Abstract
BACKGROUND AND PURPOSE
Lymphangioleiomyomatosis (LAM) is characterized by the abnormal growth of smooth muscle-like cells (LAM cells) and cystic destruction of the lung parenchyma. LAM cell-derived matrix metalloproteinases (MMPs) are thought to play a prominent role in the tissue destruction. The aim of this study was to determine whether doxycycline, a known MMP inhibitor, can inhibit LAM cell proliferation or mitochondrial function and/or modulate MMPs and their tissue inhibitors (TIMPs).
EXPERIMENTAL APPROACH
Wild-type and tuberous sclerosis complex-2 (TSC2)-null mouse embryonic fibroblasts (MEFs) were cultured in DMEM containing 10% fetal bovine serum (FBS). Human LAM cells were derived from the lungs of LAM patients and airway smooth muscle cells from control subjects. Cells were stimulated with FBS with or without doxycycline for up to 9 days. Proliferation was assessed by manual cell counts and MTT assay, MMP production by zymography and ELISA, and TIMP production using elisa.
KEY RESULTS
Doxycycline did not change FBS-induced proliferation in MEFs or human cells. However, doxycycline did reduce metabolic activity of both wild-type and TSC2-null MEFs and LAM cells, but had no effect on control cells. Furthermore, doxycycline reduced MMP-2 from MEFs and decreased active-MMP-2 from LAM cells but had no effect on TIMP-1 and TIMP-2 from human LAM cells.
CONCLUSIONS AND IMPLICATIONS
Doxycycline decreased MMP levels and cell metabolic activity, which raises the possibility of therapeutic efficacy in LAM.
Keywords: pulmonary lymphangioleiomyomatosis, doxycycline, proliferation, matrix metalloproteinase, tissue inhibitors of matrix metalloproteinase
Introduction
Lymphangioleiomyomatosis (LAM) is a rare lung disorder for which there is no current treatment other than lung transplantation and which affects women almost exclusively. LAM manifests as lesions comprised of smooth muscle-like cells (LAM cells) within the lungs, resulting in cystic destruction of the lung parenchyma and loss of pulmonary function. Although the pathogenesis of LAM remains unclear, an association with tuberous sclerosis is now widely accepted (Smolarek et al., 1998; McCormack et al., 2002). LAM is associated with the functional inactivation of the tuberous sclerosis genes, tuberous sclerosis complex 1 (TSC1) and TSC2. In particular, loss of function of TSC2, a negative regulator of the mammalian target of rapamycin (mTOR) signalling pathway, results in the phenotypic manifestation of the disease.
The LAM nodules are associated with altered extracellular matrix (ECM) proteins, in particular a decrease in collagen fibres (Merrilees et al., 2003). The degradation of ECM components is regulated by the balance between matrix metalloproteinases (MMPs) and their endogenous inhibitors, tissue inhibitors of MMPs (TIMPs). An imbalance in protease activity has been implicated in other lung disorders such as asthma and chronic obstructive pulmonary disease (Cataldo et al., 2000; Mattos et al., 2002; Ko et al., 2005). Alterations in MMP and TIMP expression are thought to play a prominent role in the tissue destruction associated with LAM. MMPs are secreted as inactive proenzymes which become active when cleaved. MMP-2 (gelatinase A) is predominantly expressed by mesenchymal cells, that is, smooth muscle cells and fibroblasts, and is up-regulated in LAM lungs (Hayashi et al., 1997). Further support for a role of MMP-2 in LAM has emerged, with increased MMP-2 secretion from an immortalized angiomyolipoma cell line derived from a LAM patient (Lee et al., 2010). In addition, MMP-9 (gelatinase B) is elevated in serum from LAM patients (Odajima et al., 2009). Furthermore, Hayashi and colleagues reported that levels of TIMP-1 and TIMP-2 were not increased in LAM cells (Hayashi et al., 1997) and because levels of TIMP-3 are decreased in LAM lungs (Zhe et al., 2003), an imbalance between MMP and TIMP levels is likely.
Drugs which block MMP expression or activity could have therapeutic potential in preventing the progressive lung destruction associated with LAM. Doxycycline, a second-generation tetracycline, is a commonly used antibiotic. However, independent of its antibiotic action it can also modulate cellular functions including proliferation (Franco et al., 2006), migration and matrix remodelling (Franco et al., 2006), through its ability to inhibit MMPs. A case report of a single LAM patient suggested that doxycycline may have therapeutic potential, as it reduced urinary MMP levels and increased lung function (forced expiratory volume in 1 s) (Moses et al., 2006). In view of this, the current study was undertaken to determine whether doxycycline could modulate LAM cell proliferation or survival and/or alter MMP and TIMP levels in both a mouse cellular model of LAM and human primary LAM-derived cells. In this study, we have demonstrated that doxycycline can decrease MMP-2 secretion and reduce metabolic activity.
Methods
Cell culture
Wild-type and TSC2-null mouse embryonic fibroblasts (MEFs), which were also negative for p53 [a gift from Dr D. Kwiatkowski (Brigham and Women's Hospital, Boston, MA, USA)], were used as a model of control and LAM cells respectively. MEFs were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37°C and 5% CO2. For all experiments, MEF cells between passage 10 and 30 were seeded at 1 × 104 cells·cm−2.
