein is incorporated at an early stage of procapsid formation. Once procapsids are closed, they become the substrate for initiation of DNA encapsidation which occurs concurrently with loss of the scaffolding protein from the procapsid interior and angularization of the capsid shell.
Figure 4.
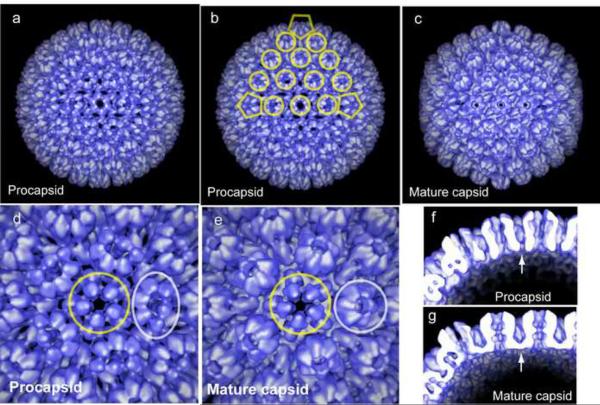
Structure of the HSV-1 procapsid compared to the mature capsid. CryoEM reconstructions are shown in surface rendering as viewed along the 2-fold axis of icosahedral symmetry (a–c). Note that the procapsid is spherical in overall shape while the mature capsid is angular. At higher magnification it can be seen that the hexons in the procapsid are oval in exterior view while those of the mature capsid are hexagonal (compare d and e). The procapsid is found to have openings in the floor layer which are closed in the mature capsid (arrows in f and g).
Once the procapsid is formed, it is packaged with the virus dsDNA genome. DNA transport into the capsid is mediated by a three-subunit complex of virus-encoded proteins called terminase [40]. As DNA enters, the scaffolding protein exits the procapsid and the overall structure angularizes to create the polyhedral shape of the mature capsid (Fig. 3). It is thought that the scaffolding protein exits the capsid by way of the capsomer channels or other openings in the procapsid structure. Detachment of the scaffolding protein from the capsid shell is promoted by a proteolytic cut near the C-terminal end of the scaffolding protein molecule catalyzed by the virus-encoded maturational protease. The same proteolytic cut is also involved in initiation of procapsid angularization [41–43].
Structural analysis of assembly components
Structural analysis of the assembly pathway has focused on the procapsid and on the maturational protease. Procapsids have been isolated from cells infected with an HSV-1 mutant, m100 [33], deficient in the maturational protease and the structure of the procapsids was determined by cryoEM [44]. Purified procapsids from the same source have been induced to mature in vitro, and cryoEM structures have been determined for intermediates in the maturation process [21**]. The resulting set of structures has been most revealing about the capsid maturation pathway. The structures have shown, for example, that the procapsid and mature capsid differ significantly in the floor layer; the floor is fully intact in the mature capsid but lacking almost entirely in the procapsid (compare Figs. 4f and 4g). Capsomers in the procapsid are connected only by the triplexes and the scaffolding protein leaving numerous openings in what will become the capsid floor. Many other differences are revealed including a “clam shell” shape to the hexons of the procapsid which mature to create the hexagonal shape found in the mature capsid (compare Figs. 4d and 4e).
The maturational protease is required for HSV-1 replication and a homolog is encoded in the genomes of all herpesviruses. Its importance for replication has made the protease the focus of intense analysis including an X-ray crystallographic structure [45]. So far, however, the structure has not suggested the nature of effective anti-herpes therapeutics directed at the protease.
Future prospects
Despite the impressive progress so far, further clarification of herpesvirus capsid structure and assembly would be most welcome. For example, higher resolution in the structures of the capsid and procapsid could reveal why some VP5 molecules are incorporated into hexons and others pentons. There is much to be learned at higher resolution about the structure of the procapsid and how it is transformed into the mature form. The mechanism of DNA encapsidation and the structure of the tegument are areas where current understanding is at a more rudimentary level. In the case of DNA packaging, the players in the process are known, the capsid, portal and three-subunit terminase. Little is known, however, about the structure of the terminase and how it interfaces with the portal. The tegument attaches directly to the capsid [46], so it is an obvious way studies of the capsid could be extended. Its more fluid organization, however, will challenge even the most advanced methods of structural analysis.
