Abstract
BACKGROUND AND PURPOSE
We have developed a strategy to target the permanently charged lidocaine derivative lidocaine N-ethyl bromide (QX-314) selectively into nociceptive sensory neurons through the large-pore transient receptor potential cation channel subfamily V (TRPV1) noxious heat detector channel. This involves co-administration of QX-314 and a TRPV1 agonist to produce a long-lasting local analgesia. For potential clinical use we propose using lidocaine as the TRPV1 agonist, because it activates TRPV1 at clinical doses.
EXPERIMENTAL APPROACH
We conducted experiments in rats to determine optimal concentrations and ratios of lidocaine and QX-314 that produce the greatest degree and duration of pain-selective block when administered nearby the sciatic nerve: reduction in the response to noxious mechanical (pinch) and to radiant heat stimuli, with minimal disruption in motor function (grip strength).
KEY RESULTS
A combination of 0.5% QX-314 and 2% lidocaine produced 1 h of non-selective sensory and motor block followed by >9 h of pain-selective block, where grip strength was unimpaired. QX-314 at this concentration had no effect by itself, while 2% lidocaine by itself produced 1 h of non-selective block. The combination of 0.5% QX-314 and 2% lidocaine was the best of the many tested, in terms of the duration and selectivity of local analgesia.
CONCLUSIONS AND IMPLICATIONS
Targeting charged sodium channel blockers into specific sets of axons via activation of differentially expressed large-pore channels provides an opportunity to produce prolonged local analgesia, and represents an example of how exploiting ion channels as a drug delivery port can be used to increase the specificity and efficacy of therapeutics.
Keywords: QX-314, TRPV1 channels, lidocaine, sodium channel blockers, long-duration analgesia, differential block, regional anaesthesia, nerve block, targeted delivery
Introduction
The most powerful known method of blocking pain while retaining consciousness is to inject local anaesthetics like lidocaine regionally into areas of the body generating pain. Lidocaine produces its local aesthetic actions by blocking voltage-gated sodium channels. Like all local anaesthetics, lidocaine has little or no selectivity among different types of sodium channels (Hille, 1977a; Schwarz et al., 1977; Liu et al., 2003; Chevrier et al., 2004; Leffler et al., 2005) and its local aesthetic action is also non-selective, blocking action potentials in all sensory, motor and autonomic fibres. In particular, it blocks both low threshold sensory axons carrying innocuous information and high threshold (nociceptor) axons that contribute to painful sensations. The net effect of a lidocaine injection close to a nerve at a therapeutically effective dose (1 to 2%, 35 to 69 mM) (Enneking et al., 2009), is complete sensory and motor block, including loss of all sensation (numbness), paralysis and abolition of autonomic function. While such an outcome may be acceptable in some settings, such as during surgery, there are many clinical situations where a selective block of some but not other axons would be more desirable. For example, block of nociceptors to produce analgesia without a loss of proprioception or motor function would enable early mobilization in patients receiving peripheral nerve block or plexus block, for example, following knee or hip joint replacement.
A further issue with regional injections of local anaesthetics is their relative short duration, limited to several hours, which is usually not enough to fully cover the normal duration of post-operative pain. Furthermore, because of lidocaine's action on central neurons and cardiac muscle, it can have major central nervous system and cardiovascular toxicity problems when administered locally at high volumes (Dillane and Finucane, 2010; Neal et al., 2010). There is therefore a need for a pharmacological therapy that has more selectivity for nociceptors, a longer duration and a reduced side effect burden.
How can a selective block of nociceptors be achieved to produce a local analgesia instead of a non-specific local anaesthesia? One way would be to selectively target those voltage-gated sodium channels expressed only or predominantly in these neurons, such as Nav1.7, 1.8 and 1.9 (Wood et al., 2004; Priest, 2009; see also Momin and Wood, 2008; Dib-Hajj et al., 2009). However, only a few subtype selective sodium channel blockers have been reported (Priest, 2009; Zhang et al., 2010), and none have been shown to produce local analgesia.
We have developed an alternative strategy, one based on targeting a sodium channel blocker to selectively enter into nociceptors and not into low threshold sensory and motor axons. This strategy is based on the location of the binding site of local anaesthetics on the inside of the pore of sodium channels, in a region involving the pore-lining S6 regions of the pseudo-subunit domains I, III and IV of the channel (Ragsdale et al., 1994; 1996; Yarov-Yarovoy et al., 2002; McNulty et al., 2007; Sheets and Hanck, 2007; Ahern et al., 2008; Muroi and Chanda, 2009). For neuronal sodium channels, local aesthetic molecules can apparently access the binding site only from the cytoplasmic end of the pore, because application of charged, membrane-impermeant derivatives of local anaesthetics have no effect if applied externally but have blocking activity if applied on the cytoplasmic side of the membrane, as first shown using lidocaine N-ethyl bromide (QX-314), a lidocaine derivative with a permanent positive charge conferred by a quaternary nitrogen (Frazier et al., 1970; Strichartz, 1973). Lidocaine itself has a tertiary nitrogen with pKa of 8.2, so that a pH of 7.4 ∼ 15% of the molecules will be in the unprotonated, uncharged state, which is highly permeable and provides rapid entry into the cell (Hille, 1977b). Once inside, protonation occurs to establish charged as well as uncharged forms of the molecule. It is likely that both charged and uncharged forms of the drug can bind and block the channels from the cytoplasmic surface, because benzocaine, an uncharged molecule similar to the uncharged form of lidocaine, blocks sodium channels nearly as potently as does lidocaine (Hille, 1977a,b; Schwarz et al., 1977; Clapham et al., 2001).
The ability of QX-314 to block from the inside but not the outside of neuronal membranes could be exploited to block only selected neurons if there were some way to allow it to enter some neurons but not others. A possible strategy to do this is to use naturally expressed large-pore ion channels as an entry port for QX-314 (or similar permanently charged sodium channel blockers) into neurons. The candidate channel we chose to investigate first was transient receptor potential cation channel subfamily V (TRPV1), a member of the large transient receptor transient receptor potential (TRP) channel family (Clapham et al., 2001). The reason for this was twofold. First, the channel has been shown to permeate large cations such as tetraethylammonium (130 Da) and N-methyl-D-glucamine (195 Da) (Hellwig et al., 2004; Oseguera et al., 2007) and surprisingly, even a very large cationic dye FM1-43 (452 Da) (Meyers et al., 2003) which, together with TRPV1's high single-channel conductance (Premkumar et al., 2002; Raisinghani et al., 2005), suggests that the channel has a large-pore, certainly large enough to permeate cationic drugs like QX-314 (263 Da). Activation of native or recombinant TRPV1 also leads to time- and agonist concentration-dependent increases in permeability to large cations like N-methyl-D-glucamine (NMDG+, 195 Da) (Chung et al., 2008). Such pore dilation also occurs for transient receptor potential subfamily A1 (TRPA1) but not transient receptor potential M8 (Chen et al., 2009). The second reason, we looked at TRPV1 is because it is a noxious heat detector (Caterina et al., 1997; Premkumar and Ahern, 2000), and is therefore almost exclusively expressed in nociceptors. Thus, if we could selectively use TRPV1 to permeate QX-314 into neurons we could potentially achieve a pain specific block.
