Abstract
Two structurally distinct peptides, angiotensin IV and LVV-haemorphin 7, both competitive high-affinity inhibitors of insulin-regulated aminopeptidase (IRAP), were found to enhance aversion-associated and spatial memory in normal rats and to improve performance in a number of memory tasks in rat deficits models. These findings provide compelling support for the development of specific, high-affinity inhibitors of the enzyme as new cognitive enhancing agents. Different classes of IRAP inhibitors have been developed including peptidomimetics and small molecular weight compounds identified through in silico screening with a homology model of the catalytic domain of IRAP. The proof of principal that inhibition of IRAP activity results in facilitation of memory has been obtained by the demonstration that the small-molecule IRAP inhibitors also exhibit memory-enhancing properties.
Keywords: memory, angiotensin IV, GLUT4, peptidomimetic, vasopressin, oxytocinase, glucose, drug development
Cognitive enhancers
Dementia is the most common medical condition involving cognitive impairment. According to the World Health Organization, 37 million people worldwide suffer from some form of dementia. It is a progressive condition primarily in old people of which Alzheimer's dementia (AD) is the most prevalent form. The incidence of mild cognitive impairment (MCI), the transitional phase between normal ageing and dementia, is on the rise, affecting 3% to 17% of people over the age of 65 years, depending on the diagnostic criteria used and type of cohort studied (Portet et al., 2006). The Peripheral and Central Nervous System Drugs Advisory Committee in the USA predicted that more than 80% of patients with MCI will go on to develop AD within 10 years at a rate of 10–15% per year. Although AD is the most common form of dementia, a range of other medical conditions can cause cognitive decline and memory impairment. These include multiple cerebral infarcts, hypoxic damage or head trauma. As a result of increases in life expectancy and average age of the general population, the prevalence of dementia and cognitive decline in the developed world has risen and will continue to rise significantly unless effective therapies are developed.
There is currently no cure for dementia – all of the prescribed medications are designed to delay the onset or manage the symptoms, which ultimately lead to improved quality of life. Most current therapies approved by the Food and Drug Administration for treating dementia and other memory deficits belong to the class of cholinesterase inhibitors with sales of the most commonly prescribed cholinesterase inhibitor, Aricept, achieving 75% of market share. These drugs act by preventing the breakdown of acetylcholine, thereby prolonging the action of the neurotransmitter and are considered first-line treatment for the cognitive symptoms of mild to moderate forms AD, but they suffer from modest efficacy (Kaduszkiewicz et al., 2005). The approval of memantine, which targets N-methyl-D-aspartate (NMDA) receptor, was granted in 2002 in Europe and 2003 in the USA. Although efficacy of memantine in the treatment of moderate to severe AD had been inconsistent (Thomas and Grossberg, 2009), this NMDA receptor antagonist was approved for this indication to avoid direct competition with the cholinesterase inhibitors. A number of novel approaches targeting central glutaminergic, serotonergic, GABAergic, adrenergic systems as well as ion channels and phosphodiesterase enzymes are currently being trialled as pro-mnemonic agents (Buccafusco, 2009).
Angiotensin IV (Ang IV) and memory
The hexapeptide Ang IV, which was initially thought of as an inactive metabolic fragment of the vasoconstrictor peptide angiotensin II, has memory-enhancing properties, improving performance in a number of memory tasks when injected in the brains of rats. The first studies demonstrated that administration of an acute dose of Ang IV into the lateral ventricle of rat brain resulted in facilitation of memory retention and retrieval in the conditioned and fear avoidance paradigms (Braszko et al., 1988; Wright et al., 1993). The mnemonic effects of Ang IV were also observed for other forms of memory. Central acute or chronic infusion of Ang IV or its more stable analogues (Norleucine Ang IV, Norleucinal Ang IV, des-Phe6-Ang IV) into rats also enhanced spatial reference memory in the Barnes, swim and radial arm mazes (Wright et al., 1999; Lee et al., 2004; Braszko et al., 2008), and spatial working memory in the spontaneous alternation plus maze task (de Bundel et al., 2009). Ang IV and des-Phe6-Ang IV were also shown to improve performance in the object recognition task as well as in the radial arm maze in rats – these effects were purported to be mediated via interactions with central dopaminergic pathways (Braszko, 2004; 2009; Braszko et al., 2008;), although the lack of specificity as to which dopamine receptor subtype is involved remains a concern (Braszko, 2010). The memory-enhancing effects of Ang IV have also been demonstrated in mice where strain differences have been reported in the response (Golding et al., 2010).
More importantly from a therapeutic viewpoint, centrally administered Ang IV or its analogues are able to reverse memory deficits induced by a broad range of experimental insults. These include four vessel occlusion rat model of global ischaemia (Wright et al., 1996), bilateral perforant pathway lesions (Wright et al., 1999), perturbations of central cholinergic systems by the muscarinic receptor antagonist scopolamine or the nicotinic receptor antagonist mecamylamine pretreatment (Pederson et al., 1998; 2001; Albiston et al., 2004; Olson et al., 2004; Olson and Cero, 2010), and chronic alcohol exposure (Wisniewski et al., 1993).
In support of its memory effects, Ang IV and its analogues have been shown to facilitate long-term potentiation (LTP) in the dentate gyrus of rats in vivo (Wayner et al., 2001) and also to enhance LTP (Kramar et al., 2001) and to attenuate alcohol-induced suppression of LTP (Wright et al., 2003) in the CA1 region of the hippocampus in vitro. Similarly, the structurally distinct decapeptide LVV-haemorphin 7 (LVV-H7), which was isolated from sheep cerebral cortex and identified through competition with radiolabelled Ang IV binding as a high-affinity ligand (Moeller et al., 1997), was able to mimic the effects of Ang IV on enhancing performance in two spatial memory tests, the Barnes circular maze (Lee et al., 2004) and the spontaneous alternation task (de Bundel et al., 2009).
Ang IV targets
It is therefore of therapeutic interest that the target protein in the brain that mediates the memory-enhancing effects of Ang IV and LVV-H7 be identified. The most compelling evidence, as outlined below, support insulin-regulated aminopeptidase (IRAP) as the target binding site of Ang IV and LVV-H7. First, both peptides are high-affinity, competitive inhibitors of IRAP (Lew et al., 2003), competing with the peptide substrates for binding to the catalytic site. The distribution of the binding site for Ang IV, characterized by high-affinity binding of radiolabelled Ang IV/Nle1Ang IV, parallels the localization of IRAP mRNA in adjacent sections of mouse brain (Albiston et al., 2001). Cells transfected with IRAP expressed a high affinity Ang IV binding site resulting in a gain of function (Albiston et al., 2001). Moreover, a dramatic loss of 125I- Nle1Ang IV binding is observed in the sections prepared from brains of the IRAP knockout mouse (Albiston et al., 2010a). As will be discussed in section 4.2, small molecule, non-peptide highly selective inhibitors of IRAP have been identified and developed using a model of the catalytic site of IRAP, recapitulating the physiological effects of the peptide inhibitors on memory and glucose uptake (Albiston et al., 2008; Fernando et al., 2008; de Bundel et al., 2009).
