Abstract
BACKGROUND AND PURPOSE
Hypoxic effects on neuronal functions vary significantly with experimental conditions, but the mechanism for this is unclear. Adenosine has been reported to play a key role in depression of neuronal activities in the CNS during acute hypoxia. Hence, we examined the effect of acute hypoxia on different spinal reflex potentials and the contribution of adenosine to them.
EXPERIMENTAL APPROACH
Spinal reflex potentials, monosynaptic reflex potential (MSR), slow ventral root potential (sVRP) and dorsal root potential (DRP), were measured in the isolated spinal cord of the neonatal rat. Adenosine release was measured by using enzymatic biosensors.
KEY RESULTS
In the spinal cord preparation isolated from postnatal day 5–8 rats at 27°C, acute hypoxia induced adenosine release and depressed three reflex potentials. However, in postnatal day 0–3 rats at 27°C, the hypoxic-induced adenosine release and depression of MSR were negligible, while the depression of sVRP and DRP were perceptible responses. In postnatal day 0–3 rats at 33°C, hypoxia evoked adenosine release and depression of MSR. An adenosine A1 receptor selective antagonist and a high [Ca2+]o, which suppressed adenosine release, abolished the hypoxic-induced depression of MSR but not those of sVRP and DRP.
CONCLUSIONS AND IMPLICATIONS
Hypoxic-induced depression of MSR depends on adenosine release, which is highly susceptible to age, temperature and [Ca2+]o. However, a large part of the depressions of DRP and sVRP are mediated via adenosine-independent mechanisms. This differential contribution of adenosine to depression is suggested to be an important factor for the variable effects of hypoxia on neuronal functions.
Keywords: adenosine, hypoxia, adenosine A1 receptors, reflex potentials, spinal cord
Introduction
Neuronal function in the CNS is extremely sensitive to decreases in partial pressure of O2. In general, hypoxia depresses neuronal activities, but its effect varies with brain region or experimental conditions (Peña and Ramirez, 2005). This variation in effect appears to be due to the differing sensitivities to hypoxia of each of the neurones that compose the neuronal networks of the CNS.
Adenosine is a central mediator of the inhibitory effect of acute hypoxia on synaptic transmission. In rat hippocampal slices, synaptic depression during acute hypoxia or ischaemia-like conditions (oxygen-glucose depletion) are largely suppressed by adenosine A1 receptor antagonists (Gribkoff and Bauman, 1992; Latini et al., 1999; Pearson et al., 2001; Martín et al., 2007) and temporally correlated with adenosine release (Latini et al., 1998; Dale et al., 2000). In rat spinal cord slices, hypoxic depression of excitatory postsynaptic current is also abolished by an adenosine A1 receptor antagonist (Park et al., 2002). In the isolated spinal cord of neonatal rat, it has been reported that hypoxia depresses the monosynaptic reflex potential (MSR), and this effect of hypoxia is significantly inhibited by an adenosine A1 receptor antagonist (Lloyd et al., 1988). Recently, we found that hypoxia induced the release of adenosine from the spinal cord (Takahashi et al., 2010). Also hypercapnia and an adenosine kinase inhibitor have been shown to release adenosine, resulting in a depression not only of the MSR but also of a slow ventral root potential (sVRP), which is associated with the nociceptive reflex (Otsuguro et al., 2006b; 2009;). However, the effects of hypoxia on sVRP remain unknown.
In the isolated spinal cord of neonatal rat, electrical stimulation of the lumber dorsal root gives rise to reflex potentials at an ipsilateral ventral and adjacent dorsal root via synaptic pathways as shown in Figure 1A (Akagi and Yanagisawa, 1987; Nussbaumer et al., 1989; Woodley and Kendig, 1991). The early part of the ventral root potentials is an MSR mediated mainly by non-NMDA receptors, which is followed by an sVRP mediated by NMDA and various metabolic receptors, such as neurokinin and metabolic glutamate receptors. The dorsal root potential (DRP) is mediated by GABAA receptors (Seno and Saito, 1985). Each reflex potential has a different sensitivity to drugs or external ions (Brockmeryer and Kendig, 1995; Kurihara and Yoshioka, 1996; Faber et al., 1997; Kubota et al., 1998; Otsuguro et al., 2005; 2006a;). In this study, we examined the effects of acute hypoxia on these different spinal reflex potentials. Our results demonstrate that hypoxia rapidly depresses not only MSR but also sVRP and DRP. The hypoxic depression of MSR reflected the hypoxia-induced release of adenosine, which was susceptible to various factors such as age, temperature and [Ca2+]o. On the other hand, the contribution of adenosine to the hypoxic depressions of sVRP and DRP was limited. The differential contribution of adenosine is suggested to be an important factor for the diversity of hypoxic responses in neuronal functions.
Figure 1.
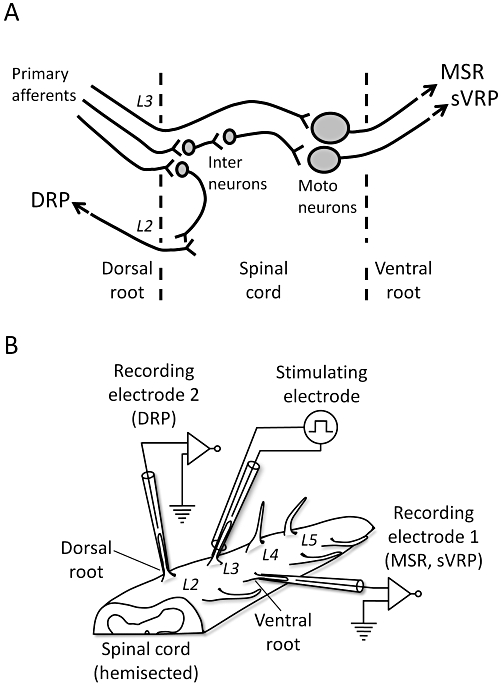
Diagram illustrating the pathways for reflex potentials and the experimental setup. (A) Diagram of the neuronal pathways for the reflex potentials evoked in the isolated spinal cord as shown in (B). The monosynaptic reflex potential (MSR) and slow ventral root potential (sVRP) evoked by stimulation of primary afferents are mediated via a monosynaptic and a polysynaptic pathway to motoneurones, respectively. The dorsal root potential (DRP) is mediated via interneurones to adjacent primary afferents. (B) The hemisected spinal cord isolated from a neonatal rat. The lumber dorsal root (L3–L5) is placed on a stimulating electrode. This picture shows a case where L3 is used for stimulation. The ipsilateral ventral root is placed on ‘recording electrode 1’ for MSR and sVRP. In a separate experiment, the adjacent dorsal root is placed on ‘recording electrode 2’ for DRP.
Methods
Preparations
All animal care and experimental procedures complied with the Guidelines of the AAALAC and SOP in Japan and were approved by the Animal Care and Use Committee of the Graduate School of Veterinary Medicine, Hokkaido University. Every effort was made to minimize animal suffering and to reduce the number of animals used. Both male and female neonatal rats (Wistar, 0–8 days old) were used.
Neonatal rats were killed by decapitation, and then the isolated spinal cord preparation was prepared as previously described (Otsuguro et al., 2006a). The hemisected spinal cord was superfused with artificial cerebrospinal fluid (ACSF) at a flow rate of approximately 2.5 mL·min−1. The temperature of the bath was monitored by a thermometer (CT-1200D, Custom, Tokyo, Japan) before and after each recording and was generally kept at 27 ± 2°C but increased to 33°C in some experiments. The composition of ACSF was as follows (mM): NaCl 138, NaHCO3 21, NaH2PO4 0.6, KCl 3.5, CaCl2 1.25, MgCl2 1.2, glucose 10, gassed with 95% O2 and 5% CO2, pH∼7.3. Hypoxic ACSF was prepared by gassing with 95% N2 and 5% CO2 (pH∼7.3) from at least 1 h before the experiments were started.
