Abstract
BACKGROUND AND PURPOSE
Many cytokines associated with autoimmune disorders and inflammation have been shown to activate the signalling kinase JAK3, implying that JAK3 plays key roles in the pathogenesis of these diseases. Therefore, investigating the alterations of JAK3 activity and the efficacy of selective JAK3 antagonists in animal models of such disorders is essential to a better understanding of the biology of JAK3 and to assess the potential clinical benefits of JAK3 inhibitors.
EXPERIMENTAL APPROACH
Through high-throughput cell-based screening using the NCI compound library, we identified NSC163088 (berberine chloride) as a novel inhibitor of JAK3. Specificity and efficacy of this compound were investigated in both cellular and animal models.
KEY RESULTS
We show that berberine chloride has selectivity for JAK3 over other JAK kinase members, as well as over other oncogenic kinases such as Src, in various cellular assays. Biochemical and modelling studies strongly suggested that berberine chloride bound directly to the kinase domain of JAK3. Also phospho-JAK3 levels were significantly increased in the synovial tissues of rat joints with acute inflammation, and the treatment of these rats with berberine chloride decreased JAK3 phosphorylation and suppressed the inflammatory responses.
CONCLUSIONS AND IMPLICATIONS
The up-regulation of JAK3/STATs was closely correlated with acute arthritic inflammation and that inhibition of JAK3 activity by JAK3 antagonists, such as berberine chloride, alleviated the inflammation in vivo.
Keywords: JAK; STAT; inflammation; small molecule inhibitor, berberine, IL-2, IL-3
Introduction
The family of Janus kinases (JAK1-3) play key roles in numerous cytokine-mediated signalling pathways (O'Shea et al., 2002; Yamaoka et al., 2004; Ghoreschi et al., 2009). JAK3 is preferentially expressed in haematopoietic cells and mediates signals by interacting with a common gamma (γc) chain shared by receptors for cytokines such as IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21, involving JAK3 function in haematopoietic development and homeostasis of the immune system (Rochman et al., 2009). Disruption of JAK3 or γc in humans and mice caused severe combined immunodeficiency disease characterized by the absence of T and NK cells and the presence of non-functional B cells (Puck et al., 1993; Macchi et al., 1995; Russell et al., 1995). Furthermore, persistent activation of JAK3 correlates with autoimmune disorders and inflammation (Papageorgiou and Wikman, 2004). Several JAK3 inhibitors have recently been developed and have been shown to function as a new class of immunosuppressive agents (Changelian et al., 2003; Kremer et al., 2009). Specifically, JAK3 antagonists such as CP-690550 reduced the severity of rheumatoid arthritis (RA) in clinical trials and significantly prolonged survival in animal models for organ transplantations. Another JAK3 inhibitor WHI-P131 effectively prevented mast cell-mediated allergic reactions as well as asthmatic responses in animal models (Malaviya et al., 1999). These findings suggest that JAK3 inhibitors have potential clinical benefits in the treatment of autoimmune disorders, organ transplant rejection and inflammation. However, many of these studies lack direct evidence that constitutively active JAK3 is involved in the progression of these disorders. Moreover, the majority of first-generation JAK3 antagonists exhibit varying degrees of inhibition of other JAKs, particularly JAK2. For example, in clinical studies of RA, patients receiving high doses of CP-690550, which has nanomolar potency against JAK3 but shows considerable affinity for JAK2 in vitro (Karaman et al., 2008), experienced a high rate of non-haematological (neurological) and haematological (anaemia and thrombocytopenia) adverse effects (Kremer et al., 2009). These effects were similar to those observed in clinical trials with JAK2 inhibitors (Verstovsek, 2009), suggesting that the CP-690550 has significant off-target effects on JAK2 in vivo. Therefore, identifying novel, highly selective JAK3 inhibitors with reduced off-target effects on other JAKs, and assessing the potential clinical benefits of those inhibitors in animal models of JAK3-mediated disorders remain an important challenge.
Here, we have identified NSC163088 (berberine chloride) as a highly selective JAK3 antagonist by means of high-throughput cell-based reporter screening of the NCI compound repository. In vitro kinase assays and a protein-compound docking simulation suggested that berberine chloride bound directly to the kinase domain of JAK3 and thus blocked JAK3 catalytic activity. Importantly, we showed that berberine chloride alleviated inflammatory responses and hyperalgesia in a rat model of carrageenan/kaolin-induced acute synovial inflammation by inhibiting JAK3.
Methods
Cell lines
32D/IL-2Rβ/6xSTAT5 cells were grown in RPMI 1640 medium containing 10% FBS, 2 mM L-glutamine, 5% WEHI-3 cell-conditioned medium and 300 µg·mL−1 hygromycin. The pro-B-cell line BaF3 stably expressing a constitutively active allele of JAK3 (JAK3V674A), the pre-T lymphoma cell line Nb2 and the multiple myeloma cell line U266 were maintained in RPMI 1640 containing 10% FBS. The Hodgkin's lymphoma cell lines L540 and HLDM-2 were maintained in RPMI 1640 containing 20% FBS. The prostate cancer cell line DU145 was maintained in DMEM containing 10% FBS.
A cell-based STAT5 reporter assay
The 32D/IL-2Rβ/6xSTAT5 reporter cells were first deprived of WEHI-3 cell-conditioned medium for 6 h. Then these cells were mixed with IL-2 (100 ng·mL−1) or IL-3 (5 ng·mL−1), and seeded into 96-well plates (2 × 104 cells per well) where each compound from the NCI diversity and mechanistic sets (http://dtp.nci.nih.gov/branches/dscb/repo_open.html) had already been aliquotted at 10 µM. The cells were then incubated for an additional 16 h in the absence of WEHI-3 cell-conditioned medium. Luciferase activity was measured using the Firefly Luciferase Assay Kit (Promega, MI).
Western blot analysis, in vitro kinase and cell viability assay
Whole-cell extracts were resolved on SDS-PAGE, transferred to nitrocellulose membrane and probed with appropriate antibodies. Antibodies specific for phospho-JAK3, JAK3, STAT3, STAT5 and Lyn were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies specific for phospho-STAT3, phospho-STAT5, JAK1, JAK2, phospho-JAK2, tyrosine kinase 2 (TYK2), phospho-TYK2, phospho-Src, Src, phospho-Lyn, phospho-Akt, Akt, phospho-ERK1/2 and ERK1/2 were purchased from Cell Signaling Technology (Cambridge, MA). Phospho-JAK1 antibody was obtained from Upstate Chemicon (Temecula, CA). For in vitro assays of JAK activity, the lysates prepared from L540 cells were pre-cleared with protein A/G-DMSO alone, berberine chloride or AG490 (LC Laboratories, Woburn, MA) for 1 h at 30°C. Kinase reactions were performed by the addition of recombinant His-tagged STAT3α (2 µg) in the absence or presence of 2 µM ATP (20 or 40 µM ATP for competition experiments) for 30 min at 30°C. The reaction products were separated by SDS-PAGE and probed with antibodies specific for phospho-STAT3, STAT3 or JAK3. For cell viability, cells (5 × 104 cells·mL−1) were treated with DMSO alone, berberine chloride or AG490 (100 µM), and incubated for the indicated time periods. The cells were harvested and viability was determined by Trypan blue exclusion. The final DMSO concentration used in all in vitro assays was 0.1%.
Modelling of JAK3-JH1 and berberine chloride complex
For the structure-based docking, we employed both AutoDock version 4 and AutoDock Vina version 1.1. The complex crystal structure between JAK3 kinase domain (JAK3-JH1) (PDB ID: 1YVJ) and the known JAK3 inhibitor CP-690550 (PDB ID: 3LXK) was used as a protein template structure. After removing the ligand and solvent molecules, AMBER software added hydrogen atoms, which was based on the PDB2PQR-determined ionizable states in Asp, Glu, His and Lys residues. The docking procedures first included the generation of 30 different conformers of berberine chloride using AMBER package. Once gaining 60 structures towards the reference template by two different methods, we clustered the resulting conformers by structural similarity that was quantified by root mean square deviation value between structures. The clusters were further sorted according to AutoDock energies. We chose the lowest energy structure in the best cluster as a final model. The values of 100 and 500 000 were the parameters for the number of individuals in population (ga_pop_size) and the maximum number of generations (ga_num_evals), respectively, for the generic algorithm in AutoDock. AutoDock Vina runs adapted default parameters. Software and scripts, written in-house, automated all the procedures.