A number of alternative proteins have been proposed as the specific binding site for Ang IV. This hexapeptide has been reported to bind, at high concentrations, to a number of other proteins including the angiotensin AT1 receptor and aminopeptidase N (APN). Indeed, some of the vasoconstrictor effects in the different vascular beds attributed to Ang IV were the result of the binding to and activation of AT1 receptors (Cheng et al., 1994; Loufrani et al., 1999). Recently, the hepatocyte growth factor (HGF) receptor C-Met was put forward as the binding site mediating the some of the effects of Ang IV – this claim is based on the structural homology of the Ang IV analogue Norleual to the hinge region of HGF (Yamamoto et al., 2010). However, the binding and actions of the native peptide, Ang IV, or other analogues were not investigated in the C-Met system. Therefore, IRAP is the only validated target for the novel pharmacological effects of Ang IV and its analogues.
IRAP
Structure
IRAP (EC 3.4.11.3) is a type II transmembrane protein that belongs to the M1 aminopeptidase, a family characterized by two distinct motifs, the HEXXH zinc-binding and the GXMEN substrate recognition sequence in its C-terminal catalytic domain (Keller et al., 1995; Rogi et al., 1996). A feature unique to IRAP amongst the aminopeptidase family is the 109 amino acid N-terminal cytoplasmic domain that contains two dileucine motifs that are preceded by acidic clusters. These are characteristic trafficking motifs. Under normal basal conditions, IRAP is present predominantly in vesicles, with the large catalytic domain located intraluminally and the N-terminal domain in the cytosol. When present at the plasma membrane, the catalytic site is exteriorized to facilitate the processing of peptide hormones that are released into the extracellular milieu.
Function
The physiological role of IRAP is not well understood. Prior to its purification from bovine adrenal membranes and its identification as a specific Ang IV binding site, the AT4 receptor (Albiston et al., 2001), IRAP was first cloned in adipocytes as the protein that accompanies the glucose transporter GLUT4 to the plasma membrane following insulin stimulation (Keller et al., 1995). The same protein was also isolated from the placenta as oxytocinase (Rogi et al., 1996), the enzyme that regulates circulating oxytocin levels during the later stages of human pregnancy.
Peptide hormone cleavage
In addition to oxytocin, IRAP has the ability to cleave the structurally similar cyclic peptide, vasopressin as well as a number of other peptide substrates including somatostatin, cholecystokinin-8, lys-bradykinin, angiotensin III, met-enkephalin, dynorphin A 1–8, neurokinin A and neuromedin B in vitro (Herbst et al., 1997; Matsumoto et al., 2001a,b; Lew et al., 2003). The physiologically relevant substrates of IRAP remain to be elucidated, although insights from the global IRAP knockout mouse suggest that vasopressin is a plausible candidate (Wallis et al., 2007). However, the major determinants as to which peptide substrates are cleaved by IRAP include the co-location of the components, either intraluminally, extracellularly or circulating in the blood stream, as well as the binding affinity of the substrate and velocity of catalysis.
Major histocompatibility class (MHC) class I antigen processing
Certain aminopeptidases appear now to play a key role in the last, yet crucial, proteolytic steps that generate small peptides for presentation onto MHC class I molecules. This allows the mature MHC–peptide complexes to be recognized by cytotoxic T lymphocytes. Close family members to IRAP, endoplasmic reticulum aminopeptidase 1 and 2 (ERAP1, ERAP2), were identified as enzymes involved in the generation of mature antigenic epitopes from peptide precursors that are delivered into the ER by a transporter associated with antigen processing (Saveanu et al., 2005). Recently, IRAP has also been implicated in the generation of antigenic peptide for cross-presentation, not in the ER but in endosomal compartments (Saveanu et al., 2009) (Segura et al., 2009) and in patterns that are distinct from those processed by the ERAPs (Georgiadou et al., 2010). Therefore, not only has a new function for IRAP been discovered but also the peptide substrate specificity is significantly broader than previously proposed (Albiston et al., 2007).
GLUT4 vesicle trafficking
IRAP was so named because the protein was first identified in close association with the facilitative glucose transporter, GLUT4, in specialized vesicles called GLUT4 specialized vesicles (GSV) found in fat and muscle cells (Keller et al., 1995). These GSVs respond to insulin receptor stimulation, translocating to the plasma membrane to facilitate glucose uptake into these cells (Bryant et al., 2002). IRAP is proposed to play a role in regulating the trafficking of GSVs because of the presence of the dileucine motifs in the cytoplasmic tail, which when injected into fat cells resulted in the translocation of the vesicles to the cell surface (Waters et al., 1997).
Insights from the IRAP knockout mice
Given the diverse functions ascribed for IRAP, the IRAP knockout (KO) mice demonstrate only modest phenotypic characteristics. In the absence of IRAP, GLUT4 levels are significantly reduced in fat and muscle (up to 80%) resulting in decreases of both basal and insulin-stimulated glucose uptake (Keller et al., 2002). However, despite the significant loss of GLUT4, IRAP KO mice are still able to maintain normal glucose levels (Keller et al., 2002). In contrast to the reported decreases in GLUT4 in the muscle and fat of IRAP KO mice (Keller et al., 2002), glucose transporter levels are not significantly altered in various brain regions, including the hippocampus and cortex, where there is almost complete co-localization between IRAP and GLUT4 in neurons (Fernando et al., 2008). Moreover, glucose uptake following potassium-induced depolarization in hippocampal slices, which is mediated by GLUT4, is the same for IRAP KO and wild-type (WT) mice (Fernando et al., 2008).
IRAP has been shown to be highly expressed in the placenta, where it is up-regulated during pregnancy and is released into the maternal circulation. Circulating IRAP has been suggested to play an important role in maintaining oxytocin levels during pregnancy to prevent the onset of premature labour and ensure adequate blood supply to the foetus. Interestingly, IRAP KO mice are able to reproduce normally, with normal litter size and frequency, and survival of pups is the same as for WT animals (Keller et al., 2002; Pham et al., 2009). However, a possible explanation for the lack of an obvious developmental phenotype following gene deletion of IRAP could be due to the fact that circulating levels of IRAP detected in the maternal blood during pregnancy have only been reported in humans (Yamahara et al., 2000). Moreover, the sequence identified as the cleavage site for the release of soluble IRAP is not conserved and is absent in most mammalian species except in higher order primates (Rosenbloom et al., 1975). Hence, the absence of circulating IRAP in mice during pregnancy is due to the inability of a secretase to cleave the membrane bound enzyme to release into the circulation (Pham et al., 2009).
Recently, Wallis et al. (2007) reported an increase in plasma vasopressin levels in IRAP KO mice, suggesting that IRAP may play a role in regulating levels of this peptide hormone. Vasopressin has been reported to have memory-enhancing properties (McEwen, 2004), which is consistent with the proposal that inhibitors of IRAP enhance memory by extending the half-life of its neuropeptide substrate, in this case, vasopressin. Along the same line, the IRAP KO mice would be expected to perform better in memory tasks; however, this was not the case. Contrary to expectation, the IRAP KO mice exhibited an age-related deficit in a spatial memory task (Albiston et al., 2010a). This surprising finding could be due to altered normal brain development as a result of the permanent germline deletion of the IRAP gene, as high levels of IRAP expression have been observed in the highly neurogenic subventricular zone of the embryonic mouse brain (Chai et al., 2001).