Electrophysiology
The spinal lumber roots were sucked into the electrodes, and the reflex potentials were extracellularly recorded as shown in Figure 1B. Suction stimulating and recording electrodes were placed on the dorsal and ipsilateral ventral roots (L3–L5) respectively. The dorsal root was stimulated every 2 min by a single square wave pulse (40 V, 200 µs), and a MSR and an sVRP were recorded from the ventral root. In separate experiments, the dorsal root was electrically stimulated (30 V, 50–100 µs), and a DRP was recorded from an adjacent dorsal root. The magnitudes of MSR and DRP were expressed as the peak amplitude (mV), and that of sVRP was expressed as the depolarization integral (mV·s) over the resting potential. The time course of these responses was expressed as a percentage of the mean of the first three responses. The effects of hypoxia for 10 min on these spinal reflex potentials were evaluated by the mean of three responses showing maximal depression during hypoxia, and the data are expressed as a percentage of the mean of three responses just before hypoxia. Although the extent of depression by hypoxia varied from preparation to preparation, it was reproducible in the same preparation. Therefore, in most of the experiments, the preparation was exposed to hypoxia twice to confirm the control response, and the reflex potentials during the second hypoxic episode were used as a control response and compared with the responses during the following hypoxic episodes under the various conditions. Electrical responses were detected with a high gain amplifier (MEZ-8300, Nihon Kohden, Tokyo, Japan). MSR was recorded using a thermal arraycorder (WR7900, Graftec, Yokohama, Japan) with a sampling time of 80 µs. DRP and sVRP were digitized by an analogue/digital converter (PowerLab, ADInstruments, Castle Hill, Australia) with a sampling time of 10 ms. Data were stored in a personal computer and analysed with LabChart 6 software (ver. 6.0, ADInstruments).
Adenosine/inosine biosensor
Hypoxia-evoked adenosine release was measured by using enzymatic biosensors (Sarissa Biomedical, Coventry, UK) according to the method of Dale et al. (2000) with some modifications. The tip of the adenosine/inosine sensor was Pt wire (50 µm diameter, 0.5 mm length) coated with active enzymes (adenosine deaminase, nucleoside phosphorylase and xanthine oxidase). This series of enzymes results in breakdown of adenosine to inosine and then inosine to hydrogen peroxide, which is oxidized on the Pt wire to yield a current. The inosine sensor lacked adenosine deaminase in its coating enzymes, resulting in breakdown of inosine, but not adenosine, to hydrogen peroxide. Therefore, the difference in the signal between the adenosine/inosine and inosine sensors is considered as adenosine signals. The null sensor lacked enzymes and was thus used to measure background signals. Each sensor was polarized to +500 mV by using a potentiostat (Model 3104, Pinnacle Technology, Lawrence, KS, USA) with software (PAL, ver. 1.5.10, Pinnacle Technology). Adenosine/inosine, inosine and null sensors were inserted into an isolated spinal cord preparation from the hemisected surface so that the entire enzyme-coating areas for each sensor were within the preparation, and then were allowed to stabilize for at least 1 h before the experiments were started. In most of the experiments, all three sensors were inserted into the dorsal horn. Adenosine/inosine and inosine sensors were calibrated with known concentrations of adenosine and inosine before and after insertion. The change in the signal of the adenosine/inosine sensor during hypoxia was expressed as Δpurine (µM) after the change in the signal of the null sensor had been subtracted.
Data analysis
Results are expressed as the mean ± SEM. The IC50 value was calculated by fitting the data to a sigmoidal logistic curve with software (Origin, ver. 7.5J, OriginLab, Northampton, MA, USA). Statistical comparisons between two groups were performed by paired or unpaired Student's t-test. For multiple comparisons, anova following Dunnett's or Tukey's test were used. A P-value of less than 0.05 was considered significant.
Materials
Adenosine, 8-cyclopentyl-1,3-dimethylxanthine (CPT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Substance P was from Peptide Institute (Minoh, Japan). Tetrodotoxin (TTX) was from Wako Pure Chemical (Osaka, Japan). All drugs were mixed in ACSF and applied to preparations. Drugs and molecular target nomenclature follows Alexander et al. (2009).
Results
Effects of hypoxia on spinal cord isolated from postnatal day 5–8 rats
To investigate the effect of hypoxia on neuronal activity, spinal cords isolated from postnatal day 5–8 (P5–8) rats were used. The dorsal root was electrically stimulated every 2 min and the preparation was exposed to hypoxic ACSF for 10 min. As shown in Figure 2A, the baseline potential of the ventral root (the resting level was expressed as a broken line) was depolarized during hypoxia. A small hyperpolarization was often observed when normoxic ACSF was re-superfused. In addition, hypoxia reversibly depressed MSR and sVRP (Figure 2B). In separate experiments, hypoxia also depolarized the baseline potential of the dorsal root (data not shown) and reversibly depressed DRP (Figure 2B). Hypoxia (10 min) repeatedly depressed these reflex potentials (Figure 2C). There was no significant difference in the hypoxia-evoked depression of MSR, sVRP and DRP among three repetitive hypoxic episodes (P > 0.05, Tukey's test; Figure 2D).
Figure 2.
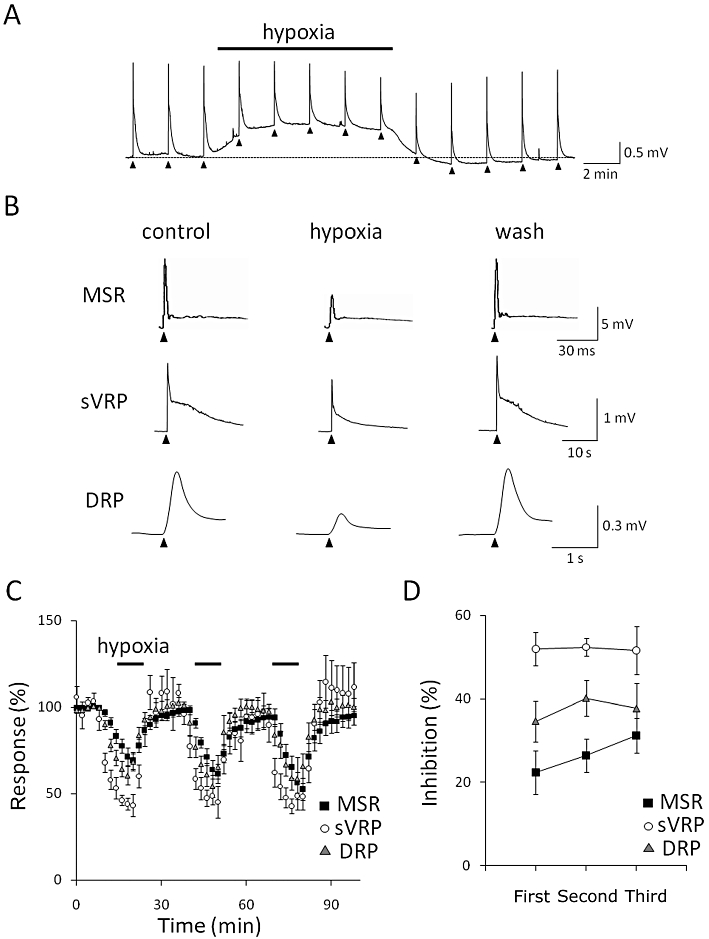
Effect of hypoxia on isolated spinal cord in neonatal rat. (A) The slow ventral root potential (sVRP) was evoked by electrical stimulation every 2 min (arrow heads). The preparation isolated from postnatal day 5–8 (P5–8) rats was exposed to hypoxia for 10 min at 27°C. The broken line shows the resting level of the ventral root potential. (B) Representative traces of monosynaptic reflex potential (MSR), sVRP and dorsal root potential (DRP). The left, middle and right traces show the responses before (control), during (hypoxia) and after hypoxia (wash) respectively. (C) The time course of the peak amplitude (MSR and DRP) and the area under the curve (sVRP) of depolarization. Each symbol and error bar represents the mean ± SEM (n = 4–6). (D) The depression of reflex potentials by repetitive exposure to hypoxia in P5–8 rats at 27°C. Each symbol and error bar represents the mean ± SEM (n = 4–6).