Carrageenan/kaolin-induced knee monoarthritis and paw hyperalgesia
All animal care and experimental procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Kyung Hee University Institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats weighing 180–200 g (6-week-old) were obtained from Charles River Laboratories (Yokohama, Japan). The rats were housed in a limited access rodent facility at 22 ± 2°C with up to five rats per polycarbonate cage. Rats in the knee monoarthritis and paw hyperalgesia experiments were divided at random into normal group (NOR, n = 7), saline-treated vehicle control group (CON, n = 7), 10 mg·kg−1 berberine chloride-treated group (Ber10, n = 9), 30 mg·kg−1 berberine chloride-treated group (Ber30, n = 9) and 50 mg·kg−1 berberine chloride-treated group (Ber50, n = 9). Prednisolone (20 mg·kg−1, p.o.) (PRE, n = 9) and celecoxib (100 mg·kg−1, p.o.) (CEL, n = 9) were given orally as positive controls in carrageenan/kaolin-induced monoarthritis and paw hyperalgesia rat models respectively. The carrageenan/kaolin-induced monoarthritic rat model has been previously described (Sluka and Westlund, 1993; Schaible et al., 1994). Arthritic inflammation was induced by a single injection of 5% carrageenan + 5% kaolin, suspended in 100 µL of pyrogen-free sterile saline, into the left tibiotarsal knee joint. Berberine chloride was dissolved in sterile saline, and the rats were injected intraperitoneally. Prednisolone and celecoxib were dissolved in an emulsion solution of 5% DMSO + 3% ethanol + 92% corn oil. The berberine chloride treatment started 1 day after carrageenan/kaolin injection and lasted once daily for 6 days. To induce paw hyperalgesia, rats were given an intraplantar injection of 1% carrageenan (0.1 mL) in the right hind paw. Food was withheld overnight and berberine chloride was intraperitoneally injected 1 h before carrageenan injection at doses of 10, 30 and 50 mg·kg−1.
Evaluation of knee arthritic and paw algesic symptoms
To evaluate the arthritic progression of carrageenan/kaolin-injected rat, two different parameters were measured daily for 6 days: knee thickness and weight distribution ratio (WDR). With progression of arthritis, redness and swelling of the knee joints and arthritic pain started to appear and reached a maximum at 1 day after carrageenan/kaolin injection. The knee thickness was measured with a dial thickness gauge. It was expressed as values relative to that obtained on day 0 when carrageenan/kaolin was injected. WDR indicating arthritic pain is the ratio of the per cent of weight carried on each hind leg in which the weight-bearing forces of both hind limbs were measured with an incapacitance meter (UGO-BASIL Biological Research Apparatus Co., Comerio-Varese, Italy). The bearing force of each hind limb was quantified by two mechanotransducers, separately placed below two hind legs: one was normal and the other was the arthritic leg. The bearing force of each hind leg was estimated as a 5 s average, and the mean bearing force was calculated from four separate experiments. The WDR percentage was calculated as % WDR = 100 × (weight borne by ipsilateral limb/total weight borne by both limbs). WDRs of the hind paws in the normal group were 50:50, indicating that 50% of the total weight was carried by each hind paw. As the pain and swelling of the ankle progressed due to developing arthritis, the weight balance was disrupted, resulting in a reduction of WDR in the arthritic leg. All behavioural tests were performed without knowledge of the treatments. At 3 h after carrageenan injection, the pain threshold was measured using a paw pressure analgesia instrument (UGO-BASIL Biological Research Apparatus Co., Comerio-Varese, Italy) for the Randall-Selitto paw test. To evaluate paw hyperalgesia, we measured the tolerance to increasing mild pressure on the affected paw between a flat surface and a blunt pointer of the instrument.
Histopathological and immunohistological analyses of knee joints
Knee joints were dissected on day 6 and the surrounding skin, tendon and ligament were removed. The solid tissues including joint bones were fixed for 5 days in 10% formalin, decalcified in CalciClear Rapid™ solution (National Diagnostics Co., Atlanta, GA) and embedded in paraffin. Coronal sections 5 mm thick were cut through the knee joint using a manual rotary microtome (Finesse 325, Thermo Shandon Inc., Pittsburgh, PA) and stained with haematoxylin and eosin (H&E) for routine histological evaluation. Paraffin tissue sections obtained from rat knees were deparaffinized in xylene. The tissue samples were then hydrated with ethanol and washed in distilled water, followed by antigen retrieval by heating with 100 mM citrate buffer (pH 6.0) at 65°C for 1–2 h. Slides were washed twice in PBST (PBS plus 0.3% Triton X-100). The samples were then blocked by incubation for 1–2 h in PBST. Primary antibodies specific for STAT3 (1:50; Cell Signaling Technology Inc., Danvers, MA), and STAT4 (1:50), phospho-JAK3 (1:50) and STAT6 (1:400; ABcam Inc., San Francisco, CA) were used. Antibodies were incubated with tissue samples overnight at 4°C in a cold chamber. After washing, the samples were incubated in the dark for 1−2 h at room temperature with secondary antibody (1:100; Alexa Fluor 647™, Invitrogen Co., Carlsbad, CA). The section samples were washed with PBST and mounted on a microslide glass with histological mounting medium (Fisher Scientific UK Ltd, Leicestershire, UK). The samples were examined with a confocal laser scanning microscope (FV10i-w-set, Olympus Co., Japan). All section samples were treated and viewed in an identical manner. For more accurate fluorescence calibration, all conditions of laser sensitivity in confocal microscope were equally manipulated. The numbers of immunopositive cells in each group were counted and calculated in three predefined square areas (0.2 mm2 each) that were prepared from at least three different tissue samples. The areas were arbitrarily selected within the folded synovium, which lines the inner surface of the fibrous outer capsule of the joint.
Statistical analysis
In animal studies, all measurements were performed by independent investigators unaware of the experimental conditions. Results in figures were expressed as mean ± standard error of means (SEM). Statistical analysis of differences among groups was carried out using one-way anova followed by Tukey's multiple comparison test. Differences were considered statistically significant at a level of P < 0.05.
Materials
The sources of the compounds used were as follows: berberine chloride was obtained from Sigma–Aldrich (MO, USA); prednisolone, λ-carrageenan and kaolin (particle size 0.1–4 µm; K7375) from Sigma–Aldrich Korea (Youngin-si, Korea); celecoxib from Pfizer Korea (Seoul, Korea); prolactin, IL-2 and IL-3 from R&D Systems Inc. (MN, USA); CP690550 from Axon Medchem BV (The Netherlands).
Results
Berberine chloride inhibits IL-2-induced STAT5 reporter activity
We previously demonstrated that in murine IL-3-dependent myeloid 32D/IL-2Rβ cells, JAK2 and JAK3 undergo transient phosphorylation in response to IL-3 and IL-2, respectively, and then subsequently activate STAT5 (Kim et al., 2010). Therefore, measuring STAT5 activity induced by IL-2 or IL-3 in the cells can faithfully monitor the activity of these kinases, and 32D/IL-2Rβ cells carrying STAT5 reporter gene (32D/IL-2Rβ/6xSTAT5) can be used as an efficacious cellular model for chemical screens to identify small molecule inhibitors selective for JAK3 over JAK2. To identify novel JAK3 inhibitors, we performed high-throughput cell-based screening using the 32D/IL-2Rβ/6xSTAT5 cells and compounds in the NCI diversity and mechanistic sets. We identified berberine chloride (NSC163088; C20H18NO4Cl; CAS no. 633-65-8) (Figure 1C) as a novel JAK3 inhibitor. Berberine chloride is an isoquinoline alkaloid derivative and has been used in traditional Chinese medicine to treat various diseases including inflammation (Imanshahidi and Hosseinzadeh, 2008; Sun et al., 2009). Berberine chloride inhibited IL-2-induced STAT5 reporter activity in a concentration-dependent manner with an IC50 value of 3.78 µM (Figure 1A). By contrast, the same compound had less effect on IL-3-induced reporter activity when compared with IL-2-induced STAT5 reporter activity (Figure 1B). The IC50 value of the compound in the IL-3-induced reporter activity was 80 µM, demonstrating that berberine chloride has almost 20-fold more selectivity for JAK3 over JAK2 in this reporter assay.
Figure 1.