The first way we examined this hypothesis was to use a combination of QX-314 and capsaicin, a TRPV1 agonist and the pungent ingredient in chilli peppers (Binshtok et al., 2007). We found that QX-314, when administered alone to dorsal root ganglion neurons, was without effect on voltage-gated sodium current, as expected. In contrast, co-application of QX-314 with capsaicin dramatically inhibited sodium current (by ∼90%), consistent with QX-314 entering the neurons through TRPV1 channels and blocking from the inside. This action completely abolished the ability to generate action potentials and was seen only in small TRPV1 expressing dorsal root ganglion (DRG) neurons, with large non-capsaicin-responsive neurons unaffected (Binshtok et al., 2007). The effect was also seen in TRPV1-expressing trigeminal ganglion neurons, where it was also shown that block of sodium current and action potentials is irreversible after washing capsaicin and QX-314, consistent with QX-314 being trapped inside the neurons after TRPV1 channels close (Kim et al., 2010).
In vivo experiments suggested that TRPV1-mediated entry of QX-314 can be used to produce nociceptor-selective block of excitability and axonal conduction. Local injection in rodents of QX-314 alone was, as expected, without effect (Binshtok et al., 2007; 2009a;). Injection of capsaicin alone subcutaneously elicited a nociceptive reaction that lasted about ∼15 min (Binshtok et al., 2007) and a similar reaction was elicited by perineural injection (Binshtok et al., 2009a), reflecting the presence of TRPV1 expression on the axons of nociceptors in peripheral nerves (Hoffmann et al., 2008). However, when QX-314 was co-applied with capsaicin, either subcutaneously or perineurally, there was a long-lasting block of heat and mechanical pain, with no block in motor function (Binshtok et al., 2007). Subsequent experiments on the jaw opening reflex confirmed the specificity of the combination for nociceptor fibres in sensory nerves, and demonstrated blockade of dental pain (Kim et al., 2010).
We interpreted these data as showing that we could indeed exploit TRPV1 as a ‘drug-delivery portal’ mechanism to target QX-314 into neurons at sufficient concentrations to block sodium currents and action potentials, with the differential expression of TRPV1 providing specificity for delivery of the drug only into nociceptors. The long duration of the effect presumably reflects trapping of QX-314 in the axon, where unlike lidocaine it cannot diffuse out the membrane and will either diffuse along the axon, or slowly be removed by exocytosis, degradation or slow leakage through channels.
While our strategy had been shown to work, there remained an important problem for its clinical exploitation. Activation of TRPV1 channels by capsaicin occurs instantly (<1 s), while entry of enough QX-314 to block action potentials takes several minutes (Binshtok et al., 2007). This delay is long enough for the capsaicin administration to produce several minutes of high-level nociceptor activation, which in humans would elicit severe burning pain (Gustafsson et al., 2009), only after which, the long-lasting pain-selective block would manifest. How to overcome this? One solution would be use non-pungent agonists of TRPV1, like eugenol (Yang et al., 2003), which is the active ingredient in oil of cloves. While we found that a combination of QX-314 and eugenol could indeed reduce sodium currents in vitro, formulation problems prevented co-application in vivo. Fortuitously, however, a concurrent study by Andreas Leffler and colleagues revealed the remarkable fact that lidocaine itself, at clinically administered concentrations (30 mM), is a TRPV1 agonist. They showed that lidocaine produced calcium influx in DRG neurons that was blocked by a TRPV1 antagonist and could activate heterologously expressed TRPV1 channels (Leffler et al., 2008). This led us to test if we could substitute lidocaine for capsaicin as a TRPV1 agonist for in vivo experiments. The results were promising, with a combination of lidocaine and QX-314 producing much longer analgesia than lidocaine alone (Binshtok et al., 2009a). In principle, the combination of lidocaine and QX-314 seems an ideal method for development of a clinical therapy using TRPV1 channels to target entry of QX-314 into nociceptors: both lidocaine and QX-314 are water soluble so there are no formulation issues, lidocaine has already been studied extensively for toxicology, and as QX-314 is a simple derivative of lidocaine, its toxicology might be expected to be generally similar. However, because of lidocaine's actions as both an indiscriminate blocker of all excitability and as a TRPV1 agonist, it is clear that a key issue in the potential clinical use of the combination of lidocaine and QX-314 is to determine optimal concentrations of the two molecules to produce long-lasting nociceptor block while minimizing the duration of motor block. A further concern is to determine whether this can be done with total concentrations of both drugs at a level likely to be acceptable from a toxicological standpoint. To address these issues, we have conducted a study, reported below, testing a range of concentrations of both agents for producing prolonged local analgesia while minimizing motor block.
Methods
Animal procedures were approved by the Committee on Research Animal Care of the Massachusetts General Hospital, Boston, MA. Male Sprague-Dawley rats were purchased from Charles River Laboratories, Inc., Wilmington, MA, USA. The rats were habituated to handling and experimental procedures for 1 week prior to testing. At the time of injection, rats were approximately 6.5 weeks old and weighed approximately 200–250 g. Each of the experiments utilized concurrent observation of a mixed cohort of three test groups (groups n = 9, cohort n = 27), with the experimenter blind to the treatments.
QX-314 bromide salt (Cat. No. L5783, Sigma, St. Louis, MO, USA) and lidocaine hydrochloride monohydrate (Cat. No. L5647, Sigma, St. Louis, MO, USA) were prepared freshly in normal saline (0.9% NaCl, 200 µL; Sigma, St. Louis, MO, USA) to the predetermined concentrations (percent weight by volume) immediately prior to injection. The pH of tested solutions ranged from 5.0 to 6.3 and was not adjusted due to the probability of rapid buffering by the pH of the extracellular fluid within tissue.
Sciatic nerve injections
Rats were lightly anaesthetized by inhalation of isoflurane (1.5–2%, in oxygen) for approximately 5–7 min, and the landmarks (greater trochanter and ischial tuberosity) of the left hind limb localized. Groups of six rats were injected with 0.2 mL of each test solution: lidocaine (1%, 1.5%, 2%), QX-314 (0.25%, 0.5%, 1%) and lidocaine mixed with QX-314 (1% lidocaine + 0.25% QX-314, 1% lidocaine + 0.5% QX-314, 1% lidocaine + 1% QX-314, 1.5% lidocaine + 0.5% QX-314, 2% lidocaine + 0.5% QX-314, 2% lidocaine + 1% QX-314). The drug was injected in immediate proximity to the sciatic nerve with a 27-gauge hypodermic needle attached to a tuberculin syringe. For the experiments described in Figure 4, QX-314 (1%) and vehicle were injected to unanaesthetized rats. The animals (n = 18) were manually restrained and sciatic injections performed as described above.
Figure 4.