We also examined whether hypoxia depressed the postsynaptic depolarizing response to substance P. Bath-applied substance P (30 and 100 nM) for 1 min depolarized the ventral root (30 nM: 0.20 ± 0.05 mV, n = 5; 100 nM: 0.32 ± 0.08 mV, n = 5). Exposure to hypoxia (10 min) significantly (P < 0.01, paired Student's t-test) suppressed the responses to substance P (30 nM: 0.05 ± 0.04 mV, n = 5; 100 nM: 0.19 ± 0.07 mV, n = 5). TTX (3 nM), which inhibited synaptic transmission by suppressing action potential generation, abolished the electrically evoked reflex potentials (data not shown) and decreased the responses to substance P (30 nM: 0.12 ± 0.06 mV, n = 4; 100 nM: 0.19 ± 0.05 mV, n = 4). In the presence of TTX, hypoxia failed to suppress the responses to substance P (30 nM: 0.09 ± 0.05 mV, n = 4; 100 nM: 0.20 ± 0.06 mV, n = 4).
Contribution of adenosine to hypoxic depression
Hypoxia results in the accumulation of adenosine in the extracellular space of the CNS, including the spinal cord (Latini and Pedata, 2001; Pearson et al., 2003). Therefore, we examined the effect of CPT, an adenosine A1 receptor selective antagonist, on the hypoxia-evoked depression of reflex potentials in P5–8 rats. The treatment with CPT (3 µM) for 20 min slightly increased MSR (123.9 ± 7.1% of control, n = 6), sVRP (126.8 ± 5.0% of control, n = 6) and DRP (104.6 ± 1.3% of control, n = 5; Figure 3A). Then, CPT almost abolished the hypoxic depression of MSR (control: 72.6 ± 6.6% inhibition, n = 6 vs. CPT: 9.6 ± 3.1% inhibition, n = 6, P < 0.01; Figure 3B). On the other hand, a large part of the depression of sVRP (control: 73.1 ± 6.0% inhibition, n = 6 vs. CPT: 59.4 ± 4.5% inhibition, n = 6, P < 0.01) and DRP (control: 42.1 ± 3.7% inhibition, n = 5 vs. CPT: 24.2 ± 2.6% inhibition, n = 5, P < 0.01, paired Student's t-test) was insensitive to CPT. A higher concentration of CPT (10 µM) or another adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine (1 µM), did not show any further inhibitory effect. Atropine (10 µM, an M receptor antagonist), bicuculline (5 µM, a GABAA receptor antagonist), strychnine (1 µM, a glycine receptor antagonist), naloxone (3 µM, an opioid receptor antagonist), NG-nitro-L-arginine methyl ester (100 µM, an NOS inhibitor), AM251 (3 µM, a CB1 receptor antagonist), ketanserin (3 µM, a 5-HT2A receptor antagonist), atipamezole (3 µM, an α2 adrenoceptor antagonist), LY341495 (1 µM, a metabolic glutamate receptor antagonist) and SCH23390 (1 µM, a D1-like receptor antagonist) also did not show any inhibitory effect on the hypoxia-evoked depression of sVRP in the presence or absence of adenosine A1 receptor antagonists (data not shown). The hypoxia-evoked depolarization of ventral root potential was not affected by CPT (control: 0.32 ± 0.06 mV, n = 6; CPT: 0.37 ± 0.21 mV, n = 6).
Figure 3.
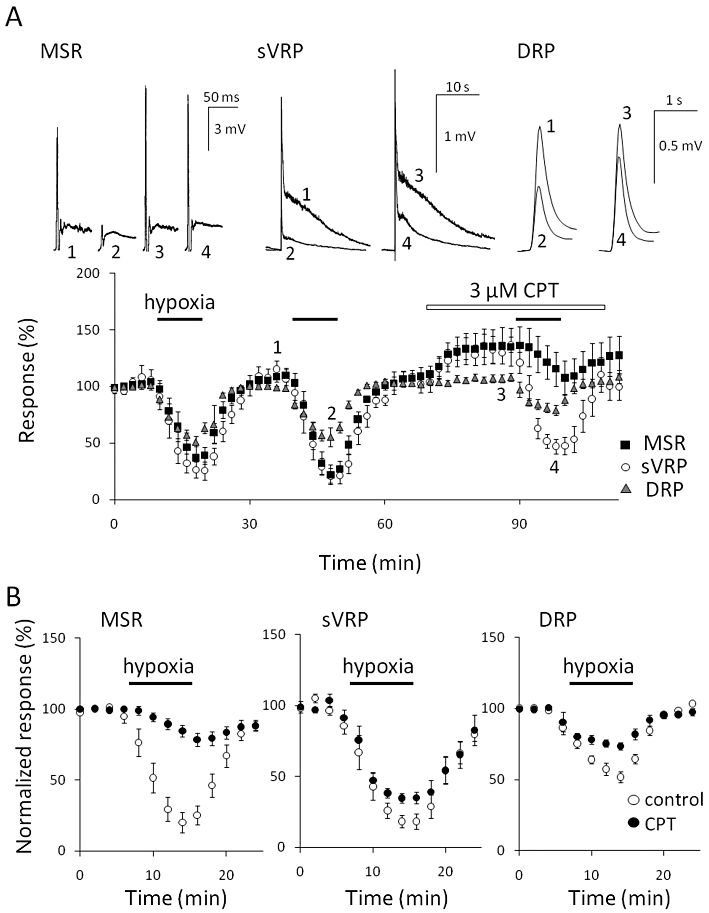
Effect of an adenosine A1 receptor selective antagonist on hypoxic depression. (A) The preparation isolated from postnatal day 5–8 (P5–8) rats was repeatedly exposed to hypoxia for 10 min each at 27°C and pretreated with 3 µM 8-cyclopentyl-1,3-dimethylxanthine (CPT) for 20 min before the third exposure. Then effects of hypoxia on monosynaptic reflex potential (MSR), slow ventral root potential (sVRP) and dorsal root potential (DRP) were observed in the presence of CPT. The number in the representative traces of reflex potentials (upper panel) correspond to those in the lower panel. (B) The effects of CPT on the hypoxia-evoked depression of MSR, sVRP and DRP in P5–8 rats at 27°C. Each symbol and error bar represents the mean ± SEM (n = 5–6).
Influence of extracellular Ca2+ on hypoxic depression
It has been reported that an increase in extracellular Ca2+ inhibits adenosine release during hypoxia (Dale et al., 2000; Takahashi et al., 2010). Therefore, the influence of high [Ca2+]o was examined in P5–8 rats. As shown in Figure 4A, the change in [Ca2+]o from 1.25 mM to 2.5 mM increased the magnitude of MSR (128.9 ± 7.3% of control, n = 6), sVRP (125.3 ± 12.0% of control, n = 6) and DRP (193.4 ± 15.9% of control, n = 5). In addition, the high [Ca2+] increased spontaneous activities of the basal ventral and DRPs as shown in the traces of sVRP of Figure 4A. A high [Ca2+]o almost abolished the hypoxic depression of MSR (1.25 mM Ca2+: 31.2 ± 4.1% inhibition, n = 6 vs. 2.5 mM Ca2+: 5.6 ± 1.0% inhibition, n = 6, P < 0.01; Figure 4B). The depression of DRP was significantly decreased by high [Ca2+]o (1.25 mM Ca2+: 23.9 ± 3.7% inhibition, n = 5 vs. 2.5 mM Ca2+: 15.5 ± 2.5% inhibition, n = 5, P < 0.05), while the depression of sVRP was not affected (1.25 mM Ca2+: 51.6 ± 5.8% inhibition, n = 6 vs. 2.5 mM Ca2+: 60.1 ± 1.4% inhibition, n = 6, P > 0.05, paired Student's t-test).
