Abstract
BACKGROUND AND PURPOSE
This study was designed to clarify mechanisms responsible for the anti-allodynic effects of duloxetine in diabetes.
EXPERIMENTAL APPROACH
The streptozotocin-induced diabetic rat model was used to compare the efficacy of duloxetine, 5-HT, the 5-HT2A receptor agonist [1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI)] and two antagonists (ketanserin and pruvanserin) on tactile allodynia.
KEY RESULTS
Systemic or intrathecal injection of duloxetine alleviated tactile allodynia in diabetic rats. The effect of systemic duloxetine was reduced by intrathecal administration of ketanserin or pruvanserin, indicating participation of spinal 5-HT2A receptors in the mechanism of action of duloxetine. In contrast to spinal delivery, systemic and local peripheral injections of ketanserin or pruvanserin alleviated tactile allodynia in diabetic rats. This effect was reversed immediately after systemic or local DOI injection.
CONCLUSIONS AND IMPLICATIONS
These results support the involvement of spinal 5-HT2A receptors in the ability of duloxetine to ameliorate painful diabetic neuropathy. Our data also suggest that the role of 5-HT2A receptors depends on the level of the neuraxis at which activation takes place, with peripheral activation contributing to tactile allodynia in diabetic rats, whereas spinal activation of this receptor alleviates tactile allodynia. The development of selective peripheral 5-HT2A receptor antagonists may offer a novel approach for the treatment of diabetic neuropathic pain.
Keywords: diabetes, duloxetine, 5-HT2A receptor, spinal cord, tactile allodynia
Introduction
Diabetes mellitus is an increasingly common chronic medical condition, affecting over 100 million people worldwide. Up to 60% of diabetic patients develop some form of peripheral neuropathy and around 20% will develop neuropathic pain, including spontaneous pain, paresthesia, dysethesia, hyperalgesia and allodynia (Boulton et al., 1983; Partanen et al., 1995). The current first-line treatments for painful diabetic neuropathy include anticonvulsants, tricyclic antidepressants, opioids and serotonin (5-HT) and noradrenaline reuptake inhibitors (Ziegler, 2009). Duloxetine (Cymbalta) is a 5-HT-noradrenaline reuptake inhibitor used to treat depression that also alleviates allodynia in several inflammatory and neuropathic pain models (Iyengar et al., 2004; Jones et al., 2005; Joshi et al., 2006; Kuhad et al., 2009; Munro, 2009; Piesla et al., 2009; Vranken et al., 2011). Clinical studies have confirmed the efficacy of duloxetine against pain in diabetic patients (Goldstein et al., 2005; Raskin et al., 2005; Wernicke et al., 2006), and the drug has been approved by the US Food and Drug Administration for management of painful diabetic neuropathy. The sites and mechanisms of action responsible for the pain-relieving effects of duloxetine in diabetes remain unclear.
Duloxetine inhibits transporters of 5-HT and noradrenaline, thereby increasing local levels of these neurotransmitters (Engleman et al., 1995; Koch et al., 2003) and promoting persistence of their actions. 5-HT exerts its action through binding to a range of receptors (Hoyer et al., 1994), including the 5-HT2A receptor (nomenclature follows Alexander et al., 2009) which has been implicated in modulation of neuropathic or inflammatory pain (Obata et al., 2000; 2001; Sasaki et al., 2001; Okamoto et al., 2002; Nitanda et al., 2005; Honda et al., 2006). Studies in animal models suggest that the nature of the involvement of the 5-HT2A receptor may be location specific. Indeed, neuropathic pain is reported to be alleviated by both activation of spinal 5-HT2A receptors and systemic administration of the 5-HT2A receptor antagonist ketanserin (Obata et al., 2001; Nitanda et al., 2005; Honda et al., 2006).
Streptozotocin-diabetic rats display evidence of sensory dysfunction, such as tactile allodynia and hyperalgesia to mechanical and thermal stimuli (Calcutt, 2004), supporting their use to investigate aetiological mechanisms of painful diabetic neuropathy and to screen for potential therapeutic agents (Calcutt and Backonja, 2007). A recent study has reported that inhibitors of 5-HT and noradrenaline reuptake can modulate diabetes-induced allodynia via a spinal mechanism of action (Ikeda et al., 2009). In the present study, we have used the streptozotocin-diabetic rat model to investigate the specific site of action of the anti-neuropathic pain properties of duloxetine and the potential role of the 5-HT2A receptor in its mechanism of action.
Methods
Animals
All animal care and experimental studies were carried out according to the protocols approved by the Institutional Animal Care and Use Committee of the University of California, San Diego. Animals were housed by two to three per cage, with free access to food and water and were maintained under conditions approved by the American Association for the Accreditation of Laboratory Animal Care. All experiments were performed using 220–250 g adult female Sprague–Dawley rats (Harlan Industries, San Diego CA, USA). Female rats were used to provide consistency with our previous studies of diabetes-induced allodynia, and we have found no sex differences in the effects of diabetes on nocifensive behaviour (Malmberg et al., 1993; Calcutt et al., 1996).
Induction of diabetes
Insulin-deficient diabetes was induced following an overnight fast by a single intraperitoneal (i.p.) injection of streptozotocin (Sigma, St. Louis, MO, USA), 50 mg·kg−1 dissolved in 0.9% sterile saline. Hyperglycaemia was confirmed using a strip-operated reflectance meter (One Touch Ultra, Life Scan Inc., Milpitas, CA, USA) in a blood sample obtained by tail prick 4 days after streptozotocin injection. The day of the experiment (between 6 and 10 weeks of diabetes), blood glucose levels were again determined and only rats with blood glucose levels ≥15 mmol·L−1 were included in the study.
Assessment of allodynia
Tactile allodynia was determined by measuring hind paw withdrawal in response to probing with a series of calibrated filaments (von Frey filaments, Stoelting, Wood Dale, IL, USA). The force applied ranged from 0.4 to 15 g. Tactile allodynia was assessed hourly for 5–6 h with an additional 30 min time point during the first hour. The 50% paw withdrawal threshold (50% PWT) was determined by using the up : down method (Chaplan et al., 1994).
Stepping and righting behaviour
Rats in all groups were observed for behavioural or motor function changes induced by the treatments. This was assessed, but not quantified, by testing the animals' ability to stand and walk in a normal posture, as proposed elsewhere (Chen and Pan, 2001).
Intrathecal (i.t.) catheterization
At 7–8 weeks of diabetes, rats were implanted with an i.t. PE-10 catheter under isoflurane anaesthesia (Yaksh and Rudy, 1976). Briefly, rats were placed in a stereotaxic head holder, and the cisternal membrane exposed. The membrane was pierced, and a PE-10 catheter (8.5 cm) passed intrathecally to the level of the lumbar enlargement. Rats were allowed to recover from surgery for at least 5 days before use. Animals showing any signs of motor impairment were killed humanely.