Primary human lung cells were obtained from lung specimens with written informed consent of the patients and with approval of the human ethics committees of The University of Sydney, the Sydney South West Area Health Service, Royal Perth Hospital, the Prince Charles Hospital, Brisbane and The Alfred Hospital, Melbourne. Smooth muscle cells were obtained from LAM patients (LAM cells) (45.0 ± 2.8 years) undergoing lung transplantation and were characterized by positive staining for human melanoma black-45 (HMB-45) (Black et al., 2005). Airway smooth muscle (ASM) cells from control subjects (50.9 ± 3.4 years) were isolated from airways from female subjects undergoing lung resection for either lung transplantation or carcinoma. Subject details are shown in Table 1. Information on patient medication was not available, but see Discussion. ASM bundles were dissected free from surrounding tissue and grown as explants as previously described (Johnson et al., 2001; Black et al., 2005). ASM cell characterization was confirmed by light microscopy and immunohistochemistry for smooth muscle α-actin and calponin expression. Cells were used between passages 4 and 8. For all experiments, human primary cells were seeded at a density of 1 × 104 cells·cm−2.
Table 1.
Subject details
| Patient number | Gender | Age | Disease | LAM/control |
|---|---|---|---|---|
| 1 | Female | 56 | LAM | LAM |
| 2 | Female | 39 | LAM | LAM |
| 3 | Female | 46 | LAM | LAM |
| 4 | Female | 48 | LAM | LAM |
| 5 | Female | 55 | LAM | LAM |
| 6 | Female | 40 | LAM | LAM |
| 7 | Female | 43 | LAM | LAM |
| 8 | Female | 33 | LAM | LAM |
| 9 | Female | 46 | Carcinoma | Control |
| 10 | Female | 38 | Carcinoma | Control |
| 11 | Female | 47 | Small cell carcinoma | Control |
| 12 | Female | 52 | Carcinoma | Control |
| 13 | Female | 52 | Emphysema | Control |
| 14 | Female | 54 | Large cell carcinoma | Control |
| 15 | Female | 67 | Non-small cell carcinoma | Control |
LAM = lymphangioleiomyomatosis.
Immunoblotting
Control and LAM cells were seeded in 75 cm2 flasks in DMEM containing 10% FBS and antibiotics (antibiotic-antimycotic) for 24 h and then were made quiescent in 0.1% bovine serum albumin (BSA) for 4 days. Cells were then rinsed with ice-cold phosphate-buffered saline (PBS) containing 200 µM Na3VO4 and 2 mM phenylmethylsulphonyl fluoride and then lysed by scraping in lysis buffer [PBS containing 1% Igepal, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS), 200 µM Na3VO4 and 0.1% Protease Inhibitor Cocktail Set III (Calbiochem, San Diego, CA, USA)]. Samples were separated by SDS-PAGE on 10% acrylamide gels and transferred to polyvinylidene fluoride membranes. The blots were then blocked in tris-buffered saline containing Tween-20 (0.05%) (TBS-T) and skim milk (5%) for 1 h. To detect phospho-p70S6 kinase (p70S6K), blots were incubated with rabbit anti-phospho-p70S6K (Thr389) (1:1000 in TBS-T containing 5% BSA) overnight at 4°C. Following washing with TBS-T, the blots were incubated with goat anti-rabbit immunoglobulin G- horse radish peroxidase (HRP) conjugated secondary antibody for 2 h at room temperature. The membrane was then washed with TBS-T and the protein was visualized using chemiluminescence (Millipore, Temecula, CA, USA) and analysed using a Kodak Image Station 4000MM and software (Version 5.0; Kodak Digital Science, New Haven, CT, USA). Membranes were stripped (0.063 M Tris pH 6.8, 2% SDS, 0.7% β-mercaptoethanol) at 50°C for 20 min, blocked with TBS-T containing 5% skim milk powder, and incubated with rabbit anti-p70S6K (1:1000) overnight or anti-GAPDH (1:5000). GAPDH bands served as a loading control.
Proliferation assay
Wild-type and TSC2-null MEFs or control and LAM cells were seeded in 12-well or 96-well tissue culture plates in DMEM containing 10% FBS and antibiotics for 24 h. MEF cells were then incubated in serum-reduced media (0.5% FBS) for 24 h before treatment with doxycycline (0.1–100 µg·mL−1) for up to 48 h and then the supernatants were collected and stored at −20°C. Human cells were made quiescent in 0.1% BSA for 24 h prior to stimulation with 10% FBS ± doxycycline (0.1–100 µg·mL−1) for up to 9 days and the supernatants were collected. Proliferation was assessed by both manual cell counts and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay as previously described (Moir et al., 2008). For the MTT assay, absorbance was measured at test wavelength 570 nm and reference wavelength 690 nm.
Lactate dehydrogenase assay
Supernatants from control (n = 1) and LAM cells (n = 2) were assayed for lactate dehydrogenase (LDH) according to the manufacturer's instructions (Sigma-Aldrich, St Louis, MO, USA) and compared with culture media in the absence of cells.
Gelatin zymography
The activity of gelatinases (MMP-2 and MMP-9) in cell culture supernatants was assessed using substrate specific zymography. Cell culture medium was size fractionated by gel electrophoresis on either an 8% or 10% SDS-polyacrylamide gel containing 0.2–1% gelatin (Sigma-Aldrich). The gel was then washed with SDS removal buffer (2.5% Triton-X 100) before incubation in enzyme activation buffer (50 mM Tris-HCl, 200 mM NaCl, 0.02% Tween-20, 5 mM CaCl2.2H2O) at 37°C overnight. The gel was then stained with Coomassie Brilliant Blue R-250 before it was destained (7% v/v acetic acid, 5% v/v methanol). MMPs were identified by comparison with the enzymatic bands generated by recombinant human MMP-2 or MMP-9. Densitometric analysis of the enzymatic bands was performed using a computer-assisted scanning and analysis system (Image station 4000MM, Kodak Digital Science).
