Abstract
Background & objectives:
We evaluated pro- and anti-oxidant disturbances in sepsis and non-sepsis burn patients with systemic inflammatory response syndrome (SIRS). Adhesion molecules and inflammation markers on leukocytes were also analyzed. We hypothesized that oxidative stress and leukocyte activation markers can lead to the severity of sepsis.
Methods:
In 28 severe sepsis and 27 acute burn injury patients blood samples were collected at admission and 4 days consecutively. Oxidative stress markers: production of reactive oxygen species (ROS), myeloperoxidase, malondialdehyde and endogenous antioxidants: plasma protein sulphydryl groups, reduced glutathione, superoxide dismutase and catalase were measured. Flow cytometry was used to determine CD11a, CD14, CD18, CD49d and CD97 adhesion molecules on leukocytes. Procalcitonin, C-reactive protein, fibrinogen, platelet count and lactate were also analyzed.
Results:
Pro-oxidant parameters were significantly elevated in sepsis patients at admission, ROS intensity increased in burn patients until the 5th day. Endogenous antioxidant levels except catalase showed increased levels after burn trauma compared to sepsis. Elevated granulocyte activation and suppressed lymphocyte function were found at admission and early activation of granulocytes caused by increasing activation/migration markers in sepsis. Leukocyte adhesion molecule expression confirmed the suppressed lymphocyte and monocyte function in sepsis.
Interpretation & conclusions:
Severe sepsis is accompanied by oxidative stress and pathological leukocyte endothelial cell interactions. The laboratory parameters used for the evaluation of sepsis and several markers of pro- and antioxidant status were different between sepsis and non-sepsis burn patients. The tendency of changes in these parameters may refer to major oxidative stress in sepsis and developing SIRS in burns.
Keywords: Leukocyte activation, oxidative stress, severe sepsis
Acute organ dysfunction in severe sepsis and septic shock are major healthcare problems, affecting a large number of individuals each year, and increasing in incidence. The mortality is on the rise with the number of organ dysfunctions reaching a tremendous 80 per cent with the complication of four organ failures1. Using early goal-dirtected treatment the mortality of sepsis could be decreased to 25 per cent2.
According to recent epidemiology data Emergency Department visits for burn injury peaked on 2.8 per 1000 United States population in 1995 and decreased to 1.6 per 1000 in 20043. The skin dysfunction or total loss may cause infections, loss of body heat and increased evaporative loss of water4. The systemic effect of burn injuries includes the release of inflammatory cytokines5. The patients may develop sepsis based on these alterations. Fitzwater et al6 reported that sepsis patients after burn injury may develop multiple organ failure on day 8th of admission on average.
Free radicals, reactive oxygen species (ROS) are formed during a variety of biochemical reactions and cellular functions, and act as pro-oxidants. The formation of free radicals is normally balanced by antioxidants. Oxidative stress (OS) results from an imbalance between formation and neutralization of free radicals. Various pathologic processes disrupt this balance by increasing the formation of free radicals or decreasing the level of available antioxidants or both. Oxidative stress is a major contributing factor to the high mortality rates associated with several inflammatory and other diseases such as severe sepsis7. Oxidative stress plays an important role in oedema formation after burn injury8.
The therapy administered in the initial “golden hours” in severe sepsis is likely to influence the outcome9. Effective antimicrobial therapies can improve the outcome of septic shock10.
The aim of the present work was to evaluate pro- and anti-oxidant disturbances in severe septic patients and compare the oxidative stress status of sepsis patients with the group of ICU treated acute non-sepsis burn injury patients, and with the healthy controls also. Burned patients were used for comparison because after burn injury long lasting systemic inflammatory response syndrome (SIRS) develops without infection and they can be regarded as non-sepsis individuals.
We also analyzed the appearance of certain adhesion molecules and inflammation markers on granulocytes, lymphocytes and monocytes, and hypothesized that oxidative stress and leukocyte activation markers could reflect evolving sepsis.
Material & Methods
Patients: The study protocol was approved by Institutional Scientific and Human Research Ethics Committee of the University of Pécs, Hungary. The patients provided a written informed consent and they were informed clearly about the details of the study and blood sampling. Twenty eight sepsis and 27 burned trauma patients were admitted consecutively to our intensive care unit at the University of Pécs between November 2006 and November 2008. The treatment of septic patients for organ failure, medication used during supportive therapy and volume resuscitation were carried out according to currently applied guidelines9. Eight sepsis patients were administered low-dose steroids (hydrocortisone) and received wide range antibiotics (mostly carbapenem), four sepsis patients received immunoglobulins9. The results of measurements were compared to age-matched healthy volunteers who were invited from the outpatient clinic of opthalmology department as controls (n=18). After informed consent we took one blood sample (2.7 ml) from each healthy control.