Western blotting
Mid-thigh sciatic nerve, L4-L5 dorsal root ganglia (DRG) and lumbar spinal cord were collected after decapitation of anaesthetized rats, homogenized in ice-cold phosphate buffered saline buffer and antiprotease, and centrifuged (14 000×g for 30 min at 4°C). Supernatant was collected and pellet was homogenized again with Chaps buffer (50 mM Tris HCl, pH 7.6, 10 mM Chaps, 0.05 mM EDTA-disodium, protease inhibitor cocktail) and centrifuged (14 000 ×g for 30 min at 4°C). Samples of total protein (10 µg) were separated on 4–12% SDS-PAGE Bis–Tris gels (Novex, Invitrogen, Carlsbad, CA, USA) and immunoblotted on nitrocellulose membrane. Membranes were incubated with anti-5-HT2A receptor (1/500, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-β-actin antibodies (1/5000, Sigma), followed by incubation with horseradish peroxidase-linked anti-rabbit secondary antibody (1/20 000, Santa Cruz Biotechnology) or anti-mouse secondary antibody (1/20 000, Santa Cruz Biotechnology). Blots were developed using an Enhanced Chemiluminescence Western-blotting protocol (Lumilight Roche Applied Science, Indianapolis, IN, USA). To detect on the same blot, 5-HT2A receptor and actin proteins that have similar molecular weights (55 vs. 43 kDa, respectively), previously anti-5-HT2A receptor bound antibodies were removed with stripping buffer (Pierce, Rockford, Il, USA) before incubation with anti-actin antibodies. Quantification of immunoreactivity was performed by densitometric scanning using Quantity One software (Bio-Rad, San Diego, CA, USA). For each animal, band intensities were normalized by calculating the ratio of the intensity of bands corresponding to 5-HT2A receptor protein to the intensity of the band corresponding to β-actin. The β-actin normalized data for each lane were expressed as a percentage of the group mean of values obtained from all control rats run on the same gel.
Administration of drugs
For local drug administration, rats received a subcutaneous (s.c.) injection (50 µL) into the dorsal surface of the hind paw of vehicle, duloxetine, DOI, ketanserin, pruvanserin or cromolyn. For systemic drug administration, drug was given in a volume of 1 mL·kg−1 for ketanserin (i.p.), pruvanserin (i.p.) or DOI (s.c.), or 2 mL·kg−1 for duloxetine (i.p.). For i.t. administration, drugs were delivered in a volume of 10 µL followed by 10 µL saline. Vehicle groups received sterile water for duloxetine; saline for DOI, ketanserin or cromolyn; sterile water containing Tween 80 for pruvanserin. PWTs were determined before and 30, 60, 120, 180, 240 and 300 min after drug administration for i.t. and local-peripheral treatments; an additional hour was recorded for systemic treatments. Doses and drug administration schedules for duloxetine, DOI, ketanserin, pruvanserin and cromolyn were based on previous reports (Koch et al., 2003; Adamec et al., 2004; Dogrul and Seyrek, 2006; Mbaki and Ramage, 2008) or our own pilot experiments.
As the aim of this study was to demonstrate that the anti-allodynic mechanism of duloxetine was mediated by 5-HT2A receptors, our schedule of administration was chosen in order to block activated 5-HT2A receptors and when the anti-allodynic effect could be reduced, rather than prevented. The time of injection of antagonists or agonists was determined by the time of peak effect of duloxetine, DOI, ketanserin or pruvanserin, depending on the route of administration. To determine the effect of duloxetine on tactile allodynia in diabetic rats, duloxetine was administered by different routes so that rats received vehicle (sterile water) or duloxetine i.p. (20 mg·kg−1), i.t. (20 µg) or locally-peripherally (50 µg). The 5-HT2A receptor antagonists ketanserin (20 µg) or pruvanserin (20 µg) were injected i.t., 210 min after systemic administration of duloxetine (20 mg·kg−1 i.p.) but these antagonists were given i.t. 90 min after duloxetine (20 µg) was administered i.t. To investigate the contribution of 5-HT2A receptors to diabetes-induced allodynia more specifically, DOI was delivered i.t. (20 µg) to rats, alone or followed by i.t. injection of either 20 µg pruvanserin or 20 µg ketanserin, 40 min after DOI. Ketanserin (1 mg·kg−1) or pruvanserin (10 mg·kg−1) were delivered i.p., alone or followed by 0.1 mg·kg−1 s.c. DOI, 150 min after ketanserin or pruvanserin. Similarly, rats received s.c. injection of ketanserin (50 µg) or pruvanserin (50 µg) into the dorsal surface of the hind paw, alone or followed by DOI (50 µg) 90 min later. To determine if ketanserin or pruvanserin acted locally, they were administered to the right paw and PWT measured in both ipsilateral and contralateral paws. Finally, cromolyn, a mast cell membrane stabilizer, was given locally at a dose of 800 ng per·paw.
Data analysis
All experimental results are given as median for non-parametric data (upper and lower cut-offs for tactile allodynia) or mean ± SEM [for area under the 50% PWT time curve (AUC)] with 4 to 15 animals per group. The mean of the 50% PWTs obtained with the von Frey filaments were used to construct PWT curves as a function of time for each group. AUC was calculated for a period of 5–6 h by the trapezoidal method, as previously described by Tallarida and Murray (1981) and calculated as: AUC = [(PWT at time 1 + PWT at time 2)/2]× (time 2–time 1) +[(PWT at time 2 + PWT at time 3)/2]× (time 3–time 2)… . One–way anova, followed by Tukey's test or t-tests were used to compare differences between treatments. Differences were considered to reach statistical significance when P < 0.05.
Materials
Streptozotocin, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI, 5-HT2A/2C receptor agonist) and ketanserin (5-HT2A/1D receptor antagonist) were purchased from Sigma. Pruvanserin [EMD 281014, selective 5-HT2A receptor antagonist (Bartoszyk et al., 2003)] was provided by Dr Gerd Bartoszyk (Merck KGaA, Darmstadt, Germany). The prescription formulation of Cymbalta (Eli Lilly, Indianapolis, IN, USA) was used as a source of duloxetine hydrochloride. Cromolyn sulphate was obtained from MP Biomedicals LLC (Solon, OH, USA). Streptozotocin, DOI, ketanserin and cromolyn were dissolved in 0.9% sterile saline. Duloxetine was dissolved in sterile water and pruvanserin was dissolved in sterile water containing Tween 80.
Results
Anti-allodynic effect of duloxetine
Streptozotocin injection resulted in hyperglycaemia within 3–4 days. The blood glucose concentration of the cohort of streptozotocin-injected rats was significantly (P < 0.001) increased (29.7 ± 0.9 mmol·L−1) compared with control rats (5.7 ± 0.2 mmol·L−1).
Studies were performed 6–10 weeks after induction of diabetes as the degree of allodynia was stable over this period. Tactile allodynia, defined as a 50% PWT < 5 g, was consistently present in streptozotocin-injected rats compared with non-diabetic rats (group median PWT = 15 g) and was significantly (P < 0.001) alleviated by systemic administration of 20 mg·kg−1 duloxetine (Figure 1). The maximal anti-allodynic effect was reached 4 h after administration, and efficacy persisted over the next 2 h (Figure 1A). Administration of duloxetine to non-diabetic rats did not affect PWT. Vehicle (sterile water) administration did not affect PWT and no changes in behavioural or motor functions, as assessed by the stepping and righting reflexes, were observed in either group at the doses employed.
Figure 1.