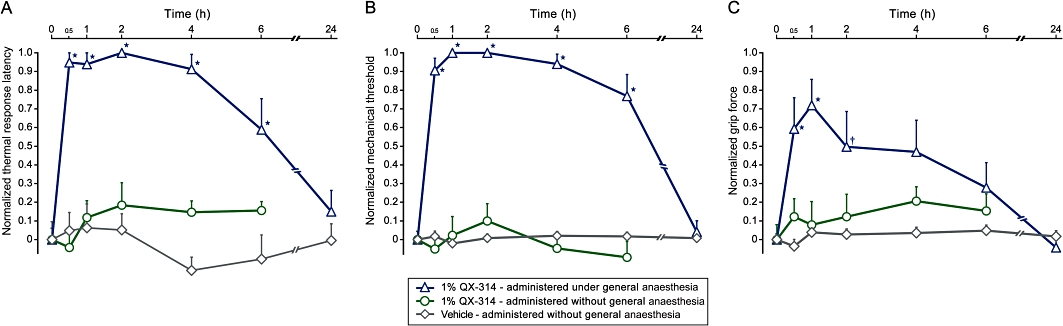
The motor and sensory block following injection of 1% lidocaine N-ethyl bromide (QX-314) is abolished when injected in the absence of general anaesthesia. Perisciatic application of 1% QX-314 alone produces prolonged elevation in thermal (radiant heat, 50°C) response latency (A), pinch tolerance threshold (B) and grip weakness (C) only while applied under isoflurane-induced general anaesthesia. Perisciatic injection of 1% QX-314 in non-anaesthetized animals did not change the responses to noxious mechanical and thermal stimuli or grip force. Application of vehicle (0.9% NaCl) administered without general anaesthesia also did not alter motor, mechanical or thermal responsiveness. Values expressed as percent of maximal block (mean ± SEM; *P < 0.01, †P < 0.01, anova followed by Dunnett's test; n = 9 for each group). All injections administered at time 0.
Two baseline readings of each test modality were taken; one at 24 h prior to injection and another immediately prior to induction of isoflurane general anaesthesia (approximately 10 min prior to injection). Additional measurements were recorded at 30 min, 60 min, and 4, 6, 9, 12 and 24 h after injection. Observer variability was determined to be insignificant by comparison of data obtained during the training period (n = 18).
Motor function
The Bioseb Grip Strength Test apparatus was used to assess changes in grasping strength of the left hind limb according to the method described by Simon et al. (Simon et al., 2004). Normal response in untreated rats ∼200 g, while the response during a complete lidocaine 2% block was ∼5 g.
Sensory function
Ugo Basile model no. 7371 was used to assess changes in thermal nociceptive response latency upon application of 52°C radiant heat at the lateral plantar surface of left hind paw according to the method described by Hargreaves et al. (Hargreaves et al., 1988). Normal response ∼16 s, cut-off 25 s.
The Bioseb Rodent Pincher Analgesia Meter was used to assess changes in mechanical nociception elicited upon pinch of the fifth proximal phalanx of the left hind paw, according to the method described by Luis-Delgado et al. (Luis-Delgado et al., 2006). Normal responses approximated 200 g in each group. Cut-off was set at 500 g and was achieved within 5 s in all animals. No damage to skin or deep tissue was evident at cut-off level.
Statistical analysis
Data is presented as mean ± SEM. Analysis of injections was done with either one-way analysis of variance (anova) followed by Dunnett's test (compared with baseline values) or two-way anova followed by Bonferroni (comparison between different treatments). To evaluate the significance of differences in the area under the curve (AUC) anova with post hoc Dunnett's test were performed. AUC were calculated as the trapezoidal area under the behavioural response score versus time curve for each behavioural modality (grip strength, pinch tolerance or thermal response latency). Scores were normalized at each time point by subtracting the group mean behavioural response score at baseline (xt = 0) from the group behavioural response score (x) and dividing by the behavioural response cut-off (xcut-off) minus group mean behavioural response score at baseline (xt = 0) as described in the following equation:
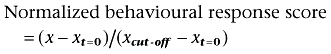 |
Results
We have previously demonstrated that the combined application of QX-314 together with lidocaine (lidocaine HCl) produces a prolonged nociceptive-selective blockade, which follows the brief non-selective effects of lidocaine (Binshtok et al., 2009a). We determined that perisciatic injection of a fixed 0.2% concentration of QX-314 together with different concentrations of lidocaine (0.5, 1, 2%) blocked the nocifensive response to pinch, an effect that persisted well beyond the duration of the transient motor block, as measured by the extensor postural thrust test. The duration of the differential block was increasingly prolonged with higher concentrations of lidocaine (Binshtok et al., 2009a).
Here, we hypothesized that by modifying the dose-ratio of QX-314 and lidocaine we could further prolong the duration of the nociceptive selective block over the motor block and thereby optimize the duration of nociceptive-specific differential block for potential clinical use. To test this we applied different dose combinations of both QX-314 and lidocaine close to the sciatic nerve of adult rats and assessed the changes in nociceptive threshold and motor strength at different time points after injection, to ascertain the particular dose combination producing an optimal duration of differential block.
Perisciatic injection of 1% lidocaine (200 µL) alone produced a short-lasting blockade of the response to noxious mechanical (pinch) and thermal (radiant heat) sensation that was no longer significant after 30 min (P < 0.01) (Figure 1A and B). The duration of motor block was coincident with the nociceptive block, lasting 30 min (Figure 1C) (P < 0.01). An increase in the concentration of lidocaine to 1.5% prolonged the duration of blockade to noxious mechanical stimuli up to 1 h, without further prolonging the duration of the blockade of response to noxious thermal stimuli or motor block. Perineural injection of 2% lidocaine alone produced sensory and motor block for 1 h (Figure 1). Thus, consistent with our previous observations, application of lidocaine in therapeutically relevant doses did not produce any differential block and its effect was relatively short lasting.
Figure 1.
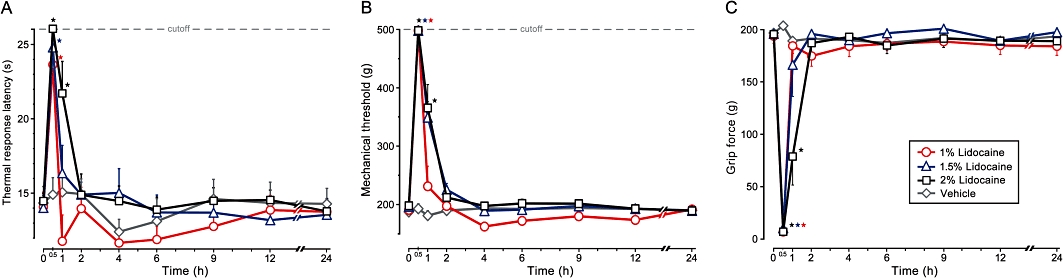
The duration of the elevation in thermal response latency (radiant heat, 50°C), pinch tolerance threshold and reduction in grip strength produced following perisciatic injection of lidocaine HCl (1%, 1.5%, 2%) and vehicle (0.9% NaCl) (mean ± SEM; *P < 0.01). All injections administered under isoflurane-induced general anaesthesia at time 0.