Figure 4.
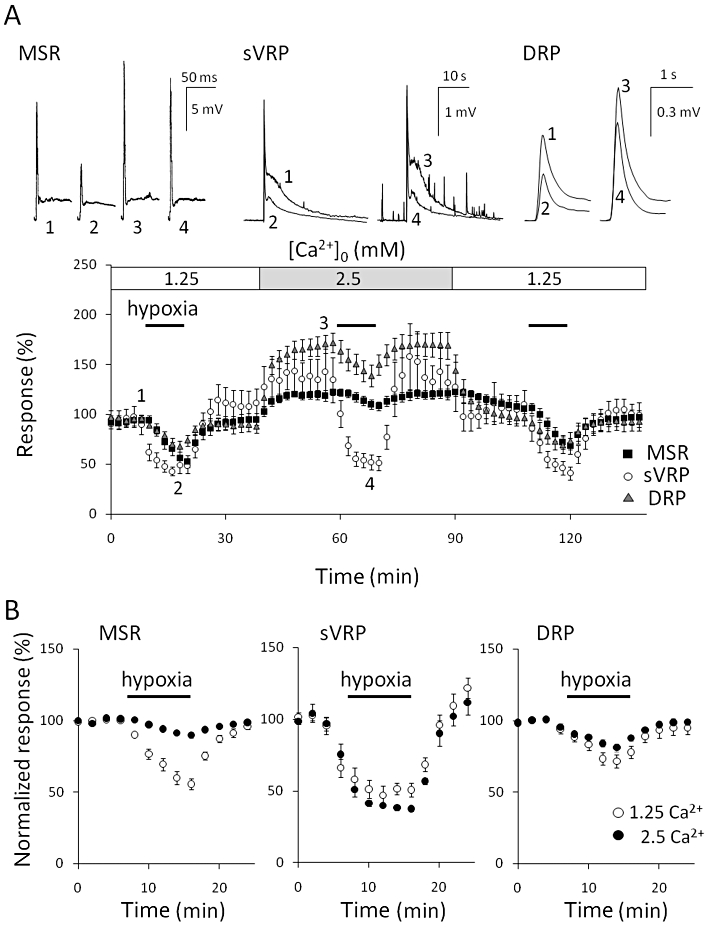
Effect of [Ca2+]o on hypoxic depression. (A) The preparation isolated from postnatal day 5–8 (P5–8) rats was repeatedly exposed to hypoxia for 10 min each at 27°C and pretreated with 2.5 mM [Ca2+]o for 20 min before the second exposure. Then the effects of hypoxia on monosynaptic reflex potential (MSR), slow ventral root potential (sVRP) and dorsal root potential (DRP) were observed in 2.5 mM [Ca2+]o. The number in the representative traces of reflex potentials (upper panel) correspond to those in the lower panel. (B) The hypoxia-evoked depression of MSR, sVRP and DRP in 1.25 and 2.5 mM [Ca2+]o in P5–8 rats at 27°C. Each symbol and error bar represents the mean ± SEM (n = 5–6).
Age-dependent depression during hypoxia
In the rat hippocampus, it has been reported that the influence of hypoxia/ischaemia varies with age (Ben-Ari, 1992; Yager and Thornhill, 1997). We therefore examined the effect of hypoxia on reflex potentials in spinal cord isolated from younger neonatal rats. In the spinal cords from postnatal day 0–3 (P0–3) rats, little hypoxia-evoked depression of MSR was observed (2.9 ± 1.6% inhibition, n = 8), while the depression of DRP was small but detectable (11.5 ± 3.3% inhibition, n = 11; Figure 5). In contrast to MSR, sVRP was markedly depressed by hypoxia in the spinal cords from P0–3 rats, although the inhibitory effect of hypoxia in P0–3 (30.5 ± 8.7% inhibition, n = 9) was less than that in P5–8 rats (57.9 ± 10.8% inhibition, n = 9, P < 0.01 vs. P0–3, unpaired Student's t-test).
Figure 5.
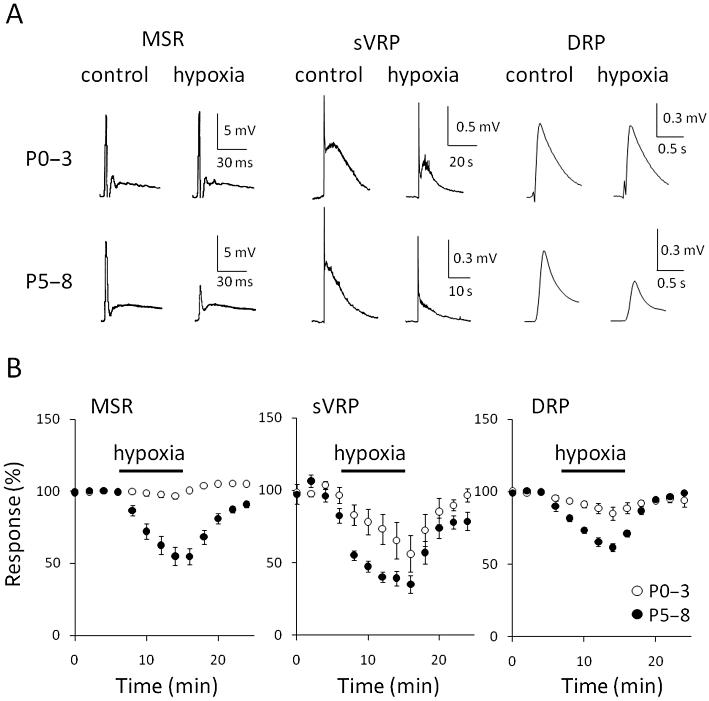
Effect of age on hypoxic-induced depression. (A) Representative traces of monosynaptic reflex potential (MSR), slow ventral root potential (sVRP) and dorsal root potential (DRP) in postnatal day 0–3 (P0–3, upper panel) and 5–8 rats (P5–8, lower panel), both before (control) and during hypoxia (hypoxia) at 27°C. (B) The hypoxia-evoked depression of MSR, sVRP and DRP in P0–3 and P5–8 rats at 27°C. Each symbol and error bar represents the mean ± SEM (n = 8–11).
The effects of adenosine on three spinal reflex potentials were examined in P0–3 and P5–8 rats. Adenosine (0.01 µM–1 mM) was cumulatively applied to the spinal cords for 10 min at each concentration. All three reflex potentials in both P0–3 and P5–8 rats were depressed by adenosine in a concentration-dependent manner (Figure 6). The IC50 value for sVRP was the smallest among the three reflex potentials in both P0–3 and P5–8 rats, and that for MSR in P0–3 rats were significantly smaller than that in P5–8 rats (P0–3: 47.5 ± 4.6 µM, n = 6 vs. P5–8: 186.4 ± 22.6 µM, n = 6, P < 0.01, unpaired Student's t-test) but not for sVRP (P0–3: 5.7 ± 2.4 µM, n = 6 vs. P5–8: 36.8 ± 17.2 µM, n = 6) and DRP (P0–3: 149.0 ± 14.9 µM, n = 5 vs. P5–8: 236.9 ± 50.4 µM, n = 5). These results indicate that the lack of hypoxic depression of MSR in P0–3 is not due to the failure of downstream mechanisms of adenosine A1 receptor activation.
Figure 6.