Total MMP-2, TIMP-1 and TIMP-2 elisa s
Total MMP-2, TIMP-1 and TIMP-2 in cell culture supernatants were assessed using commercially available elisa kits according to the manufacturer's protocols (R&D Systems, Minneapolis, MN, USA; RayBiotech, Norcross, GA, USA). Absorbance was measured at a wavelength of 450 nm and corrected for reference wavelength 570 nm. The limits of detection were 0.78 ng·mL−1 for total MMP-2, 0.03 ng·mL−1 for mouse MMP-2 and 31.25 pg·mL−1 for TIMP-1 and TIMP-2.
Statistical analysis
Data were expressed as mean ± standard error of the mean obtained from cells cultured from n subjects and analysed using Student's paired t-test or one-way repeated anova with Bonferroni correction post hoc where appropriate. A probability (P) value of less than or equal to 0.05 was considered significant.
Materials
Tissue culture reagents were purchased from Invitrogen (Carlsbad, CA, USA). Doxycycline hyclate and the LDH assay were obtained from Sigma-Aldrich. Recombinant human MMP-2 and MMP-9 were purchased from EMD Biosciences (La Jolla, CA, USA). Phospho-p70S6K and p70S6K antibodies were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA); GAPDH were from Millipore and HRP-conjugated secondary antibodies were from Dako (Glostrup, Denmark). Coomassie Brilliant Blue R-250 was purchased from Progen (Queensland, Australia).
Results
Characterization of LAM cells
LAM cells were previously identified by positive staining for HMB-45 (see Black et al., 2005). In addition, LAM cells isolated from four individual patients were compared with those from four control subjects for up-regulation of phospho-p70S6K (Thr389). Comparison of control versus LAM cells showed increased levels of phospho-p70S6K in LAM-derived cells compared with control (P < 0.05; Figure 1).
Figure 1.
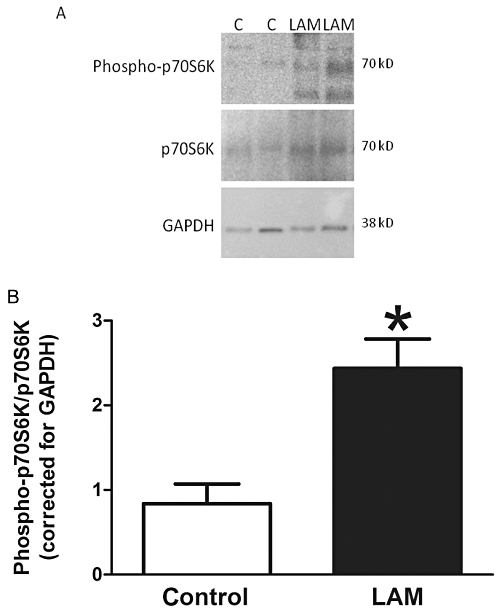
Phospho-p70S6K levels in control (n = 4) and lymphangioleiomyomatosis (LAM) (n = 4)-derived cells. (A) Representative Western blot of phospho-p70S6K, p70S6K and GAPDH. (B) Mean data ± SEM. *Significant difference from control; C = control.
Doxycycline does not inhibit proliferation of TSC2-null MEFs or LAM cells
Treatment of wild-type (n = 3) and TSC2-null MEFs (n = 3) with doxycycline (0.1–100 µg·mL−1) had no significant effect on cell number when compared with the mitogenic stimulus FBS (Figures 2A,B, P > 0.05). FBS induced proliferation of control and LAM cells (Figure 2C,2D); however, proliferation was not significantly greater in the LAM cells than in the control cells (day 7; LAM 4.6 × 104± 1.3 × 104 cells·mL−1, n = 5; control 4.0 × 104± 1.6 × 104 cells·mL−1; P > 0.05). Doxycycline treatment for up to 7 days had no effect on the proliferation of control cells (n = 5; Figure 2C) or LAM cells (n = 5; Figure 2D) as determined from manual cell counts (P > 0.05). However, doxycycline decreased MTT reduction to formazan in wild-type (100 µg·mL−1 doxycycline; n = 5, P≤ 0.05, Figure 3A) and TSC2-null MEFs (30 & 100 µg·mL−1 doxycycline; n = 6, P≤ 0.05; Figure 3B). In the human cells, doxycycline had no significant effect on the metabolic activity of FBS-treated control cells, as assessed by MTT assay (Figure 3C), but the metabolic activity of LAM cells was decreased by doxycycline treatment (30 and 100 µg·mL−1) for 7 days (approximately 23% and 32%, respectively; Figure 3D) and 9 days (approximately 32% and 44%, respectively; data not shown). Furthermore, doxycycline for 3, 5 or 7 days (0.1–100 µg·mL−1) did not increase cytoplasmic release of LDH into the supernatant when compared with FBS alone, as assessed using the LDH assay (n = 1 control, n = 2 LAM; data not shown).
Figure 2.