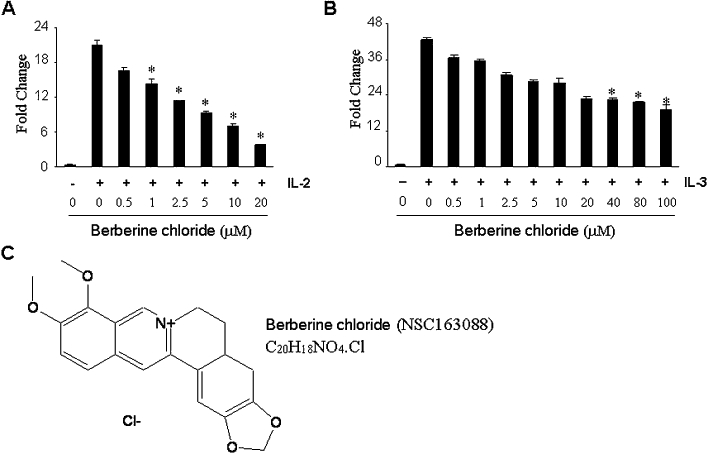
Berberine chloride inhibits activation of STAT5 following IL-2 treatment. In 32D/IL-2Rβ/6xSTAT5 cells, berberine chloride inhibits the reporter activity by IL-2 with an IC50 value of 3.78 µM (A) but has little inhibitory effect on the reporter activity by IL-3, with an IC50 value of 80 µM (B). Results are shown as the mean of five independent experiments (±SD indicated by error bar). *P < 0.001, significantly different from IL-2- or IL-3-treated reporter cells in the absence of NSC163088. (C) The chemical structure of berberine chloride (NSC163088).
Berberine chloride inhibits IL-2-induced JAK3 phosphorylation
As induction of the STAT5 reporter activity by IL-2 is JAK3-dependent, we hypothesized that berberine chloride would block the activation of JAK3 and subsequently that of STAT5. To test this hypothesis, we monitored the levels of phosphorylated JAK3 in 32D/IL-2Rβ/6xSTAT5 cells after treatment with IL-2 in the absence or presence of berberine chloride. Phospho-JAK3 was barely detectable in the cells without IL-2, but its levels were substantially increased after IL-2 treatment (Figure 2A). Berberine chloride efficiently blocked the phosphorylation of JAK3 and STAT5 by IL-2 in a concentration-dependent manner (Figure 2A). By contrast, we found no significant inhibitory effects of this reagent on phospho-JAK2 and -STAT5 following IL-3 treatment at the concentrations up to 10 µM (Figure 2B). We further evaluated the specificity of berberine chloride for JAK3 using the rat pre-T lymphoma cell line Nb2 and the human myeloma cell line U266. In Nb2 cells, JAK2 is phosphorylated by prolactin treatment, whereas JAK3 becomes phosphorylated upon IL-2 stimulation (Kim et al., 2010). Subsequently STAT5 becomes phosphorylated after either prolactin/JAK2 or IL-2/JAK3. While phospho-JAK3 and phospho-JAK2 were nearly undetectable in Nb2 cells in the absence of stimulation, their levels were increased in response to IL-2 and prolactin stimulation respectively (Figure 2C and D). Berberine chloride blocked IL-2-induced phospho-JAK3 and -STAT5, both of which were almost undetectable at 3 µM berberine (Figure 2C). By contrast, this compound failed to inhibit prolactin-induced JAK2 and STAT5 phosphorylation at concentrations up to 10 µM (Figure 2D). The selective effect of berberine chloride on JAK3-dependent signalling was further examined in U266 cells, in which JAK1 and TYK2 are transiently phosphorylated after interferon-α (IFN-α) (Figure 2E) (David et al., 1996). However, treatment of U266 cells with up to 10 µM berberine chloride did not affect the phosphorylation of either JAK1 or TYK2 following IFN-α stimulation. Consistent with these results, the phosphorylation of STAT1, a key downstream substrate of IFN-α, was not diminished by berberine chloride (Figure 2E). These findings suggest that berberine chloride exerts substantially greater inhibition of JAK3 than of the other members of the JAK family.
Figure 2.
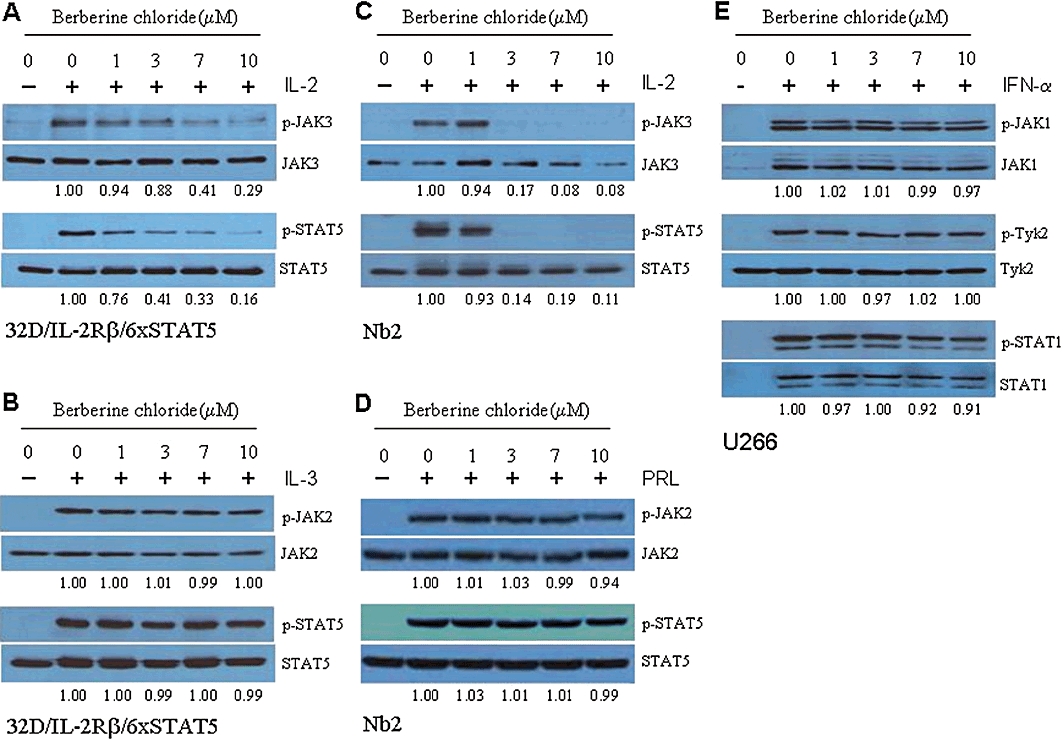
Berberine chloride inhibits the phosphorylation of JAK3 in response to IL-2 treatment. In 32D/IL-2Rβ/6xSTAT5 cells, berberine chloride decreases IL-2-induced phospho-JAK3/STAT5 levels (A), but fails to inhibit IL-3-induced phospho-JAK2/STAT5 (B). Treatment of rat pre-T lymphoma Nb2 cells with berberine chloride inhibits IL-2-induced JAK3/STAT5 phosphorylation (C), but fails to affect prolactin (PRL)-induced JAK2/STAT5 phosphorylation (D). (E) Treatment of human myeloma U266 cells with berberine chloride at concentrations up to 10 µM did not affect IFN-α-induced phosphorylation of JAK1 and TYK2, as well as their key downstream target STAT1.
Berberine chloride inhibits persistently active JAK3
We further assessed the selectivity of berberine chloride for JAK3 using cancer cell lines that contain constitutively active JAKs. The growth of murine pro-B Ba/F3-JAK3V674A cells is IL-3-independent after transduction of a JAK3 (V674A) allele, which encodes a dominant-active kinase (Choi et al., 2007). Ba/F3-JAK3V674A cells contain activated JAK3 and JAK1 but not activated JAK2 (Figure 3A). Hodgkin's lymphoma L540 cells have high levels of phospho-JAK3 but undetectable levels of phospho-JAK1 and -JAK2 (Figure 3B). Conversely, Hodgkin's lymphoma HLDM-2 cells and prostate cancer DU145 cells exhibit high levels of phospho-JAK1 and -JAK2 but not phospho-JAK3 (Figure 3C and D). Treatment of Ba/F3-JAK3V674A cells or L540 cells with berberine chloride inhibited phospho-JAK3 levels in a concentration-dependent manner, with a significant reduction occurring at 3 µM (Figure 3A and B, lanes 5–6). By contrast, even at a 10 µM concentration, this compound did not alter phospho-JAK1 and -JAK2 levels in Ba/F3-JAK3V674A, HDLM-2 and DU145 cells (Figure 3A, C and D, lanes 1–4). To assess the functional outcome of this inhibition, we monitored the activation of STAT3 or STAT5 in these four cell lines after treatment with this compound. Berberine chloride inhibited phospho-STAT5 and -STAT3 in Ba/F3-JAK3V674A cells and L540 cells, respectively, both of which harbour activated JAK3 (Figure 3A and B, lanes 7–8). In contrast, even at a 10 µM concentration, berberine chloride did not inhibit the phosphorylation of STAT3 in HDLM-2 and DU145 cells, which lack persistently active JAK3 (Figure 3C and D, lanes 7–8). As expected, the pan-JAK inhibitor AG490 profoundly reduced the phosphorylation levels of all JAKs and both STAT3 and STAT5 in these cells (Figure 3A–D, lanes 1–8). These data indicate that berberine chloride specifically inhibits JAK3 activity after cytokine administration or as a result of an activating mutation.