Although the role of IRAP in peptide trimming for MHC class I antigen presentation has also been studied in the IRAP KO mice, its physiological significance is far from resolved. In human dendritic cells, IRAP is thought to play a role in trimming peptides for antigen cross-presentation in an alternate pathway, in endosomal compartments rather than in the endoplasmic reticulum (Saveanu et al., 2009; Segura et al., 2009). IRAP deficiency was shown to compromise cross-presentation and not endogenous presentation (Saveanu et al., 2009) but only in inflammatory dendritic cells (Segura et al., 2009).
Mechanisms of action
The distribution of IRAP in the brain provides valuable insights into its physiological role, with high concentrations of the enzyme present in regions involved in processing cognitive function including the prefrontal and entorhinal cortices, hippocampus, basal forebrain and amygdala (Fernando et al., 2005). IRAP is found predominantly in neurons where the enzyme occurs intracellularly in large dense core vesicles, in the endoplasmic reticulum and golgi (Fernando et al., 2007). The factor(s) that stimulate the translocation of IRAP to the plasma membrane in neurons have not been elucidated, although activation of adenylyl cyclase by dibutyryl cyclic AMP has been shown to result in the mobilization of IRAP to the neuronal cell surface (Fernando et al., 2008). Although inhibitors of IRAP facilitate both short- and long-term memory, as well as have a beneficial impact on learning, the physiological function of IRAP in the brain is unknown (Albiston et al., 2007). Based on the known functions of IRAP in the periphery, we propose two potential mechanisms of action by which IRAP inhibitors may enhance memory.
Aminopeptidase activity
Although modulation of neuropeptide degradation by IRAP inhibitors is the most straightforward proposition, it is unclear which of the IRAP substrates are clearly associated with memory, and studies looking at changes in neuropeptide levels following inhibitor treatment are limited and technically challenging. IRAP is capable of degrading a range of neuropeptides in vitro (section 3.2.). Oxytocin and vasopressin seem unlikely candidates as they are associated with social behaviour and anxiety, although it is interesting to note that a role for oxytocin in face recognition has recently been proposed (Rimmele et al., 2009). Beyer and coworkers demonstrated utilizing microdialysis, that systemic and local intracerebral injection of a stable analogue of Ang IV elevated extracellular levels of oxytocin in the rat amygdala resulting in anxiolytic effects that were blocked by an oxytocin receptor antagonist (Beyer et al., 2010).
Another potential IRAP substrate, somatostatin, has recently been demonstrated to play a role in memory processing via action on the somatostatin receptor subtype 3 (Einstein et al., 2010). Although critical for object recognition, the authors did not observe an effect on spatial memory (Einstein et al., 2010). In complementary studies, it was demonstrated that intracerebroventricular (i.c.v.) injection of Ang IV protected rats against pilocarpine-induced seizures, potentially by elevating the concentration of somatostatin-14 in the brain since the anti-seizure activity was blocked by concomitant administration of a somatostatin receptor-2 antagonist (Stragier et al., 2006).
As described on the previous page, IRAP was recently demonstrated to play a role in the immune system by trimming peptides for cross-presentation via MHCI molecules. What is thought provoking about this discovery is the emerging role of immune proteins in brain development and synaptic plasticity (reviewed in Boulanger, 2009). MHCI genes and associated proteins are expressed in a number of regions in the adult brain including the hippocampus and cortex, with a subset of MHCI genes regulated by CREB in hippocampal neurons (Corriveau et al., 1998) (Barco et al., 2005). Because over 50 MHCI proteins are in the mouse, insights into the function of these proteins in the central nervous system (CNS) have been obtained from studies using β2-microglobulin/transporter associated with antigen processing (β2M/TAP) double knockout mice that are MHCI deficient. The adult β2M/TAP knockout mice demonstrate altered synaptic plasticity with enhanced NMDA receptor-dependent LTP and absence of long-term depression (LTD) in the CA1 region of the hippocampus (Huh et al., 2000). However, analyses of the performance of these mice in memory and learning tasks have not been reported. Potentially, IRAP inhibitors in the CNS could alter the processing of these antigens for presentation by MHCI molecules in neurons, which could then impact on synaptic plasticity. Facilitation of LTP by IRAP inhibitors has been reported, as discussed previously.
Regulation of glucose uptake
IRAP co-localizes with GLUT4 in neurons in regions of the brain associated with cognition, raising the question as to whether there is an analogous system in neurons as found in adipocytes and muscle cells. IRAP inhibitors could facilitate memory by potentiating glucose uptake into neurons. The key role glucose plays in memory formation is well established. Exogenous glucose administration has been shown to facilitate memory in rodents (Lee et al., 1988; Ragozzino et al., 1998) and to reverse memory deficits in animals (Parent et al., 1997; Parkes and White, 2000) and age-related deficits in humans (Korol and Gold, 1998; Ragozzino et al., 1998). Increased cognitive load results in a decrease in extracellular glucose levels in the hippocampus (McNay et al., 2000), and this depletion can be reversed by peripheral glucose administration (McNay et al., 2000). The decrease in extracellular glucose levels in the hippocampus during the performance of cognitive tasks is the result of increased glucose uptake into neurons facilitated by GLUT4. Exogenous glucose administration facilitate memory in rodents, possibly via the activation of the mTOR pathway (Dash et al., 2006). Infusions of glucose also reverse memory deficits induced by the GABA receptor agonist muscimol (Parent et al., 1997) in rats.
In brain regions that are important in memory processing, such as in the pyramidal neurons of the hippocampus, IRAP is found in the same vesicles as GLUT4 (Figure 1) (Fernando et al., 2008). Utilizing hippocampal slices, we demonstrated that IRAP inhibitors increased the amount of glucose taken up into active neurons via GLUT4 (Albiston et al., 2008; Fernando et al., 2008). This finding supports the hypothesis that the close association of IRAP and GLUT4 is functional in neurones and suggests a potential mechanism of action for memory enhancement by IRAP inhibitors. However, in whole animal studies, we have been unable to correlate the memory-enhancing effects of IRAP inhibitors with increased glucose utilisation. Acute i.c.v. administrations of IRAP inhibitors in rats enhance spatial working memory observed in the plus maze spontaneous alternation task (Albiston et al., 2008; de Bundel et al., 2009). Although extracellular hippocampal glucose levels decreased significantly when the animals explored the plus maze, indicating increased glucose uptake by the hippocampus, IRAP inhibitors did not potentiate this effect (de Bundel et al., 2009).
Figure 1.
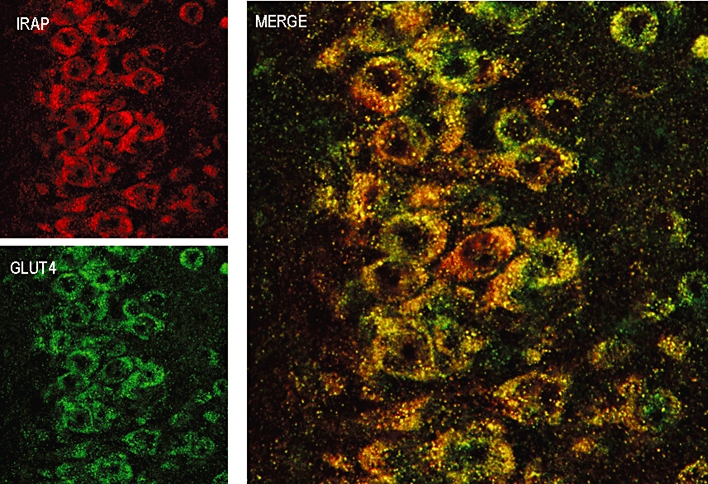
IRAP (top left panel) and GLUT4 (bottom left panel) immunostaining in pyramidal neurones in the CA3 region of the hippocampus with the extent of co-localization shown in the merged image (right panel).
