Abstract
Background & objectives:
The P300 wave is an event related potential (ERP) elicited by infrequent, task-relevant stimuli and appeared at about 300 ms, represents higher cognitive function of information processing, working memory or stimulus categorization. Hypobaric hypoxia deteriorates the cognitive function during the short term stay (days to few weeks) at high altitude. The present study was carried out to evaluate the P300 responses during long duration stay (1 month and 6 months) at high altitude (HA, 4115 m) in a sample of Indian lowlanders.
Methods:
The study was carried out on 18 healthy male volunteers at sea level (SL). The volunteers were stage inducted to 4115 m altitude in the Eastern Himalayas. The P300 was recorded after 1 and 6 months of their stay at HA.
Results:
The latencies of peaks N100, P200 and N200 waves did not show any significant changes after 1 and 6 months of stay at HA as compared to SL. The P300 latency was significantly delayed after 1 month and further delayed after 6 month of residence at 4115 m. The P200 and P300 amplitudes did not show any changes.
Interpretation & conclusions:
The increase in P300 latency indicated that long duration of stay at high altitude slows the stimulus evaluation processes. The observations suggest that hypoxia causes slowing of the signal processing at HA. The magnitude of the effects of hypobaric hypoxia may be dependent upon the duration of residence at high altitude.
Keywords: Cognition, ERP, high altitude, hypobaric hypoxia, P300
Event related potentials (ERPs) are objective tools for detecting cognitive impairment1. The P300 event related potential is a positive potential that occurs approximately 300 miliseconds (ms) after presentation of a stimulus, requiring detection, counting or cognitive processing by the participant2,3. Researchers often rely on measurement of the P300 to examine event related potentials, especially with regard to decision making. The waveform can be used as a measure for the efficacy of various treatments on cognitive function because cognitive impairment is often correlated with modification in the P300. Some researchers have suggested its use as a clinical marker for precisely this reason. There is a broad range of uses for the P300 in scientific research, ranging from study of depression and drug addiction to anxiety disorders4.
Cognitive deterioration during rapid ascent to high altitude (HA) has been investigated using various types of neurophysiological tests5,6. ERPs are good index which objectively quantify the level of cognitive impairment as compared to other psychometric tests employed for assessing cognitive functions7–9. The ERPs give an idea about the time course of information processing including expectancy, attention, cognition search, decision making and memorization10.
Previous studies have employed gas breathing mixture to assess the effect of hypoxia on ERP6. A rapid ascent to simulated 4300 m altitude led to an increase in P300 latency and prolongation of reaction time while the amplitude of P300 decreased6. A similar experiment using a low oxygen breathing mixture reported an interaction of hypoxia and stimulus intensity for reaction time, N200 latency and P300 latency5. Our earlier studies carried out after 1st and 3rd week of induction to altitude of 3500 m in Western Himalayas revealed a prolongation in latency of P30011. Another study on days 6 and 8 at 3200 and 4300 m respectively in Eastern Himalayas also showed the same trend12. However, there is no information on ERP on long term residence at high altitude. The present study was aimed to evaluate changes with long term residence (1 and 6 months) at HA on P300 response. It was hypothesized that changes in P300 response would occur after 1 month and 6 months of residence at 4115 m and these would depend upon the duration of residence at HA.
Material & Methods
The study was carried out on 18 healthy male volunteers from Indian Army in the age group of 22-30 yr. They were lowlanders from north India with no previous exposure to high altitude and about to be posted to HA [T R Junction (4115 m), Eastern Command, North Sikkim] for the next 2-3 yr. The volunteers were selected from Army Headquarters, Delhi, specially for this study in 2006. They were free from any neurological disorder, sleep-related and cardiac problems and diabetes. All the volunteers had thorough ENT check-up including audiometry to rule out any ear pathology affecting hearing. They were not taking any sort of psychotropic, psychoactive or other drugs during the course of the study.
All volunteers were explained the purpose and relevance of the study and written individual informed consent was obtained. The entire protocol was approved by Ethics Committee of the Institute (Defence Institute of Physiology & Allied Sciences, Delhi). In the first trial, the ERPs were recorded at sea level (SL) where the oxygen saturation was found to be 98 ± 1 per cent. The subjects were transported by road from SL to 3200 m altitude in the Eastern Himalayas, where they stayed for six days. Then they were inducted by road to 4115 m (oxygen saturation was 86±2%), where they were stationed for the next 6 months. The second and third trials were carried out at 4115 m after 1 and 6 months of their stay, respectively.