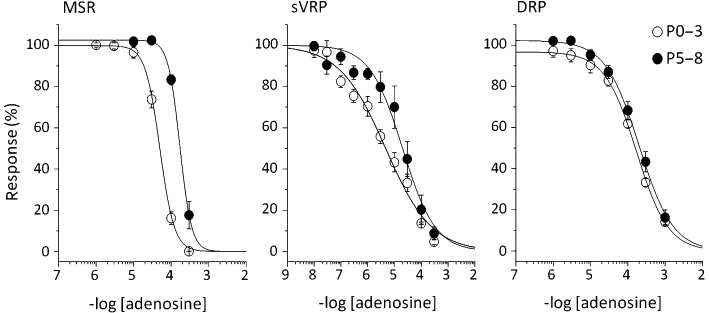
Concentration-dependent depression by adenosine in postnatal day 0–3 (P0–3) and 5–8 (P5–8) rats. Adenosine was cumulatively applied to spinal cords isolated from P0–3 and 5–8 (P5–8) rats at 27°C. Concentration-response curves for monosynaptic reflex potential (MSR), slow ventral root potential (sVRP) and dorsal root potential (DRP) in the presence of adenosine. Each symbol and error bar represents the mean ± SEM (n = 5–6).
Measurement of purines by biosensors
We investigated the effects of hypoxia on the extracellular concentration of purines using the biosensors. In P5–8 rats at 27°C, the exposure of spinal cords to hypoxia for 20 min gradually increased signal currents from both adenosine/inosine and inosine but not null sensors in the dorsal horn of the spinal cord (Figure 7A). The time course of the sensor response was slower than that for the inhibition of reflex potentials by hypoxia. The difference in the signal between the adenosine/inosine and inosine sensors, which was considered to represent the adenosine signal, was quite small, if any, indicating that most of the signal from the adenosine/inosine sensor originated from inosine. This result is consistent with our previous findings, obtained by use of HPLC, that most of the adenosine is converted to inosine by endogenous adenosine deaminase and that the amount of inosine released during hypoxia is about 10-fold greater than that of adenosine in neonatal rat spinal cord (Takahashi et al., 2010). Therefore, the changes in the signal from adenosine/inosine sensors during hypoxia at 10 and 20 min were expressed as Δpurine, and these sensors were calibrated with known concentrations of inosine (Figure 7B). In P0–3 rats at 27°C, hypoxia failed to increase the signal currents from all sensors. These results indicate that hypoxia increases the extracellular purine concentration in P5–8, but not in P0–3 rats at 27°C. We also inserted the sensors into both the dorsal and ventral horn of a preparation. There was no difference in the signals between them (data not shown).
Figure 7.
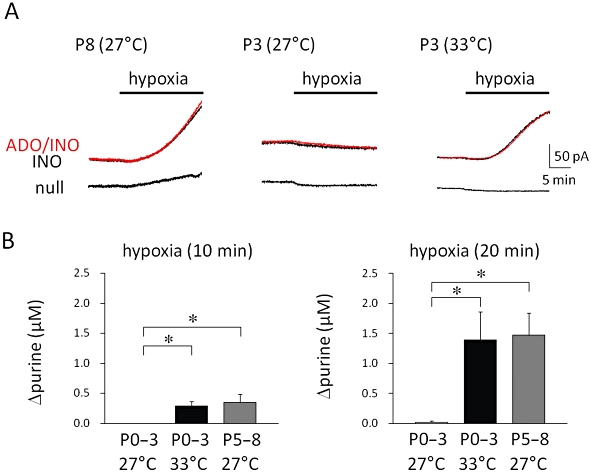
Adenosine release from spinal cord during hypoxia. Adenosine/inosine (ADO/INO), inosine (INO) and null sensors were inserted into the dorsal horn of the spinal cord and then hypoxia was applied for 20 min. (A) Representative traces of signal current for each sensor observed in a postnatal day 8 (P8) rat at 27°C (left panel), postnatal day 3 (P3) rats at 27°C (middle panel) and 33°C (right panel) are shown. (B) The hypoxia-evoked change in adenosine/inosine concentration (Δpurine) at 10 min (left panel) and 20 min (right panel) after hypoxia in postnatal day 0–3 (P0–3) rats at 27 and 33°C and in postnatal day 5–8 (P5–8) rats at 27°C. Each symbol and error bar represents the mean ± SEM (n = 5–6). *P < 0.05 versus P0–3 rats at 27°C (Dunnett's test).
Temperature-dependent depression during hypoxia
Next we examined the influence of temperature on the hypoxia-evoked depression in P0–3 rats. The increase in temperature from 27 to 33°C decreased the magnitude of MSR, sVRP and DRP by 8.9 ± 8.9% (n = 5), 45.0 ± 8.5 % (n = 5) and 11.7 ± 6.3% (n = 6) respectively (Figure 8A). As shown in Figure 8, the hypoxic depression of MSR, which was only just detectable at 27°C (5.3 ± 1.2% inhibition, n = 5), appeared at 33°C (67.9 ± 11.8% inhibition, n = 5, P < 0.01 vs. 27°C). The increase in temperature significantly enhanced the hypoxic depression of sVRP (27°C: 44.6 ± 7.0% inhibition, n = 5 vs. 33°C: 63.9 ± 7.8% inhibition, n = 6, P < 0.05) and DRP (27°C: 7.6 ± 1.8% inhibition, n = 6 vs. 33°C: 18.8 ± 2.9% inhibition, n = 6, P < 0.01). At 33°C, the treatment with CPT (3 µM) for 20 min slightly increased MSR (115.7 ± 4.0% of control, n = 5), sVRP (122.7 ± 14.6% of control, n = 5) and DRP (111.3 ± 3.1% of control, n = 6). Then, CPT almost abolished the hypoxic depression of MSR (4.8 ± 2.0% inhibition, n = 5, P < 0.01 vs. 33°C) and significantly reversed the hypoxic depression of DRP (6.8 ± 0.9% inhibition, n = 6, P < 0.01 vs. 33°C) but not that of sVRP (55.5 ± 4.3% inhibition, n = 6, P > 0.05 vs. 33°C, Dunnett's test). Similar to the depression of MSR, the hypoxia-evoked increase in purine concentration, which was only just detectable at 27°C, emerged at 33°C (Figure 7). We could not measure the purine concentration in P5–8 rats at 33°C because the signal current from the sensors did not become steady even more than 2 h after the insertion.
Figure 8.
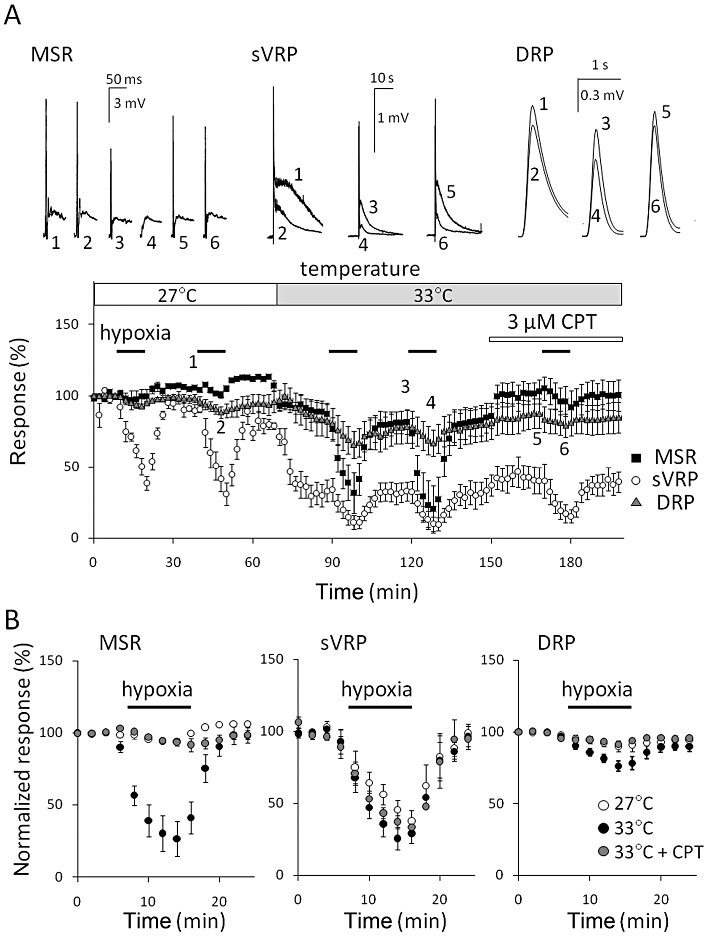
Effect of temperature on hypoxic-induced depression. (A) The preparation isolated from postnatal day 0–3 (P0–3) rats was repeatedly exposed to hypoxia for 10 min each. Temperature was elevated from 27 to 33°C 20 min before the third exposure. Then, the preparation was pretreated with 3 µM 8-cyclopentyl-1,3-dimethylxanthine (CPT) 20 min before the fifth exposure at 33°C. The number in the representative traces of monosynaptic reflex potential (MSR), slow ventral root potential (sVRP) and dorsal root potential (DRP) (upper panel) correspond to those in the lower panel. (B) The hypoxia-evoked depression of MSR, sVRP and DRP in P0–3 rats at 27 and 33°C in the presence and absence of 3 µM CPT. Each symbol and error bar represents the mean ± SEM (n = 5–6).