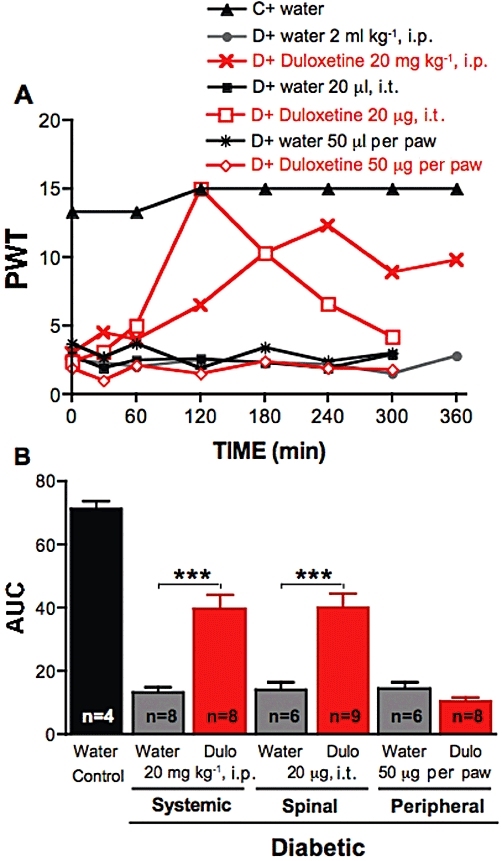
Effect of duloxetine on tactile allodynia in diabetic rats. (A) Tactile responses, represented as 50% paw withdrawal threshold (PWT), for control rats (C) receiving i.p. injection of water, for diabetic rats (D) receiving i.p. injection of water or duloxetine at 20 mg·kg−1, intrathecal (i.t.) injection of water or duloxetine (20 µg), or local injection in the paw of water or duloxetine (50 µg). Data are expressed as median, n = 4–9 per·group as indicated on bar. (B) Area under the 50% PWT curve (AUC) from 0 to 300 min for control rats receiving water, diabetic rats receiving water or duloxetine (Dulo) by systemic, spinal or peripheral route as indicated on graph. Data are expressed as mean + SEM, ***P < 0.001 using unpaired Student's t-test against vehicle treated animals for each route of administration.
Duloxetine was also delivered to diabetic rats by local paw injection or directly to the spinal cord to clarify potential sites of action. Intrathecal delivery of 20 µg duloxetine alleviated tactile allodynia in diabetic rats, with the maximal effect reached at 2 h (Figure 1A). Local administration of duloxetine to the paw (50 µg per paw) did not alter tactile allodynia in diabetic rats (Figure 1), although a slight decrease of PWT was noticeable in the first hour after duloxetine administration, but did prompt flinching of the injected paw, implying pro-nociceptive properties. To determine how systemic duloxetine might exert its spinal anti-nociceptive properties, duloxetine (20 mg·kg−1) was given i.p., followed 210 min later by an i.t. injection of 20 µg ketanserin, a 5-HT2A/1D receptor antagonist. Similar experiments were performed with a selective 5-HT2A receptor antagonist, pruvanserin (Bartoszyk et al., 2003). The alleviation of allodynia induced by systemic duloxetine was reversed by i.t. ketanserin or pruvanserin, indicating participation of spinal 5-HT2A receptors in the mechanism of action of duloxetine (Figure 2). Similarly, the antiallodynic effect after i.t. administration of duloxetine was reversed by i.t. ketanserin or pruvanserin administration (Figure 3).
Figure 2.
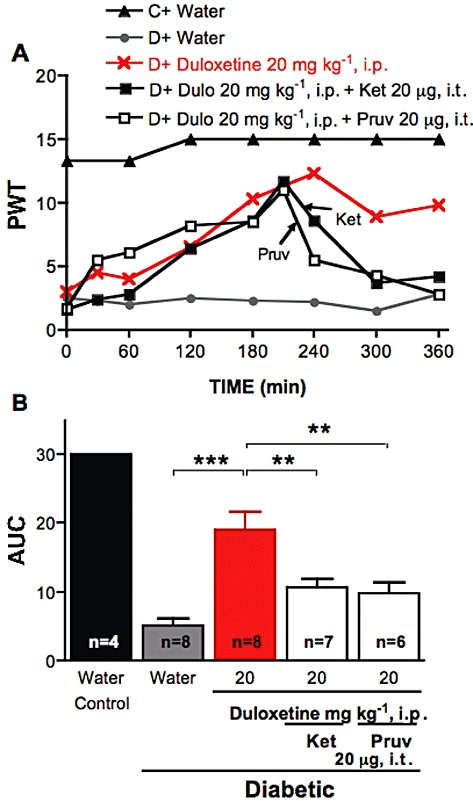
Effect of ketanserin or pruvanserin on the anti-allodynic effects of systemic duloxetine. (A) Tactile responses, represented as 50% paw withdrawal threshold (PWT), for control rats (C) receiving water and for diabetic rats (D) receiving i.p. injection of water, duloxetine at 20 mg·kg−1 or duloxetine (Dulo) followed 210 min later by i.t. injection of ketanserin (Ket, 20 µg) or pruvanserin (Pruv, 20 µg). Arrows indicate the time of injection of ketanserin (Ket) or pruvanserin (Pruv). Data are expressed as median, n = 4–8 per group as indicated on bar. (B) Area under the 50% PWT curve (AUC) from 240 to 360 min for control rats receiving water, diabetic rats receiving i.p. injection of water, duloxetine alone or duloxetine followed by i.t. injection of ketanserin (Ket) or pruvanserin (Pruv) as indicated on graph. Data are expressed as mean + SEM. **P < 0.01, ***P < 0.001 using one-way anova followed by Tukey's post hoc test.
Figure 3.
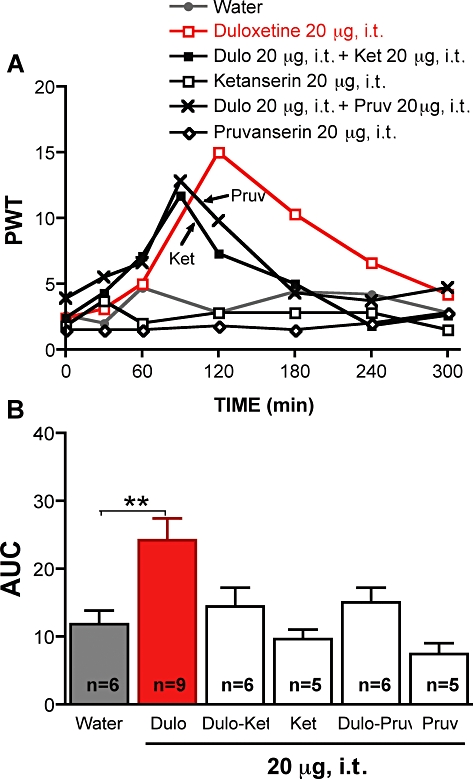
Effect of ketanserin or pruvanserin on the anti-allodynic effects of duloxetine given i.t.. (A) Tactile responses, represented as 50% paw withdrawal threshold (PWT) for diabetic rats receiving i.t. injection of water, duloxetine (20 µg), ketanserin (20 µg), pruvanserin (20 µg) or duloxetine followed 90 min later by i.t. injection of ketanserin (Ket, 20 µg) or pruvanserin (Pruv, 20 µg). Arrows indicate the time of injection of ketanserin (Ket) or pruvanserin (Pruv). Data are expressed as median, n = 5–9 per group as indicated on bar. (B) Area under the 50% PWT curve (AUC) from 90 to 300 min for diabetic rats receiving i.t. injection of water, duloxetine alone (Dulo) or duloxetine followed by i.t. injection of ketanserin (Ket) or pruvanserin (Pruv) as indicated on graph. Data are expressed as mean + SEM. **P < 0.01 using one-way anova followed by Tukey's post hoc test.
Effects of 5-HT2A receptor agonist and antagonist in diabetic rats
Intrathecal injection of the 5-HT2A/2C receptor agonist DOI (20 µg) induced a rapid alleviation of tactile allodynia in diabetic rats, with peak efficacy occurring 1–2 h after injection (Figure 4A). Neither pruvanserin nor ketanserin nor vehicle (saline) alone had any effect (Figure 4). The anti-allodynic effect of DOI was reversed by subsequent i.t. administration of ketanserin (20 µg) or pruvanserin (20 µg), given 40 min after DOI. These results suggest that 5-HT2A receptor activation is anti-allodynic at the level of the spinal cord.
Figure 4.