Recording of P300: The P300 response was recorded using the standard auditory oddball paradigm (Nicolet, USA). The volunteer was instructed to lie down and relax on a bed in a dimly lit room and instructed to fix the eyes on a particular spot on the roof to avoid any electro-ocular artifacts. The ground electrode was placed at FPz. Two reference electrodes were placed one on the left ear lobe (A1) and the other on right ear lobe (A2). P300 response was recorded from the vertex (Cz & Pz) in response to stimuli presented monaurally through head phones. The input impedance of electrodes was kept below 5 kΩ. The frequencies of rare and frequent stimulus were 750 Hz and 2 KHz respectively and rate of presentation was 0.9 Hz. Alternating tone bursts with 100 μs duration and 80 dB intensity were used randomly. The peak latencies of N100, P200, N200 and P300 and amplitudes of wave P200 and P300 of target stimuli (rare) were calculated.
Statistical analyses: Values were expressed as mean ± SE. Analysis for statistical significance of changes during the successive episode (SL, 1 & 6 months) was performed using one way analysis of variance (ANOVA) for repeated measures. If there was any significant change in ANOVA, scheffe's multiple comparison F test13 was used for post-hoc analysis (SPSS 10.0). Statistical significance was fixed at P<0.05.
Results & Discussion
The latencies of N100, P200 and N200 wave did not show any significant change after one and six months stay at HA compared to SL values. P300 wave latency recorded a significant delay after one and six months as compared to sea level (SL). The latency of P300 wave at SL was 295.33 ± 3.716 msec, it significantly increased to 307.17 ± 4.329 msec after 1 month (P<0.05) and to 311.83 ± 4.486 msec after six months (P<0.01) at 4115 m. However, there was no significant difference between 1 and 6 months values of P300 latency. The amplitude of P200 (AMP-2) and P300 (AMP-3) peak did not show any significant change (Table, Fig).
Table.
Effect of high altitude (4115 m) on P300 wave latency
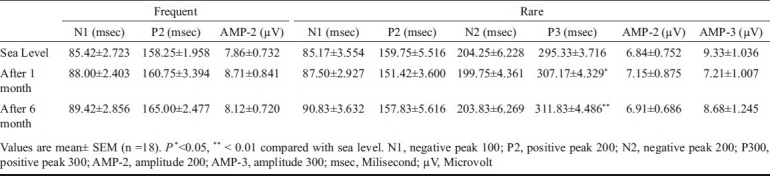
Fig.

Representative waveform depicting the prolongation of P300 latency at 1 and 6 months of residence at 4115 m, whereby indicating towards furthering of deleterious effect of hypoxia with respect to time of stay at high altitude. However, amplitude of P200 and P300 peaks did not show any significant change indicating no compensatory mechanism in force. N1, negative peak 100; P2, positive peak 200; N2, negative peak 200; P300, positive peak 300.
In the present study, delay in P300 wave latency was observed after one month and 6 months of stay at 4115 m. A study by Hayashi et al14 has reported a prolongation in P300 but no change in N100 response latency after two hours of exposure to a simulated high altitude of 4500 m. Our earlier study carried out at altitude in 1st and 3rd week after induction to 3500 m showed an increase in latency of P30011. Another study also showed prolonged P300 latencies at 3200 and 4300 m on day 6 and day 8, respectively12.
Event related potentials give information about the time course of information processing including expectancy, attention, cognition and working memory. The long-latency ERP components are sensitive to the same factors that affect mental performance i.e., attention, task difficulty, memory load. The latency of the P300 response depends upon the speed by which the information content of relevant stimulus is being evaluated10. It is these aspects which are ready to be affected by hypobaric hypoxia.
Specific cognitive processes are associated with specific components of ERP. The P300 component, occurring approximately 300 msec subsequent to the presentation of a stimulus must be counted, and recognized15. It reflects a later, rather than earlier, more controlled and voluntary memory and decision-making capacity. In the present study, only the P300 component was significantly altered at 1 and 6 months at 4115 m showing a decrement in the mentioned cognitive capacities at HA which not only persisted for the duration of the study i.e., 6 months but also became more severe at 6 months as compared to 1 month. This is an agreement with other studies at HA on various aspects of performance16,17.