Figure 3.
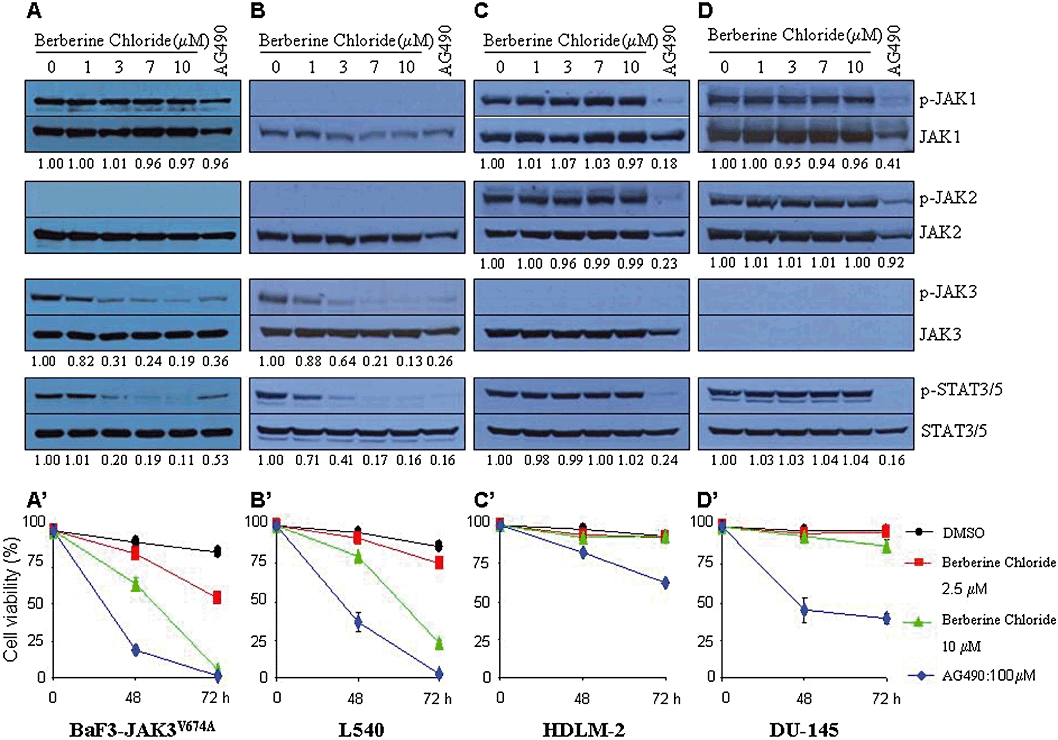
Berberine chloride selectively inhibits persistently active JAK3. BaF3-JAK3V674A (A), L540 (B), HDLM-2 (C) and DU145 (D) cells were cultured for 16 h in the presence of DMSO alone, berberine chloride or the pan-JAK inhibitor AG490 (150 µM). Whole-cell extracts were processed for Western blot analysis using antibodies specific for the molecules indicated. Treatment with berberine chloride did not alter the levels of phospho-JAK1 and phospho-JAK2 (A, C and D, lanes 1–4), but it did potently inhibit phospho-JAK3 (A and B, lanes 5–6). This compound decreased the phosphorylation of STATs only in cell lines (i.e. BaF3-JAK3V674A and L540), which express persistently active JAK3 (A–D, lanes 7–8). In contrast, AG490 decreased the phosphorylation of all JAK kinases (and subsequently of all STATs) tested in four cell lines (A–D, lanes 1–8). (A′–D′) Treatment with berberine chloride affects cell viability in BaF3-JAK3V674A (A′) and L540 (B′) cells that express constitutively active JAK3, but not in HLDM-2 (C′) and DU145 (D′) cells that do not harbour constitutively active JAK3. By contrast, AG490 reduces cell viability in all cell lines tested. Results are shown as the mean of three independent experiments (±SD indicated by error bar).
Berberine chloride inhibits the viability of cancer cells with constitutively active JAK3
Small molecule inhibitors of JAK/STAT signalling have been shown to repress cell proliferation by affecting cell viability in several cancer cell lines, suggesting the critical role of JAK/STAT signalling in their proliferation (Bharti et al., 2003; Blaskovich et al., 2003; Lipka et al., 2008). As berberine chloride selectively inhibited JAK3, we hypothesized that treatment with our compound would affect cell viability only in cancer cells that express constitutively active JAK3. Indeed, berberine chloride decreased cell viability only in Ba/F3-JAK3V674A and L540 cells, which contain persistent JAK3 activation (Figure 3A′ and B′), but not in HDLM-2 and DU145, which lack persistently active JAK3 (Figure 3C′ and D′). As expected, AG490 reduced cell viability in all cell lines tested (Figure 3A′–D′).
Berberine chloride directly blocks JAK3 kinase activity
To obtain insight into the molecular mechanism of berberine chloride to inhibit JAK3, we performed in vitro kinase assays on JAK3 immunoprecipitates using recombinant STAT3α as a substrate. JAK3 immunoprecipitates efficiently phosphorylated STAT3α in the absence of berberine chloride. However, this compound inhibited JAK3 kinase activity in a concentration-dependent manner, suggesting that berberine chloride may bind directly to JAK3 and suppress its catalytic activity (Figure 4A). By contrast, we did not detect any inhibitory effect of berberine chloride on the kinase activities of JAK1 and JAK2 in kinase assays at concentrations up to 10 µM (data not shown). Increasing the concentration of free ATP in the reaction blocked the ability of berberine chloride to inhibit JAK3 kinase activity, demonstrating that berberine chloride is an ATP-competitive JAK3 inhibitor (Figure 4B). To predict whether berberine chloride may bind directly to the JAK3 kinase domain, we used AutoDock version 4 and AutoDock Vina version 1.1 to develop a structure model for the interaction between berberine chloride and the kinase domain of JAK3 (JAK3-JH1; PDB ID: 1YVJ). The model structure of berberine chloride in complex with JAK3-JH1 domain revealed the contacts with the side-chain atoms of Lys-831, Val-860, Met-878, Tyr-880, Leu-932 and Asp-943 of the kinase domain (Figure 4C). While hydrophobic interactions were dominant, the side chains of Lys-831 and Asp-943 were also involved in the hydrophilic contacts with the −OCH3 moiety of berberine chloride. The AutoDock-calculated binding free energy between JAK3-JH1 and berberine chloride is −9.65 kcal·mol−1, which is comparable to that of between JAK3-JH1 and the known JAK3 inhibitor CP-690550 (PDB ID: 3LXK) (−9.23 kcal·mol−1; Figure 4D). These data suggest that berberine chloride may bind to the kinase domain of JAK3.
Figure 4.