Discussion and conclusions
Hypoxic depression of MSR via adenosine A1 receptors
Hypoxia increases extracellular adenosine level in rat hippocampal slices (Dale et al., 2000) and isolated spinal cords (Takahashi et al., 2010). In the rat hippocampus, the depression of synaptic transmission during brief periods of hypoxia is greatly reduced by adenosine A1 receptor antagonists (Fowler, 1989; Katchman and Hershkowitz, 1993; Martín et al., 2007). In addition, the hypoxic depression of MSR is abolished by the blockade of adenosine A1 receptors in the isolated spinal cord of postnatal day 6–10 rats (Lloyd et al., 1988). In the present study, CPT also reversed the hypoxic depression of MSR by more than 80% and hypoxia increased the signal currents from the adenosine/inosine sensors in P5–8 rats, suggesting that hypoxia released adenosine and thus depressed MSR via adenosine A1 receptors. However, the time course of the signal currents was much slower than that of MSR inhibition by hypoxia. It is likely that a small negative shift of signal currents observed with hypoxic ACSF (Dale et al., 2000) make it difficult to correctly detect the onset of increase in purine. Adenosine is believed to depress synaptic transmission by presynaptically inhibiting Ca2+ channels or postsynaptically activating K+ channels via adenosine A1 receptors (de Mendonça et al., 2000; Wardas, 2002). MSR is mediated via a monosynaptic pathway from primary afferent endings to motoneurones. Hypoxia does not seem to impair the postsynaptic activity of motoneurons as it failed to depress the response to substance P in the presence of TTX, reflecting the direct effect on motoneurons. Thus, it is possible that MSR is depressed by a presynaptic inhibitory effect of adenosine on transmitter release from primary afferent endings.
The [Ca2+]o in CSF is around 1.25 mM (Hunter and Smith, 1960) and would further decrease during hypoxia (Martin et al., 1994). At 2.5 mM [Ca2+]o, the hypoxic depression of MSR was completely masked. It has been reported that elevating the electrochemical gradient driving the Ca2+ influx counteracts the depression of MSR (Czéh and Somjen, 1990). In our study, the high [Ca2+]o increased the spontaneous neuronal activities and augmented the magnitude of reflex potentials under normoxic conditions. Therefore, it is likely that high [Ca2+]o enhances Ca2+ influx and thus synaptic transmission. However, an increase in the driving force of Ca2+ does not seem to be a major factor in its inhibitory effect on hypoxic depression, because the hypoxic depression of sVRP was not attenuated by the increase in [Ca2+]o. High [Ca2+]o inhibits the increase in extracellular adenosine during hypoxia in rat hippocampal slices (Dale et al., 2000) and cortical astrocytes (Martín et al., 2007).This is the case in the spinal cord (Takahashi et al., 2010). In contrast, the removal of external Ca2+ enhances adenosine release during hypoxia or ischaemia-like conditions (Pedata et al., 1993; Frenguelli et al., 2007; Takahashi et al., 2010). From these results, it is hypothesized that Ca2+ limits the adenosine release during hypoxia and thus the depression of MSR. This idea is supported by the finidng that the hypoxic depression of sVRP, largely independent of adenosine release, is mostly unaffected by the increase in [Ca2+]o. The inhibitory effects of Ca2+ on adenosine release remain unclear. The manipulation of external Ca2+ concentration also changes the amount of inosine release in proportion to that of adenosine release (Pedata et al., 1993; Takahashi et al., 2010), suggesting that the activity of adenosine deaminase is unchanged. Therefore, it is speculated that Ca2+ inhibits adenosine production by acting on processes upstream of adenosine deaminase. Alternatively Ca2+ may inhibit the purine transport mechanisms.
The susceptibility of MSR to hypoxia drastically changed during the neonatal period. In postnatal day 6–10 rats, it has been reported that hypoxia rapidly depresses MSR (Lloyd et al., 1988), which is consistent with our results in P5–8 rats. However, in P0–3 rats, hypoxia failed to depress MSR although bath-applied adenosine more potently depressed MSR in P0–3 than in P5–8 rats. This resistance of MSR to hypoxia in younger rats is suggested to be due to a lack of adenosine release, as hypoxic adenosine release was negligible in these rats. The influence of hypoxia/ischaemia varies with age (Ben-Ari, 1992; Yager and Thornhill, 1997). During fetal and postnatal development, there are marked conversions of some factors, such as the types of haemoglobins and expression pattern of adenosine kinase (Iwahara et al., 1996; Studer et al., 2006). In P0–3 rats, the CPT-sensitive hypoxic depression of MSR at a higher temperature was associated with hypoxic adenosine release. It is therefore suggested that the increase in temperature results in an increase in the release and/or intracellular production of adenosine because acute hypoxia releases adenosine per se (Takahashi et al., 2010).
Reflex potentials were depressed by an increase in temperature alone. This effect does not seem to be due to a change in the extracellular adenosine concentration because there was little difference in the effect of CPT on reflex potentials during normoxia between 27 and 33°C. In rat hippocampus, it is reported that increasing the temperatures from 32.5 to 38.5°C causes adenosine release, which depresses synaptic transmission (Masino and Dunwiddie, 1999; Masino et al., 2001), while the basal concentration of extracellular adenosine at 21°C is twice that at 32°C (Dunwiddie and Diao, 2000), suggesting a U-shaped temperature–response curve for extracellular adenosine that is lowest at around 32°C. If this is the case in the spinal cord, it is also likely that the change of temperature from 27 to 33°C does not increase or rather decrease the extracellular adenosine concentration. In experiments using the isolated spinal cord of rats, it is known that the optimum temperature for maximal amplitude and stable recording of MSR is around 25°C, and that both lower and higher temperatures reduce MSR (Harada and Takahashi, 1983), although the mechanism for this remains unclear (Deshpande and Warnick, 1988). Temperature influences various aspects of neuronal function (Thompson et al., 1985; Borst and Sakmann, 1998; Kullmann and Asztely, 1998; Volgushev et al., 2000), making its effect on synaptic transmission a complex matter.