The N100 and P200 are considered indices of early attention capacities related to stimulus processing18, while N200 component is considered an index of stimulus detection19. The N100, P200 and N200 may reflect the stage of early attention (automatic or obligatory) to the stimuli. Since the latencies and amplitudes of N100, P200 and N200 remained unaltered in the present study, it can be assumed that the capacities reflected by these components are less sensitive to hypobaric hypoxia.
There were individual differences in the response recorded at HA in 18 volunteers. Of the 18 participants, there was an increase in P300 wave latency in 12 volunteers after one month and six months of stay at HA, while 6 volunteers recorded no significant changes in P300 wave latency at 1 / 6 months. A previous study20 reported similar individual differences in performance of a reaction time (RT) task with no change in N100 and N200 components of ERP at simulated HA of 6000 m. The difference in response may be attributed to different arousal levels of the participants as measured by EEG in another study21.
AMP-3 did not show any significant change at high altitude. It has been reported that P300 amplitude depends upon the selective attention of the volunteer; it is greater for attended rather than unattended target stimuli, greater with better motivation and task priority22. It is larger for relevant stimuli; it is reduced in distraction or multi task paradigms where attention of the participant to the relevant stimulus is reduced23. In a study, the amplitude was reported to increase when participants were told that their performance was being monitored and would be compared to others24. In the present study possibly the volunteers may have put in an extra effort to attend to the odd-ball paradigm task due to which the amplitude may not have altered significantly, as reported in a recent study following sleep deprivation25.
The central mechanisms responsible for the effect of hypoxia on cognitive performance are not clear. It is likely that the direct effects of mild transient hypoxia on brain causes variations in the level of specific neurotransmitters involved in cognitive processes because the experimental studies on rats showed alterations in synthesis of monoamines under simulated hypoxia26. Alterations in norepinephrine, serotonin, dopamine and enzyme tyrosine hydroxylase, choline acetyl transferase and glutamine acid decarboxylase were found in our recent studies on exposure to simulated hypobaric hypoxia in rat cerebral cortex, pons, midbrain and hypothalamus (unpublished data). Acetylcholine (Ach) is another neurotransmitter involved in the regulation of learning and memory process26. The rates of synthesis of serotonin, dopamine and the amino acids are reported to be sensitive to hypoxia by other workers27.
It may be inferred that hypoxia at HA may tantamount to being one of the factor such as decrement of cognitive processing speed and/or sensitivity of the brainstem neurons to auditory input among others to cause disturbance in cognitive function which manifests as prolongation in P300 latency.
Acknowledgments
The authors express their gratitude to all the volunteers who participated in the study.
References
- 1.Golob EJ, Johnson JK, Starr A. Auditory event-related potentials during target detection are abnormal in mild cognitive impairment. Clin Neurophysiol. 2002;113:151–61. doi: 10.1016/s1388-2457(01)00713-1. [DOI] [PubMed] [Google Scholar]
- 2.Salisbury DF, Rutherford B, Shenton ME, McCarley RW. Button-pressing affects P300 amplitude and scalp topography. Clin Neurophysiol. 2001;112:1676–84. doi: 10.1016/s1388-2457(01)00607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9:456–79. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hansenne M. The P300 cognitive event-related potential. II. The Inter individual variability and clinical application in psychopathology. Neurophysiol Clin. 2000;30:211–31. doi: 10.1016/s0987-7053(00)00224-0. [DOI] [PubMed] [Google Scholar]
- 5.Fowler B, Prlic H. A comparison of visual and auditory reaction time and P300 latency threshold to acute hypoxia. Aviat Space Environ Med. 1995;66:645–50. [PubMed] [Google Scholar]