Injection of vehicle (0.9% NaCl) did not cause any significant changes in motor and nociceptive function (Figure 1). Similarly, injection of 0.5% QX-314 alone did not change the responses to noxious mechanical and thermal stimuli, and did not produce motor weakness (reduced grip strength) (Figure 2).
Figure 2.
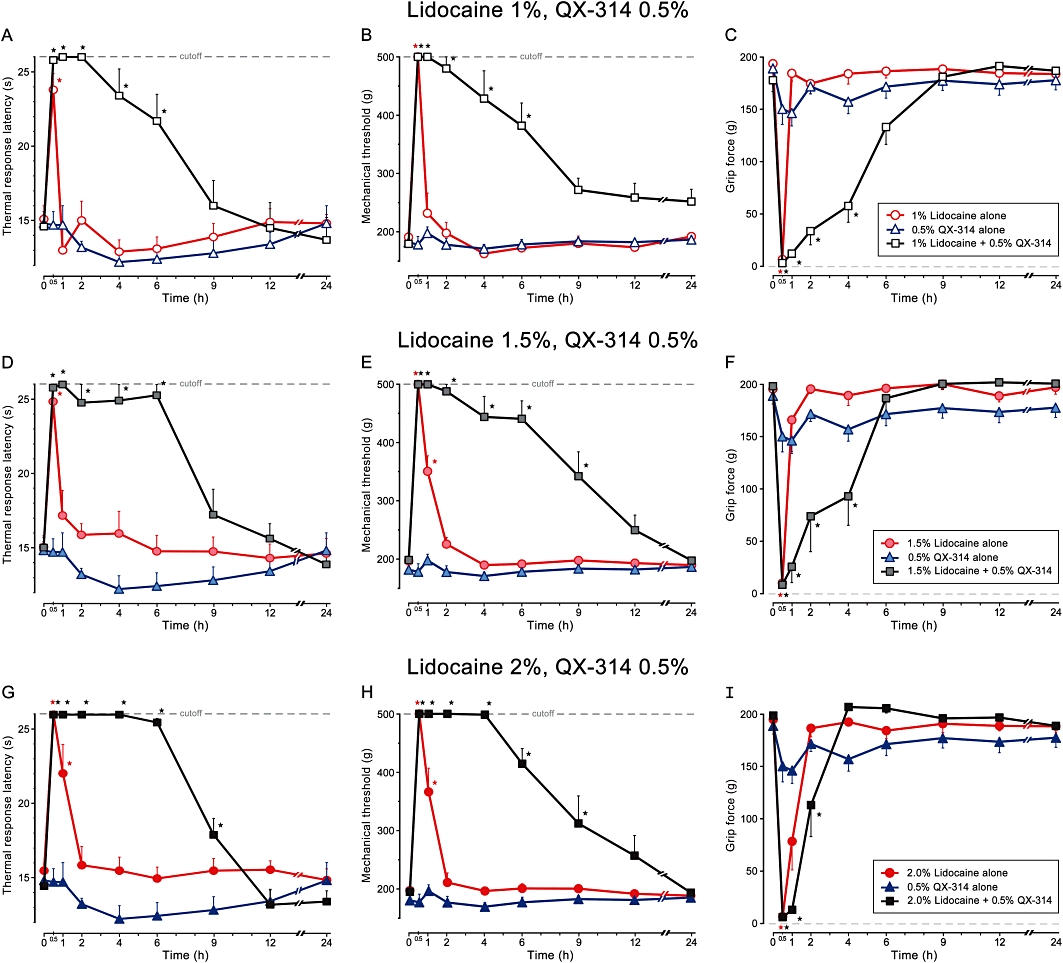
The duration of the elevation in thermal (radiant heat, 50°C) response latency (A, D and G), pinch tolerance threshold (B, E and H), and reduction in grip strength (C, F and I) produced following perisciatic (200 µL) injection of lidocaine alone (1%, 1.5% or 2%), 0.5% lidocaine N-ethyl bromide (QX-314) alone, or 0.5% QX-314 administered together with lidocaine (1%, 1.5% or 2%) (mean ± SEM; *P < 0.01). All injections administered under isoflurane-induced general anaesthesia at time 0.
When combined with lidocaine, QX-314 prolonged the duration of both sensory and motor block for all doses of lidocaine tested. All examined combinations of lidocaine and QX-314 resulted in a sensory block that exceeded the duration of the motor block. The duration of the differential block, however, varied according to the dose of both lidocaine and QX-314.
Co-application of 0.5% QX-314 with 1% lidocaine produced a blockade of the responses to noxious thermal and mechanical stimuli that was significant for 6 h (P < 0.01), and a motor block that was significant for 4 h (P < 0.01), resulting in 2 h of differential block (Figure 2A–C). Injection of 1.5% of lidocaine together with 0.5% QX-314 further prolonged the blockade of the response to noxious mechanical stimuli to 9 h (P < 0.01) (Figure 2E). The duration of the blockade of response to noxious thermal stimuli was prolonged to 6 h (P < 0.01) (Figure 2E), while the duration of the motor block remained similar to that produced by the combination of 1% lidocaine and 0.5% QX-314 (Figure 2C and F).
Increasing the concentration of lidocaine to 2%, while maintaining QX-314 concentration at 0.5%, prolonged the duration of blockade to noxious thermal and mechanical stimuli to 9 h (P < 0.01), thus inducing a nociceptive block that persisted 8 h beyond the blockade produced by the injection of 2% lidocaine alone (Figure 2G and H). Surprisingly, the duration of motor block resulting from injection of 2% lidocaine together with 0.5% QX-314 lasted only 1 h longer than the motor block induced by 2% lidocaine alone (Figure 2I). The duration of this motor block was considerably shorter than the motor block produced by corresponding combinations containing lower concentrations of lidocaine (Figures 1 and 2C, F and I).
The combination of 0.5% QX-314 (which has no significant effect when administered on its own) with 2% lidocaine (which has a brief non-selective action when administered on its own) produces a long-lasting decrease in pinch sensitivity (pinch) and noxious thermal (radiant heat) responsiveness. Addition of 0.5% QX-314 to 2% lidocaine has a minimal effect on grip strength versus 2% lidocaine alone. AUC analysis demonstrated that the effect of this particular combination of lidocaine and QX-314 on radiant heat response latency [(see Methods for the details of the normalization method) normalized AUC (nAUC) Lidocaine 2% + QX-314 0.5% = 8; nAUC lidocaine 2% = 1.1; nAUC QX-314 0.5% = 0.23] and pinch tolerance (nAUC Lidocaine 2% + QX-314 0.5% = 9.2; nAUC lidocaine 2% = 1.4; nAUC QX-314 0.5% = 0.3) is much greater than the additive effects of the two drugs administered individually, but the effect on the grip strength (nAUC Lidocaine 2% + QX-314 0.5% = 2.1; nAUC lidocaine 2% = 1.7; nAUC QX-314 0.5% = 1.7) is less than the additive effects of the two drugs administered individually (Figure 3).
Figure 3.