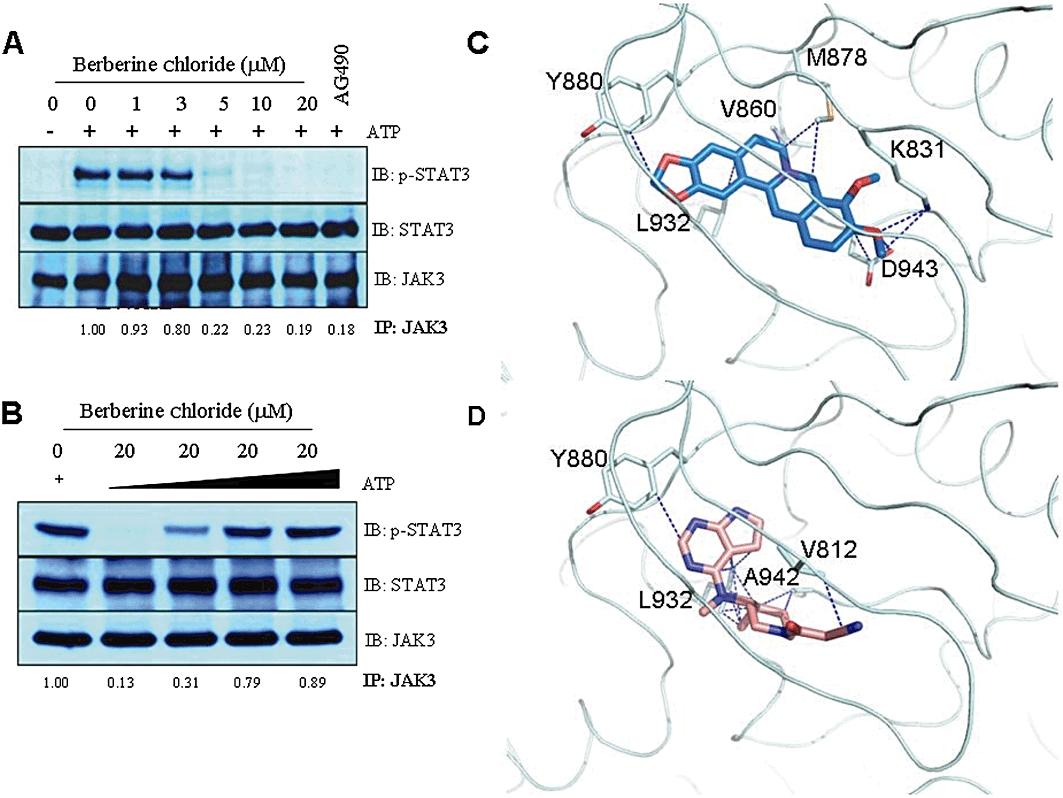
Berberine chloride directly binds to JAK3. (A) JAK3 immunoprecipitates prepared from the lysates of L540 cells were pre-incubated with DMSO alone, berberine chloride or AG490 (100 µM) for 1 h. Kinase reactions were subsequently performed by the addition of recombinant STAT3α as a substrate and 2 µM ATP for 30 min at 30°C. The reaction products were processed for Western blotting and probed with antibodies specific for phospho-STAT3, STAT3 and JAK3. (B) JAK3 kinase assays were performed in the presence of excess ATP. Note that berberine chloride is an ATP-competitive JAK3 inhibitor. (C) Predicted binding model of berberine chloride to the JAK3 kinase domain. (D) Binding mode of CP-690550 and JAK3 kinase domain in the complex structure (PDB ID: 3LXK). In (C) and (D), only the residues that contact berberine chloride and CP-690550 with side-chain atoms are labelled. These contacts were selected only when two atoms in protein and ligand are located within 3.5 Å.
Berberine chloride alleviates oedema and pain in a rat model of carrageenan/kaolin-induced monoarthritis
Many cytokines and growth factors associated with inflammation and arthritis have been shown to activate the JAK/STAT pathway, suggesting that this pathway plays key roles in the pathogenesis of inflammatory diseases (Malemud, 2010). While recently developed JAK3 inhibitors have anti-inflammatory and anti-arthritic activities (Kudlacz et al., 2008; Milici et al., 2008; West, 2009; Coombs et al., 2010; Lin et al., 2010), these studies did not provide the direct evidence of decreases in JAK3 activity following drug administration in vivo. We assessed whether berberine chloride was efficacious in a rat model of carrageenan/kaolin-induced acute synovial inflammation (Sluka and Westlund, 1993; Schaible et al., 1994). In our preliminary study, we found that co-injection of carrageenan with kaolin at higher doses is more effective than carrageenan alone in sustaining inflammation and pain without significant decline caused by early resolution in the rats (data not shown). Therefore, a mixture of 5% carrageenan and 5% kaolin was injected to aggravate and maintain the arthritic symptoms for a week. Rats injected in the knee joint of left hind limb with carrageenan/kaolin exhibited redness (as measured by visual inspection), swelling (as assessed by knee thickness) and pain (as monitored by weight distribution per leg) that reached a maximum at 1 day after injection. By contrast, untreated rats exhibited none of these symptoms (Figure 5A and B). Beginning at 1 day after injection of carrageenan/kaolin, rats were injected with either vehicle (saline) alone or berberine chloride at three doses (10, 30 or 50 mg·kg−1). The thickness of the inflamed knee at day 6 in rats treated with 30 or 50 mg·kg−1 berberine chloride was reduced by 25% or 47%, respectively, compared with that of in saline-treated rats (Figure 5A). We next examined the effect of berberine chloride on arthritic pain by measuring weight bearing (WDR) in the two hind legs of carrageenan/kaolin-injected rats. At day 0, no significant difference in the WDR was found among the experimental groups, as animals carry 50% of the weight on each hind leg (WDR = 50:50) (Figure 5B). However, significant changes to the ratio were observed in the carrageenan/kaolin-injected control rats which were treated with saline, and the WDR reached a maximum of 28:72 at day 6. By contrast, the WDR was significantly reduced in animals treated with berberine chloride at all doses tested. Notably, the effect of 50 mg·kg−1 berberine chloride on arthritic pain was even more pronounced than that of the positive control anti-inflammatory agent, prednisolone, a corticosteroid (Figure 5B). We also monitored hyperalgesia in rats by injecting 1% carrageenan to the plantar skin surface and analysed the pain tolerance using a semi-automatic Randall-Selitto test apparatus. We observed that rats treated with 30 mg·kg−1 or 50 mg·kg−1 berberine chloride showed significantly higher tolerance to the pressure on the affected paw than did those treated with saline alone (Figure 5C). This suggests that berberine chloride, at least at the doses tested, exerted an analgesic effect that was in fact better than that observed with celecoxib, a non-steroidal anti-inflammatory drug used as a positive control.
Figure 5.
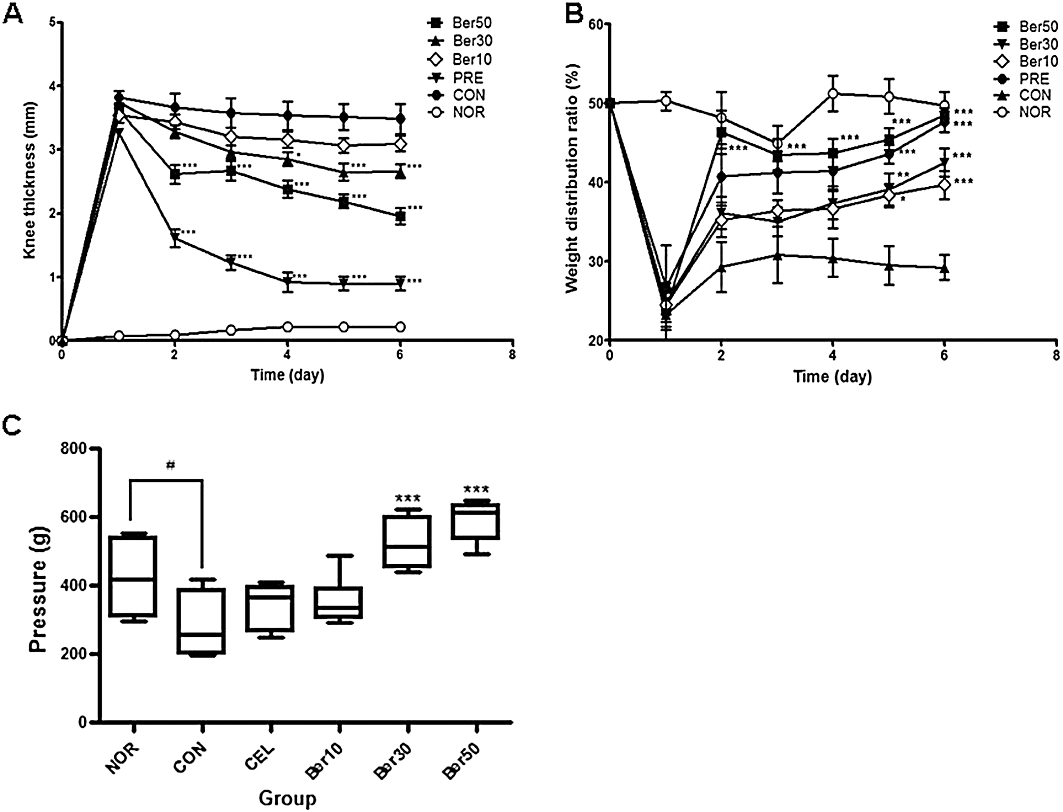
Berberine chloride alleviates oedema and pain in monoarthritic rats. Treatment with berberine chloride reduces knee thickness (A) and weight distribution ratio between two hind limbs (B) in carrageenan/kaolin-induced monoarthritic rats. (C) Berberine chloride exerts analgesic effects on paw hyperalgesia (paw pressure vocalization) in a rat model of carrageenan-induced paw hyperalgesia. NOR (normal group), CON (saline-treated vehicle control group), Ber10 (10 mg·kg−1 berberine chloride-treated group, i.p.), Ber30 (30 mg·kg−1), Ber50 (50 mg·kg−1), PRE (Prednisolone; 20 mg·kg−1, p.o.) and CEL (celecoxib; 100 mg·kg−1, p.o.). #P < 0.05 versus NOR group, and *P < 0.05, **P < 0.01, ***P < 0.005 versus CON group. Results are shown as the mean experiments (±SEM indicated by error bar) (one-way anova followed by Tukey's post-test).