IRAP inhibitors
The acute and chronic effects of IRAP inhibitors on facilitating different forms of memory have been demonstrated by a number of laboratories (section on Ang IV). However, the mechanisms by which these inhibitors enhance memory in normal animals and reverse performance deficits in experimental models of memory loss are yet to be elucidated. Regardless, the robustness of the memory effect of these inhibitors reaffirms IRAP as a suitable target for the development of new classes of cognitive enhancers.
Peptidomimetic IRAP inhibitors
As a tool for pharmacological studies or as a lead of a potential therapeutic, Ang IV, like many peptides, is compromised by its susceptibility to degradation in vivo and lack of efficacy when administered peripherally. These have been the primary issues considered in the structural modification of the parent peptide. The inclusion of unusual amino acids has seen some success with peptide sequences based on Ang IV including Nle1Ang IV, mentioned earlier. Most recently, Lukaszuk and coworkers generated an analogue with an N-terminal β2-homovaline residue and a C-terminal β3-homophenylalanine in the Ang IV sequence yielding a peptidomimetic (AL-11) of significantly longer in vivo half-life (Lukaszuk et al., 2008). In addition, replacement of the C-terminal His-Pro segment by a constrained Trp-Gly motif (AL-40) yielded increased potency with selectivity over APN and AT1 receptor (Lukaszuk et al., 2009).
Extending the work of Taisho Pharmaceuticals (Kobori et al., 2000), a range of cyclized analogues of Ang IV have also been developed. The most recent disulfide cyclised tripeptide analogues of Ang IV described retained low nanomolar Kivalues (Andersson et al., 2010). Compared with earlier attempts (Axén et al. 2006; 2007; Andersson et al., 2008), a critical substitution of Tyr2 for a β3-homotyrosine residue, the use of homocysteine residues and the inclusion of a C-terminal aminomethylphenylacetic acid residue yielded peptides that essentially share only the phenolic side chain of the original peptide. However, the compounds still show low metabolic stability and in vivo efficacy has not been investigated, showing the challenges still present in the development of peptidomimetic inhibitors.
In some respects, the conundrum that remains with these peptides is what distinguishes IRAP inhibitors from substrates. A listing of the identified substrates and inhibitors of IRAP shows elements that may yet be useful in inhibitor design. Most significantly, the IRAP substrates oxytocin, vasopressin and CCK8 possess a tyrosine residue in position 2, although there is a broad substrate specificity exemplified by the role of IRAP in trimming peptides for MHC I presentation. The presence of a pivotal tyrosine residue near the NH2 terminus is also present for peptide IRAP inhibitors (Figure 2). Both vasopressin and oxytocin have been shown to be substrates as a cyclic disulfide, while somatostatin cleavage stops at the terminal disulfide, and calcitonin and endothelins are not substrates. This shows that there may be an element of conformational recognition by IRAP. On the other hand, as far back as 1961, IRAP (as oxytocinase) activity was found to be inhibited when oxytocin was linearized by benzylation of cysteines, or desulphurization to yield alanine residues (Berankova and Sorm, 1961).
Figure 2.
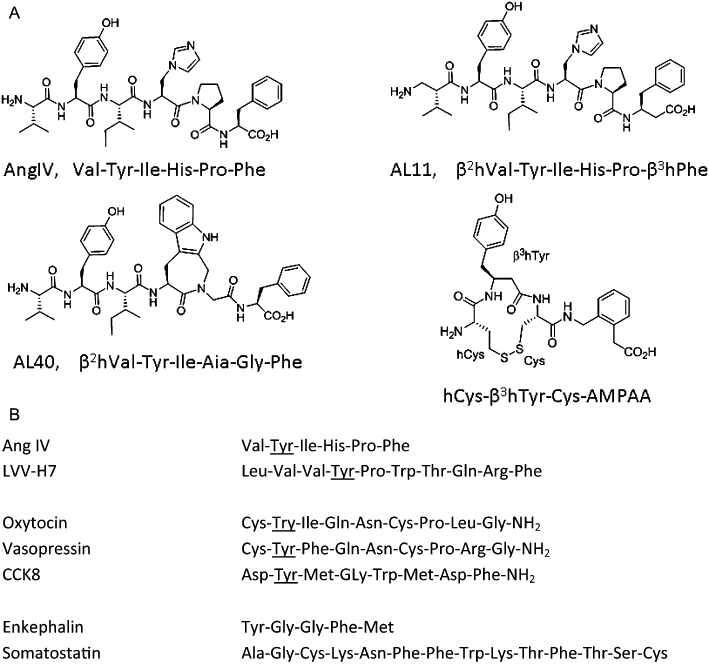
Peptide inhibitors of IRAP. (A) Structures of peptide inhibitors and analogues. (B) Comparison of inhibitor and substrate sequences.
Benzopyran-based IRAP inhibitors
Identification
Although the crystal structure of IRAP has not been obtained, the structures of a number of M1 aminopeptidase family members including human leukotriene A4 hydrolase (LTA4H) (Thunnissen et al., 2001), bacterial APN (Addlagatta et al., 2006) and PfA-M1 aminopeptidase (McGowan et al., 2009) have been solved. Using the crystal structure of LTA4H, a homology model of the catalytic site of IRAP was developed and employed in an in silico screen for potential IRAP inhibitors (Albiston et al., 2008).
A library of more than 1.5 million commercially available compounds were screened, compounds that had predicted high affinity for IRAP purchased, and assessed for their ability to inhibit IRAP activity. Subsequent sequential analogue identification screens with the hit compounds as templates led to the identification of a family of nanomolar affinity benzopyran-based IRAP inhibitors. Three of the compounds, HFI-419 (ethyl 2-acetylamino-7-hydroxy-4-pyridin-3-yl-4H-chromene-3-carboxylate), the quinoline analogue HFI-435 and the hybrid molecule HFI-437 (ethyl 2-acetylamino-7-hydroxy-4-quinolin-3-yl-4H-chromene-3-carboxylate), prepared as racemates, exhibited Ki values of 420, 360 and 20 nM respectively (Figure 3). All three compounds demonstrated selectivity for IRAP (Albiston et al., 2008) in contrast to the peptide inhibitors Ang IV and LVV-H7. I.c.v. administration of HFI-419 demonstrated memory-enhancing effects in two memory paradigms (Albiston et al., 2008), significantly improving performance in the novel object recognition and spontaneous alternation task. The performance of rats treated with HFI-419 in the spatial working memory spontaneous alternation task, exhibited a bell-shaped dose–response curve (Albiston et al., 2008) and paralleled the responses to the peptide IRAP inhibitors, Ang IV and LVV-H7 (de Bundel et al., 2009).
Figure 3.