Adenosine A1 receptor-independent depression during hypoxia
In contrast to its effect on MSR, CPT did not have a marked inhibitory effect on the hypoxic depression of sVRP and DRP. This was unexpected because sVRP was the most sensitive to exogenous adenosine in the present study. However, the possibility that CPT failed to inhibit the effect of endogenous adenosine cannot be completely excluded, but CPT does abolish the depression of sVRP when endogenous adenosine is released by an adenosine kinase inhibitor (Otsuguro et al., 2006b). In this study, CPT and high [Ca2+]o increased MSR and sVRP, indicating that not only MSR but also sVRP was under the control of tonic inhibition by adenosine. As the high [Ca2+]o-evoked increase in the reflex potentials appears to be larger than that induced by CPT, it is speculated to be due to an increase in the driving force of Ca2+, in addition to the inhibition of adenosine release, as described above. Therefore, the lack of sensitivity of the hypoxic-induced sVRP depression to CTP suggests that a significant accumulation of adenosine does not occur near to the site generating sVRP. There was no difference in the hypoxia-induced purine increase between the dorsal and ventral horn in this study. More detailed analysis is needed to determine the release site of adenosine.
The MSR is evoked by direct transmissions from primary afferent fibres to motoneurons in the ventral horn, whereas DRP and sVRP are evoked by transmissions from primary afferent fibres to interneurons in the dorsal horn. It is likely that hypoxia decreases the activities of interneurons in the dorsal horn in an adenosine A1 receptor-independent manner. Alternatively, hypoxia may selectively suppress the transmitter release from the endings of primary afferents, which project into interneurons at the dorsal horn but not into motoneurones, because the primary afferent neurons projecting into the dorsal horn have different transmitters and receptors from those into the ventral horn (Garry et al., 1989; Gold et al., 1996; Petruska et al., 2000).
Several studies have been done on the contribution of inhibitory transmitters besides adenosine to hypoxic/ischaemic depression in the CNS (de Mendonça and Ribeiro, 1997; Coelho et al., 2000; Deshpande and Jha, 2004; Youssef et al., 2007). However, the antagonists of various inhibitory transmitters used in the present study had no effect on the hypoxia-induced depression. As hypoxia reportedly modulates the activity of several channels (Nieber, 1999; Peña and Ramirez, 2005), the activities of such channels expressed in primary afferent endings or interneurones of the dorsal horn may be directly affected by hypoxia. It is speculated that the adenosine A1 receptor-independent mechanism is less susceptible to environmental conditions, at least compared with adenosine release, and that this gives rise to the different responses to hypoxia among neuronal pathways in the spinal cord.
In conclusion, the contribution of adenosine to hypoxia-induced depression differs among neuronal pathways in the spinal cord. This feature is important for the differences in hypoxic effects, because hypoxia-induced adenosine release is highly susceptible to various conditions, such as age, temperature and [Ca2+]o.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
Glossary
Abbreviations
- ACSF
artificial cerebrospinal fluid
- CPT
8-cyclopentyl-1,3-dimethylxanthine
- DRP
dorsal root potential
- MSR
monosynaptic reflex potential
- sVRP
slow ventral root potential
Conflict of interest
None.
References
- Akagi H, Yanagisawa M. GABAergic modulation of a substance P-mediated reflex of slow time course in the isolated rat spinal cord. Br J Pharmacol. 1987;91:189–197. doi: 10.1111/j.1476-5381.1987.tb08998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 4th edn. Br J Phamacol. 2009;158:S1–S254. [Google Scholar]
- Ben-Ari Y. Effects of anoxia and aglycemia on the adult and immature hippocampus. Biol Neonate. 1992;62:225–230. doi: 10.1159/000243875. [DOI] [PubMed] [Google Scholar]
- Borst JGG, Sakmann B. Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem. J Physiol. 1998;506:143–157. doi: 10.1111/j.1469-7793.1998.143bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmeryer DM, Kendig JJ. Selective effects of ketamine on amino acid-mediated pathways in neonatal rat spinal cord. Br J Anaesth. 1995;74:79–84. doi: 10.1093/bja/74.1.79. [DOI] [PubMed] [Google Scholar]
- Coelho JE, de Mendonça A, Ribeiro JA. Presynaptic inhibitory receptors mediate the depression of synaptic transmission upon hypoxia in rat hippocampal slices. Brain Res. 2000;869:158–165. doi: 10.1016/s0006-8993(00)02381-7. [DOI] [PubMed] [Google Scholar]
- Czéh G, Somjen GG. Hypoxic failure of synaptic transmission in the isolated spinal cord, and the effects of divalent cations. Brain Res. 1990;527:224–233. doi: 10.1016/0006-8993(90)91141-3. [DOI] [PubMed] [Google Scholar]
- Dale N, Pearson T, Frenguelli BG. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J Physiol. 2000;526:143–155. doi: 10.1111/j.1469-7793.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande SB, Jha A. Aglycemia and ischemia depress monosynaptic and polysynaptic reflexes in neonatal rat spinal cord in vitro by involving different types of 5-hydroxytryptamine receptors. Neurosci Lett. 2004;372:167–172. doi: 10.1016/j.neulet.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Deshpande SB, Warnick JE. Temperature-dependence of reflex transmission in the neonatal rat spinal cord, in vitro: influence on strychnine- and bicuculline-sensitive inhibition. Neuropharmacology. 1988;27:1033–1037. doi: 10.1016/0028-3908(88)90064-0. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L. Regulation of extracellular adenosine in rat hippocampal slices is temperature dependent: role of adenosine transporters. Neuroscience. 2000;95:81–88. doi: 10.1016/s0306-4522(99)00404-2. [DOI] [PubMed] [Google Scholar]
- Faber ES, Chambers JP, Brugger F, Evans RH. Depression of A and C fibre-evoked segmental reflexes by morphine and clonidine in the in vitro spinal cord of the neonatal rat. Br J Pharmacol. 1997;120:1390–1396. doi: 10.1038/sj.bjp.0701064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JC. Adenosine antagonists delay hypoxia-induced depression of neuronal activity in hippocampal brain slice. Brain Res. 1989;490:378–384. doi: 10.1016/0006-8993(89)90258-8. [DOI] [PubMed] [Google Scholar]
- Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem. 2007;10:1400–1413. doi: 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry MG, Miller KE, Seybold VS. Lumbar dorsal root ganglia of the cat: a quantitative study of peptide immunoreactivity and cell size. J Comp Neurol. 1989;284:36–47. doi: 10.1002/cne.902840104. [DOI] [PubMed] [Google Scholar]
- Gold MS, Dastmalchi S, Levine JD. Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience. 1996;71:265–275. doi: 10.1016/0306-4522(95)00433-5. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Bauman LA. Endogenous adenosine contributes to hypoxic synaptic depression in hippocampus from young and aged rats. J Neurophysiol. 1992;68:620–628. doi: 10.1152/jn.1992.68.2.620. [DOI] [PubMed] [Google Scholar]
- Harada Y, Takahashi T. The calcium component of the action potential in spinal motoneurones of the rat. J Physiol. 1983;335:89–100. doi: 10.1113/jphysiol.1983.sp014521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter G, Smith HV. Calcium and magnesium in human cerebrospinal fluid. Nature. 1960;186:161–162. doi: 10.1038/186161a0. [DOI] [PubMed] [Google Scholar]
- Iwahara S, Abe Y, Okazaki T. Identification of five embryonic hemoglobins of rat and ontogeny of their constituent globins during fetal development. J Biochem. 1996;119:360–366. doi: 10.1093/oxfordjournals.jbchem.a021248. [DOI] [PubMed] [Google Scholar]