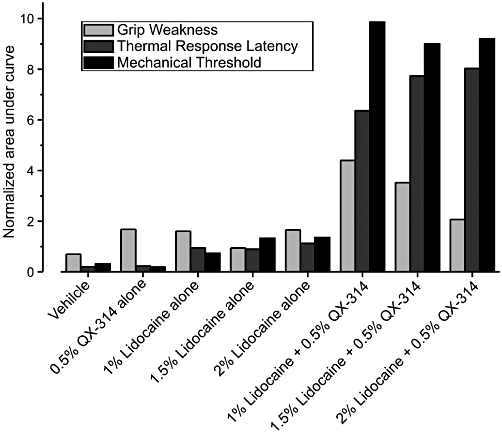
Analysis of the change in grip strength, thermal (radiant heat, 50°C) response latency and pinch tolerance threshold produced following perisciatic injection of varying doses of lidocaine N-ethyl bromide (QX-314) (0%, 0.5%) and lidocaine (0%, 1%, 2%) expressed as total area under the curve (AUC). Note that the value of AUC representing change in pinch tolerance threshold in the group receiving a combined dose of 0.5% QX-314 + 2% lidocaine, is greater than the combined values of corresponding AUCs from the group receiving 0.5% QX-314 alone plus the AUC from the group receiving 2.0% lidocaine alone. Similarly, the AUC value representing change in thermal latency in the group receiving a combined dose of 0.5% QX-314 + 2% lidocaine, is much greater than the combined values of corresponding AUCs from the group receiving 0.5% QX-314 alone plus the AUC from the group receiving 2.0% lidocaine alone. Conversely, the value of AUC representing grip strength in the group receiving a combined dose of 0.5% QX-314 + 2% lidocaine, is less than the combined values of grip strength AUCs from the group receiving 0.5% QX-314 alone plus the grip strength AUC from the group receiving 2.0% lidocaine alone.
Because the optimal lidocaine concentration for sciatic nerve block is similar between rat and humans (Nakamura et al., 2003), and in order to limit potential lidocaine toxicity that may arise from addition of a second lidocaine-based agent such as QX-314, we did not exceed the clinically recommended concentration range (1–2%) for optimal single-injection sciatic nerve block in humans (Enneking et al., 2009). We thus decided to increase the concentration of QX-314 in combination with clinically relevant doses of lidocaine (1%, 1.5%, 2%). Increasing the concentration of QX-314 from 0.5% to 1% did not further enhance the duration of differential block. Specifically, the application of 1% lidocaine together with 1% QX-314 prolonged the duration of thermal nociceptive block to 9 h (radiant heat; P < 0.01) and mechanical nociceptive block to 12 h (P < 0.01; pinch), but also prolonged the motor block to 6 h (P < 0.01) (Figure S1). Injection of 2% lidocaine and 1% QX-314 produced 12 h of sensory block (P < 0.01) and 9 h of motor block (P < 0.01) (data not shown).
Surprisingly, application of 1% QX-314 alone (i.e. without lidocaine) produced a differential sensory block characterized by a reduction of noxious mechanical threshold persisting for 12 h (P < 0.05) and a blockade of the response to noxious thermal stimuli lasting for 6 h (P < 0.01). The injected animals also demonstrated a motor weakness that continued for 2 h (P < 0.05) (Figure 4). Because the present experiments were all performed under isoflurane-induced general anaesthesia to facilitate perisciatic nerve injections, we hypothesized that the isoflurane-mediated activation of TRPV1 and/or TRPA1 (Harrison and Nau, 2008; Matta et al., 2008) may permit QX-314 entry into nociceptors at QX-314 concentrations greater than or equal to 1%.
To determine whether the appearance of a non-selective block by high doses of QX-314 administered on its own was a consequence of the isoflurane general anaesthesia, we conducted a series of experiments where the perisciatic injection of QX-314 (1%) was performed in the absence of isoflurane general anaesthesia. The sensory and motor blocking effects of 1% QX-314 administered alone in the presence of isoflurane were totally abolished in the absence of general anaesthesia (Figure 4), indicating that isoflurane can induce a means of entry for high concentrations of QX-314 into axons. The sensory blockade produced by QX-314 under general anaesthesia at concentrations exceeding 1% suggests that isoflurane mediated activation of TRPV1 and/or TRPA1 may provide a passage for QX-314 into nociceptors. However, QX-314 alone at high doses in the presence of isoflurane also produced a motor block implying some action on channels expressed by motor axons. While the results of such non-anaesthetized groups are of obvious mechanistic interest, the stress induced by conscious perisciatic injections, requiring restraint, together with lack of a clinical correlate, convinced us that broader studies of perisciatic injections in absence of general anaesthesia were not warranted, as our prime effort was focused on finding maximal differential block even when administered under general anaesthesia, for potential clinical exploitation. We conclude therefore, that a combination of 0.5% QX-314 and 2% lidocaine is the optimal concentration and ratio for producing the longest-duration differential block.
Discussion and conclusions
Regional anaesthesia with local anaesthetic agents has the great advantage over general anaesthesia of targeting treatment to the affected site, whether by local tissue/perineural injection or epidural/intrathecal delivery, thus avoiding or minimizing systemic side effects. Although very successful for many surgical interventions (Hogan et al., 2009; Fredrickson et al., 2010; Hawkins, 2010; Murray et al., 2010; Scott, 2010) as well treatment of some chronic pain conditions (Dillane and Tsui, 2010; Power et al., 2010), the non-selective action of currently available sodium channel blockers means that a block of motor, sensory and autonomic function inevitably occurs, even if only analgesia is required. Our strategy of using large-pore channels to deliver sodium channel blockers into nociceptors (Binshtok et al., 2007) provides an alternative approach. In its ideal form, this approach incorporates both a TRPV1 agonist and a permanently charged sodium channel blocker such as QX-314 to produce a block only of nociceptors (Binshtok et al., 2007). However, patients would simply not tolerate the initial pain that would be produced by injection of a TRPV1 agonist like capsaicin prior to production of the nociceptor block.
As an alternative strategy, we have chosen to activate TRPV1 using lidocaine because its activation of TRPV1 channels (Leffler et al., 2008) – although substantial at clinically used doses (>5 mM) – is masked within seconds by its sodium channel blocking action so that only a very transient burning sensation is experienced (Davies, 2003; Vossinakis et al., 2004). While co-administration of lidocaine with QX-314 can target QX-314 through TRPV1 into nociceptor neurons in culture (unpublished observations), this is obviously at the expense of an initial period of non-selective block (Binshtok et al., 2009a), as demonstrated by the short-lasting reduction in grip strength in the current experiments. However, the early non-selective block produced by the lidocaine is followed by a much longer period of differential block due to the distribution of QX-314 into nociceptors, where the response to noxious mechanical and thermal stimuli is very substantially reduced, even after motor function has fully recovered. This profile of short non-selective block followed by a prolonged pain-selective block produced by the lidocaine/QX-314 combination may have utility for many surgical procedures. For example, the initial non-selective block would be advantageous during surgery, while the longer-lasting local analgesia would be beneficial during the postsurgical period; a long-lasting effect that is absent when lidocaine is administered alone. Clinically, such long-lasting local post-operative analgesia with intact motor function could contribute to more rapid mobilization and decreased requirements for intra/post-operative opioids, both of which would be valuable to patients and caregivers, particularly in an outpatient surgical setting, because it could allow earlier hospital discharge and better pain control. More generally, the inherent advantages of early mobilization would likely prove beneficial following any surgery of the upper or lower extremity as well as in dental surgical procedures.