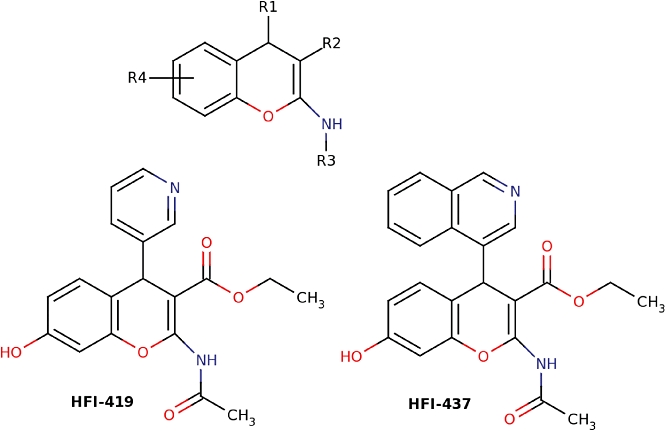
Structures of benzopyran-based inhibitors.
Computational docking of the inhibitors into a molecular model of IRAP
Docking studies were used to provide a detailed view of how the inhibitors are likely to bind to IRAP, which will be important in guiding ongoing medical chemistry programmes. Unexpectedly, the docking results revealed two alternate binding conformations for these structurally analogous inhibitors but indicated in both cases that Phe544 would provide a hydrophobic packing point at one side of the active site (Albiston et al., 2010b), and that the inhibitors interacted with the Zn atom. It should be noted that in the docking studies the S-isomer was predicted as the preferred binding mode in all examples, irrespective of the pose.
In the binding pose adopted by the pyridinyl derivatives, HFI-142 and HFI-419, a ring stack is predicted between the benzopyran moieties of the compounds and Phe544 (Albiston et al., 2010b) (Figure 4A). This binding pose contains numerous other interactions including a hydrogen bond from the hydroxyl moiety of the inhibitors to Glu295 and van der Waals interactions involving Gln293, Pro296, Glu426, Ala427, Leu483 and Ile540.
Figure 4.
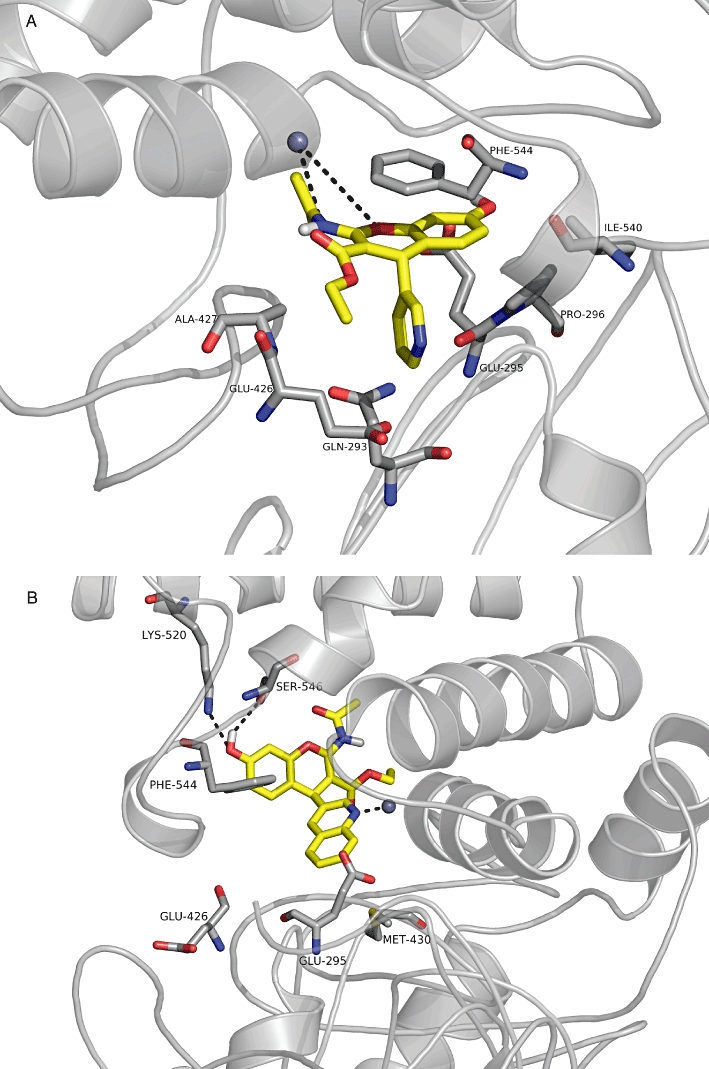
Docking of the benzopyran-based inhibitors. (A) HFI-419 docked into the IRAP model. The residues are discussed above in the main text. (B) Compound HFI-437 docked into the IRAP model. The residues are discussed above in the main text. This figure is rotated 90° along the y-axis from Figure 4A.
The quinolines (HFI-435 and HFI-437) are not able to adopt the aforementioned binding mode for the pyridinyl compounds as the quinoline group is too large to enter the small polar pocket formed by Glu426 and Glu293 (Figure 4B). Alternatively, they adopt a mode which allows a stronger interaction with the Zn ion through the quinoline nitrogen. The quinoline compounds are predicted to be more active than the pyridinyl compounds due to the more favourable coordination with the Zn ion, along with the hydrogen bonding network between the hydroxyl moiety of the inhibitors and Ser546 and Lys520, and more van der Waals contacts, for example, between the quinoline ring and the side chain of Met430 and between the ethyl ester with Ile461.
Future directions
From this overview of IRAP inhibitors, it is apparent that there are three key issues requiring resolution. (i) Elucidation of the mechanism of action by which IRAP inhibitors facilitate memory. (ii) The need to develop IRAP inhibitors that are effective following peripheral administration. Both the peptide and benzopyran based inhibitors have undergone medicinal chemical alerations, but to date only efficacy via i.c.v. administration has been reported. (iii) Are IRAP inhibitors an effective treatment for memory loss resulting from AD or aging? Studies utilizing animal models will help to address this issue. Progress in any of these areas is likely to result in a clearer understanding of the role of IRAP in the CNS.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia (NHMRC) (development grant 520695 and project grant 502602), Neurosciences Victoria, and Alzheimer's Drug Discovery Foundation USA. MWP is an Australian Research Council Federation Fellow and an NHMRC Honorary Fellow. SYC is an NHMRC Senior Research Fellow. The authors would like to thank Prof Frederick Mendelsohn for his support and contribution to the project.
Glossary
Abbreviations
- AD
Alzheimer's dementia
- Ang
IV, angiotensin IV
- APN
aminopeptidase N
- β2 m/TAP
β2-microglobulin/transporter associated with antigen processing
- CREB
cyclicAMP responsive element binding protein
- ERAP
endoplasmic reticulum associated aminopeptidase
- GLUT4
glucose transporter 4
- GSV
GLUT4 specialized vesicles
- HGF
hepatocyte growth factor
- IRAP
insulin-regulated aminopeptidase
- KO
knockout
- LTD
long-term depression
- LTP
long-term potentiation
- LVV-H7
LVV-haemorphin 7
- MCI
mild cognitive impairment
- MHC
major histocompatibility complex
- mTOR
mammalian target of rapamycin
- WT
wild type
Conflict of interests
The authors declare that there is no conflict of interest for this paper.