Berberine chloride has anti-inflammatory property in monoarthritic rats
To further evaluate the anti-inflammatory effect of berberine chloride, knee joint tissues obtained from each experimental group were examined by H&E staining. Rats injected with carrageenan/kaolin and treated with saline alone exhibited thicker synovial membranes, more pannus formation and more infiltrated immune cells into the synovial membrane than those treated with berberine chloride (Figure 6A–E). The degree of inflammation in five specimens from each experimental group was scored by three independent pathologists, unaware of the treatments given to the rats. The scores were based on the thickness of synovial membrane (Figure 6; blue thick lines), the number of infiltrated immune cells (Figure 6; black arrows in black squares) and the growth of pannus and cartilage-pannus junction (Figure 6; black lines in red squares). All three ranked the samples in a similar manner and their averaged inflammation score indicates that berberine chloride effectively reduced the histological signs of carrageenan/kaolin-induced synovial inflammation, in a dose-dependent manner (Figure 6F).
Figure 6.
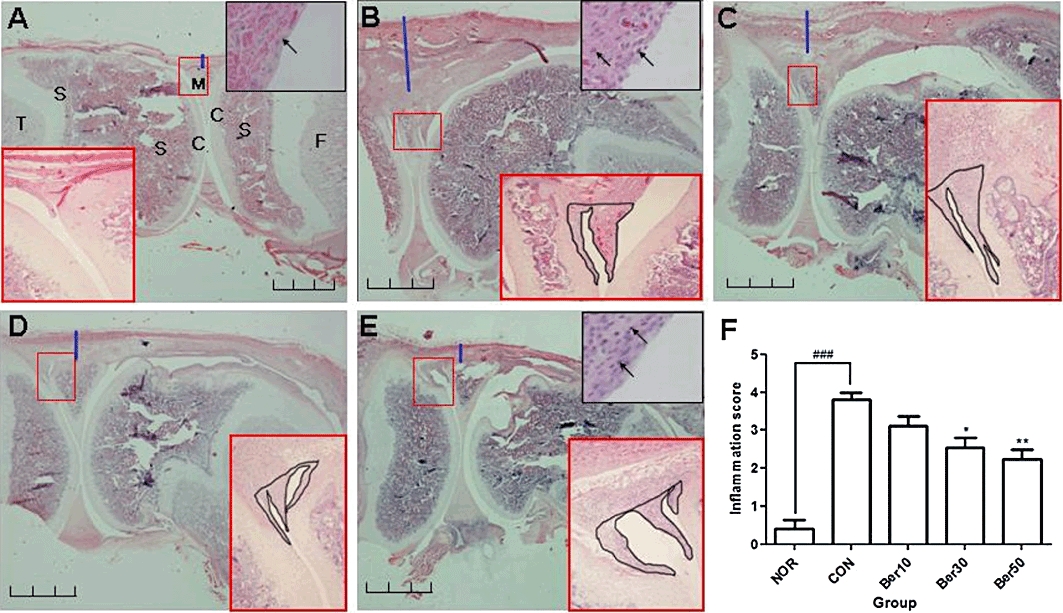
Berberine chloride reduces histological signs of inflammation. Histological section of knee joints of normal (A), saline-treated monoarthritic (B), 10 mg·kg−1 berberine chloride-treated (C), 30 mg·kg−1 berberine chloride-treated (D) and 50 mg·kg−1 berberine chloride-treated (E) monoarthritic rats. Tissue structure was visualized by haematoxylin and eosin (H&E) staining (10×). Black scale bar indicates 2 mm. The arthritic symptoms were evaluated by scoring the degree of inflammation in H&E histological sections of knee joints (F). Extent of inflammation was scored on a scale of 0–4, based on the degree of thickness of synovium tissue (blue thick line), pannus and cartilage-pannus junction (black line in red square), and cellular infiltration into the synovium tissue (black arrow), where 0 = normal (no infiltrates), 1 = minimal inflammation, 2 = mild inflammation, 3 = moderate inflammation and 4 = severe inflammation. Enlarged histological figures in black squares in the upper right corners indicate inflammatory cell infiltration (A, B, E). Small red squares in H&E staining are magnified in the right or left corner (100×). C: cartilage, S: subchondral bone, F: femur, T: tibia, M: meniscus. ###P < 0.005 versus NOR group, and *P < 0.05, **P < 0.01 versus CON group. Results are shown as the mean experiments (±SEM indicated by error bar) (one-way anova followed by Tukey's post-test).
Berberine chloride inhibits JAK3 phosphorylation in the synovial tissues of arthritic rats
To demonstrate that persistently active JAK3 contributes to the progression of carrageenan/kaolin-induced acute inflammation and that the inhibitory effect of berberine chloride on the inflammation results from JAK3 inhibition, we monitored, by immunofluorescence, the levels of phospho-JAK3, STAT6, STAT4 and phospho-STAT3 in the synovial tissues. Basal levels of phospho-JAK3 were observed in the synoviocytes of a normal knee joint. However, the number of phospho-JAK3-positive cells, as well as the intensity of phospho-JAK3 levels, was significantly increased in inflammatory cells from carrageenan/kaolin-injected, saline-treated rats. By contrast, the number of phospho-JAK3-positive cells was decreased by almost 50% in monoarthritic rats treated with 50 mg·kg−1 berberine chloride. Furthermore, the intensity of phospho-JAK3 levels was also dramatically decreased in samples from carrageenan/kaolin-injected, berberine chloride-treated rats (Figure 7A1–A4). We also observed a dramatic increase in the expression of STAT4 and STAT6 in saline-treated monoarthritic rats compared with that in normal rats. These data are consistent with previous reports that STAT4 and STAT6 levels are increased in the synovial tissue of RA patients (Walker and Smith, 2005; Walker et al., 2006a,b; 2007;), However, this up-regulation of STAT4 and STAT6 in monoarthritic rats was diminished by administration of berberine chloride (Figure 7B1–C4). Interestingly, phospho-STAT3-positive cells were also increased in the synovial tissues of monoarthritic rats, and treatment of these rats with our compound decreased the number of cells positive for phospho-STAT3 (Figure 7D1–D4). These results suggested that the JAK3/STAT pathway contributed to the pathogenesis of carrageenan/kaolin-induced inflammation and that berberine chloride alleviated inflammatory responses by inhibiting JAK3.
Figure 7.
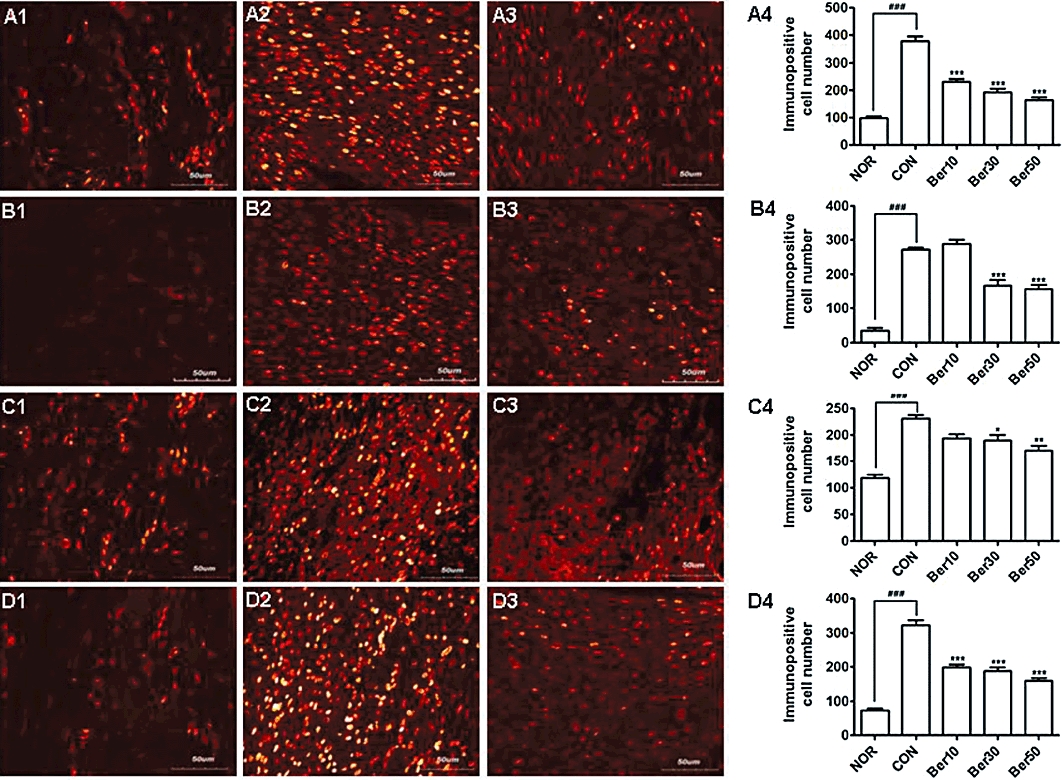
Berberine chloride inhibits JAK3 phosphorylation in the synovial tissue of monoarthritic rats. Immunohistochemical investigations of phospho-JAK3 (A1–A4), STAT6 (B1–B4), STAT4 (C1–C4) or phospho-STAT3 (D1–D4) in the synovial tissues of normal NOR; A1, B1, C1 and D1), saline-treated monoarthritic (CON; A2, B2, C2 and D2) or 50 mg·kg−1 berberine chloride-treated (A3, B3, C3 and D3) monoarthritic rat knee joint. Cells immunopositive for phospho-JAK3 (A4), STAT6 (B4), STAT4 (C4) or phospho-STAT3 (D4) were counted. The cells were counted in predefined square areas (0.2 mm2 each) of the coronal sections, and assessed independently by three investigators who were unaware of the treatments. ###P < 0.005 versus NOR group, and *P < 0.05, **P < 0.01, ***P < 0.005 versus CON group. Results are shown as the mean experiments (±SEM indicated by error bar) (one-way anova followed by Tukey's post-test).