Sepsis criteria: The diagnosis of sepsis was based on the ACCP/SCCM Consensus guideline11 whereas severe sepsis was approved by current score systems: New Simplified Acute Physiology Score (SAPS II)12, Sequential Organ Failure Assessment (SOFA) score13 and Multiple Organ Dysfunction Score (MODS)14. The MODS and SOFA scores were measured on the first, third, and fifth day.
Inclusion and exclusion criteria: Inclusion criteria for sepsis patients were two or more organ dysfunctions, hypoperfusion abnormality, or sepsis-induced hypotension15 and a higher than 2 ng/ml serum procalcitonin level. Exclusion criteria were the presence of any kind of baseline haematological disease, cytostatic treatment in the last 30 days, high dose steroid therapy, disseminated intravascular coagulation (DIC) score of ≥ 516, preterminal state and absence of consent to the study.
Inclusion criteria for burn trauma patients were the presence of flame burn injury affecting at least 20 per cent of body surface area (BSA) and in-hospital fluid resuscitation started within 3 hours after burn injury. BSA was estimated by the Lund–Browder chart17. If inhalation injury was suspected, bronchoscopy was performed for verification. Exclusion criteria included cytostatic treatment in the last one month, presence of haematological disease, medication influencing the inflammatory state (e.g., chronic use of steroids) extreme burn severity (BSA > 90% or Baux index > 120).
Blood sampling: Blood samples were collected via a radial artery cannula on admission and on the mornings of the next 4 consecutive days. Beside the daily standard laboratory tests (blood gas, blood cell counts, serum electrolytes, renal functions, etc.) the following parameters were measured: C-reactive protein (CRP, reference value /rv/: <10 mg/l), procalcitonin (PCT, rv: <0.5 ng/ml), lactate (rv: 0.63 - 2.44 mmol/l), fibrinogen (rv: 1.7 - 4 g/l). The blood test measurements were carried out at the Institute of Laboratory Medicine, University of Pécs.
Oxidative stress measurements: The measurements of oxidative stress and leukocyte activation markers were analyzed at the Department of Surgical Research and Techniques, University of Pécs using the methods as described earlier18. Blood samples for malondialdehyde (MDA) and myeloperoxidase (MPO) measurements were stored at –80°C and analyzed in a single batch at the end of the study. Malondialdehyde, a marker of lipid peroxidation was determined with Ohkawa method19. Free radical (ROS) generating capacity in whole blood was measured with a Whole Blood Lumi-aggregometer (Chrono-Log, Model 560, USA). This chemiluminescense method is based upon the reaction of luminol with free radicals. Phorbol-12-myristate-13-acetate (PMA) was used to induce free radical production and the resulting light output was recorded on a chart recorder (Chrono-Log, Model 707, USA). The peak value of free radical production was calculated from the recorded curve, and the results were related to the white blood cell (WBC) counts. The slope of steep elevation in radical production was also determined. Plasma myeloperoxidase level which is a sign of neutrophil granulocyte activation in plasma was measured spectophotometrically.
Endogenous antioxidant measurements: Both plasma protein sulphydryl groups (PSH) and reduced glutathione (GSH) were determined with Ellman's reagent20. Superoxide dismutase (SOD) was determined with the method of Misra and Fridovich21. Catalase enzyme activity in whole blood was determined by the method of Aebi22.
Leukocyte surface marker expression: Flow cytometry was used to analyze all adhesion molecules (CD11a, CD11b, CD18, CD49d), lipopolysaccharide (LPS) receptor CD14, and leukocyte activation marker CD97 expression on leukocytes18. Mouse anti- human monoclonal antibodies CD11a, CD11b, CD18, CD49d, CD14, and CD97 (BD Pharmingen, USA) conjugated with FITC or phycoerythrin were used for immunofluorescence staining of leukocytes. Cell immunofluorescence and light scatter data were acquired on a FACSCalibur (Becton Dickinson, New Jersey, USA) flow cytometer and analyzed by Cellquest software (Becton Dickinson, New Jersey, USA). Mouse isotype controls (BD Pharmingen, USA) were used to determine the non-specific background fluorescence. Binding of antibodies to leukocytes was quantified as the mean channel fluorescence in arbitrary units that exceeded non-specific background fluorescence. The values for healthy controls are listed in Table 1.