- Katchman AN, Hershkowitz N. Adenosine antagonists prevent hypoxia-induced depression of excitatory but not inhibitory synaptic currents. Neurosci Lett. 1993;159:123–126. doi: 10.1016/0304-3940(93)90814-2. [DOI] [PubMed] [Google Scholar]
- Kubota K, Fujibayashi K, Saito K. Enhancing effect of dimethyl sulfoxide on nociceptive transmission in isolated spinal cord of newborn rat. Eur J Pharmacol. 1998;351:173–179. doi: 10.1016/s0014-2999(98)00313-6. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F. Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. Trends Neurosci. 1998;21:8–14. doi: 10.1016/s0166-2236(97)01150-8. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Yoshioka K. The excitatory and inhibitory modulation of primary afferent fibre-evoked responses of ventral roots in the neonatal rat spinal cord exerted by nitric oxide. Br J Pharmacol. 1996;118:1743–1753. doi: 10.1111/j.1476-5381.1996.tb15600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Latini S, Bordoni F, Corradetti R, Pepeu G, Pedata F. Temporal correlation between adenosine outflow and synaptic potential inhibition in rat hippocampal slices during ischemia-like conditions. Brain Res. 1998;794:325–328. doi: 10.1016/s0006-8993(98)00304-7. [DOI] [PubMed] [Google Scholar]
- Latini S, Bordoni F, Pedata F, Corradetti R. Extracellular adenosine concentrations during in vitro ischaemia in rat hippocampal slices. Br J Pharmacol. 1999;127:729–739. doi: 10.1038/sj.bjp.0702591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd HGE, Spence I, Johnston GAR. Involvement of adenosine in synaptic depression induced by a brief period of hypoxia in isolated spinal cord of neonatal rat. Brain Res. 1988;462:391–395. doi: 10.1016/0006-8993(88)90571-9. [DOI] [PubMed] [Google Scholar]
- Martín ED, Fernández M, Perea G, Pascual O, Haydon PG, Araque A, et al. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- Martin RL, Lloyd HGE, Cowan AI. The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci. 1994;17:251–257. doi: 10.1016/0166-2236(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Masino SA, Dunwiddie TV. Temperature-dependent modulation of excitatory transmission in hippocampal slices is mediated by extracellular adenosine. J Neurosci. 1999;19:1932–1939. doi: 10.1523/JNEUROSCI.19-06-01932.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Latini S, Bordoni F, Pedata F, Dunwiddie TV. Changes in hippocampal adenosine efflux, ATP levels, and synaptic transmission induced by increased temperature. Synapse. 2001;41:58–64. doi: 10.1002/syn.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mendonça A, Ribeiro JA. Contribution of metabotropic glutamate receptors to the depression of excitatory postsynaptic potentials during hypoxia. Neuroreport. 1997;8:3667–3671. doi: 10.1097/00001756-199712010-00003. [DOI] [PubMed] [Google Scholar]
- de Mendonça A, Sebastião AM, Ribeiro JA. Adenosine: does it have a neuroprotective role after all? Brain Res Rev. 2000;33:258–274. doi: 10.1016/s0165-0173(00)00033-3. [DOI] [PubMed] [Google Scholar]
- Nieber K. Hypoxia and neuronal function under in vitro conditions. Pharmacol Ther. 1999;82:71–86. doi: 10.1016/s0163-7258(98)00061-8. [DOI] [PubMed] [Google Scholar]
- Nussbaumer JC, Yanagisawa M, Otsuka M. Pharmacological properties of a C-fibre response evoked by saphenous nerve stimulation in an isolated spinal cord-nerve preparation of the newborn rat. Br J Pharmacol. 1989;98:373–382. doi: 10.1111/j.1476-5381.1989.tb12607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuguro K, Yasutake S, Ohta T, Ito S. Effects of opioid receptor and α2-adrenoceptor agonists on slow ventral root potentials and on capsaicin and formalin tests in neonatal rats. Dev Brain Res. 2005;158:50–58. doi: 10.1016/j.devbrainres.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Otsuguro K, Ohta T, Ito S. Zinc modulates primary afferent fiber-evoked responses of ventral roots in neonatal rat spinal cord in vitro. Neuroscience. 2006a;138:281–291. doi: 10.1016/j.neuroscience.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Otsuguro K, Yamaji Y, Ban M, Ohta T, Ito S. Involvement of adenosine in depression of synaptic transmission during hypercapnia in isolated spinal cord of neonatal rats. J Physiol. 2006b;574:835–847. doi: 10.1113/jphysiol.2006.109660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuguro K, Ban M, Ohta T, Ito S. Roles of purines in synaptic modulation evoked by hypercapnia in isolated spinal cord of neonatal rat in vitro. Br J Pharmacol. 2009;156:1167–1177. doi: 10.1111/j.1476-5381.2009.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YK, Jung SJ, Kwak J, Kim J. Effect of hypoxia on excitatory transmission in the rat substantia gelatinosa neurons. Biochem Biophys Res Commun. 2002;295:929–936. doi: 10.1016/s0006-291x(02)00790-8. [DOI] [PubMed] [Google Scholar]
- Pearson T, Nuritova F, Caldwell D, Dale N, Frenguelli BG. A depletable pool of adenosine in area CA1 of the rat hippocampus. J Neurosci. 2001;21:2298–2307. doi: 10.1523/JNEUROSCI.21-07-02298.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T, Currie AJ, Etherington LAV, Gadalla AE, Damian K, Llaudet E, et al. Plasticity of purine release during cerebral ischemia: clinical implications? J Cell Mol Med. 2003;7:362–375. doi: 10.1111/j.1582-4934.2003.tb00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedata F, Latini S, Pugliese AM, Pepeu G. Investigations into the adenosine outflow from hippocampal slices evoked by ischemia-like conditions. J Neurochem. 1993;61:284–289. doi: 10.1111/j.1471-4159.1993.tb03566.x. [DOI] [PubMed] [Google Scholar]
- Peña F, Ramirez JM. Hypoxia-induced changes in neuronal network properties. Mol Neurobiol. 2005;32:251–283. doi: 10.1385/MN:32:3:251. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol. 2000;84:2365–2379. doi: 10.1152/jn.2000.84.5.2365. [DOI] [PubMed] [Google Scholar]
- Seno N, Saito K. The development of the dorsal root potential and the responsiveness of primary afferent fibers to γ-aminobutyric acid in the spinal cord of rat fetuses. Dev Brain Res. 1985;349:11–16. doi: 10.1016/0165-3806(85)90127-0. [DOI] [PubMed] [Google Scholar]
- Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, et al. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142:125–137. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Otsuguro K, Ohta T, Ito S. Adenosine and inosine release during hypoxia in the isolated spinal cord of neonatal rats. Br J Pharmacol. 2010;161:1806–1816. doi: 10.1111/j.1476-5381.2010.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Masukawa LM, Prince DA. Temperature dependence of intrinsic membrane properties and synaptic potentials in hippocampal CA1 neurons in vitro. J Neurosci. 1985;5:817–824. doi: 10.1523/JNEUROSCI.05-03-00817.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev M, Vidyasagar TR, Chistiakova M, Yousef T, Eysel UT. Membrane properties and spike generation in rat visual cortical cells during reversible cooling. J Physiol. 2000;522:59–76. doi: 10.1111/j.1469-7793.2000.0059m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardas J. Neuroprotective role of adenosine in the CNS. Pol J Pharmacol. 2002;54:313–326. [PubMed] [Google Scholar]
- Woodley SJ, Kendig JJ. Substance P and NMDA receptors mediate a slow nociceptive ventral root potential in neonatal rat spinal cord. Brain Res. 1991;559:17–21. doi: 10.1016/0006-8993(91)90281-y. [DOI] [PubMed] [Google Scholar]
- Yager JY, Thornhill JA. The effect of age on susceptibility to hypoxic-ischemic brain damage. Neurosci Biobehav Rev. 1997;21:167–174. doi: 10.1016/s0149-7634(96)00006-1. [DOI] [PubMed] [Google Scholar]
- Youssef FF, Hormuzdi SG, Irving AJ, Frenguelli BG. Cannabinoid modulation of neuronal function after oxygen/glucose deprivation in area CA1 of the rat hippocampus. Neuropharmacology. 2007;52:1327–1335. doi: 10.1016/j.neuropharm.2006.12.003. [DOI] [PubMed] [Google Scholar]