Highlights
-
-
X-ray crystallographic structure determined for part of the HSV-1 major capsid protein.
-
-
HSV-1 capsid assembly occurs by way of a procapsid intermediate as observed in dsDNA bacteriophage.
-
-
use of cryoelectron microscopy to identify steps in the procapsid maturation pathway.
-
-
HSV-1 portal identified at a unique capsid vertex.
-
-
portal involved in initiation of HSV-1 capsid assembly.
Acknowledgements
For help with the graphics we gratefully acknowledge James Conway, Benes Trus and Bernard Heymann. Work in our laboratory is supported by NIH award AI-041644.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Homa FL, Brown JC. Capsid assembly and DNA packaging in herpes simplex virus. Reviews in Medical Virology. 1997;7:107–122. doi: 10.1002/(sici)1099-1654(199707)7:2<107::aid-rmv191>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Rixon FJ. Structure and assembly of herpesviruses. Seminars in Virol. 1993;4:135–144. [Google Scholar]
- 3.Booy FP, Newcomb WW, Trus BL, Brown JC, Baker TS, Steven AC. Liquid-crystalline, phage-like packing of encapsidated DNA in herpes simplex virus. Cell. 1991;64:1007–1015. doi: 10.1016/0092-8674(91)90324-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou ZH, Prasad BVV, Jakana J, Rixon FJ, Chiu W. Protein subunit structures in herpes simplex virus A-capsid determined from 400kV spot-scan electron cryomicroscopy. J. Mol. Biol. 1994;242:456–469. doi: 10.1006/jmbi.1994.1594. [DOI] [PubMed] [Google Scholar]
- 5.Zhou ZH, Chiu W, Haskell K, Spears H, Jr., Jakana J, Rixon FJ, Scott LR. Refinement of herpesvirus B-capsid structure on parallel supercomputers. Biophys. J. 1998;74:576–588. doi: 10.1016/S0006-3495(98)77816-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **6.Zhou ZH, Dougherty M, Jakana J, He J, Rixon FJ, Chiu W. Seeing the herpesvirus capsid at 8.5 A. Science. 2000;288:877–880. doi: 10.1126/science.288.5467.877. [DOI] [PubMed] [Google Scholar]; The highest resolution yet obtained for a herpesvirus capsid. Alpha helices can be seen in the structure, and the structure has been used to validate crystallographic information about the VP5 upper domain and ideas about the relationship between the floor domain and the capsids of dsDNA bacteriophage.
- 7.Newcomb WW, Trus BL, Booy FP, Steven AC, Wall JS, Brown JC. Structure of the herpes simplex virus capsid: molecular composition of the pentons and the triplexes. J. Mol. Biol. 1993;232:499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- 8.Trus BL, Cheng N, Newcomb WW, Homa FL, Brown JC, Steven AC. Structure and polymorphism of the UL6 portal protein of herpes simplex virus type 1. J. Virol. 2004;78:12668–12671. doi: 10.1128/JVI.78.22.12668-12671.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rochat RH, Liu X, Murata K, Nagayama K, Rixon FJ, Chiu W. Seeing the portal in herpes simplex virus type 1 B capsids. J. Virol. 2011;85:1871–1874. doi: 10.1128/JVI.01663-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardone G, Winkler DC, Trus BL, Cheng N, Heuser JE, Newcomb WW, Brown JC, Steven AC. Visualization of the herpes simplex virus portal in situ by cryo-electron tomography. Virol. 2006 doi: 10.1016/j.virol.2006.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conway JF, Cockrell SK, Copeland AM, Newcomb WW, Brown JC, Homa FL. Labeling and localization of the herpes simplex virus capsid protein UL25 and its interaction with the two triplexes closest to the penton. J. Mol. Biol. 2010;397:575–586. doi: 10.1016/j.jmb.2010.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *12.Trus BL, Newcomb WW, Cheng N, Cardone G, Marekov L, Homa FL, Brown JC, Steven AC. Allosteric signaling and a nuclear exit strategy: binding of UL25/UL17 heterodimers to DNA-Filled HSV-1 capsids. Mol. Cell. 2007;26:479–489. doi: 10.1016/j.molcel.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; First reported structure of the CCSC including information about its protein composition.