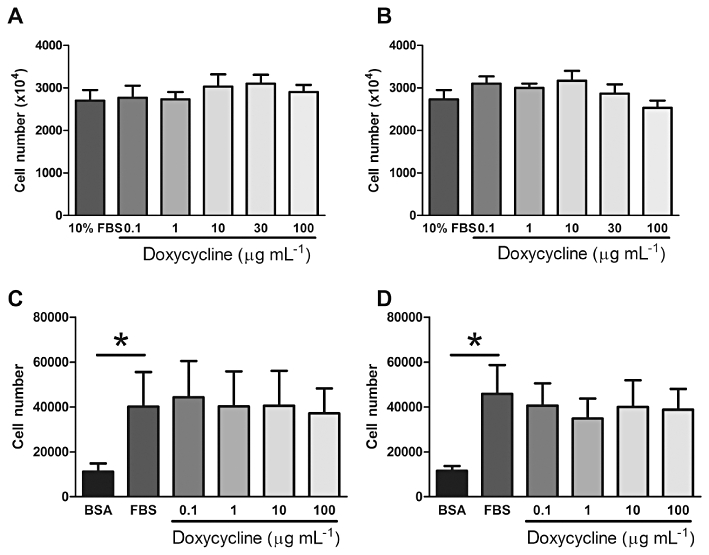
Cell number of wild-type (A, n = 3) and TSC2-null (B, n = 3) MEFs (day 2) and control (C, n = 5) and LAM cells (D, n = 5) in the presence or absence of doxycycline (0.1–100 µg·mL−1). *Significant difference from unstimulated (BSA), P≤ 0.05 one-way repeated measures anova. BSA, bovine serum albumin; LAM, lymphangioleiomyomatosis; MEF, mouse embryonic fibroblast; TSC, tuberous sclerosis complex.
Figure 3.
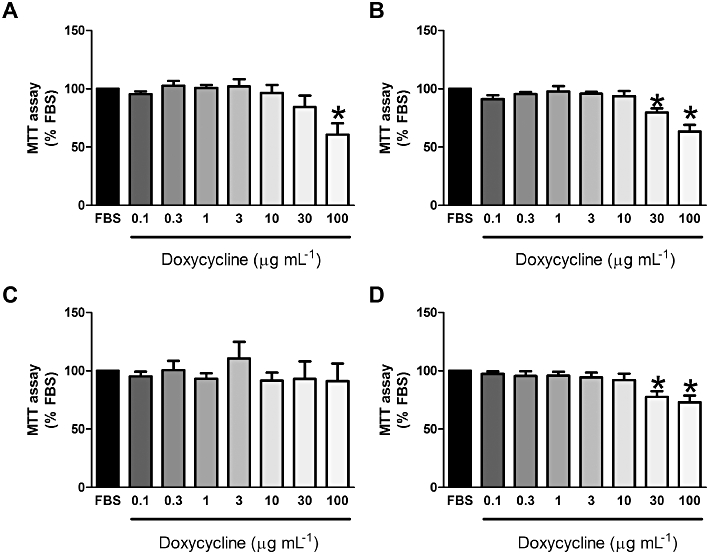
MTT reduction assay of wild-type (A; n = 5), TSC2-null (B; n = 5) MEFs and control (C; n = 7) and LAM (D; n = 8) cells in the presence or absence of doxycycline. *Significant difference from FBS, P≤ 0.05 one-way repeated measures anova. FBS, fetal bovine serum; LAM, lymphangioleiomyomatosis; MEF, mouse embryonic fibroblast; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium; TSC, tuberous sclerosis complex.
MMP production
A 2.3-fold increase in MMP-2 secretion was observed in TSC2-null MEFs compared with the wild-type controls over 24 h (TSC2-null n = 6, wild type n = 6; P≤ 0.05, Figure 4). Although there was an absence of active-MMP-2, both wild-type and TSC2-null MEFs produced pro- and intermediate-MMP-2. Higher levels of pro- and intermediate-MMP-2 were present in TSC2-null compared with wild-type MEFs (n = 6, P < 0.05). Levels of intermediate MMP-2 in both cell types were also significantly greater than pro-MMP-2 (data not shown).
Figure 4.
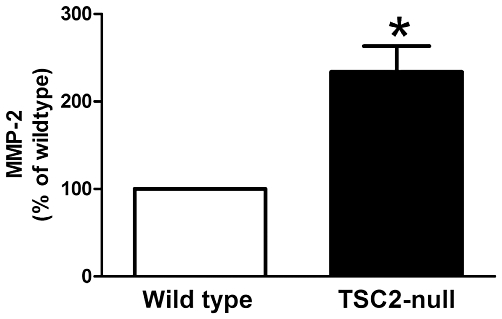
TSC2-null MEFs (n = 6) secrete more MMP-2 than wild-type MEFs (n = 6) as assessed using elisa. *P≤ 0.05, t-test. MEF, mouse embryonic fibroblast; MMP, matrix metalloproteinase; TSC, tuberous sclerosis complex.
Control cells and LAM cells constitutively (in the absence of a stimulus) produced both pro- and active-MMP-2 (Figure 5A). Total MMP-2 levels increased over the 7 day time period examined in both cell types (P < 0.05 compared with 3 days; Figure 5B). The stimulation of control and LAM cells with the mitogenic stimulus FBS increased MMP-2 over time (P < 0.05 compared with 3 days; Figure 5C). Unstimulated LAM and control cells produced low but increasing levels of MMP-9 over the 7 day period (Figure 5A); however, these were not significantly different between cell types. In the presence of FBS, levels of MMP-9 were not greater than those present in the media alone (data not shown) and hence were not examined further.
Figure 5.