Table I.
Oxidative stress markers and endogenous antioxidant levels in healthy controls (n=18)
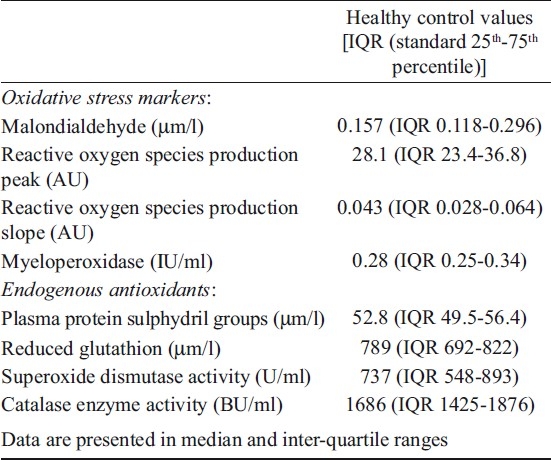
Statistical analysis: Data were expressed as median and inter-quartile range [IQR (standard 25th-75th percentile)]. Mann-Whitney test was used to compare the values of the two groups, and P<0.05 was considered significant.
Results
The demographic and characteristic parameters of patients are shown in Table II. Sepsis patients showed a steadily elevated white blood cell count during the 5 day period. Patients affected by thermal injury showed a constant decrease. A significant difference was observed in white blood cell count between the sepsis patients and burn trauma group on day 2 (P<0.05) and from day 3 until day 5 after admission (Fig. 1A, P<0.001). Severe granulocytosis 92.6 per cent (IQR 87.4-95.0), and lymphocytopenia 4.6 per cent (IQR 3.1-8.8) were observed on admission in sepsis patients which represented remarkably by the neutrophil/lymphocyte ratio. This ratio was different in sepsis patients compared to burn trauma group in all measured time (Fig. 1B, P<0.01). Platelet count levels were in the low-normal or low range during the study period in both groups but burn trauma patients showed significantly higher platelet count in the first two consecutive days (Fig. 1C, P<0.05).
Table II.
Demographic and characteristic data of sepsis study group and burn trauma controls
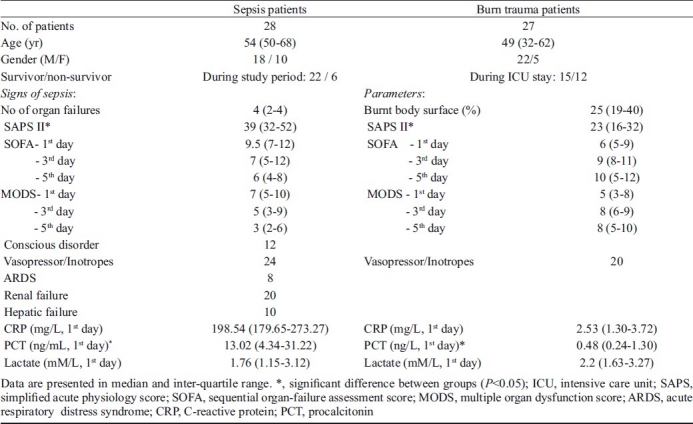
Fig. 1.