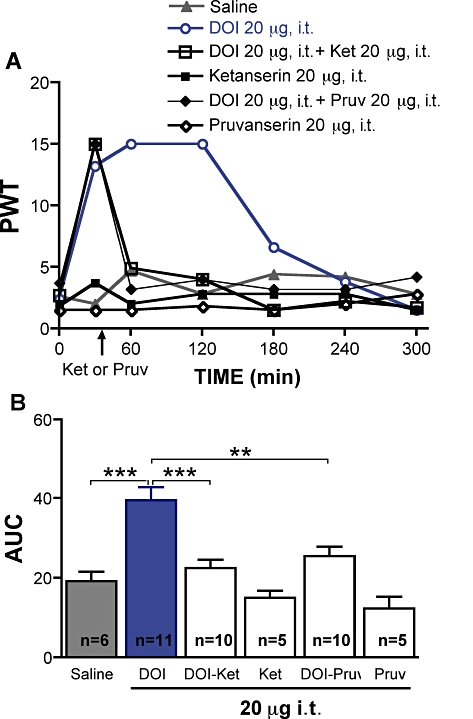
Effect of spinal delivery of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI), ketanserin or pruvanserin on tactile allodynia in diabetic rats. (A) Tactile responses, represented as 50% paw withdrawal threshold (PWT), for diabetic rats receiving intrathecal injection of saline, DOI (20 µg), ketanserin (20 µg), pruvanserin (20 µg) or DOI followed 40 min later by i.t. injection of ketanserin (Ket, 20 µg), or pruvanserin (Pruv, 20 µg). Data are expressed as median, n = 5–11 per group as indicated on bar. (B) Area under the 50% PWT curve (AUC) from 0 to 300 min for diabetic rats receiving i.t. injection of saline or 5-HT2A agonist and/or antagonist, as indicated on graph. Data are expressed as mean + SEM. **P < 0.01, ***P < 0.001 using one-way anova followed by Tukey's post hoc test.
Systemic (1 or 10 mg·kg−1 i.p.) and local (50 µg per paw) injection of the 5-HT2A receptor antagonists ketanserin or pruvanserin significantly alleviated tactile allodynia in streptozotocin-diabetic rats (P < 0.001, Figures 5 and 6). The systemic and local anti-allodynic effects of ketanserin or pruvanserin were reversed immediately after systemic (0.1 mg·kg−1 s.c.) or local (50 µg per paw) injection of DOI (Figures 5 and 6). Systemic (0.1 mg·kg−1 s.c.) or local (50 µg per paw) administration of DOI did not modify tactile responses in either control (data not shown) or diabetic rats (Figures 5 and 6), although a slight decrease of PWT was noticeable for the first hour after DOI administration in the paw of diabetic rats. These results indicate a pro-allodynic role of 5-HT2A receptor activation in the periphery in diabetic rats. Local administration of the mast cell stabilizer cromolyn (800 µg per paw) did not inhibit allodynia in diabetic rats (Figure 6A, B).
Figure 5.
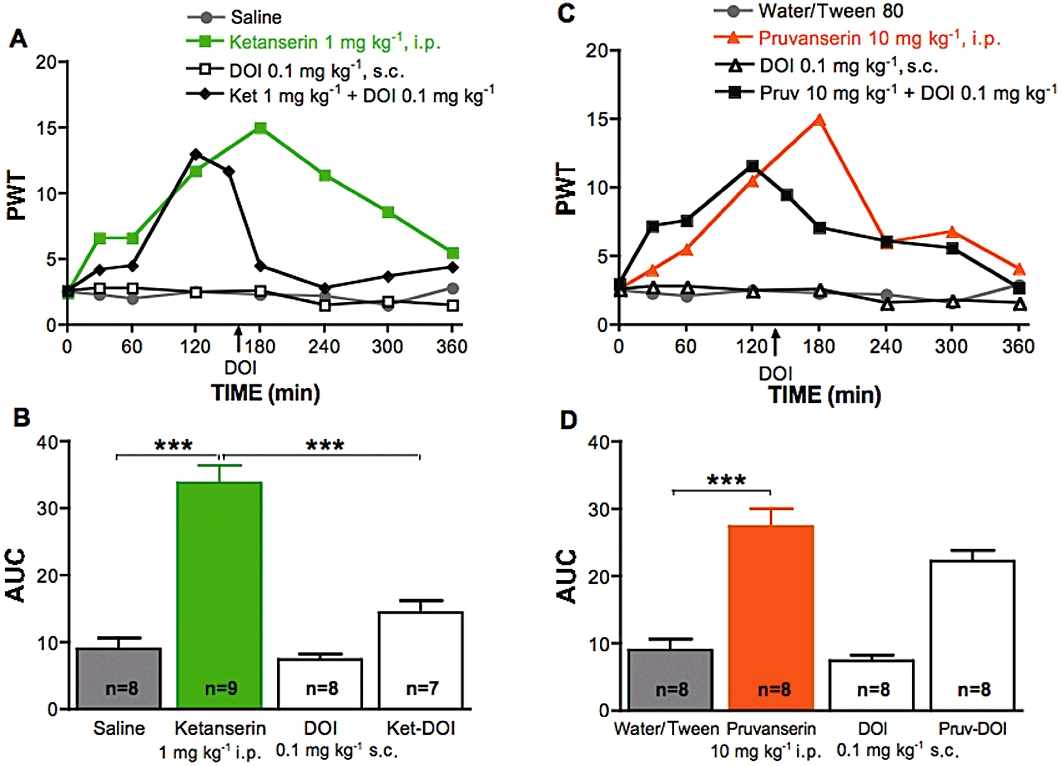
Effect of systemic delivery of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI), ketanserin or pruvanserin on tactile allodynia in diabetic rats. (A) Tactile responses, represented as 50% paw withdrawal threshold (PWT), for diabetic rats receiving i.p. injection of saline, ketanserin (1 mg·kg−1), s.c. injection of DOI (0.1 mg·kg−1) or i.p. injection of ketanserin (Ket, 1 mg·kg−1) followed 150 min later by subcutaneous injection of DOI (0.1 mg·kg−1). Data are expressed as median, n = 7–9 per group as indicated on bar. (B) Area under the 50% PWT curve (AUC) from 150 to 360 min for diabetic rats receiving systemic injection of saline, ketanserin alone, with DOI or DOI alone, as indicated on graph. Data are expressed as mean + SEM. ***P < 0.001 using one-way anova followed by Tukey's post hoc test. (C) Tactile responses, represented as 50% paw withdrawal threshold (PWT), for diabetic rats receiving i.p. injection of water containing Tween80, pruvanserin (10 mg·kg−1), s.c. injection of DOI (0.1 mg·kg−1) or i.p. injection of pruvanserin (Pruv, 10 mg·kg−1) followed 150 min later by subcutaneous injection of DOI (0.1 mg·kg−1). Data are expressed as median, n = 7–9 per group as indicated on bar. (D) Area under the 50% PWT curve (AUC) from 150 to 360 min for diabetic rats receiving systemic injection of water containing Tween 80 or pruvanserin alone, followed by DOI or DOI alone, as indicated on graph. Data are expressed as mean + SEM. ***P < 0.001 using one-way anova followed by Tukey's post hoc test.
Figure 6.