- *13.Coller KE, Lee JI, Ueda A, Smith GA. The capsid and tegument of the alphaherpesviruses are linked by an interaction between the UL25 and VP1/2 proteins. J. Virol. 2007;81:11790–11797. doi: 10.1128/JVI.01113-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration that a prominent tegument protein, UL36, binds to the HSV-1 capsid by way of the CCSC.
- 14.Booy FP, Trus BL, Newcomb WW, Brown JC, Conway JF, Steven AC. Finding a needle in a haystack: Detection of a small protein (the 12-kDa VP26) in a large complex (the 200-MDa capsid of herpes simplex virus) Proc. Natl. Acad. Sci. USA. 1994;91:5652–5656. doi: 10.1073/pnas.91.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wingfield PT, Stahl SJ, Thomsen DR, Homa FL, Booy FP, Trus BL, Steven AC. Hexon-only binding of VP26 reflects differences between the hexon and penton conformations of VP5, the major capsid protein of herpes simplex virus. J. Virol. 1997;71:8955–8961. doi: 10.1128/jvi.71.12.8955-8961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou ZH, He J, Jakana J, Tatman JD, Rixon FJ, Chiu W. Assembly of VP26 in herpes simplex virus-1 inferred from structures of wild-type and recombinant capsids. Nature Struct.Biol. 1995;2:1026–1030. doi: 10.1038/nsb1195-1026. [DOI] [PubMed] [Google Scholar]
- **17.Bowman BR, Baker ML, Rixon FJ, Chiu W, Quiocho FA. Structure of the herpesvirus major capsid protein. EMBO J. 2003;22:757–765. doi: 10.1093/emboj/cdg086. [DOI] [PMC free article] [PubMed] [Google Scholar]; This was the first X-ray crystallographic structure of a region of a herpesvirus major capsid protein. A novel protein fold was revealed.
- *18.Baker ML, Jiang W, Rixon FJ, Chiu W. Common ancestry of herpesviruses and tailed DNA bacteriophages. J Virol. 2005;79:14967–14970. doi: 10.1128/JVI.79.23.14967-14970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Creative use of structural analysis to deduce evolutionary relationships linking animal viruses and bacteriophage. This was a most influential contribution.
- 19.Bamford DH, Grimes JM, Stuart DI. What does structure tell us about virus evolution? Curr. Opin. Struct. Biol. 2005;15:655–663. doi: 10.1016/j.sbi.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Spencer JV, Newcomb WW, Thomsen DR, Homa FL, Brown JC. Assembly of the herpes simplex virus capsid: pre-formed triplexes bind to the nascent capsid. J. Virol. 1998;72:3944–3951. doi: 10.1128/jvi.72.5.3944-3951.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Heymann JB, Cheng N, Newcomb WW, Trus BL, Brown JC, Steven AC. Dynamics of herpes simplex virus capsid maturation visualized by time lapse cryo-electron microscopy. Nat. Struct. Biol. 2003;10:334–341. doi: 10.1038/nsb922. [DOI] [PubMed] [Google Scholar]; CryoEM was used skillfully and creatively to determine the structures of intermediates between the HSV-1 procapsid and mature capsid. Many interesting features of the transition were revealed.
- **22.Newcomb WW, Juhas RM, Thomsen DR, Homa FL, Burch AD, Weller SK, Brown JC. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J. Virol. 2001;75:10923–10932. doi: 10.1128/JVI.75.22.10923-10932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; The UL6 protein encoded by HSV-1 was found to have the same structure as bacteriophage portals. The finding suggested that the mechanism of DNA encapsidation is the same in herpesviruses as it is in dsDNA phage.