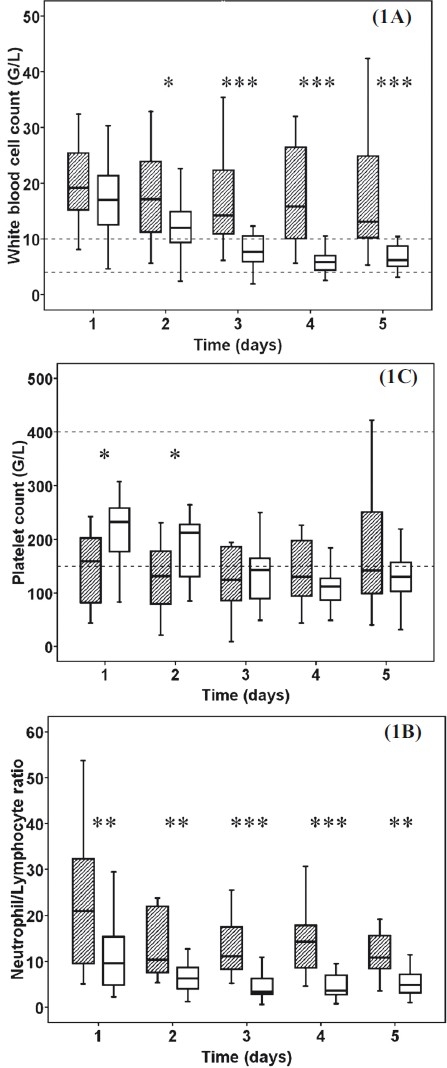
White blood cell count (1A), the ratio of neutrophils and lymphocytes (1B) and platelet count (1C) in severe sepsis and burn patients. Striped boxes show the data of sepsis patients, clear boxes represent the burn trauma patients. Broken lines show the normal ranges/local laboratory range. (Pvalues *<0.05; **<0.01; ***< 0.001, G/L means 106/L).
Procalcitonin levels were significantly elevated in severe sepsis patients compared to control on 1st, 2nd (P< 0.001), 3rd (P <0.05) days (Fig. 2A). While sepsis patients showed a steady decrease in PCT level in patients suffering from burn injury a slight increase developed until 4th day. C-reactive protein levels were different between the two groups on 1st (P< 0.001), 2nd, 4th, and 5th (P< 0.01) days (Fig. 2B). After thermal injury, CRP showed a remarkable increase in opposition to the moderate decrease in severe sepsis. Fibrinogen levels were above normal range in both groups except for the 1st day in burn patients. Although the severe sepsis group had significantly higher levels of fibrinogen on 1st (P<0.001) and 2nd (P<0.05) days, the burn trauma patients had elevated levels on the 5th (P<0.05) day compared to sepsis patients (Fig. 2C). Median lactate levels remained within the normal range during our study period in both groups (Fig. 2D).
Fig. 2.
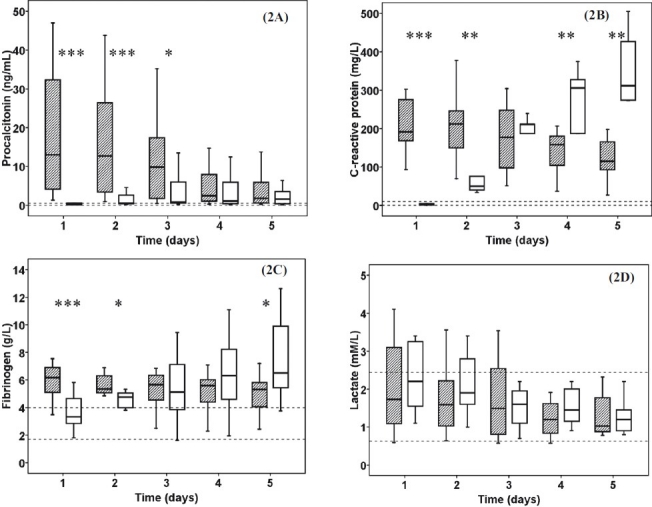
The procalcitonin (2A), C-reactive protein (2B), fibrinogen (2C) and lactate (2D) levels in severe sepsis and burn trauma patients. Striped boxes show the data of sepsis patients, clear boxes represent the thermal injury patients. Broken lines show the normal ranges/local laboratory range. (Pvalues *<0.05; **<0.01; ***< 0.001).
The plasma MDA concentration was elevated in both groups during the 5 day study period. In sepsis patients a significant increase of MDA levels developed compared to burn trauma patients on the 2nd (P<0.05) day (Fig. 3A). Activity of myeloperoxidase was higher in sepsis patients on the 1st, 2nd (P<0.05), 3rd (P<0.01) days (Fig. 3B). The PMA stimulated ROS production in whole blood was increased in sepsis patients on 1st (P<0.05) and 2nd (P< 0.01) days. Patients showed a steady but insignificant elevation in inducible ROS production after burn trauma (Fig. 3C). The maximum intensity of ROS production was continuously higher and showed significant differences on 4thand 5th (P<0.05) days after admission compared to sepsis group (Fig. 3D).
Fig. 3.