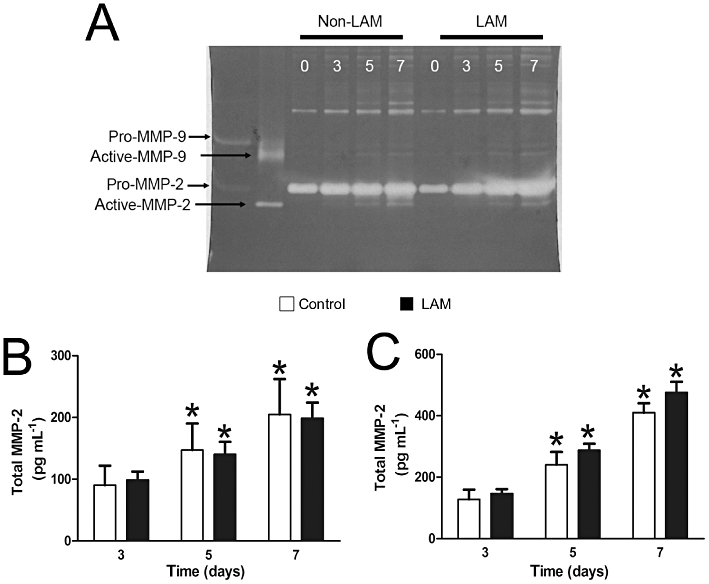
Representative gelatin zymogram showing constitutive production of MMP-2 and MMP-9 by LAM and control cells. Cells were incubated in 0.1% BSA for up to 7 days (as indicated). MMP-2 and MMP-9 expression was detected by enzymatic digestion of the gel (white bands) (A). Total MMP-2 expression from unstimulated (B) and FBS-stimulated (C) cells detected using elisa. Mean data ± SEM from control (n = 4) and LAM cells (n = 6–7). *Significant difference from day 3, P≤ 0.05 two-way repeated measures anova. BSA, bovine serum albumin; FBS, fetal bovine serum; LAM, lymphangioleiomyomatosis; MMP, matrix metalloproteinase.
Doxycycline decreased MMP-2
Doxycycline significantly decreased total MMP-2 secretion at 30 and 100 µg·mL−1 in wild-type MEFs (P≤ 0.05; Figure 6A). Similarly, TSC2-null MEFs had decreased MMP-2 secretion with doxycycline (100 µg·mL−1) (P≤ 0.05; Figure 6B).
Figure 6.
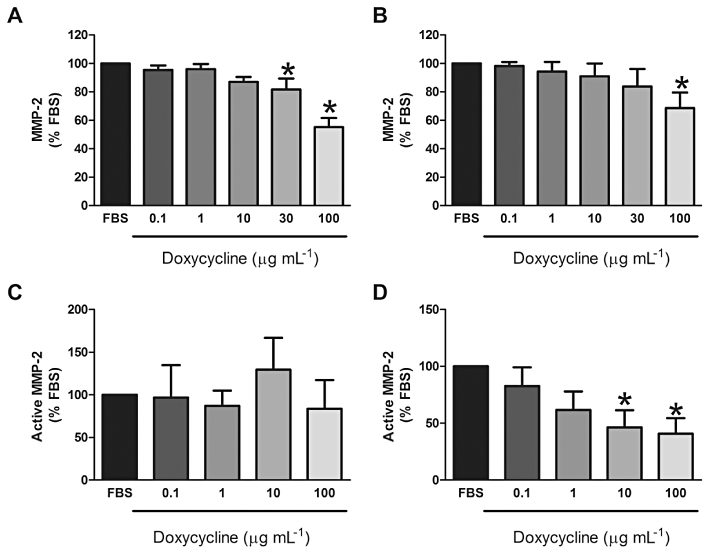
Levels of active-MMP-2 from wild-type (A; n = 6) and TSC2-null MEFs (B; n = 6) or control (C; n = 5) and LAM cells (D; n = 5) following treatment with doxycycline for 2 days (A,B) or 5 days (C,D). Data expressed as a percentage of FBS-stimulated response. *Significant difference from FBS P≤ 0.05 one-way repeated measures anova. FBS, fetal bovine serum; LAM, lymphangioleiomyomatosis; MEF, mouse embryonic fibroblast; MMP, matrix metalloproteinase; TSC, tuberous sclerosis complex.
Treatment with doxycycline for 5 days decreased total MMP-2 secretion from both control (74%; n = 4; P < 0.05) and LAM (45%; n = 6–7; P < 0.05) cells (data not shown). Doxycycline treatment for 5 days had no effect on active MMP-2 levels secreted by control cells (n = 5, P > 0.05; Figure 6C); however, it decreased levels of active MMP-2 in supernatants from LAM cells (10, 100 µg·mL−1; n = 5; P < 0.05) (Figure 6D). Doxycycline treatment for 7 days had no significant effect on active MMP-2 levels (data not shown).
Doxycycline had no effect on TIMPs
Control and LAM cells produced TIMP-1 and TIMP-2 in response to FBS, although there was no significant difference between cell types. Treatment with doxycycline had no effect on TIMP-1 secretion from control or LAM cells [Day 5; Control: FBS 560 ± 80 ng·mL−1 FBS + doxycycline (100 µg·mL−1) 879 ± 193 ng·mL−1, n = 6; LAM: FBS 567 ± 210 ng·mL−1 FBS + doxycycline (100 µg·mL−1) 572 ± 125 ng·mL−1, n = 7; P > 0.05]. Similarly, doxycycline had no effect on TIMP-2 secretion from control or LAM cells [Day 5; Control: FBS 90 ± 25 ng·mL−1 FBS + doxycycline (100 µg·mL−1) 97 ± 21 ng·mL−1, n = 6; LAM: FBS 110 ± 49 ng·mL−1 FBS + doxycycline (100 µg·mL−1) 85 ± 19 ng·mL−1, n = 7; P > 0.05].
Discussion
LAM is a destructive lung disorder affecting almost exclusively females and for which there is no current treatment. MMPs have been implicated in the pathophysiology of the disease, with increased MMP-1, −2 and −9 in LAM tissue (Hayashi et al., 1997). In the present study, we showed that TSC2-null MEFs secreted more MMP-2 than wild-type cells, and that the non-specific MMP inhibitor doxycycline decreased MMP-2 secretion. Furthermore, human LAM cells secreted MMP-2 and this was also decreased by doxycycline. In addition, doxycycline decreased the metabolic activity of both MEFs and LAM-derived cells but had no effect on cell proliferation.