In the present experiments, we set out to systematically identify the optimal concentration and ratio of lidocaine and QX-314 for producing prolonged local analgesia. We found, however, that QX-314 when administered alone under inhalational (isoflurane) anaesthesia begins to produce an effect on its own at high concentrations (1%, ∼35 mM and higher), as has been reported previously (Lim et al., 2007). When we tested administration of QX-314 alone in the absence of the general aesthetic isoflurane, this action disappeared. We conclude that the TRP activation that has been reported for isoflurane and other general aesthetic agents (Cornett et al., 2008; Matta et al., 2008; Satoh and Yamakage, 2009), is likely sufficient to permit some QX-314 entry into nociceptors when administered alone at high enough concentrations, something also reported by other investigators (Ries et al., 2009). What action isoflurane has on motor axons to allow QX-314 entry needs to be explored. At 0.5% (17 mM) QX-314, we found no effect though, even in the presence of isoflurane, and therefore consider this concentration to be a suitable dose for maximizing selectivity even in the presence of general anaesthetics (Figure S1).
QX-314, when injected intrathecally in mice at concentrations of 5 to 10 mM, has been found to produce marked irritation and death in some animals (Schwarz et al., 2010), something never seen when it is injected subcutaneously or perineurally at very high doses (Lim et al., 2007; Ries et al., 2009). The intrathecal effect of QX-314 administered alone may represent the action of extracellular QX-314 on some other target present on central neurons. One known effect of extracellular QX-314 is to block nicotinic ACh receptors. Conceivably, this could reduce inhibitory synaptic activity in the spinal cord, which is enhanced by nicotinic receptor stimulation (Takeda et al., 2003; 2007;). In any case, if the irritant effect of intrathecal QX-314 is duplicated in primates it would obviously preclude intrathecal use of QX-314 in patients; and, to avoid any risk of inadvertent intrathecal injection, would also preclude epidural administration. In our experience, neither subcutaneous injection nor perineural administration of QX-314 at concentrations up to 2% (68 mM) even at high volumes produced any observable adverse effects in mice and rats.
Increasing the concentration of lidocaine from 0.5 to 2.0% markedly increased the duration of analgesia to mechanical and heat stimuli when combined with 0.5% QX-314. Although lidocaine is used clinically at concentrations up to 4%, it has both a risk of direct neural toxicity (Lirk et al., 2007; Perez-Castro et al., 2009; Werdehausen et al., 2009) and systemic CNS/CVS side effects (Dillane and Finucane, 2010; Neal et al., 2010), that are particularly evident at higher doses. Moreover, current clinical standards recommend a lidocaine concentration of 1–2% as optimal for sciatic nerve block (Enneking et al., 2009). We therefore decided that 2% lidocaine (69 mM) would be the maximal dose used in the present study. Leffler et al. demonstrated that lidocaine, at this concentration, also activates the TRPA1 channel – another nociceptive specific transducer that involved in detection of noxious cold and various harmful chemicals (Leffler et al., 2008). We recently demonstrated that the lidocaine-mediated activation of TRPA1 channels provides an additional pathway for QX-314 entry (Binshtok et al., 2009b). We also demonstrated that the lidocaine/QX-314-mediated effect on mechanical threshold was partially abolished in TRPA1 knockout mice (Binshtok et al., 2009b). These findings suggest that the lidocaine/QX-314 effect on mechanical threshold is partially mediated via TRPA1 channels. Surprisingly, the combination of QX-314 and lidocaine produced an increase in the duration of the motor block compared with lidocaine alone, even though TRPV1 and TRPA1 are not expressed in motor neurons. This may indicate that lidocaine acts on some other large-pore channel to facilitate QX-314 entry into motor axons. The non-selective block decreased, however, at the highest dose of lidocaine used (2%), providing the longest ‘pain-specific’ phase (9 h) after the initial short non-selective phase (1 h). This long-lasting differential effect may have considerable clinical utility because pain alone is blocked for 90% of the total time.
These data clearly suggest therefore, that a combination of 0.5% QX-314 and 2% lidocaine may be optimally suited for peripheral nerve block in human patients, offering the best compromise of long analgesia over short motor block. Because the duration of perineural lidocaine anaesthesia in rodents (<1 h) is considerably shorter than that found in humans (Lemke and Dawson, 2000; Berberich et al., 2009; O'Donnell et al., 2010), it will be interesting to determine if the lidocaine (2%) QX-314 (0.5%) combination, when administered in humans, produces an even longer local analgesic phase than the 9 h seen in the rat. This should be readily detectable in Phase 1b studies in human volunteers. Whether there is a differential change over time in local levels of lidocaine and QX-314 in patients because of their different lipid solubility will need to be explored.
In conclusion, we describe here a preclinical study specifically designed to translate the strategy of targeting sodium channel blockers into nociceptors in a manner applicable for clinical use. The next step, assuming that there are no toxicological issues with the local injection of QX-314 in combination with lidocaine in peripheral tissue or nerves, will be testing this combination in human volunteers and patients to determine the nature, selectivity, depth and duration of sensory and motor block. If the clinical data match the preclinical findings reported here, the combination of lidocaine and its quaternary derivative QX-314 in an injectable formulation may be a useful addition for regional pain control, producing a longer and more selective action than existing local aesthetic agents.
Acknowledgments
This study was supported by a research grant from Endo Pharmaceuticals who have licensed the technology, which was invented by Bruce Bean and Clifford Woolf, from Harvard University and the Massachusetts General Hospital. Additional support was obtained from the NIH (C. J. W. and B. B., NS039518 and NS064274). We thank David Segal for technical assistance and Peter Gerner for useful comments and advice.