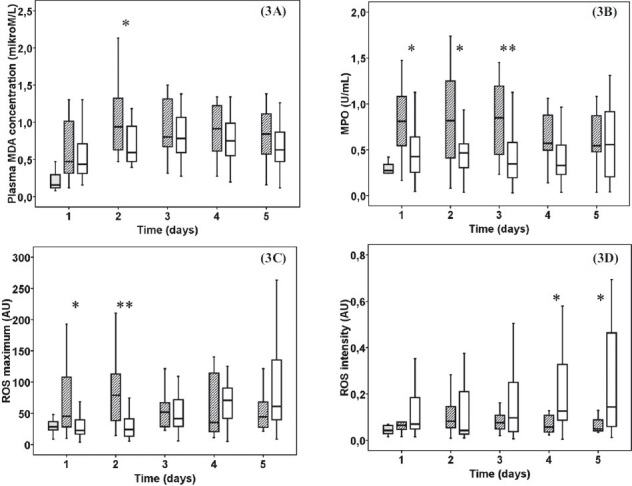
Oxidative stress markers: Plasma malondialdehyde (3A), myeloperoxidase (3B), peak value of reactive oxigen species production (ROS) (3C) and the intensity of ROS production (3D). Striped boxes show the data of sepsis patients, clear boxes represent the thermal injury patients, gray boxes show the normal healthy controls. (Pvalues *<0.05; **<0.01). AU, arbitrary unit.
The haemolysate GSH measurements revealed significantly elevated levels in burn trauma patients on 1st (P< 0.001) and 3rd (P<0.05) days (Fig. 4A) while PSH levels were higher only on 1st (P<0.05) day (Fig. 4B). Superoxide dismutase enzyme activity showed no significant alteration among the control, sepsis and thermal injury groups (Fig. 4C). Catalase activity was increased in sepsis patients on the 1st (P< 0.05) day compared to burn trauma group (Fig. 4D).
Fig. 4.
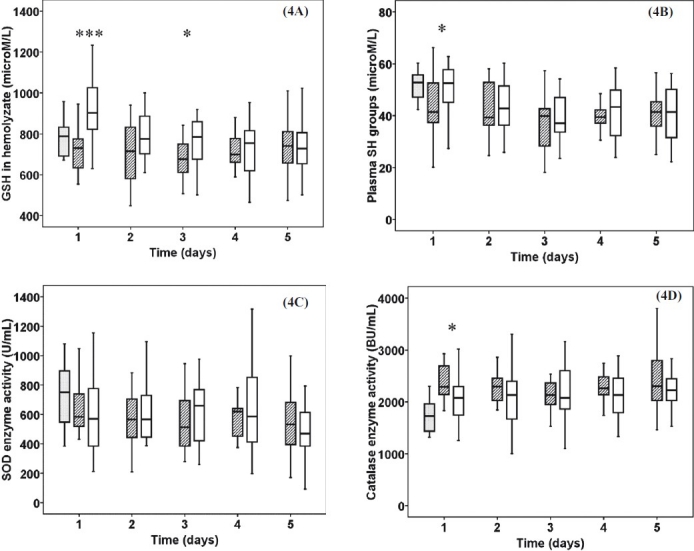
Antioxidant status: Reduced glutathione (GSH) (4A), plasma protein sulphydryl groups (PSH) (4B), superoxide dismutatase (SOD) activity (4C), catalyse enzyme activity (4D) in haemolyzate. Striped boxes show the data of sepsis patients, clear boxes represent the thermal injury patients, gray boxes show the normal healthy controls. (Pvalues *<0.05; ***< 0.001). BU, Bergmeyer unit.
Sepsis patients had remarkably elevated granulocyte CD11a expression on 1st (P<0.05) and 2nd (P<0.01) days (Fig. 5A). The granulocyte CD18 expression increased in the severe sepsis group on 1st (P<0.01) day (Fig. 5B). The CD49d expression on granulocytes was significantly higher in sepsis patients on 1st (P<0.01) day but increased in burn trauma patients until the 4th (P<0.05) day (Fig. 5C). Granulocyte CD97 expression was significantly elevated in sepsis patients on 1st, 2nd (P<0.001) and 3rd (P<0.05) days, with a moderate nonsignificant increase after thermal injury (Fig. 5D).
Fig. 5.
