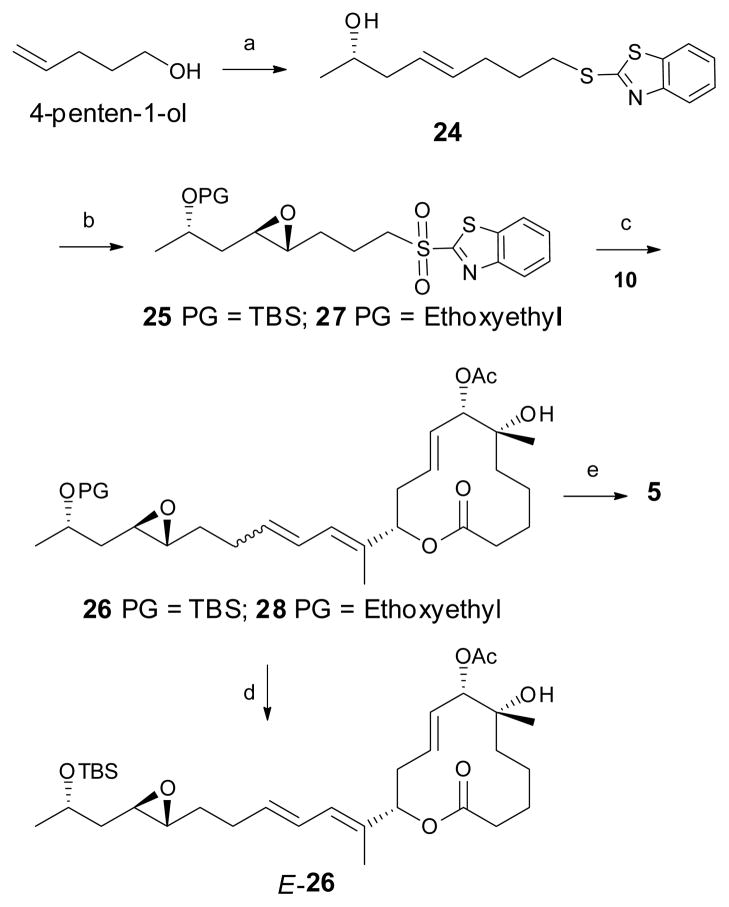
Scheme 4.
The synthesis of C15–C22 unit, fragment coupling and the synthesis of simplified pladienolide analogs E-26 and 5. Reagents and conditions: a) i. 2-mercaptobenzothiazole, TPP, DIAD, 90%; ii. (S)-4-penten-2-ol, 2nd generation Grubbs catalyst, 60%; b) i. (NH3)6Mo7(H2O)4, H2O2, EtOH, 72%; ii. Shi epoxidation catalyst, oxone, K2CO3, 59%; iii. TBSOTf, 2,6-lutidine, 86% for compound 25; Ethyl vinyl ether, PPTS, CH2Cl2, 85% for compound 27; c) 10, NaHMDS, THF, E:Z 72:28, 54% for compound 26; mixture of stereoisomers, 38% for compound 28; d) SFC; e) 28, PPTS, MeOH, E:Z 72:28, 25%. (The reaction sequence with ethoxyethyl protecting group are presented in italics).