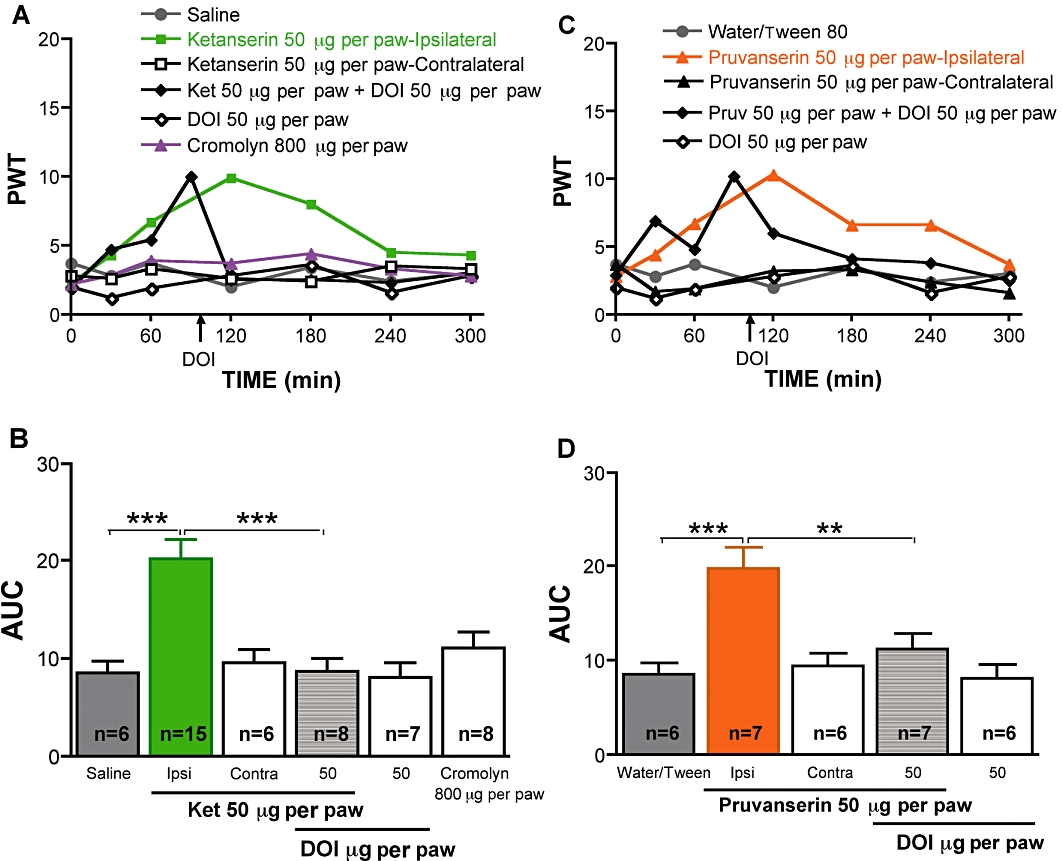
Effect of local peripheral delivery of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI), ketanserin or pruvanserin on tactile allodynia in diabetic rats. (A) Tactile responses, represented as 50% paw withdrawal threshold (PWT), for diabetic rats receiving local injection in the paw of saline, ketanserin (50 µg, ipsilateral), ketanserin (contralateral), DOI (50 µg, ipsilateral), cromolyn (800 µg), or ketanserin (Ket) followed 90 min later by paw injection of DOI (50 µg). Data are expressed as median, n = 6–15 per group as indicated on bar. (B) Area under the 50% PWT curve (AUC) from 120 to 300 min for diabetic rats receiving injections in the paw of saline, 5-HT2A antagonist ipsilaterally, contralaterally, or followed by paw injection of DOI and for diabetic rats receiving DOI alone or cromolyn alone, as indicated on graph. Data are expressed as mean + SEM. ***P < 0.001 using one-way anova followed by Tukey's post hoc test. (C) Tactile responses, represented as 50% paw withdrawal threshold (PWT), for diabetic rats receiving local injection in the paw of water containing Tween 80, pruvanserin (50 µg, ipsilateral), pruvanserin (contralateral), DOI (50 µg, ipsilateral), or pruvanserin (Pruv, followed 90 min later by paw injection of DOI (50 µg,). Data are expressed as median, n = 6–7 per group as indicated on bar. (B) Area under the 50% PWT curve (AUC) from 120 to 300 min for diabetic rats receiving injection in the paw of water containing Tween 80, 5-HT2A antagonist ipsilaterally, contralaterally, followed by paw injection of DOI or DOI alone, as indicated on graph. Data are expressed as mean + SEM. **P < 0.01, ***P < 0.001 using one-way anova followed by Tukey's post hoc test.
5-HT2A receptor protein expression in diabetic rat tissues
Quantification of 5-HT2A receptor protein levels by Western blot indicated no change in spinal cord, DRG or sciatic nerve of diabetic rats compared with controls (Figure 7).
Figure 7.
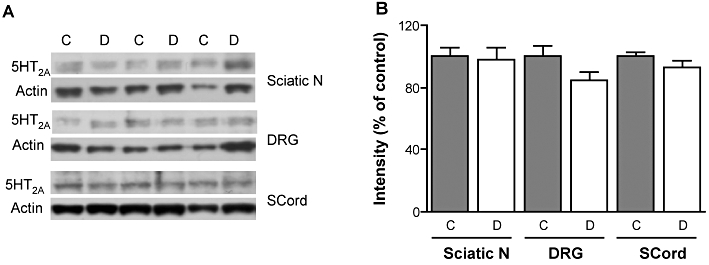
Western blot analysis of 5-HT2A receptor protein levels in rat sciatic nerve, dorsal root ganglia and spinal cord. (A) Representative Western blots and (B) densitometric quantification for 5-HT2A receptor protein (55 kDa) and β-actin (43 kDa) in the sciatic nerve (Sciatic N), dorsal root ganglia (DRG) and spinal cord (SCord) of control (C) and streptozotocin-diabetic (D) rats. Data are expressed as mean + SEM, n = 5–7 per group.
Discussion
Duloxetine is a potent and selective 5-HT and noradrenaline reuptake inhibitor with little or no affinity for 5-HT, noradrenaline, dopamine, acetylcholine or opioid receptors (Bymaster et al., 2001). It is widely used as an antidepressant with a mechanism of action based on its ability to inhibit 5-HT and noradrenaline transporters (Koch et al., 2003) and therefore, to increase extracellular monoamine levels in the brain (Engleman et al., 1995; Koch et al., 2003). Duloxetine is also one of the first prescription drugs approved by the US Food and Drug Administration for the management of the pain associated with diabetic neuropathy (Lunn et al., 2009). The mechanism of action by which duloxetine alleviates pain is considered to be distinct from its actions as an anti-depressant (Perahia et al., 2006). In patients suffering from pain and major depressive disorder, duloxetine treatment resulted in significant improvement of pain symptoms without changes in depressive symptoms, suggesting different mechanisms for relief of pain and depression (Brannan et al., 2005). In addition, duloxetine showed efficacy in diabetic patients suffering from peripheral painful neuropathy without depression (Goldstein et al., 2005; Raskin et al., 2005). The precise mechanism of pain relief is not clear, although the enhanced involvement of 5-HT and noradrenaline in descending inhibitory pathways or direct action on neuronal Na+ currents have been evoked as plausible mechanisms (Millan, 2002; Thor et al., 2007; Wang et al., 2010).