References
- Addlagatta A, Gay L, Matthews BW. Structure of aminopeptidase N from Escherichia coli suggests a compartmentalized, gated active site. Proc Natl Acad Sci U S A. 2006;103:13339–13344. doi: 10.1073/pnas.0606167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albiston AL, McDowall SG, Matsacos D, Sim P, Clune E, Mustafa T, et al. Evidence that the angiotensin IV (AT4) receptor is the enzyme insulin regulated aminopeptidase. J Biol Chem. 2001;276:48263–48266. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- Albiston AL, Pederson ES, Burns P, Purcell B, Wright JW, Harding JW, et al. Reversal of scopolamine-induced memory deficits by LVV-hemorphin 7 in rats in the passive avoidance and Morris water maze paradigms. Behav Brain Res. 2004;154:239–243. doi: 10.1016/j.bbr.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Albiston AL, Peck GR, Yeatman HR, Fernando R, Ye S, Chai SY. Therapeutic targeting of insulin-regulated aminopeptidase: heads and tails? Pharmacol Ther. 2007;116:417–427. doi: 10.1016/j.pharmthera.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Albiston AL, Morton CJ, Ng HL, Pham V, Yeatman HR, Ye S, et al. Identification and characterization of a new cognitive enhancer based on inhibition of insulin-regulated aminopeptidase. Faseb J. 2008;22:4209–4217. doi: 10.1096/fj.08-112227. [DOI] [PubMed] [Google Scholar]
- Albiston AL, Fernando RN, Yeatman HR, Burns P, Ng L, Daswani D, et al. Gene knockout of insulin-regulated aminopeptidase: loss of the specific binding site for angiotensin IV and age-related deficit in spatial memory. Neurobiol Learn Mem. 2010a;93:19–30. doi: 10.1016/j.nlm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Albiston AL, Pham V, Ye S, Ng L, Lew RA, Thompson PE, et al. Phenylalanine-544 plays a key role in substrate and inhibitor binding by providing a hydrophobic packing point at the active site of insulin-regulated aminopeptidase. Mol Pharmacol. 2010b;78:600–607. doi: 10.1124/mol.110.065458. [DOI] [PubMed] [Google Scholar]
- Andersson H, Demaegdt H, Vauquelin G, Lindeberg G, Karlén A, Hallberg M. Ligands to the (IRAP)/AT4 receptor encompassing a 4-hydroxydiphenylmethane scaffold replacing Tyr2. Bioorg Med Chem. 2008;16:6924–6935. doi: 10.1016/j.bmc.2008.05.046. [DOI] [PubMed] [Google Scholar]
- Andersson H, Demaegdt H, Vauquelin G, Lindeberg G, Karlen A, Hallberg M, et al. Disulfide cyclized tripeptide analogues of angiotensin IV as potent and selective inhibitors of insulin-regulated aminopeptidase (IRAP) J Med Chem. 2010;53:8059–8071. doi: 10.1021/jm100793t. [DOI] [PubMed] [Google Scholar]
- Axén A, Lindeberg G, Demaegdt H, Vauquelin G, Karlen A, Hallberg M. Cyclic insulin-regulated aminopeptidase (IRAP)/AT4 receptor ligands. J Pept Sci. 2006;12:705–713. doi: 10.1002/psc.782. [DOI] [PubMed] [Google Scholar]
- Axén A, Andersson H, Lindeberg G, Rönnholm H, Kortesmaa J, Demaegdt H, et al. Small potent ligands to the insulin-regulated aminopeptidase (IRAP)/AT(4) receptor. J Pept Sci. 2007;13:434–444. doi: 10.1002/psc.859. [DOI] [PubMed] [Google Scholar]
- Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, et al. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Berankova Z, Sorm F. Characterization of purified preparations of serum oxytocinase. Coll Czech Chem Commun. 1961;26:2557–2561. [Google Scholar]
- Beyer CE, Dwyer JM, Platt BJ, Neal S, Luo B, Ling HP, et al. Angiotensin IV elevates oxytocin levels in the rat amygdala and produces anxiolytic-like activity through subsequent oxytocin receptor activation. Psychopharmacology (Berl) 2010;209:303–311. doi: 10.1007/s00213-010-1791-1. [DOI] [PubMed] [Google Scholar]
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Braszko JJ. Involvement of D1 dopamine receptors in the cognitive effects of angiotensin IV and des-Phe6 angiotensin IV. Peptides. 2004;25:1195–1203. doi: 10.1016/j.peptides.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Braszko JJ. Dopamine D4 receptor antagonist L745,870 abolishes cognitive effects of intracerebroventricular angiotensin IV and des-Phe(6)-Ang IV in rats. Eur Neuropsychopharmacol. 2009;19:85–91. doi: 10.1016/j.euroneuro.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Braszko JJ. Participation of D 1-4 dopamine receptors in the pro-cognitive effects of angiotensin IV and des-Phe 6 angiotensin IV. Neurosci Biobehav Rev. 2010;34:343–350. doi: 10.1016/j.neubiorev.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Braszko JJ, Kupryszewski G, Witczuk B, Wisniewski K. Angiotensin II-(3-8)-hexapeptide affects motor activity, performance of passive avoidance and a conditioned avoidance response in rats. Neuroscience. 1988;27:777–783. doi: 10.1016/0306-4522(88)90182-0. [DOI] [PubMed] [Google Scholar]
- Braszko JJ, Wielgat P, Walesiuk A. Effect of D(3) dopamine receptors blockade on the cognitive effects of angiotensin IV in rats. Neuropeptides. 2008;42:301–309. doi: 10.1016/j.npep.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ. Emerging cognitive enhancing drugs. Expert Opin Emerg Drugs. 2009;14:577–589. doi: 10.1517/14728210903257796. [DOI] [PubMed] [Google Scholar]
- de Bundel D, Smolders I, Yang R, Albiston AL, Michotte Y, Chai SY. Angiotensin IV and LVV-Haemorphin 7 enhance spatial working memory in rats: effects on hippocampal glucose levels and blood flow. Neurobiol Learn Mem. 2009;92:19–26. doi: 10.1016/j.nlm.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Chai SY, Lee JH, Matscos D, McDowall SG, Mendelsohn FAO, Robel P, et al. Angiotensin AT4 receptor distribution in mouse brain and its possible role in facilitation of spatial memory. J Neurochem. 2001;78(Suppl 1):15. [Google Scholar]
- Cheng DY, DeWitt BJ, Dent EL, Nossaman BD, Kadowitz PJ. Analysis of responses to angiotensin IV in the pulmonary vascular bed of the cat. Eur J Pharmacol. 1994;261:223–227. doi: 10.1016/0014-2999(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J Neurosci. 2006;26:8048–8056. doi: 10.1523/JNEUROSCI.0671-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein EB, Patterson CA, Hon BJ, Regan KA, Reddi J, Melnikoff DE, et al. Somatostatin signaling in neuronal cilia is critical for object recognition memory. J Neurosci. 2010;30:4306–4314. doi: 10.1523/JNEUROSCI.5295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando R, Larm J, Albiston AL, Chai SY. Distribution and cellular localization of the insulin regulated aminopeptidase (IRAP) in the rat central nervous system. J Comp Neurol. 2005;487:372–390. doi: 10.1002/cne.20585. [DOI] [PubMed] [Google Scholar]