- 6.Wesensten NJ, Crowley J, Balkin T, Kamimori G, Iwanyk E, Pearson N, et al. Effects of simulated high altitude exposure on long-latency event related brain potentials and performance. Aviat Space Environ Med. 1993;64:30–6. [PubMed] [Google Scholar]
- 7.Alexander MP, Geschwinal N. Dementia in the elderly. In: Albert ML, editor. Clinical neurology of aging. New York: Oxford University Press; 1984. pp. 259–76. [Google Scholar]
- 8.Katzman R, Terry RD, Bick KL. Alzheimer's disease: Senile dementia and related disorders. New York: Raven Press; 1978. [Google Scholar]
- 9.Tandon OP, Mahajan A. Averaged evoked potentials: event related potentials (ERPs) and their applications. Indian J Physiol Pharmacol. 1999;43:425–34. [PubMed] [Google Scholar]
- 10.Licht R, Homberg V. An introduction of methodology, Psychophysiological significance and clinical application. In: Colon EJ, Visser SL, editors. Evoked potentials manual. Netharlands: Kluwer; 1990. pp. 327–53. [Google Scholar]
- 11.Singh SB, Thakur L, Anand JP, Panjwani U, Yadav D, Selvamurthy W. Effect of high altitude (HA) on event related brain potentials. Indian J Physiol Pharmacol. 2003;47:52–8. [PubMed] [Google Scholar]
- 12.Singh SB, Thakur L, Anand JP, Yadav D, Amitab, Banerjee PK. Effect of chronic hypobaric hypoxia on components of the human event- related potential. Indian J Med Res. 2004;120:94–9. [PubMed] [Google Scholar]
- 13.Kendall MG, Sturt A. The advanced theory of statistics. III. New York: Hofner Press; 1973. [Google Scholar]
- 14.Hayashi R, Matsuzawa Y, Kubo K, Kobayashi T. Effect of simulated high altitude on event-related potential (P300) and auditory brain-stem responses. Clin Neurophysiol. 2005;116:1471–6. doi: 10.1016/j.clinph.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Fowler B, Kelso B. The effects of hypoxia on components of the human event-related potential and relationship to reaction time. Aviat Space Environ Med. 1992;63:510–6. [PubMed] [Google Scholar]
- 16.Fulco CS, Cymerman A. Human performance and acute hypoxia. In: KB Pandolf, Sawka MN, Gonzalez RR., editors. Human performance physiology and environmental medicine at terrestrial extremes. Indianapolis: Benchmark Press; 1988. pp. 467–95. [Google Scholar]
- 17.Hackett PH, Roach RC, Sutton JR. Management of wilderners and environmental emergencies. 2nd ed. St Louis: C.V. Mosby Company; 1989. High altitude medicine; pp. 1–34. [Google Scholar]
- 18.Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 19.Naatanen R. Processing negativity- an evoked potential reflection of selective attention. Psychol Bull. 1982;92:605–40. doi: 10.1037/0033-2909.92.3.605. [DOI] [PubMed] [Google Scholar]
- 20.Kida M, Imai A. Cognitive performance and event-related brain potentials under simulated high altitudes. J Appl Physiol. 1993;74:1735–41. doi: 10.1152/jappl.1993.74.4.1735. [DOI] [PubMed] [Google Scholar]
- 21.Kraaier V, Van Huffelen AC, Wieneke GH. Quantitative EEG changes due to hypobaric hypoxia in normal subjects. Electroencephalogr Clin Neurophysiol. 1988;69:303–12. doi: 10.1016/0013-4694(88)90002-8. [DOI] [PubMed] [Google Scholar]
- 22.Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–77. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- 23.Nash AJ, Fernandez M. P300 and allocation of attention in dual-task. Int J Psychophysiol. 1996;23:171–80. doi: 10.1016/s0167-8760(96)00049-9. [DOI] [PubMed] [Google Scholar]
- 24.Carrillo-de-la-Pena MT, Cadaveira F. The effect of motivational instructions on P300 amplitude. Neurophysiol Clin. 2000;30:232–9. doi: 10.1016/s0987-7053(00)00220-3. [DOI] [PubMed] [Google Scholar]
- 25.Panjwani U, Ray K, Chatterjee A, Bhaumik S, Kumar S. Electrophysiological correlates of cognition improve with nap during sleep deprivation. Eur J Appl Physiol. 2010;108:549–56. doi: 10.1007/s00421-009-1222-3. [DOI] [PubMed] [Google Scholar]
- 26.Freeman GB, Gibson GF. Dopamine, acetylcholine, and glutamate interactions in aging.b0 ehavioral and neurochemical correlates. Ann NY Acad Sci. 1988;515:191–202. doi: 10.1111/j.1749-6632.1988.tb32984.x. [DOI] [PubMed] [Google Scholar]
- 27.Freeman GB, Nielsen P, Gibson GE. Monoamine neurotransmitter metabolism and locomotor activity during chemical hypoxia. J Neurochem. 1986;46:733–8. doi: 10.1111/j.1471-4159.1986.tb13033.x. [DOI] [PubMed] [Google Scholar]