Discussion
Here, we identified berberine chloride as a lead compound, showing increased selective inhibition of JAK3 over other JAK family members. Berberine chloride inhibited both cytokine-induced and persistently active JAK3 in various cellular assays and blocked the catalytic activity of JAK3, possibly by directly binding to the kinase domain. Importantly, the IC50 value of berberine chloride in IL-2- and IL-3-induced reporter activity was 3.78 µmol·L−1 and 80 µmol·L−1, respectively, in the assay using 32D/IL-2Rβ/6xSTAT5 cells. This selectivity is comparable to that of the JAK3 inhibitor CP-690550, which has previously shown 20-fold greater selectivity for JAK3 over JAK2 in ex vivo JAK3 kinase assay (Changelian et al., 2003). Furthermore, berberine chloride exhibited increased selectivity for JAK3 over other oncogenic pathway components. Berberine chloride did not reduce the levels of phospho-Lyn in L540 and HDLM-2 cells or the levels of phospho-Src in MDA-MB-468 and DU145 cells at all concentration tested (Figure S1). In addition, this compound did not alter the levels of phospho-Akt and phospho-ERK1/2 in any of these cell lines (Figure S1). Although the specificity of berberine chloride for JAK3 over other oncogenic kinases still needs to be fully examined by evaluating its effects on a larger panel of tyrosine and serine/threonine kinases in vitro, our findings strongly suggest that it selectively inhibits JAK3.
Notably, berberine chloride showed potent anti-inflammatory activity and analgesic properties in a rat model of monoarthritis. Many cytokines, including IFN-γ, IL-2, IL-4, IL-6, IL-10, IL-12, IL-15, all of which are thought to have significant roles in inflammation and/or RA, mediate their biological effects through activation of the JAK/STAT pathway (Kisseleva et al., 2002; Walker and Smith, 2005). Consistent with this, small molecules that inhibit JAK3 attenuate psoriasiform skin inflammation and allergic pulmonary inflammation in mice, and reduce the severity and clinical scores of RA in humans and animals (Kudlacz et al., 2008; Milici et al., 2008; West, 2009; Coombs et al., 2010; Lin et al., 2010). Here, we provided direct evidence that the JAK/STAT signalling was involved in the progression of inflammation in vivo. Our immunohistochemical analysis showed that the levels of phospho-JAK3 were significantly increased in the synovial tissues of monoarthritic rats, and treatment of these rats with berberine chloride inhibited the up-regulation of phospho-JAK3 in a dose-dependent manner. The arthritic rats also displayed increased levels of phospho-STAT3, STAT4 and STAT6, and treatment of these rats with berberine chloride efficiently inhibited the up-regulation of those molecules. STAT1 expression and activity have previously been shown to be increased in the synovium of RA patients (van der Pouw Kraan et al., 2003; Kasperkovitz et al., 2004). Furthermore, STAT5 has been known to be activated by cytokines, including IL-2, IL-7, GM-CSF and IFN-α/β, expressed in RA (Gadina et al., 2001; Kisseleva et al., 2002). Although the expression of STAT1 and STAT5 remains to be determined in the synovial tissues of the monoarthritic rats, these observations suggest that STAT1 and/or STAT5 may also contribute to the progression of inflammatory arthritis. Nonetheless, our findings strongly suggest that JAK3/STAT signalling is closely correlated with inflammation.
Berberine, an isoquinoline alkaloid derived from medicinal plants used extensively in traditional Chinese and Ayurvedic medicine, has been known to have multiple pharmacological effects on several human diseases including metabolic disorders, microorganism infection, a wide variety of neoplasms and inflammation, but its mechanism of action is yet fully understood (Bhadra and Kumar, 2010). Interestingly, recent studies have shown that berberine and/or its derivatives can efficiently reduce inflammation through several distinct mechanisms, such as by down-regulating COX-2 (Kuo et al., 2004), promoting AMP-activated protein kinase (AMPK) activity (Jeong et al., 2009) or inhibiting NF-κB activation (Lee et al., 2010), in various cellular and animal models of inflammation. Therefore, there is a possibility that the anti-inflammatory effect of berberine chloride in monoarthritic rats also resulted from changes in activity of other inflammation-related molecules such as COX-2 and NF-κB. To rule out this possibility, it would be necessary to assess whether co-administration of berberine chloride with the inhibitors of AMPK signalling including Ara A and Compound C or with the agonists of NF-κB pathway in a rat model of carrageenan/kaolin-induced acute synovial inflammation can affect the anti-inflammatory effect of berberine chloride alone. Nonetheless, we clearly demonstrated that treatment with berberine chloride significantly decreases the up-regulation of phospho-JAK3 in a rat model of acute synovial inflammation, suggesting that at least in part JAK3 signalling contributes to the pathogenesis of the inflammation and inhibition of JAK3 activity can lead to a reduction of the inflammation.
Furthermore, our findings provide new insight into the action of berberine to inhibit inflammation. Chronic inflammation has been reported to contribute to the pathogenesis of numerous human diseases, including allergy, asthma, autoimmune disorders and diabetes, and thus anti-inflammatory agents have many potential clinical benefits (Germolec et al., 2010). However, such drugs often display a risk of gastrointestinal toxicity as a result of required long-term administration. Therefore, there is a great need to develop safe and effective new drugs. Berberine is a low molecular weight, non-steroid, compound and is already commercially available as a natural dietary supplement. Importantly, the potential clinical benefits of berberine have already been evaluated in various studies using human subjects, and it has been shown to be safe in the majority of clinical trials (Wu et al., 2005; Liu et al., 2008; Wang et al., 2009). In conclusion, our study suggests that JAK3/STAT signalling plays an important role in the progression of inflammation and inhibition of the activity can alleviate the inflammatory responses in vivo.
Acknowledgments
We thank Dr C. Clevenger for Nb2 cells, Dr H. Mano for BaF3-JAKV674A cells, and Drs A.D. Keegan and W. Leonard for 32D/IL-2Rβ cells. We also thank Drs J. Johnson and R.J. Schultz from the NCI Developmental Therapeutics Program for providing the diversity and mechanistic sets of compounds. This work was supported by Korean National Research Foundation (Grant No. R11-2005-014; D.-H. Hahm) and Children's Cancer Fund (Millwood, NY; G.-H. Baeg).