In this study, we investigated both TSC2-null and wild-type MEFs, an established cell model of LAM and control cells respectively. In addition, we isolated LAM cells from the explanted lungs of LAM patients and compared these with ASM cells from resected or explanted lungs from approximately age-matched female patients. The LAM cells isolated were positive for HMB-45 (Black et al., 2005), an accepted marker of LAM cells. Furthermore, phospho-p70S6K levels have been reported to be elevated in LAM cells (Goncharova et al., 2002) and, in the current study, phospho-p70S6K levels were greater in the cell cultures examined from four LAM patients compared with those from four control subjects. Together, these data would strongly suggest that the cells isolated from LAM subjects and used in the present study were LAM cells.
There are some limitations which must be considered when interpreting the findings of the present study. The ideal control cell for LAM is not clear; however, we chose to use ASM cells isolated from non-LAM female subjects. Lung tissue was removed from the control subjects for other lung disorders (refer to Table 1). A potential confounding factor may be the smoking history of the patients. Five of the seven control patients were either smokers or ex-smokers; however, although this information was not available for all of the patients with LAM, four of the patients were non-smokers. As patients with end-stage LAM (as our donors were) have severe respiratory difficulties and often require supplemental oxygen, it is unlikely that the remaining patients were heavy smokers. We recognize that this is not ‘ideal’ when examining MMP expression, as MMPs have been reported to be elevated in both carcinoma and emphysema; however, this potential bias would in fact have decreased the observed differences between LAM and control samples, rather than enhancing the differences. Furthermore, to limit the impact of disease characteristics in control cells from carcinoma patients, the ASM was isolated from macroscopically normal regions distant to the tumour. Additionally, whether any medications taken by the patients prior to surgery affected the metabolic function of the cells in vitro is unknown. However, we would expect such effects to be minimal because the cells in vitro have been passaged.
LAM is associated with cystic airspace enlargement, similar to that seen in other diseases, including chronic obstructive pulmonary disease. It is proposed that these changes are associated with an imbalance of MMPs and TIMPs. MMPs are proteolytic enzymes capable of degrading most ECM proteins. Immunohistochemical studies have previously demonstrated abundant staining for MMP-2 and MMP-9 in LAM lesions (Hayashi et al., 1997; Matsui et al., 2000), and it has been postulated that these enzymes may be, at least in part, responsible for the altered structure of the lung tissue in LAM. In the present study, both wild-type and TSC-null MEFs produced MMP-2. In addition, LAM and control cells produced and activated the gelatinases, MMP-2 and MMP-9. MMP-2 and MMP-9 are capable of degrading collagen, in particular collagen type IV and denatured type I collagen (gelatin), as well as degrading elastic fibres, and thus an imbalance in MMP activity may contribute to the loss of elastic fibres associated with LAM (Merrilees et al., 2003). Furthermore, although we did not pursue MMP-9 production, it was interesting to find that unstimulated cells produced MMP-9 over the 7 day culture period, whereas, in the presence of the mitogenic stimulus FBS, which contains MMP-9, neither LAM nor control cells produced MMP-9.
MMP-2 expression is abundant in LAM tissue (Hayashi et al., 1997) and we found that TSC2-null MEFs produced significantly more MMP-2 than wild-type MEFs. This is in agreement with Lee and colleagues who reported that TSC2-null cells secrete more MMP-2 than TSC2-positive cells (a model of control cells) (Lee et al., 2010). In contrast, we did not find a significant difference in the levels of MMP-2 secreted by LAM and control cells and this may be related to species differences. In our study, the majority of MMP-2 from MEFs was an intermediate form, whereas active MMP-2 was detected from human cells. Similarly, Chang and colleagues showed an absence of active-MMP-2 from ELT3 cells (a TSC2-null smooth muscle derived cell line from an Eker rat spontaneous uterine leiomyoma) which appear to lack the autocatalytic mechanism and in turn fail to completely activate MMP-2 (Chang et al., 2010).
MMP expression and activity is regulated at multiple levels, including gene transcription, activation of the latent form and by its natural inhibitors, TIMPs. In the present study, the tetracycline derivative, doxycycline, which has been shown to have biological activity independent of its anti-microbial activity, decreased total MMP-2 levels from MEFs and both control and LAM-derived cells. Furthermore, doxycycline decreased active-MMP-2 levels in LAM cell supernatants. This reduction in MMP levels was not due to a decrease in cell number as shown by manual cell counts nor was there any increase in LDH release which would indicate cell death. However, doxycycline did reduce cellular metabolic activity, as assessed by the MTT assay. Recent work by Chang and colleagues also observed that at doxycycline concentrations of greater than 25 µg·mL−1, there was a trend towards decreased MMP-2 levels from ELT3 cells; however, this was not significant when corrected using the MTT assay as a surrogate marker for cell number (Chang et al., 2010). In other studies, tetracyclines have been reported to inhibit mitochondrial function (Rubins et al., 2001) as well as collagen degradation (Franco et al., 2006) and angiogenesis (Tamargo et al., 1991). Mitochondria play a major role in cell function and targeting the mitochondria has been the focus of many anti-cancer therapies. The MTT assay is a mitochondrial assay and hence the reduction in mitochondrial function observed in the present study following doxycycline treatment may be beneficial in halting the disease progression.
MMPs are secreted as pro-enzymes which become active when cleaved. Once in this active form, MMPs can degrade ECM proteins, thus the ability to reduce active-MMP-2 levels may be of particular benefit in disease treatment. It is also interesting to note that doxycycline had no effect on TIMP-1 or TIMP-2 levels which are natural inhibitors of MMPs. These inhibitors have been reported to be unchanged in LAM. Thus, the ability of doxycycline to reduce MMPs but not TIMPs may restore the balance.