Glossary
Abbreviation
- QX-314
lidocaine N-ethyl bromide
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 The duration of change in grip strength (blue triangles), thermal (radiant heat, 50°C) response latency (red circles) and pinch tolerance threshold (black squares) produced following perisciatic injection of vehicle (0.9% NaCl), 1% lidocaine, 2% lidocaine, 0.5% lidocaine N-ethyl bromide (QX-314), 1% QX-314, combined doses of 0.5% QX-314 + 1% lidocaine, 0.5% QX-314 + 2% lidocaine, 1% QX-314 + 1% lidocaine and 1% QX-314 + 2% lidocaine.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Ahern CA, Eastwood AL, Dougherty DA, Horn R. Electrostatic contributions of aromatic residues in the local anesthetic receptor of voltage-gated sodium channels. Circ Res. 2008;102:86–94. doi: 10.1161/CIRCRESAHA.107.160663. [DOI] [PubMed] [Google Scholar]
- Berberich G, Reader A, Drum M, Nusstein J, Beck M. A prospective, randomized, double-blind comparison of the anesthetic efficacy of two percent lidocaine with 1:100,000 and 1:50,000 epinephrine and three percent mepivacaine in the intraoral, infraorbital nerve block. J Endod. 2009;35:1498–1504. doi: 10.1016/j.joen.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Gerner P, Oh SB, Puopolo M, Suzuki S, Roberson DP, et al. Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology. 2009a;111:127–137. doi: 10.1097/ALN.0b013e3181a915e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok AM, et al. Activation of TRPA1 As Well As TRPV1 Channels by Lidocaine Allows Entry of QX-314 into Nociceptors to Produce A Pain Selective Sodium Channel Block. Chikago, IL: Society for Neuroscience Meeting; 2009b. [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chen J, Kim D, Bianchi BR, Cavanaugh EJ, Faltynek CR, Kym PR, et al. Pore dilation occurs in TRPA1 but not in TRPM8 channels. Mol Pain. 2009;5:3. doi: 10.1186/1744-8069-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier P, Vijayaragavan K, Chahine M. Differential modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by the local anesthetic lidocaine. Br J Pharmacol. 2004;142:576–584. doi: 10.1038/sj.bjp.0705796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Guler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci. 2008;11:555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- Cornett PM, Matta JA, Ahern GP. General anesthetics sensitize the capsaicin receptor transient receptor potential V1. Mol Pharmacol. 2008;74:1261–1268. doi: 10.1124/mol.108.049684. [DOI] [PubMed] [Google Scholar]
- Davies RJ. Buffering the pain of local anaesthetics: a systematic review. Emerg Med (Fremantle) 2003;15:81–88. doi: 10.1046/j.1442-2026.2003.00413.x. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Binshtok AM, Cummins TR, Jarvis MF, Samad T, Zimmermann K. Voltage-gated sodium channels in pain states: role in pathophysiology and targets for treatment. Brain Res Rev. 2009;60:65–83. doi: 10.1016/j.brainresrev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Dillane D, Finucane BT. Local anesthetic systemic toxicity. Can J Anaesth. 2010;57:368–380. doi: 10.1007/s12630-010-9275-7. [DOI] [PubMed] [Google Scholar]
- Dillane D, Tsui BC. From basic concepts to emerging technologies in regional anesthesia. Curr Opin Anaesthesiol. 2010;23:663–669. doi: 10.1097/ACO.0b013e32833d9513. [DOI] [PubMed] [Google Scholar]
- Enneking F, Wedel D, Horlocker T. The Lower Extremity: Somatic Blockade. 4th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- Frazier DT, Narahashi T, Yamada M. The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J Pharmacol Exp Ther. 1970;171:45–51. [PubMed] [Google Scholar]
- Fredrickson MJ, Krishnan S, Chen CY. Postoperative analgesia for shoulder surgery: a critical appraisal and review of current techniques. Anaesthesia. 2010;65:608–624. doi: 10.1111/j.1365-2044.2009.06231.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson H, Akesson J, Lau CL, Williams D, Miller L, Yap S, et al. A comparison of two formulations of intradermal capsaicin as models of neuropathic pain in healthy volunteers. Br J Clin Pharmacol. 2009;68:511–517. doi: 10.1111/j.1365-2125.2009.03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harrison N, Nau C. Sensitization of nociceptive ion channels by inhaled anesthetics–a pain in the gas? Mol Pharmacol. 2008;74:1180–1182. doi: 10.1124/mol.108.051615. [DOI] [PubMed] [Google Scholar]
- Hawkins JL. Epidural analgesia for labor and delivery. N Engl J Med. 2010;362:1503–1510. doi: 10.1056/NEJMct0909254. [DOI] [PubMed] [Google Scholar]
- Hellwig N, Plant TD, Janson W, Schafer M, Schultz G, Schaefer M. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J Biol Chem. 2004;279:34553–34561. doi: 10.1074/jbc.M402966200. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977a;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The pH-dependent rate of action of local anesthetics on the node of Ranvier. J Gen Physiol. 1977b;69:475–496. doi: 10.1085/jgp.69.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann T, Sauer SK, Horch RE, Reeh PW. Sensory transduction in peripheral nerve axons elicits ectopic action potentials. J Neurosci. 2008;28:6281–6284. doi: 10.1523/JNEUROSCI.1627-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MV, Grant RE, Lee L., Jr Analgesia for total hip and knee arthroplasty: a review of lumbar plexus, femoral, and sciatic nerve blocks. Am J Orthop (Belle Mead NJ) 2009;38:E129–E133. [PubMed] [Google Scholar]
- Kim HY, Kim K, Li HY, Chung G, Park CK, Kim JS, et al. Selectively targeting pain in the trigeminal system. Pain. 2010;150:29–40. doi: 10.1016/j.pain.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler A, Herzog RI, Dib-Hajj SD, Waxman SG, Cummins TR. Pharmacological properties of neuronal TTX-resistant sodium channels and the role of a critical serine pore residue. Pflugers Arch. 2005;451:454–463. doi: 10.1007/s00424-005-1463-x. [DOI] [PubMed] [Google Scholar]
- Leffler A, Fischer MJ, Rehner D, Kienel S, Kistner K, Sauer SK, et al. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J Clin Invest. 2008;118:763–776. doi: 10.1172/JCI32751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke KA, Dawson SD. Local and regional anesthesia. Vet Clin North Am Small Anim Pract. 2000;30:839–857. doi: 10.1016/s0195-5616(08)70010-x. [DOI] [PubMed] [Google Scholar]
- Lim TK, Macleod BA, Ries CR, Schwarz SK. The quaternary lidocaine derivative, QX-314, produces long-lasting local anesthesia in animal models in vivo. Anesthesiology. 2007;107:305–311. doi: 10.1097/01.anes.0000270758.77314.b4. [DOI] [PubMed] [Google Scholar]
- Lirk P, Haller I, Colvin HP, Frauscher S, Kirchmair L, Gerner P, et al. In vitro, lidocaine-induced axonal injury is prevented by peripheral inhibition of the p38 mitogen-activated protein kinase, but not by inhibiting caspase activity. Anesth Analg. 2007;105:1657–1664. doi: 10.1213/01.ane.0000286171.78182.e2. Table of contents. [DOI] [PubMed] [Google Scholar]