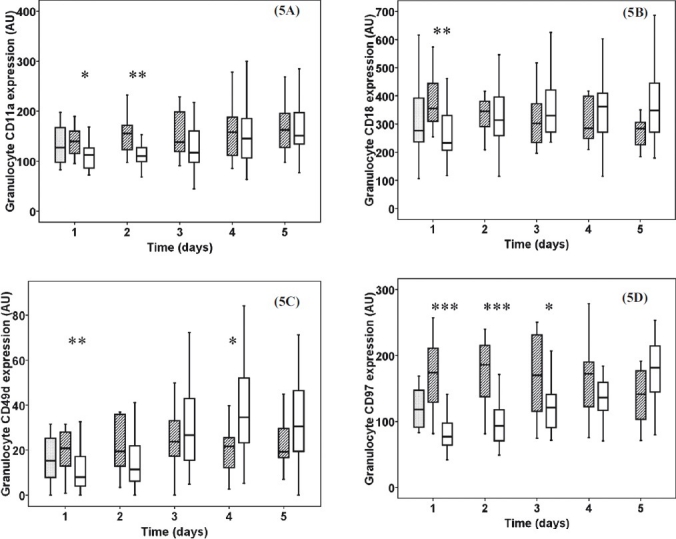
Granulocyte CD11a (5A), CD18a (5B) CD49d (5C) and CD97 (5D) expression in severe sepsis, burn injury and normal control patients. Striped boxes show the data of sepsis patients, clear boxes represent the thermal injury patients, gray boxes show the normal healthy controls. (Pvalues *<0.05; **<0.01; ***< 0.001). AU, arbitrary unit.
Lymphocyte surface CD18 expression was elevated in burn trauma patients on the 1st (P<0.05), 2nd (P<0.01) and 3rd (P<0.05) days ( Fig. 6A). The CD97 expression in lymphocytes was higher in sepsis patients on 1st, 3rd (P<0.01) and 4th (P<0.05) days (Fig. 6B). In burn trauma patients monocyte CD18 expression was increased on the 2nd, 3rd, 4th (P<0.01) and 5th (P<0.05) days of admission (Fig. 6C) while CD14 was elevated on the 1st (P<0.05), 2nd (P<0.01), 3rd (P<0.001), 5th (P< 0.05) days (Fig. 6D).
Fig. 6.
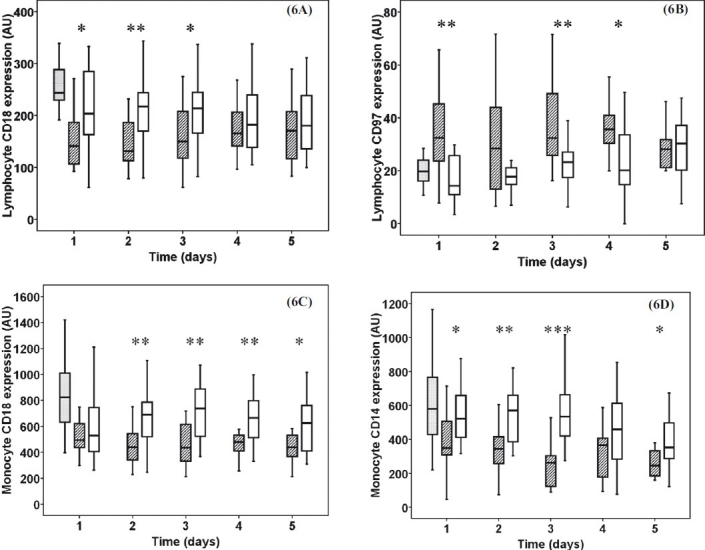
Lymphocyte CD18 (6A) and CD97 (6B) expression, monocyte CD18 (6C) and CD14 expression (6D). Striped boxes show the data of sepsis patients, clear boxes represent the thermal injury patients, gray boxes show the normal healthy controls. (Pvalues *<0.05; **<0.01; ***< 0.001). AU, arbitrary unit.
Discussion
Oxidative stress plays an important role in sepsis23. Previous studies confirmed severe oxidative stress in sepsis patients demonstrating reduced plasma, total antioxidant capacity, and elevated levels of malondialdehyde and 4-hydroxynonenal24.