The cystic destruction of the lung associated with LAM involves the abnormal growth of LAM cells. We have previously reported no difference in the rate of proliferation of LAM and control cells (Black et al., 2005), as assessed using tritiated thymidine. In the present study, we confirmed our previous findings using manual cell counts. Our findings are in contrast to those of Goncharova and colleagues who reported that both unstimulated and PDGF-stimulated LAM cells exhibited increased DNA synthesis, a marker of proliferation, compared with human lung fibroblasts, human bronchial fibroblasts and human vascular smooth muscle cells (Goncharova et al., 2006). However, there are some differences between the studies. As previously discussed (Black et al., 2005), phenotypic diversity may account for the differences in proliferation observed. The expression of HMB-45 has been reported to negatively correlate with levels of the proliferating cell nuclear antigen (Matsumoto et al., 1999). In the current study, LAM cells were positive for HMB-45, an acceptable marker of LAM cells, whereas in the study by Goncharova et al. (2006), cells were not immunoreactive for HMB-45. These differences may account for the lack of hyper-proliferation observed in the present study.
Although there is no current treatment for LAM, inhibitors of mTOR, such as rapamycin, have been proposed as therapeutic agents. Despite its ability to inhibit cell proliferation (Goncharova et al., 2002; Black et al. 2005) rapamycin targets only the mTOR pathway, thus other pathways up-regulated in LAM are not affected. Lee et al. (2010) reported that enhanced MMP-2 secretion from TSC2-null angiomyolipoma cells was rapamycin insensitive, thus suggesting that rapamycin treatment alone may be inadequate to control disease progression. In the present study, high concentrations of doxycycline (10–100 µg·mL−1) reduced active-MMP-2 levels and metabolic activity, thus suggesting that doxycycline may also be beneficial in treating LAM. However, it should be noted that these concentrations may be well above the concentrations achieved in the plasma in patients treated with doxycycline (100 mg twice daily), which have been reported to be in the range 1.8–9.4 µg·mL−1 (Prall et al., 2002). Furthermore, while we have focused on the effect of doxycycline on MMP-2 levels, we acknowledge that doxycycline may also inhibit other MMPs, such as MMP-1, MMP-8 and MMP-13 (Hanemaaijer et al., 1998; Li et al., 2003; Hashimoto et al., 2005). These MMPs were however not the focus of the present study.
In summary, doxycycline decreased active-MMP-2 secretion from MEFs and LAM cells and reduced metabolic function of these cells, suggesting potential therapeutic efficacy in LAM patients.
Acknowledgments
The authors acknowledge the collaborative effort of the cardiopulmonary transplant team and pathologists at St Vincent's Hospital; Sydney, The Alfred Hospital, Melbourne; the Royal Perth Hospital, Perth; The Prince Charles Hospital, Brisbane; and the thoracic physicians and pathologists at Royal Prince Alfred, Concord and Strathfield Private Hospitals and Rhodes Pathology, Sydney. We also acknowledge Joanne Thompson, Pablo Britos and Qi Ge for their technical assistance. This work was supported by the National Health and Medical Research Council, Australia, LAM Australia and LAM Australasia Research Alliance. J.K. Burgess is supported by a National Health & Medical Research Council R. Douglas Wright Fellowship #402835. V.P. Krymskaya is supported in part by a grant from the NIH/NHLBI RO1 HL090829. J.L. Black is supported by a National Health & Medical Research Council Senior Principal Research Fellowship #571098.
Glossary
Abbreviations
- ASM
airway smooth muscle
- BSA
bovine serum albumin
- DMEM
Dulbecco's modified Eagle's medium
- ECM
extracellular matrix
- FBS
fetal bovine serum
- HMB-45
human melanoma Black-45
- LAM
lymphangioleiomyomatosis
- LDH
lactate dehydrogenase
- MEF
mouse embryonic fibroblast
- MMP
matrix metalloproteinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
- mTOR
mammalian target of rapamycin
- PBS
phosphate-buffered saline
- SDS
sodium dodecyl sulfate
- TIMP
tissue inhibitor of matrix metalloproteinase
- TSC
tuberous sclerosis complex
Conflicts of interest
The authors have no conflict of interests to declare.