- 23.Newcomb WW, Homa FL, Brown JC. Herpes simplex virus capsid structure: DNA packaging protein UL25 is located on the external surface of the capsid near the vertices. J Virol. 2006;80:6286–6294. doi: 10.1128/JVI.02648-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurlow JK, Murphy M, Stow ND, Preston VG. Herpes simplex virus type 1 DNA-packaging protein UL17 is required for efficient binding of UL25 to capsids. J Virol. 2006;80:2118–2126. doi: 10.1128/JVI.80.5.2118-2126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cockrell SK, Huffman JB, Toropova K, Conway JF, Homa FL. Residues of the UL25 Protein of Herpes Simplex Virus That Are Required for Its Stable Interaction with Capsids. J. Virol. 2011;85:4875–4887. doi: 10.1128/JVI.00242-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowman BR, Welschhans RL, Jayaram H, Stow ND, Preston VG, Quiocho FA. Structural characterization of the UL25 DNA-packaging protein from herpes simplex virus type 1. J Virol. 2006;80:2309–2317. doi: 10.1128/JVI.80.5.2309-2317.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng L, Ryazantsev S, Sun R, Zhou ZH. Three-dimensional visualization of gammaherpesvirus life cycle in host cells by electron tomography. Structure. 2010;18:47–58. doi: 10.1016/j.str.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **28.Thomsen DR, Roof LL, Homa FL. Assembly of herpes simplex virus (HSV) intermediate capsids in insect cells infected with recombinant baculoviruses expressing HSV capsid proteins. J. Virol. 1994;68:2442–2457. doi: 10.1128/jvi.68.4.2442-2457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper and reference 29 below were the first to demonstrate that morphologically normal HSV-1 capsids can be assembled in insect cells multiply infected with recombinant baculoviruses encoding HSV-1 capsid proteins. Studies with this system were highly revealing about the mechanism of capsid formation.
- **29.Tatman JD, Preston VG, Nicholson P, Elliott RM, Rixon FJ. Assembly of herpes simplex virus type 1 capsids using a panel of recombinant baculoviruses. J. Gen. Virol. 1994;75:1101–1113. doi: 10.1099/0022-1317-75-5-1101. [DOI] [PubMed] [Google Scholar]; See reference 28 above.
- 30.Newcomb WW, Homa FL, Thomsen DR, Ye Z, Brown JC. Cell-free assembly of the herpes simplex virus capsid. J. Virol. 1994;68:6059–6063. doi: 10.1128/jvi.68.9.6059-6063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **31.Newcomb WW, Homa FL, Thomsen DR, Trus BL, Cheng N, Steven AC, Booy FP, Brown JC. Assembly of the herpes simplex virus procapsid from purified components and identification of small complexes containing the major capsid and scaffolding proteins. J. Virol. 1999;73:4239–4250. doi: 10.1128/jvi.73.5.4239-4250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]; Studies described here demonstrated that HSV-1 capsids can be assembled in vitro from purified HSV-1 capsid proteins only. Host-encoded proteins are not required to form structurally normal capsids.
- 32.Preston VG, Coates JAV, Rixon FJ. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J. Virol. 1983;45:1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao M, Matusick-Kumar L, Hurlburt W, DiTusa SF, Newcomb WW, Brown JC, McCannIII PJ, Deckman I, Colonno RJ. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J. Virol. 1994;68:3702–3712. doi: 10.1128/jvi.68.6.3702-3712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Newcomb WW, Homa FL, Thomsen DR, Booy FP, Trus BL, Steven AC, Spencer JV, Brown JC. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid assembly. J. Mol. Biol. 1996;263:432–446. doi: 10.1006/jmbi.1996.0587. [DOI] [PubMed] [Google Scholar]; Characterization of intermediates in the HSV-1 capsid assembly pathway as observed with extracts derived from rBV-infected insect cells. The assembly pathway shown in Fig. 3 was derived in large part from this study.
- *35.Newcomb WW, Thomsen DR, Homa FL, Brown JC. Assembly of the herpessimplex virus capsid: identification of soluble scaffold-portal complexes and their role in formation of portal-containing capsids. J. Virol. 2003;77:9862–9871. doi: 10.1128/JVI.77.18.9862-9871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration that HSV-1 capsids are assembled from small complexes of the major capsid and scaffolding proteins.