- Fernando RN, Luff SE, Albiston AL, Chai SY. Sub-cellular localization of insulin-regulated membrane aminopeptidase, IRAP, to vesicles in neurons. J Neurochem. 2007;102:967–976. doi: 10.1111/j.1471-4159.2007.04659.x. [DOI] [PubMed] [Google Scholar]
- Fernando RN, Albiston AL, Chai SY. The insulin-regulated aminopeptidase IRAP is colocalised with GLUT4 in the mouse hippocampus – potential role in modulation of glucose uptake in neurones? Eur J Neurosci. 2008;28:588–598. doi: 10.1111/j.1460-9568.2008.06347.x. [DOI] [PubMed] [Google Scholar]
- Georgiadou D, Hearn A, Evnouchidou I, Chroni A, Leondiadis L, York IA, et al. Placental leucine aminopeptidase efficiently generates mature antigenic peptides in vitro but in patterns distinct from endoplasmic reticulum aminopeptidase 1. J Immunol. 2010;185:1584–1592. doi: 10.4049/jimmunol.0902502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding BJ, Overall AD, Brown G, Gard PR. Strain differences in the effects of angiotensin IV on mouse cognition. Eur J Pharmacol. 2010;641:154–159. doi: 10.1016/j.ejphar.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Herbst JJ, Ross SA, Scott HM, Bobin SA, Morris NJ, Lienhard GE, et al. Insulin stimulates cell surface aminopeptidase activity toward vasopressin in adipocytes. Am J Physiol. 1997;272(4 Pt 1):E600–E606. doi: 10.1152/ajpendo.1997.272.4.E600. [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt H, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. Br Med J. 2005;331:321–327. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SR, Scott HM, Mastick CC, Aebersold R, Lienhard GE. Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vesicles. J Biol Chem. 1995;270:23612–23618. doi: 10.1074/jbc.270.40.23612. [DOI] [PubMed] [Google Scholar]
- Keller SR, Davis AC, Clairmont KB. Mice deficient in the insulin-regulated membrane aminopeptidase show substantial decreases in glucose transporter GLUT4 levels but maintain normal glucose homeostasis. J Biol Chem. 2002;277:17677–17686. doi: 10.1074/jbc.M202037200. [DOI] [PubMed] [Google Scholar]
- Kobori T, Goda K, Sugimoto K, Ota T, Tomisawa K. US Patent 5439919. Taisho Pharmaceutical Co., Ltd; 2000. Amino compounds and angiotensin IV receptor agonists. [Google Scholar]
- Korol DL, Gold PE. Glucose, memory, and aging. Am J Clin Nutr. 1998;67:764S–771S. doi: 10.1093/ajcn/67.4.764S. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Armstrong DL, Ikeda S, Wayner MJ, Harding JW, Wright JW. The effects of angiotensin IV analogs on long-term potentiation within the CA1 region of the hippocampus in vitro. Brain Res. 2001;897:114–121. doi: 10.1016/s0006-8993(01)02100-x. [DOI] [PubMed] [Google Scholar]
- Lee MK, Graham SN, Gold PE. Memory-enhancement with post-training intraventricular glucose injections in rats. Behav Neurosci. 1988;102:591–595. doi: 10.1037//0735-7044.102.4.591. [DOI] [PubMed] [Google Scholar]
- Lee J, Albiston AL, Allen AM, Mendelsohn FAO, Ping SE, Barrett GL, et al. Effect of intracerebroventricular injection of AT4 receptor ligands, Nle1-angiotensin IV and LVv-hemorphin 7, on spatial learning in rats. Neuroscience. 2004;124:341–349. doi: 10.1016/j.neuroscience.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Lew RA, Mustafa T, Ye S, McDowall SG, Chai SY, Albiston AL. Angiotensin AT4 ligands are potent, competitive inhibitors of insulin regulated aminopeptidase (IRAP) J Neurochem. 2003;86:344–350. doi: 10.1046/j.1471-4159.2003.01852.x. [DOI] [PubMed] [Google Scholar]
- Loufrani L, Henrion D, Chansel D, Ardaillou R, Levy BI. Functional evidence for an angiotensin IV receptor in rat resistance arteries. J Pharmacol Exp Ther. 1999;291:583–588. [PubMed] [Google Scholar]
- Lukaszuk A, Demaegdt H, Szemenyei E, Tóth G, Tymecka D, Misicka A, et al. Beta-homo-amino acid scan of angiotensin IV. J Med Chem. 2008;51:2291–2296. doi: 10.1021/jm701490g. [DOI] [PubMed] [Google Scholar]
- Lukaszuk A, Demaegdt H, Feytens D, Vanderheyden P, Vauquelin G, Tourwé D. The replacement of His(4) in angiotensin IV by conformationally constrained residues provides highly potent and selective analogues. J Med Chem. 2009;52:5612–5618. doi: 10.1021/jm900651p. [DOI] [PubMed] [Google Scholar]
- McEwen BB. De Wied and colleagues IV: research into mechanisms of action by which vasopressin and oxytocin influence memory processing. Adv Pharmacol. 2004;50:177–225. doi: 10.1016/S1054-3589(04)50005-4. [DOI] [PubMed] [Google Scholar]
- McGowan S, Porter CJ, Lowther J, Stack CM, Golding SJ, Skinner-Adams TS, et al. Structural basis for the inhibition of the essential Plasmodium falciparum M1 neutral aminopeptidase. Proc Natl Acad Sci U S A. 2009;106:2537–2542. doi: 10.1073/pnas.0807398106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci U S A. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Hattori A, Mizutani S, Tsujimoto M. Cleavage of peptide hormones by placental leucine aminopeptidase/oxytocinase. In: Mizutani, et al., editors. Cell-Surface Aminopeptidases: Basic and Clinical Aspects. Nagoya: Elsevier Science BV; 2001a. pp. 295–299. [Google Scholar]
- Matsumoto H, Nagasaka T, Hattori A, Rogi T, Tsuruoka N, Mizutani S, et al. Expression of placental leucine aminopeptidase/oxytocinase in neuronal cells and its action on neuronal peptides. Eur J Biochem. 2001b;268:3259–3266. doi: 10.1046/j.1432-1327.2001.02221.x. [DOI] [PubMed] [Google Scholar]
- Moeller I, Lew RA, Mendelsohn FA, Smith AI, Brennan ME, Tetaz TJ, et al. The globin fragment LVV-hemorphin-7 is an endogenous ligand for the AT4 receptor in the brain. J Neurochem. 1997;68:2530–2537. doi: 10.1046/j.1471-4159.1997.68062530.x. [DOI] [PubMed] [Google Scholar]
- Olson ML, Cero IJ. Intrahippocampal Norleucine1-Angiotensin IV mitigates scopolamine-induced spatial working memory deficits. Peptides. 2010;31:2209–2215. doi: 10.1016/j.peptides.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Olson ML, Olson EA, Qualls JH, Stratton JJ, Harding JW, Wright JW. Norleucine1-angiotensin IV alleviates mecamylamine-induced spatial memory deficits. Peptides. 2004;25:233–241. doi: 10.1016/j.peptides.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Parent MB, Leaurey PT, Wilkniss S, Gold PE. Intraseptal infusions of muscimol impair spontaneous alternation performance: infusions of glucose into the hippocampus, but not the medial septum, reverse the deficit. Neurobiol Learn Mem. 1997;68:75–85. doi: 10.1006/nlme.1997.3769. [DOI] [PubMed] [Google Scholar]