References
- Black JL, Ge Q, Boustany S, Johnson PRA, Poniris MH, Glanville AR, et al. In vitro studies of lymphangioleiomyomatosis. Eur Respir J. 2005;26:569–576. doi: 10.1183/09031936.05.00016905. [DOI] [PubMed] [Google Scholar]
- Cataldo D, Munaut C, Noel A, Frankenne F, Bartsch P, Foidart JM, et al. MMP-2- and MMP-9-linked gelatinolytic activity in the sputum from patients with asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 2000;123:259–267. doi: 10.1159/000024452. [DOI] [PubMed] [Google Scholar]
- Chang WY, Clements D, Johnson SR. Effect of doxycycline on proliferation, MMP production and adhesion in LAM related cells. Am J Physiol Lung Cell Mol Physiol. 2010;299:L393–L400. doi: 10.1152/ajplung.00437.2009. [DOI] [PubMed] [Google Scholar]
- Franco C, Ho B, Mulholland D, Hou G, Islam M, Donaldson K, et al. Doxycycline alters vascular smooth muscle cell adhesion, migration, and reorganization of fibrillar collagen matrices. Am J Pathol. 2006;168:1697–1709. doi: 10.2353/ajpath.2006.050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis. J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- Goncharova EA, Goncharov DA, Spaits M, Noonan D, Talovskaya E, Eszterhas A, et al. Abnormal smooth muscle cell growth in lymphangioleiomyomatosis (LAM): role for tumor suppressor TSC2. Am J Respir Cell Mol Biol. 2006;34:561–572. doi: 10.1165/rcmb.2005-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanemaaijer R, Visser H, Koolwijk P, Sorsa T, Salo T, Golub LM, et al. Inhibition of MMP synthesis by doxycycline and chemically modified tetracyclines (CMTs) in human endothelial cells. Adv Dent Res. 1998;12:114–118. doi: 10.1177/08959374980120010301. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Matsumoto MM, Li JF, Lawton MT, Young WL. Suppression of MMP-9 by doxycycline in brain arteriovenous malformations. BMC Neurol. 2005;5:1. doi: 10.1186/1471-2377-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Fleming MV, Stetler-Stevenson WG, Liotta LA, Moss J, Ferrans VJ, et al. Immunohistochemical study of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in pulmonary lymphangioleiomyomatosis. Hum Pathol. 1997;28:1071–1078. doi: 10.1016/s0046-8177(97)90061-7. [DOI] [PubMed] [Google Scholar]
- Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, et al. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164:474–477. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- Ko FW, Diba C, Roth M, McKay K, Johnson PR, Salome C, et al. A comparison of airway and serum matrix metalloproteinase-9 activity among normal subjects, asthmatic patients, and patients with asthmatic mucus hypersecretion. Chest. 2005;127:1919–1927. doi: 10.1378/chest.127.6.1919. [DOI] [PubMed] [Google Scholar]
- Lee PS, Tsang SW, Moses MA, Trayes-Gibson Z, Hsiao LL, Jensen R, et al. Rapamycin-insensitive up-regulation of MMP2 and other genes in tuberous sclerosis complex 2-deficient lymphangioleiomyomatosis-like cells. Am J Respir Cell Mol Biol. 2010;42:227–234. doi: 10.1165/rcmb.2009-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Shang TY, Kim HS, Solomon A, Lokeshwar BL, Pflugfelder SC. Regulated expression of collagenases MMP-1, -8, and -13 and stromelysins MMP-3, -10, and -11 by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:2928–2936. doi: 10.1167/iovs.02-0874. [DOI] [PubMed] [Google Scholar]
- McCormack F, Brody A, Meyer C, Leonard J, Chuck G, Dabora S, et al. Pulmonary cysts consistent with lymphangioleiomyomatosis are common in women with tuberous sclerosis: genetic and radiographic analysis. Chest. 2002;121:61S. [PubMed] [Google Scholar]
- Matsui K, Takeda K, Yu ZX, Travis WD, Moss J, Ferrans VJ. Role for activation of matrix metalloproteinases in the pathogenesis of pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med. 2000;124:267–275. doi: 10.5858/2000-124-0267-RFAOMM. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Horiba K, Usuki J, Chu SC, Ferrans VJ, Moss J. Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 1999;21:327–336. doi: 10.1165/ajrcmb.21.3.3693. [DOI] [PubMed] [Google Scholar]
- Mattos W, Lim S, Russell R, Jatakanon A, Chung KF, Barnes PJ. Matrix metalloproteinase-9 expression in asthma: effect of asthma severity, allergen challenge, and inhaled corticosteroids. Chest. 2002;122:1543–1552. doi: 10.1378/chest.122.5.1543. [DOI] [PubMed] [Google Scholar]
- Merrilees MJ, Hankin EJ, Black JL, Beamont B. Matrix proteoglycans and remodelling of interstitial lung tissue in lymphangioleiomyomatosis. J Pathol. 2003;203:653–660. doi: 10.1002/path.1577. [DOI] [PubMed] [Google Scholar]
- Moir LM, Burgess JK, Black JL. Transforming growth factor beta(1) increases fibronectin deposition through integrin receptor alpha(5)beta(1) on human airway smooth muscle. J Allergy Clin Immunol. 2008;121:1034–1039 e4. doi: 10.1016/j.jaci.2007.12.1159. [DOI] [PubMed] [Google Scholar]
- Moses MA, Harper J, Folkman J. Doxycycline treatment for lymphangioleiomyomatosis with urinary monitoring for MMPs. N Engl J Med. 2006;354:2621–2622. doi: 10.1056/NEJMc053410. [DOI] [PubMed] [Google Scholar]
- Odajima N, Betsuyaku T, Nasuhara Y, Inoue H, Seyama K, Nishimura M. Matrix metalloproteinases in blood from patients with LAM. Respir Med. 2009;103:124–129. doi: 10.1016/j.rmed.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Prall AK, Longo GM, Mayhan WG, Waltke EA, Fleckten B, Thompson RW, et al. Doxycycline in patients with abdominal aortic aneurysms and in mice: comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg. 2002;35:923–929. doi: 10.1067/mva.2002.123757. [DOI] [PubMed] [Google Scholar]
- Rubins JB, Charboneau D, Alter MD, Bitterman PB, Kratzke RA. Inhibition of mesothelioma cell growth in vitro by doxycycline. J Lab Clin Med. 2001;138:101–106. doi: 10.1067/mlc.2001.116591. [DOI] [PubMed] [Google Scholar]
- Smolarek TA, Wessner LL, McCormack FX, Mylet JC, Menon AG, Henske EP. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998;62:810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamargo RJ, Bok RA, Brem H. Angiogenesis inhibition by minocycline. Cancer Res. 1991;51:672–675. [PubMed] [Google Scholar]
- Zhe X, Yang Y, Jakkaraju S, Schuger L. TIMP-3 downregulation in lymphangioleiomyomatosis: potential consequence of abnormal SRF expression. Am J Respir Cell Mol Biol. 2003;28:504–511. doi: 10.1165/rcmb.2002-0124OC. [DOI] [PubMed] [Google Scholar]