Several studies have shown that systemic duloxetine is anti-nociceptive in models of traumatic and inflammatory pain (Iyengar et al., 2004; Bomholt et al., 2005; Jones et al., 2005; Joshi et al., 2006; Munro, 2009; Piesla et al., 2009), but efficacy of duloxetine in models of painful diabetic neuropathy has been restricted to a recent report of the alleviation of thermal hyperalgesia in mice (Kuhad et al., 2009). In the present study, we have shown that systemic and spinal, but not peripheral, administration of duloxetine alleviated tactile allodynia in diabetic rats, in the absence of effects on motor function. Maximal efficacy was similar when duloxetine was delivered either systemically or spinally, with the latter showing a predictably faster onset. Duloxetine may therefore replenish depleted 5-HT levels in the CNS of diabetic rats (Sandrini et al., 1997). Our results are consistent with the alleviation of mechanical hyperalgesia after i.t. injection of 5-HT in diabetic rats (Bardin et al., 2000), the attenuation of thermal hyperalgesia in diabetic mice by the 5-HT reuptake inhibitor fluoxetine (Anjaneyulu and Chopra, 2004) and a report which showed that other 5-HT reuptake inhibitors, selective or non-selective, showed anti-allodynic effects in diabetic rats after spinal delivery (Ikeda et al., 2009). The ability of spinally delivered ketanserin or pruvanserin, a selective 5-HT2A receptor antagonist, to block the anti-allodynic effects of duloxetine also indicates that the mechanism of action by which systemic duloxetine alleviated painful diabetic neuropathy included enhancement of 5-hydroxytryptaminergic inhibitory systems operating through spinal 5-HT2A receptors. Involvement of spinal 5-HT2A receptor activation in alleviation of neuropathic pain has been demonstrated in nerve injury models (Obata et al., 2001; Honda et al., 2006) and in formalin-evoked hyperalgesia (Sasaki et al., 2001). Alleviation of allodynia after spinal delivery of 5-HT2A agonist suggests possible impairment of the spinal 5-HT2A receptor that could contribute to spinal disinhibition and subsequent pain-associated behaviours, as it does in other models of neuropathic pain (Dubner and Ren, 1999). However, 5-HT2A receptor protein levels were unchanged in spinal cord from diabetic rats. Similarly, no changes were observed in the peripheral nervous system.
Although enhanced stimulation of spinal 5-HT2A receptors alleviated allodynia in diabetic rats, we also found that the effects of 5-HT2A receptor stimulation and blockade were reversed when drugs were administered in the periphery. Indeed, peripheral and systemic administration of ketanserin or pruvanserin alleviated tactile allodynia in diabetic rats, and in both paradigms the effect was reversed by DOI. In addition, direct injection of duloxetine or DOI into the paw of diabetic rats produced a slight decrease of PWT in the first hour after administration. Our data implicate activation of peripheral 5-HT2A receptors in the allodynia of diabetic rats and are in agreement with the reported excitatory role of the peripheral 5-HT2A receptor in other pain modality models such as neuropathic or inflammatory pain models (Obata et al., 2000; Sasaki et al., 2001; Okamoto et al., 2002; Nitanda et al., 2005). The peripheral sources of 5-HT include mast cells, platelets and endothelial cells (Dray, 1995; Millan, 1999), while 5-HT2A receptors are located on endothelial cells (Martin, 1994), Schwann cells (Yoder et al., 1997; Gaietta et al., 2003), the perikarya of sensory neurons in the DRG (Okamoto et al., 2002; Van Steenwinckel et al., 2009), and in axons of both myelinated and unmyelinated fibres (Carlton and Coggeshall, 1997; Okamoto et al., 2002). The release of 5-HT from mast cells is specific to rodents (Sjoerdsma et al., 1957; Benditt et al., 1963), and we have excluded these cells as a source of the diabetes-induced, 5-HT-mediated allodynia by using the mast cell stabilizer cromolyn. Endothelial cells are another potential source of 5-HT, as they are perturbed by diabetes (Cameron et al., 2001) and may release 5-HT into the endoneurial environment. Platelets abnormalities that occur in diabetes (Glassman, 1993) may also contribute to the peripheral pathogenesis of diabetic painful neuropathy via increased 5-HT release (Pietraszek et al., 1992; Malyszko et al., 1994). Alternatively, a number of cell types in the skin also produce 5-HT (Nordlind et al., 2008). Investigating the location and mechanisms by which a peripheral 5-HT2A receptor-mediated process contributes to allodynia in diabetic rats awaits future studies.
Duloxetine is a balanced 5-HT and noradrenaline reuptake inhibitor (Bymaster et al., 2001), therefore, activation of the descending noradrenergic pathway may also play a role in the anti-allodynic effects of duloxetine. Our data showed that, in our rat model, duloxetine acts predominantly via activation of 5-HT2A receptors. Indeed, the effect of the non-selective 5-HT2A receptor antagonist, ketanserin, parallels that of the selective antagonist, pruvanserin and their effects were reversed by DOI, a 5-HT2A/2C agonist. An additional support for the minor role of noradrenaline, but a major role of 5-HT and 5-HT2A receptors in both diabetic painful neuropathy and the mechanism of action of duloxetine, is the rapid and complete reversal of the anti-allodynic effect by the 5-HT2A receptor antagonists. In contrast, in normal rats, duloxetine suppressed spinal hyperactivity triggered by prostaglandin E2, and that effect was blocked by a combination of 5-HT1B/1D and α2-adrenoceptor antagonists (Tsukamoto et al., 2010), implying a role for these receptors in the antinociceptive effect of duloxetine. Although we cannot totally exclude noradrenaline or other possible mechanisms, such as blockade of Na+ currents (Wang et al., 2010), 5-HT1B/1D and α2-adrenoceptors (Tsukamoto et al., 2010), from playing a role in the anti-allodynic effect of duloxetine, our data suggest that duloxetine alleviated allodynia in diabetic rat model mainly via the indirect activation of 5-HT2A receptors in the spinal cord.
In summary, we have identified spinal 5-HT2A receptors as a transduction system for the anti-allodynic and anti-hyperalgesic properties of duloxetine in diabetic rats. Our results also indicate that the role of the 5-HT2A receptor in diabetes-induced allodynia and hyperalgesia depends on the level of the neuroaxis, with peripheral activation of the 5-HT2A receptor contributing to tactile allodynia, whereas spinal activation of the receptor alleviates tactile allodynia. This is consistent with the known properties of 5-HT, whereby it can exert either algesic or analgesic effects depending on the site of action and the receptor subtype activated (Eide and Hole, 1993). These findings may also explain why, in contrast to acute effects in our rat study, patients with painful diabetic neuropathy noticed improvement of pain after at least 1 week of daily duloxetine administration (Goldstein et al., 2005). Patients receive duloxetine via the oral route, limiting the amount of drug gaining access to the spinal cord, while in our rat study, duloxetine was injected systemically or directly to the spinal cord, bypassing the metabolism that follows oral administration and also the location-dependent actions of 5-HT. The combination of a 5-HT2A receptor-mediated peripheral pro-allodynic effect with depletion of central 5-HT (Sandrini et al., 1997; Padayatti and Paulose, 1999) and consequent impairment of 5-HT2A receptor-mediated spinal inhibition may together promote pain states during diabetes. Peripherally restricted, selective 5-HT2A receptor antagonists could offer a novel approach for the treatment of diabetic neuropathic pain.
Acknowledgments
This work was supported by a JDRF Career Development Award (CGJ), a post-doctoral fellowship from the UC Mexus-Conacyt Program (TM-Z) and NIH grant DK057629 (to Dr Nigel Calcutt). The authors would like to thank Drs Nigel Calcutt, Fred Esch and Melissa Zolodz for their advice and assistance with these studies. The authors would also like to thank Dr Gerd Bartoszyk (Merck KGaA) for providing pruvanserin.
Glossary
Abbreviations
- AUC
area under the 50% PWT time curve
- ECL
enhanced chemiluminescence
- DOI
1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride
- DRG
dorsal root ganglia
- i.t.
intrathecal
- PBS
phosphate buffered saline buffer
- PWT
paw withdrawal threshold
Conflict of interest
The authors state no conflict of interest.