- Parkes M, White KG. Glucose attenuation of memory impairments. Behav Neurosci. 2000;114:307–319. [PubMed] [Google Scholar]
- Pederson ES, Harding JW, Wright JW. Attenuation of scopolamine-induced spatial learning impairments by an angiotensin IV analog. Regul Pept. 1998;74:97–103. doi: 10.1016/s0167-0115(98)00028-7. [DOI] [PubMed] [Google Scholar]
- Pederson ES, Krishnan R, Harding JW, Wright JW. A role for the angiotensin AT4 receptor subtype in overcoming scopolamine-induced spatial memory deficits. Regul Pept. 2001;102:147–156. doi: 10.1016/s0167-0115(01)00312-3. [DOI] [PubMed] [Google Scholar]
- Pham V, Burns P, Albiston AL, Yeatman HR, Ng L, Diwakarla S, et al. Reproduction and maternal behaviour in insulin regulated aminopeptidase (IRAP) knockout mice. Peptides. 2009;30:1861–1865. doi: 10.1016/j.peptides.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Portet F, Ousset PJ, Visser PJ, Frisoni GB, Nobili F, Scheltens P, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer's Disease. J Neurol Neurosurg Psychiatry. 2006;77:714–718. doi: 10.1136/jnnp.2005.085332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Pal SN, Unick K, Stefani MR, Gold PE. Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. J Neurosci. 1998;18:1595–1601. doi: 10.1523/JNEUROSCI.18-04-01595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogi T, Tsujimoto M, Nakazato H, Mizutani S, Tomoda Y. Human placental leucine aminopeptidase/oxytocinase. A new member of type II membrane-spanning zinc metallopeptidase family. J Biol Chem. 1996;271:56–61. doi: 10.1074/jbc.271.1.56. [DOI] [PubMed] [Google Scholar]
- Rosenbloom AA, Sack J, Fisher DA. The circulating vasopressinase of pregnancy: species comparison with radioimmunoassay. Am J Obstet Gynecol. 1975;121:316–320. doi: 10.1016/0002-9378(75)90005-8. [DOI] [PubMed] [Google Scholar]
- Saveanu L, Carroll O, Lindo V, Del Val M, Lopez D, Lepelletier Y, et al. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat Immunol. 2005;6:689–697. doi: 10.1038/ni1208. [DOI] [PubMed] [Google Scholar]
- Saveanu L, Carroll O, Weimershaus M, Guermonprez P, Firat E, Lindo V, et al. IRAP identifies an endosomal compartment required for MHC class I cross-presentation. Science. 2009;325:213–217. doi: 10.1126/science.1172845. [DOI] [PubMed] [Google Scholar]
- Segura E, Albiston AL, Wicks IP, Chai SY, Villadangos JA. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc Natl Acad Sci U S A. 2009;106:20377–20381. doi: 10.1073/pnas.0910295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragier B, Clinckers R, Meurs A, De Bundel D, Sarre S, Ebinger G, et al. Involvement of the somatostatin-2 receptor in the anti-convulsant effect of angiotensin IV against pilocarpine-induced limbic seizures in rats. J Neurochem. 2006;98:1100–1113. doi: 10.1111/j.1471-4159.2006.03942.x. [DOI] [PubMed] [Google Scholar]
- Thomas SJ, Grossberg GT. Memantine: a review of studies into its safety and efficacy in treating Alzheimer's disease and other dementias. Clin Interv Aging. 2009;4:367–377. doi: 10.2147/cia.s6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunnissen MM, Nordlund P, Haeggstrom JZ. Crystal structure of human leukotriene A(4) hydrolase, a bifunctional enzyme in inflammation. Nat Struct Biol. 2001;8:131–135. doi: 10.1038/84117. [DOI] [PubMed] [Google Scholar]
- Wallis MG, Lankford MF, Keller SR. Vasopressin is a physiological substrate for the insulin-regulated aminopeptidase IRAP. Am J Physiol Endocrinol Metab. 2007;293:E1092–E1102. doi: 10.1152/ajpendo.00440.2007. [DOI] [PubMed] [Google Scholar]
- Waters SB, D'Auria M, Martin SS, Nguyen C, Kozma LM, Luskey KL. The amino terminus of insulin-responsive aminopeptidase causes Glut4 translocation in 3T3-L1 adipocytes. J Biol Chem. 1997;272:23323–23327. doi: 10.1074/jbc.272.37.23323. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Armstrong DL, Phelix CF, Wright JW, Harding JW. Angiotensin IV enhances LTP in rat dentate gyrus in vivo. Peptides. 2001;22:1403–1414. doi: 10.1016/s0196-9781(01)00475-2. [DOI] [PubMed] [Google Scholar]
- Wisniewski K, Borawska M, Car H. The effect of angiotensin II and its fragments on post-alcohol impairment of learning and memory. Pol J Pharmacol. 1993;45:23–29. [PubMed] [Google Scholar]
- Wright JW, Miller-Wing AV, Shaffer MJ, Higginson C, Wright DE, Hanesworth JM, et al. Angiotensin II(3-8) (ANG IV) hippocampal binding: potential role in the facilitation of memory. Brain Res Bull. 1993;32:497–502. doi: 10.1016/0361-9230(93)90297-o. [DOI] [PubMed] [Google Scholar]
- Wright JW, Clemens JA, Panetta JA, Smalstig EB, Weatherly LA, Kramar EA, et al. Effects of LY231617 and angiotensin IV on ischemia-induced deficits in circular water maze and passive avoidance performance in rats. Brain Res. 1996;717:1–11. doi: 10.1016/0006-8993(95)01454-3. [DOI] [PubMed] [Google Scholar]
- Wright JW, Stubley L, Pederson ES, Kramar EA, Hanesworth JM, Harding JW. Contributions of the brain angiotensin IV-AT4 receptor subtype system to spatial learning. J Neurosci. 1999;19:3952–3961. doi: 10.1523/JNEUROSCI.19-10-03952.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Kramár EA, Myers ED, Davis CJ, Harding JW. Ethanol-induced suppression of LTP can be attenuated with an angiotensin IV analog. Regul Pept. 2003;113:49–56. doi: 10.1016/s0167-0115(02)00302-6. [DOI] [PubMed] [Google Scholar]
- Yamahara N, Nomura S, Suzuki T, Itakura A, Ito M, Okamoto T, et al. Placental leucine aminopeptidase/oxytocinase in maternal serum and placenta during normal pregnancy. Life Sci. 2000;66:1401–1410. doi: 10.1016/s0024-3205(00)00451-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto BJ, Elias PD, Masino JA, Hudson BD, McCoy AT, Anderson ZJ, et al. The angiotensin IV analog Nle-Tyr-Leu-psi-(CH2-NH2)3-4-His-Pro-Phe (norleual) can act as a hepatocyte growth factor/c-Met inhibitor. J Pharmacol Exp Ther. 2010;333:161–173. doi: 10.1124/jpet.109.161711. [DOI] [PMC free article] [PubMed] [Google Scholar]