Glossary
Abbreviations
- AMPK
AMP-activated protein kinase
- COX-2
cyclooxygenaxse-2
- GM-CSF
granulocyte macrophage colony-stimulating factor
- IC50
the half maximal inhibitory concentration
- IFN
interferon
- IL
interleukin
- JAK
janus kinase
- RA
rheumatoid arthritis
- STAT
signal transducer and activator of transcription
- TYK2
tyrosine kinase 2
- WDR
weight distribution ratio
Conflict of interest
The authors have nothing to declare.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Berberine chloride shows selectivity for JAK3 over other oncogenic pathway components. Treatment with berberine chloride did not reduce the levels of phospho-Lyn in L540 and HDLM-2 cells or the levels of phospho-Src in MDAMB-468 and DU145 cells at all concentration tested. In addition, this compound did not alter the levels of phospho-Akt and phospho-ERK1/2 in any of these cell lines.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Bhadra K, Kumar GS. Therapeutic potential of nucleic acid-binding isoquinoline alkaloids: binding aspects and implications for drug design. Med Res Rev. 2010 doi: 10.1002/med.20202. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, et al. Prevention of organ allograft rejection by a specific janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- Choi YL, Kaneda R, Wada T, Fujiwara S, Sod M, Watanabe H, et al. Identification of a constitutively active mutant of JAK3 by retroviral expression screening. Leuk Res. 2007;31:203–209. doi: 10.1016/j.leukres.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Coombs JH, Bloom BJ, Breedveld FC, Fletcher MP, Gruben D, Kremer JM, et al. Improved pain, physical functioning and health status in patients with rheumatoid arthritis treated with CP-690,550, an orally active janus kinase (JAK) inhibitor: results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2010;69:413–416. doi: 10.1136/ard.2009.108159. [DOI] [PubMed] [Google Scholar]
- David M, Petricoin E, 3rd, Larner AC. Activation of protein kinase A inhibits interferon induction of the Jak/Stat pathway in U266 cells. J Biol Chem. 1996;271:4585–4588. doi: 10.1074/jbc.271.9.4585. [DOI] [PubMed] [Google Scholar]
- Gadina M, Hilton D, Johnston JA, Morinobu A, Lighvani A, Zhou YJ, et al. Signaling by type I and II cytokine receptors: ten years after. Curr Opin Immunol. 2001;13:363–373. doi: 10.1016/s0952-7915(00)00228-4. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Frawley RP, Evans E. Markers of inflammation. Methods Mol Biol. 2010;598:53–73. doi: 10.1007/978-1-60761-401-2_5. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–E964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Kasperkovitz P, Verbeet N, Smeets T, van Rietschoten JG, Kraan MC, van der Pouw Kraan TC, et al. Activation of the STAT1 pathway in rheumatoid arthritis. Ann Rheum Dis. 2004;63:233–239. doi: 10.1136/ard.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BH, Jee JG, Yin CH, Sandoval C, Jayabose S, Kitamura D, et al. NSC114792, a novel small molecule identified through structure-based computational database screening, selectively inhibits JAK3. Mol Cancer. 2010;9:36. doi: 10.1186/1476-4598-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–1905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- Kudlacz E, Conklyn M, Andresen C, Whitney-Pickett C, Changelian P. The JAK-3 inhibitor CP-690550 is a potent anti-inflammatory agent in a murine model of pulmonary eosinophilia. Eur J Pharmacol. 2008;582:154–161. doi: 10.1016/j.ejphar.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Lee IA, Hyun YJ, Kim DH. Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-κB activation. Eur J Pharmacol. 2010;648:162–170. doi: 10.1016/j.ejphar.2010.08.046. [DOI] [PubMed] [Google Scholar]
- Lin TH, Hegen M, Quadros E, Nickerson-Nutter CL, Appell KC, Cole AG, et al. Selective functional inhibition of JAK3 kinase is sufficient for efficacy in collagen induced arthritis in mice. Arthritis Rheum. 2010;62:2283–2293. doi: 10.1002/art.27536. [DOI] [PubMed] [Google Scholar]
- Lipka DB, Hoffmann LS, Heidel F, Markova B, Blum MC, Breitenbuecher F, et al. LS104, a non-ATP-competitive small-molecule inhibitor of JAK2, is potently inducing apoptosis in JAK2V617F-positive cells. Mol Cancer Ther. 2008;7:1176–1184. doi: 10.1158/1535-7163.MCT-07-2215. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu H, Zhang C, Cheng Y, Hu L, Meng X, et al. Protective effects of berberine on radiation-induced lung injury via intercellular adhesion molecular-1 and transforming growth factor-beta-1 in patients with lung cancer. Eur J Cancer. 2008;44:2425–2432. doi: 10.1016/j.ejca.2008.07.040. [DOI] [PubMed] [Google Scholar]
- Macchi P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, et al. Mutations of jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Zhu D, Dibirdik I, Uckun FM. Targeting janus kinase 3 in mast cells prevents immediate hypersensitivity reactions and anaphylaxis. J Biol Chem. 1999;274:27028–27038. doi: 10.1074/jbc.274.38.27028. [DOI] [PubMed] [Google Scholar]
- Malemud CJ. Suppression of autoimmune arthritis by small molecule inhibitors of the JAK/STAT pathway. Pharmaceuticals. 2010;3:1446–1455. doi: 10.3390/ph3051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milici AJ, Kudlacz EM, Audoly L, Zwillich S, Changelian P. Cartilage preservation by inhibition of janus kinase 3 in two rodent models of rheumatoid arthritis. Arthritis Res Ther. 2008;10:R14. doi: 10.1186/ar2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- Papageorgiou AC, Wikman LE. Is JAK3 a new drug target for immunomodulation-based therapies? Trends Pharmacol Sci. 2004;25:558–562. doi: 10.1016/j.tips.2004.09.008. [DOI] [PubMed] [Google Scholar]
- van der Pouw Kraan TC, van Gaalen FA, Kasperkovitz PV, Verbeet N, Smeets T, Kraan M, et al. Rheumatoid arthritis is a heterogenous disease. Evidence for differences in the activation of the STAT1 pathway between tissues. Arthritis Rheum. 2003;48:2132–2145. doi: 10.1002/art.11096. [DOI] [PubMed] [Google Scholar]
- Puck JM, Deschenes SM, Porter JC, Dutra AS, Brown CJ, Willard HF, et al. The interleukin-2 receptor gamma chain maps to Xq13.1 and is mutated in X-linked severe combined immunodeficiency, SCIDX1. Hum Mol Genet. 1993;2:1099–1104. doi: 10.1093/hmg/2.8.1099. [DOI] [PubMed] [Google Scholar]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Freudenberger U, Neugebauer V, Stiller RU. Intraspinal release of immunoreactive calcitonin gene-related peptide during development of inflammation in the joint in vivo–a study with antibody microprobes in cat and rat. Neuroscience. 1994;62:1293–1305. doi: 10.1016/0306-4522(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Westlund KN. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain. 1993;55:367–377. doi: 10.1016/0304-3959(93)90013-F. [DOI] [PubMed] [Google Scholar]
- Sun Y, Xun K, Wang Y, Chen X. A systematic review of the anticancer properties of berberine, a natural product from chinese herbs. Anticancer Drugs. 2009;20:757–769. doi: 10.1097/CAD.0b013e328330d95b. [DOI] [PubMed] [Google Scholar]
- Verstovsek S. Therapeutic potential of JAK2 inhibitors. Hematology Am Soc Hematol Educ Program Book. 2009;2009:636–642. doi: 10.1182/asheducation-2009.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JG, Smith MD. The jak-STAT pathway in rheumatoid arthritis. J Rheumatol. 2005;32:1650–1653. [PubMed] [Google Scholar]
- Walker JG, Ahern MJ, Coleman M, Weedon H, Papangelis V, Beroukas D, et al. Changes in synovial tissue jak-stat expression in rheumatoid arthritis in response to successful DMARD treatment. Ann Rheum Dis. 2006a;65:1558–1564. doi: 10.1136/ard.2005.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JG, Ahern MJ, Coleman M, Weedon H, Papangelis V, Beroukas D, et al. Expression of Jak3, STAT1, STAT4, and STAT6 in inflammatory arthritis: unique Jak3 and STAT4 expression in dendritic cells in seropositive rheumatoid arthritis. Ann Rheum Dis. 2006b;65:149–156. doi: 10.1136/ard.2005.037929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JG, Ahern MJ, Coleman M, Weedon H, Papangelis V, Beroukas D, et al. Characterisation of a dendritic cell subset in synovial tissue which strongly expresses Jak/STAT transcription factors from patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:992–999. doi: 10.1136/ard.2006.060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Yang Z, Xu MG, Chen L, Wang Y, Su C, et al. Berberine-induced decline in circulating CD31+/CD42- microparticles is associated with improvement of endothelial function in humans. Eur J Pharmacol. 2009;614:77–83. doi: 10.1016/j.ejphar.2009.04.037. [DOI] [PubMed] [Google Scholar]
- West K. CP-690550, a JAK3 inhibitor as an immunosuppressant for the treatment of rheumatoid arthritis, transplant rejection, psoriasis and other immune-mediated disorders. Curr Opin Investig Drugs. 2009;10:491–504. [PubMed] [Google Scholar]
- Wu X, Li Q, Xin H, Yu A, Zhong M. Effects of berberine on the blood concentration of cyclosporin A in renal transplanted recipients: clinical and pharmacokinetic study. Eur J Clin Pharmacol. 2005;61:567–572. doi: 10.1007/s00228-005-0952-3. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Saharinen P, Pesu M, Holt VE, 3rd, Silvennoinen O, O'Shea JJ. The janus kinases (jaks) Genome Biol. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