References
- Adamec R, Creamer K, Bartoszyk GD, Burton P. Prophylactic and therapeutic effects of acute systemic injections of EMD 281014, a selective serotonin 2A receptor antagonist on anxiety induced by predator stress in rats. Eur J Pharmacol. 2004;504:79–96. doi: 10.1016/j.ejphar.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br. J. Pharmacol. (4th edn.) 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjaneyulu M, Chopra K. Fluoxetine attenuates thermal hyperalgesia through 5-HT1/2 receptors in streptozotocin-induced diabetic mice. Eur J Pharmacol. 2004;497:285–292. doi: 10.1016/j.ejphar.2004.06.063. [DOI] [PubMed] [Google Scholar]
- Bardin L, Schmidt J, Alloui A, Eschalier A. Effect of intrathecal administration of serotonin in chronic pain models in rats. Eur J Pharmacol. 2000;409:37–43. doi: 10.1016/s0014-2999(00)00796-2. [DOI] [PubMed] [Google Scholar]
- Bartoszyk GD, van Amsterdam C, Bottcher H, Seyfried CA. EMD 281014, a new selective serotonin 5-HT2A receptor antagonist. Eur J Pharmacol. 2003;473:229–230. doi: 10.1016/s0014-2999(03)01992-7. [DOI] [PubMed] [Google Scholar]
- Benditt EP, Holcenberg H, Lagunoff D. The role of serotonin (5-hydroxytryptamine) in mast cells. Ann N Y Acad Sci. 1963;103:179–184. doi: 10.1111/j.1749-6632.1963.tb53697.x. [DOI] [PubMed] [Google Scholar]
- Bomholt SF, Mikkelsen JD, Blackburn-Munro G. Antinociceptive effects of the antidepressants amitriptyline, duloxetine, mirtazapine and citalopram in animal models of acute, persistent and neuropathic pain. Neuropharmacology. 2005;48:252–263. doi: 10.1016/j.neuropharm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Boulton AJ, Armstrong WD, Scarpello JH, Ward JD. The natural history of painful diabetic neuropathy–a 4-year study. Postgrad Med J. 1983;59:556–559. doi: 10.1136/pgmj.59.695.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan SK, Mallinckrodt CH, Brown EB, Wohlreich MM, Watkin JG, Schatzberg AF. Duloxetine 60 mg once-daily in the treatment of painful physical symptoms in patients with major depressive disorder. J Psychiatr Res. 2005;39:43–53. doi: 10.1016/j.jpsychires.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, Shaw JL, Thompson L, Nelson DL, et al. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25:871–880. doi: 10.1016/S0893-133X(01)00298-6. [DOI] [PubMed] [Google Scholar]
- Calcutt NA. Experimental models of painful diabetic neuropathy. J Neurol Sci. 2004;220:137–139. doi: 10.1016/j.jns.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Calcutt NA, Backonja MM. Pathogenesis of pain in peripheral diabetic neuropathy. Curr Diab Rep. 2007;7:429–434. doi: 10.1007/s11892-007-0072-9. [DOI] [PubMed] [Google Scholar]
- Calcutt NA, Jorge MC, Yaksh TL, Chaplan SR. Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: effects of insulin, aldose reductase inhibition and lidocaine. Pain. 1996;68:293–299. doi: 10.1016/s0304-3959(96)03201-0. [DOI] [PubMed] [Google Scholar]
- Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Coggeshall RE. Immunohistochemical localization of 5-HT2A receptors in peripheral sensory axons in rat glabrous skin. Brain Res. 1997;763:271–275. doi: 10.1016/s0006-8993(97)00489-7. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Spinal endogenous acetylcholine contributes to the analgesic effect of systemic morphine in rats. Anesthesiology. 2001;95:525–530. doi: 10.1097/00000542-200108000-00039. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Seyrek M. Systemic morphine produce antinociception mediated by spinal 5-HT7, but not 5-HT1A and 5-HT2 receptors in the spinal cord. Br J Pharmacol. 2006;149:498–505. doi: 10.1038/sj.bjp.0706854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75:125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- Dubner R, Ren K. Endogenous mechanisms of sensory modulation. Pain. 1999;(Suppl 6):S45–S53. doi: 10.1016/S0304-3959(99)00137-2. [DOI] [PubMed] [Google Scholar]
- Eide PK, Hole K. The role of 5-hydroxytryptamine (5-HT) receptor subtypes and plasticity in the 5-HT systems in the regulation of nociceptive sensitivity. Cephalalgia. 1993;13:75–85. doi: 10.1046/j.1468-2982.1993.1302075.x. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Perry KW, Mayle DA, Wong DT. Simultaneous increases of extracellular monoamines in microdialysates from hypothalamus of conscious rats by duloxetine, a dual serotonin and norepinephrine uptake inhibitor. Neuropsychopharmacology. 1995;12:287–295. doi: 10.1016/0893-133X(94)00093-F. [DOI] [PubMed] [Google Scholar]
- Gaietta GM, Yoder EJ, Deerinck T, Kinder K, Hanono A, Han A, et al. 5-HT2a receptors in rat sciatic nerves and Schwann cell cultures. J Neurocytol. 2003;32:373–380. doi: 10.1023/B:NEUR.0000011331.58835.fd. [DOI] [PubMed] [Google Scholar]
- Glassman AB. Platelet abnormalities in diabetes mellitus. Ann Clin Lab Sci. 1993;23:47–50. [PubMed] [Google Scholar]
- Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116:109–118. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Honda M, Uchida K, Tanabe M, Ono H. Fluvoxamine, a selective serotonin reuptake inhibitor, exerts its antiallodynic effects on neuropathic pain in mice via 5-HT2A/2C receptors. Neuropharmacology. 2006;51:866–872. doi: 10.1016/j.neuropharm.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Ikeda T, Ishida Y, Naono R, Takeda R, Abe H, Nakamura T, et al. Effects of intrathecal administration of newer antidepressants on mechanical allodynia in rat models of neuropathic pain. Neurosci Res. 2009;63:42–46. doi: 10.1016/j.neures.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 2004;311:576–584. doi: 10.1124/jpet.104.070656. [DOI] [PubMed] [Google Scholar]
- Jones CK, Peters SC, Shannon HE. Efficacy of duloxetine, a potent and balanced serotonergic and noradrenergic reuptake inhibitor, in inflammatory and acute pain models in rodents. J Pharmacol Exp Ther. 2005;312:726–732. doi: 10.1124/jpet.104.075960. [DOI] [PubMed] [Google Scholar]
- Joshi SK, Hernandez G, Mikusa JP, Zhu CZ, Zhong C, Salyers A, et al. Comparison of antinociceptive actions of standard analgesics in attenuating capsaicin and nerve-injury-induced mechanical hypersensitivity. Neuroscience. 2006;143:587–596. doi: 10.1016/j.neuroscience.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Koch S, Hemrick-Luecke SK, Thompson LK, Evans DC, Threlkeld PG, Nelson DL, et al. Comparison of effects of dual transporter inhibitors on monoamine transporters and extracellular levels in rats. Neuropharmacology. 2003;45:935–944. doi: 10.1016/s0028-3908(03)00268-5. [DOI] [PubMed] [Google Scholar]
- Kuhad A, Bishnoi M, Chopra K. Anti-nociceptive effect of duloxetine in mouse model of diabetic neuropathic pain. Indian J Exp Biol. 2009;47:193–197. [PubMed] [Google Scholar]