In opposition with the burn group, severe sepsis patients had elevated leukocyte count and percentage distribution of granulocytes and lymphocytes on admission, typical findings of the presence of serious infection. The decreased lymphocyte count suggested the margination and extravasation of these cells25. The initially elevated inflammatory markers (PCT, CRP, and fibrinogen) proved the severity of sepsis26. The elevatation of CRP and fibrinogen levels from day 3 and normal PCT and lactate levels indicate that burn patients did not have sepsis but a systemic inflammation had developed. We are aware of the importance of lactate clearance in the survival of sepsis27, but we found no significant difference between the two patient groups in lactate levels.
Oxidative stress caused by sepsis induces a concomitant imbalance between the production of free radicals and endogenous available antioxidants24. Our results from the comparison of sepsis and burn patients proved that sepsis is associated with more pronounced free radical production than SIRS alone.
The decreased erythrocyte GSH and plasma SH group content in sepsis patients show the depletion of anti-oxidant resources on admission caused by prolonged OS, while acute burn patients gradually developed oxidative stress during our study period. Although GSH levels were decreased in sepsis patients on admission, our results cannot support Cherian's former findings on the non-significant change in erythrocyte GSH, SOD and thiobarbituric acid reactive substance levels in sepsis compared to controls based on the adaptive response of the body to combat the OS28. In accordance with the results of Kapoor we have found increased MDA production in sepsis patients during our study period29, but the catalase enzyme activity showed difference only on the 1st day between burned and sepsis patients.
Our study attempted a novel approach in comparing the changes in leukocyte adhesion molecule and inflammation marker expression in severe sepsis and burn trauma patients. Ibbotson et al found, that not only increased β2-integrin (CD11a/CD18) activity, but a functional presence of α4-integrin (CD49d/CD29) on sepsis human neutrophil granulocytes greatly enhances the adhesion of these cells in sepsis30. Sepsis patients showed more elevated expression of granulocyte surface markers in the initial phase of the study and our data confirms these findings at the time of admission. Severe acute burn patients show a slight increase in granulocyte adhesion molecule expression on the following days. The increased expression of the CD97 molecule suggests the early activation of granulocytes in sepsis patients31.
Contrary to neutrophils the lymphocytes and monocytes shows reduced CD18 expression compared to burn group. The reduced CD14 expression in sepsis patients correlate with the findings of Brunialti32. Our data suggests that although sepsis patients have increased granulocyte adhesion (CD11a, CD18, CD49D) on the first day, lymphocyte (CD18), monocyte (CD18, CD14) adhesion molecules were depressed. These findings suggest that both lymphocyte and monocyte function are suppressed in sepsis conditions although we found increased CD97 expression in lymphocytes, which refer to systemic inflammation.
We have found a moderate decrease of MODS and SOFA scores during our study period (Table II) which was parallel with the improving clinical status of our sepsis patients. The increase in the above clinical scores in burn patients suggests the deterioration of SIRS.
The role of oxidative stress in the development of sepsis is still unclear. In our study we compared the pro-oxidant/antioxidant status of patients with severe sepsis to another ICU-treated non-sepsis group. The markers of pro-oxidant status in sepsis patients showed severe OS and diminished antioxidant capacity on admission while we demonstrated a progressive development of OS in burn patients. The increased presence of activation/migration markers in sepsis patients suggests the early activation of granulocytes in the course of sepsis. Leukocyte adhesion molecule expression confirmed the suppressed lymphocyte and monocyte function in sepsis. Detailed study of oxygen radical-mediated mechanisms may lead to improved therapies in the treatment of critically ill patients.
Acknowledgments
This work was supported by the Hungarian Scientific Research Fund OTKA K060227 grant. The authors acknowledge Professor Naranjan Dhalla for giving support, and thank the laboratory assistants and the nurses of the intensive care unit at the department of Anaesthesia and Intensive Therapy for their enormous contribution.