- 36.Singer GP, Newcomb WW, Thomsen DR, Homa FL, Brown JC. Identification of a region in the herpes simplex virus scaffolding protein required for interaction with the portal. J. Virol. 2005;79:1–11. doi: 10.1128/JVI.79.1.132-139.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang K, Baines JD. Domain within herpes simplex virus 1 scaffold proteins required for interaction with portal protein in infected cells and incorporation of the portal vertex into capsids. J. Virol. 2008;82:5021–5030. doi: 10.1128/JVI.00150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huffman JB, Newcomb WW, Brown JC, Homa FL. Amino acids 143 to 150 of the herpes simplex virus type 1 scaffold protein are required for the formation of portal-containing capsids. J. Virol. 2008;82:6778–6781. doi: 10.1128/JVI.00473-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **39.Newcomb WW, Homa FL, Brown JC. Involvement of the portal at an early step in herpes simplex virus capsid assembly. J Virol. 2005;79:10540–10546. doi: 10.1128/JVI.79.16.10540-10546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study clarified how virus capsids can be formed with the portal at a single capsid site. The portal is incorporated by a mechanism unique to the initiation step of assembly.
- 40.Yang K, Homa F, Baines JD. Putative terminase subunits of herpes simplex virus 1 form a complex in the cytoplasm and interact with portal protein in the nucleus. J. Virol. 2007;81:6419–6433. doi: 10.1128/JVI.00047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu F, Roizman B. Characterization of the protease and other products of amino-terminus-proximal cleavage of the herpes simplex virus 1 UL26 protein. J Virol. 1993;67:1300–1309. doi: 10.1128/jvi.67.3.1300-1309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matusick-Kumar L, Newcomb WW, Brown JC, McCann PJ, Hurlburt W, Weinheimer SP, Gao M. The C-terminal 25 amino acids of the protease and its substrate ICP35 of herpes simplex virus type 1 are involved in formation of sealed capsids. J. Virol. 1995;69:4347–4356. doi: 10.1128/jvi.69.7.4347-4356.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kennard J, Rixon FJ, McDougall IM, Tatman JD, Preston VG. The 25 amino acid residues at the carboxyl terminus of the herpes simplex virus type 1 UL26.5 protein are required for the formation of the capsid shell around the scaffold. J. Gen. Virol. 1995;76:1611–1621. doi: 10.1099/0022-1317-76-7-1611. [DOI] [PubMed] [Google Scholar]
- 44.Newcomb WW, Trus BL, Cheng N, Steven AC, Sheaffer AK, Tenney DJ, Weller SK, Brown JC. Isolation of herpes simplex virus procapsids from cells infected with a protease-deficient mutant virus. J. Virol. 2000;74:1663–1673. doi: 10.1128/jvi.74.4.1663-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoog SS, Smith WW, Qiu X, Janson CA, Hellmig B, McQueney MS, O'Donnell K, O'Shannessy D, DiLella AG, Debouck C, Abdel-Meguid SS. Active site cavity of herpesvirus proteases revealed by the crystal structure of herpes simplex virus protease/inhibitor complex. Biochem. 1997;36:14023–14029. doi: 10.1021/bi9712697. [DOI] [PubMed] [Google Scholar]
- 46.Grunewald K, Desai P, Winkler DC, Heymann JB, Belnap DM, Baumeister W, Steven AC. Three-dimensional structure of herpes simplex virus from cryo-electron tomography. Science. 2003;302:1396–1398. doi: 10.1126/science.1090284. [DOI] [PubMed] [Google Scholar]
- 47.Gan L, Speir JA, Conway JF, Lander G, Cheng N, Firek BA, Hendrix RW, Duda RL, Liljas L, Johnson JE. Capsid conformational sampling in HK97 maturation visualized by X-ray crystallography and cryo-EM. Structure. 2006;14:1655–1665. doi: 10.1016/j.str.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Chen DH, Baker ML, Hryc CF, DiMaio F, Jakana J, Wu W, Dougherty M, Haase-Pettingell C, Schmid MF, Jiang W, Baker D, King JA, Chiu W. Structural basis for scaffolding-mediated assembly and maturation of a dsDNA virus. Proc. Natl. Acad. Sci. U.S.A. 2011;108:1355–1360. doi: 10.1073/pnas.1015739108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agirrezabala X, Martin-Benito J, Valle M, Gonzalez JM, Valencia A, Valpuesta JM, Carrascosa JL. Structure of the connector of bacteriophage T7 at 8A resolution: structural homologies of a basic component DNA translocating machinery. J. Mol. Biol. 2005;347:895–902. doi: 10.1016/j.jmb.2005.02.005. [DOI] [PubMed] [Google Scholar]