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy or chronic pain. Cochrane Database Syst Rev. 2009;4:CD007115. doi: 10.1002/14651858.CD007115.pub2. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL, Calcutt NA. Anti-nociceptive effects of the GM1 ganglioside derivative AGF 44 on the formalin test in normal and streptozotocin-diabetic rats. Neurosci Lett. 1993;161:45–48. doi: 10.1016/0304-3940(93)90136-9. [DOI] [PubMed] [Google Scholar]
- Malyszko J, Urano T, Knofler R, Taminato A, Yoshimi T, Takada Y, et al. Daily variations of platelet aggregation in relation to blood and plasma serotonin in diabetes. Thromb Res. 1994;75:569–576. doi: 10.1016/0049-3848(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Martin GR. Vascular receptors for 5-hydroxytryptamine: distribution, function and classification. Pharmacol Ther. 1994;62:283–324. doi: 10.1016/0163-7258(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Mbaki Y, Ramage AG. Investigation of the role of 5-HT2 receptor subtypes in the control of the bladder and the urethra in the anaesthetized female rat. Br J Pharmacol. 2008;155:343–356. doi: 10.1038/bjp.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Munro G. Pharmacological assessment of the rat formalin test utilizing the clinically used analgesic drugs gabapentin, lamotrigine, morphine, duloxetine, tramadol and ibuprofen: influence of low and high formalin concentrations. Eur J Pharmacol. 2009;605:95–102. doi: 10.1016/j.ejphar.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Nitanda A, Yasunami N, Tokumo K, Fujii H, Hirai T, Nishio H. Contribution of the peripheral 5-HT 2A receptor to mechanical hyperalgesia in a rat model of neuropathic pain. Neurochem Int. 2005;47:394–400. doi: 10.1016/j.neuint.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Nordlind K, Azmitia EC, Slominski A. The skin as a mirror of the soul: exploring the possible roles of serotonin. Exp Dermatol. 2008;17:301–311. doi: 10.1111/j.1600-0625.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- Obata H, Saito S, Ishizaki K, Goto F. Antinociception in rat by sarpogrelate, a selective 5-HT(2A) receptor antagonist, is peripheral. Eur J Pharmacol. 2000;404:95–102. doi: 10.1016/s0014-2999(00)00522-7. [DOI] [PubMed] [Google Scholar]
- Obata H, Saito S, Sasaki M, Ishizaki K, Goto F. Antiallodynic effect of intrathecally administered 5-HT(2) agonists in rats with nerve ligation. Pain. 2001;90:173–179. doi: 10.1016/s0304-3959(00)00401-2. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Imbe H, Morikawa Y, Itoh M, Sekimoto M, Nemoto K, et al. 5-HT2A receptor subtype in the peripheral branch of sensory fibers is involved in the potentiation of inflammatory pain in rats. Pain. 2002;99:133–143. doi: 10.1016/s0304-3959(02)00070-2. [DOI] [PubMed] [Google Scholar]
- Padayatti PS, Paulose CS. Alpha2 adrenergic and high affinity serotonergic receptor changes in the brain stem of streptozotocin-induced diabetic rats. Life Sci. 1999;65:403–414. doi: 10.1016/s0024-3205(99)00261-1. [DOI] [PubMed] [Google Scholar]
- Partanen J, Niskanen L, Lehtinen J, Mervaala E, Siitonen O, Uusitupa M. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:89–94. doi: 10.1056/NEJM199507133330203. [DOI] [PubMed] [Google Scholar]
- Perahia DG, Pritchett YL, Desaiah D, Raskin J. Efficacy of duloxetine in painful symptoms: an analgesic or antidepressant effect? Int Clin Psychopharmacol. 2006;21:311–317. doi: 10.1097/01.yic.0000224782.83287.3c. [DOI] [PubMed] [Google Scholar]
- Piesla MJ, Leventhal L, Strassle BW, Harrison JE, Cummons TA, Lu P, et al. Abnormal gait, due to inflammation but not nerve injury, reflects enhanced nociception in preclinical pain models. Brain Res. 2009;1295:89–98. doi: 10.1016/j.brainres.2009.07.091. [DOI] [PubMed] [Google Scholar]
- Pietraszek MH, Takada Y, Takada A, Fujita M, Watanabe I, Taminato A, et al. Blood serotonergic mechanisms in type 2 (non-insulin-dependent) diabetes mellitus. Thromb Res. 1992;66:765–774. doi: 10.1016/0049-3848(92)90052-c. [DOI] [PubMed] [Google Scholar]
- Raskin J, Pritchett YL, Wang F, D'Souza DN, Waninger AL, Iyengar S, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6:346–356. doi: 10.1111/j.1526-4637.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- Sandrini M, Vitale G, Vergoni AV, Ottani A, Bertolini A. Streptozotocin-induced diabetes provokes changes in serotonin concentration and on 5-HT1A and 5-HT2 receptors in the rat brain. Life Sci. 1997;60:1393–1397. doi: 10.1016/s0024-3205(97)00084-2. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Ishizaki K, Obata H, Goto F. Effects of 5-HT2 and 5-HT3 receptors on the modulation of nociceptive transmission in rat spinal cord according to the formalin test. Eur J Pharmacol. 2001;424:45–52. doi: 10.1016/s0014-2999(01)01117-7. [DOI] [PubMed] [Google Scholar]
- Sjoerdsma A, Waalkes TP, Weissbach H. Serotonin and histamine in mast cells. Science. 1957;125:1202–1203. doi: 10.1126/science.125.3259.1202. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations with Computer Programs. New York; Heidelberg; Berlin: Springer-Verlag; 1981. [Google Scholar]
- Thor KB, Kirby M, Viktrup L. Serotonin and noradrenaline involvement in urinary incontinence, depression and pain: scientific basis for overlapping clinical efficacy from a single drug, duloxetine. Int J Clin Pract. 2007;61:1349–1355. doi: 10.1111/j.1742-1241.2007.01433.x. [DOI] [PubMed] [Google Scholar]
- Tsukamoto M, Kiso T, Shimoshige Y, Aoki T, Matsuoka N. Spinal mechanism of standard analgesics: evaluation using mouse models of allodynia. Eur J Pharmacol. 2010;634:40–45. doi: 10.1016/j.ejphar.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Van Steenwinckel J, Noghero A, Thibault K, Brisorgueil MJ, Fischer J, Conrath M. The 5-HT2A receptor is mainly expressed in nociceptive sensory neurons in rat lumbar dorsal root ganglia. Neuroscience. 2009;161:838–846. doi: 10.1016/j.neuroscience.2009.03.087. [DOI] [PubMed] [Google Scholar]
- Vranken JH, Hollmann MW, van der Vegt MH, Kruis MR, Heesen M, Vos K, et al. Duloxetine in patients with central neuropathic pain caused by spinal cord injury or stroke: a randomized, double-blind, placebo-controlled trial. Pain. 2011;152:267–273. doi: 10.1016/j.pain.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Wang SY, Calderon J, Kuo Wang G. Block of neuronal Na+ channels by antidepressant duloxetine in a state-dependent manner. Anesthesiology. 2010;113:655–665. doi: 10.1097/ALN.0b013e3181e89a93. [DOI] [PubMed] [Google Scholar]
- Wernicke JF, Pritchett YL, D'Souza DN, Waninger A, Tran P, Iyengar S, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67:1411–1420. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yoder EJ, Tamir H, Ellisman MH. Serotonin receptors expressed by myelinating Schwann cells in rat sciatic nerve. Brain Res. 1997;753:299–308. doi: 10.1016/s0006-8993(96)01411-4. [DOI] [PubMed] [Google Scholar]
- Ziegler D. Painful diabetic neuropathy: advantage of novel drugs over old drugs? Diabetes Care. 2009;32(Suppl 2):S414–S419. doi: 10.2337/dc09-S350. [DOI] [PMC free article] [PubMed] [Google Scholar]