References
- 1.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29(7 Suppl):S109–16. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 3.Fagenholz PJ, Sheridan RL, Harris NS, Pelletier AJ, Camargo CA. National study of emergency department visits for burn injuries, 1993 to 2004. J Burn Care Res. 2007;28:681–90. doi: 10.1097/BCR.0B013E318148C9AC. [DOI] [PubMed] [Google Scholar]
- 4.Maike K, David HH, Lars PK, Manfred F, Marc GJ. Pathophysiology of burns. Wien Med Wochenschr. 2009;159:327–36. doi: 10.1007/s10354-009-0651-2. [DOI] [PubMed] [Google Scholar]
- 5.Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol. 1993;23:21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- 6.Fitzwater J, Purdue GF, Hunt JL, O’Keefe GE. The risk factors and time course of sepsis and organ dysfunction after burn trauma. J Trauma. 2003;54:959–66. doi: 10.1097/01.TA.0000029382.26295.AB. [DOI] [PubMed] [Google Scholar]
- 7.Everton Andrades M, Ritter C, Dal-Pizzol F. The role of free radicals in sepsis development. Front Biosci (Elite Ed) 2009;1:277–87. doi: 10.2741/E27. [DOI] [PubMed] [Google Scholar]
- 8.Demling RH. The burn edema process: Current concepts. J Burn Care Rehab. 2005;26:207–27. [PubMed] [Google Scholar]
- 9.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 11.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 12.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. doi: 10.1001/jama.270.24.2957. Erratum in: JAMA 1994; 4 : 1321. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 14.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–52. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 16.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–30. [PubMed] [Google Scholar]
- 17.Malic CC, Karoo RO, Austin O, Phipps A. Resuscitation burn card--a useful tool for burn injury assessment. Burns. 2007;33:195–9. doi: 10.1016/j.burns.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Mühl D, Füredi R, Cristofari J, Ghosh S, Bogár L, Borsiczki B, et al. Evaluation of oxidative stress in the thrombolysis of pulmonary embolism. J Thromb Thrombolys. 2006;22:221–8. doi: 10.1007/s11239-006-9035-2. [DOI] [PubMed] [Google Scholar]
- 19.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Sedlak J, Lindsay RH. Estimation of total protein-bound and non-protein sulphydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 21.Misra HP, Fridovich I. The role of superoxide anion in the antioxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;27:3170–5. [PubMed] [Google Scholar]
- 22.Aebi H. Catalase in vitro. Method Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 23.Crimi E, Sica V, Slutsky AS, Zhang H, Williams-Ignarro S, Ignarro LJ, et al. Role of oxidative stress in experimental sepsis and multisystem organ dysfunction. Free Radic Res. 2006;40:665–72. doi: 10.1080/10715760600669612. [DOI] [PubMed] [Google Scholar]
- 24.Alonso de Vega JM, Díaz J, Serrano E, Carbonell LF. Oxidative stress in critically ill patients with systemic inflammatory response syndrome. Crit Care Med. 2002;30:1782–6. doi: 10.1097/00003246-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–96. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 26.Hatherill M, Tibby SM, Turner C, Ratnavel N, Murdoch IA. Procalcitonin and cytokine levels: relationship to organ failure and mortality in pediatric septic shock. Crit Care Med. 2000;28:2591–4. doi: 10.1097/00003246-200007000-00068. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–42. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 28.Cherian S, Jameson S, Rajarajeswari C, Helena V, Latha L, Anu Rekha MR, et al. Oxidative stress in sepsis in children. Indian J Med Res. 2007;125:143–8. [PubMed] [Google Scholar]
- 29.Kapoor K, Basu S, Das BK, Bhatia BD. Lipid peroxidation and antioxidants in neonatal septicemia. J Trop Pediatr. 2006;52:372–5. doi: 10.1093/tropej/fml013. [DOI] [PubMed] [Google Scholar]
- 30.Ibbotson GC, Doig C, Kaur J, Gill V, Ostrovsky L, Fairhead T, et al. Functional alpha4-integrin: a newly identifi ed pathway of neutrophil recruitment in critically ill septic patients. Nat Med. 2001;7:465–70. doi: 10.1038/86539. [DOI] [PubMed] [Google Scholar]
- 31.Leemans JC, Velde AA, Florquin S, Bennink RJ, Bruin K, van Lier RAW, et al. The Epidermal Growth Factor-Seven Transmembrane (EGF-TM7) receptor cd0 97 is required for neutrophil migration and host defense. J Immunol. 2004;172:1125–31. doi: 10.4049/jimmunol.172.2.1125. [DOI] [PubMed] [Google Scholar]
- 32.Brunialti MKC, Martins PS, Barbosa de Carvalho H, Machado FR, Barbosa LM, Salomao R. Clinical Aspects Tlr2, Tlr4, Cd14, Cd11b, and Cd11c expressions on monocytes surface and cytokine production in patients with sepsis, severe sepsis, and septic shock. Shock. 2006;25:351–7. doi: 10.1097/01.shk.0000217815.57727.29. [DOI] [PubMed] [Google Scholar]