- Liu H, Atkins J, Kass RS. Common molecular determinants of flecainide and lidocaine block of heart Na+ channels: evidence from experiments with neutral and quaternary flecainide analogues. J Gen Physiol. 2003;121:199–214. doi: 10.1085/jgp.20028723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis-Delgado OE, Barrot M, Rodeau JL, Schott G, Benbouzid M, Poisbeau P, et al. Calibrated forceps: a sensitive and reliable tool for pain and analgesia studies. J Pain. 2006;7:32–39. doi: 10.1016/j.jpain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A. 2008;105:8784–8789. doi: 10.1073/pnas.0711038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty MM, Edgerton GB, Shah RD, Hanck DA, Fozzard HA, Lipkind GM. Charge at the lidocaine binding site residue Phe-1759 affects permeation in human cardiac voltage-gated sodium channels. J Physiol. 2007;581(Pt 2):741–755. doi: 10.1113/jphysiol.2007.130161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, et al. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momin A, Wood JN. Sensory neuron voltage-gated sodium channels as analgesic drug targets. Curr Opin Neurobiol. 2008;18:383–388. doi: 10.1016/j.conb.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Muroi Y, Chanda B. Local anesthetics disrupt energetic coupling between the voltage-sensing segments of a sodium channel. J Gen Physiol. 2009;133:1–15. doi: 10.1085/jgp.200810103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JM, Derbyshire S, Shields MO. Lower limb blocks. Anaesthesia. 2010;65(Suppl. 1):57–66. doi: 10.1111/j.1365-2044.2010.06240.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Popitz-Bergez F, Birknes J, Strichartz GR. The critical role of concentration for lidocaine block of peripheral nerve in vivo: studies of function and drug uptake in the rat. Anesthesiology. 2003;99:1189–1197. doi: 10.1097/00000542-200311000-00028. [DOI] [PubMed] [Google Scholar]
- Neal JM, Bernards CM, Butterworth JFT, Di Gregorio G, Drasner K, Hejtmanek MR, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152–161. doi: 10.1097/AAP.0b013e3181d22fcd. [DOI] [PubMed] [Google Scholar]
- O'Donnell B, Riordan J, Ahmad I, Iohom G. A clinical evaluation of block characteristics using one milliliter 2% lidocaine in ultrasound-guided axillary brachial plexus block. Anesth Analg. 2010;111:808–810. doi: 10.1213/ANE.0b013e3181e79965. [DOI] [PubMed] [Google Scholar]
- Oseguera AJ, Islas LD, Garcia-Villegas R, Rosenbaum T. On the mechanism of TBA block of the TRPV1 channel. Biophys J. 2007;92:3901–3914. doi: 10.1529/biophysj.106.102400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Castro R, Patel S, Garavito-Aguilar ZV, Rosenberg A, Recio-Pinto E, Zhang J, et al. Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108:997–1007. doi: 10.1213/ane.0b013e31819385e1. [DOI] [PubMed] [Google Scholar]
- Power I, McCormack JG, Myles PS. Regional anaesthesia and pain management. Anaesthesia. 2010;65(Suppl. 1):38–47. doi: 10.1111/j.1365-2044.2009.06202.x. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Agarwal S, Steffen D. Single-channel properties of native and cloned rat vanilloid receptors. J Physiol. 2002;545(Pt 1):107–117. doi: 10.1113/jphysiol.2002.016352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest BT. Future potential and status of selective sodium channel blockers for the treatment of pain. Curr Opin Drug Discov Devel. 2009;12:682–692. [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci U S A. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisinghani M, Pabbidi RM, Premkumar LS. Activation of transient receptor potential vanilloid 1 (TRPV1) by resiniferatoxin. J Physiol. 2005;567(Pt 3):771–786. doi: 10.1113/jphysiol.2005.087874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries CR, Pillai R, Chung CC, Wang JT, MacLeod BA, Schwarz SK. QX-314 produces long-lasting local anesthesia modulated by transient receptor potential vanilloid receptors in mice. Anesthesiology. 2009;111:122–126. doi: 10.1097/ALN.0b013e3181a9160e. [DOI] [PubMed] [Google Scholar]
- Satoh J, Yamakage M. Desflurane induces airway contraction mainly by activating transient receptor potential A1 of sensory C-fibers. J Anesth. 2009;23:620–623. doi: 10.1007/s00540-009-0786-8. [DOI] [PubMed] [Google Scholar]
- Schwarz W, Palade PT, Hille B. Local anesthetics. Effect of pH on use-dependent block of sodium channels in frog muscle. Biophys J. 1977;20:343–368. doi: 10.1016/S0006-3495(77)85554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz SK, Cheung HM, Ries CR, Lee SM, Wang JT, MacLeod BA. Lumbar intrathecal administration of the quaternary lidocaine derivative, QX-314, produces irritation and death in mice. Anesthesiology. 2010;113:438–444. doi: 10.1097/ALN.0b013e3181dfd31b. [DOI] [PubMed] [Google Scholar]
- Scott NB. Wound infiltration for surgery. Anaesthesia. 2010;65(Suppl. 1):67–75. doi: 10.1111/j.1365-2044.2010.06241.x. [DOI] [PubMed] [Google Scholar]
- Sheets MF, Hanck DA. Outward stabilization of the S4 segments in domains III and IV enhances lidocaine block of sodium channels. J Physiol. 2007;582(Pt 1):317–334. doi: 10.1113/jphysiol.2007.134262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D, Seznec H, Gansmuller A, Carelle N, Weber P, Metzger D, et al. Friedreich ataxia mouse models with progressive cerebellar and sensory ataxia reveal autophagic neurodegeneration in dorsal root ganglia. J Neurosci. 2004;24:1987–1995. doi: 10.1523/JNEUROSCI.4549-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz GR. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973;62:37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda D, Nakatsuka T, Papke R, Gu JG. Modulation of inhibitory synaptic activity by a non-alpha4beta2, non-alpha7 subtype of nicotinic receptors in the substantia gelatinosa of adult rat spinal cord. Pain. 2003;101:13–23. doi: 10.1016/s0304-3959(02)00074-x. [DOI] [PubMed] [Google Scholar]
- Takeda D, Nakatsuka T, Gu JG, Yoshida M. The activation of nicotinic acetylcholine receptors enhances the inhibitory synaptic transmission in the deep dorsal horn neurons of the adult rat spinal cord. Mol Pain. 2007;3:26. doi: 10.1186/1744-8069-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossinakis IC, Stavroulaki P, Paleochorlidis I, Badras LS. Reducing the pain associated with local anaesthetic infiltration for open carpal tunnel decompression. J Hand Surg Br. 2004;29:399–401. doi: 10.1016/j.jhsb.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Werdehausen R, Fazeli S, Braun S, Hermanns H, Essmann F, Hollmann MW, et al. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth. 2009;103:711–718. doi: 10.1093/bja/aep236. [DOI] [PubMed] [Google Scholar]
- Wood JN, Boorman JP, Okuse K, Baker MD. Voltage-gated sodium channels and pain pathways. J Neurobiol. 2004;61:55–71. doi: 10.1002/neu.20094. [DOI] [PubMed] [Google Scholar]
- Yang BH, Piao ZG, Kim YB, Lee CH, Lee JK, Park K, et al. Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res. 2003;82:781–785. doi: 10.1177/154405910308201004. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, McPhee JC, Idsvoog D, Pate C, Scheuer T, Catterall WA. Role of amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel alpha subunit in voltage-dependent gating and drug block. J Biol Chem. 2002;277:35393–35401. doi: 10.1074/jbc.M206126200. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Shieh CC, Chapman ML, Matulenko MA, Hakeem AH, Atkinson RN, et al. A-887826 is a structurally novel, potent and voltage-dependent Na(v)1.8 sodium channel blocker that attenuates neuropathic tactile allodynia in rats. Neuropharmacology. 2010;59:201–207. doi: 10.1016/j.neuropharm.2010.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


