Abstract
Background & objectives:
The human system possesses antioxidants that act harmoniously to neutralize the harmful oxidants. This study was aimed to evaluate the serum total antioxidant capacity (TAC) as a single parameter in Eales’ disease (ED) and in an acute inflammatory condition such as uveitis and in cataract which is chronic, compared to healthy controls.
Methods:
The TAC assay was done spectrophotometrically in the serum of Eales’ disease cases (n=20) as well as in other ocular pathologies involving oxidative stress namely, uveitis and cataract (n=20 each). The oxidative stress measured in terms of TBARS, was correlated with the TAC. Individual antioxidants namely vitamin C, E and glutathione were also estimated and correlated with TAC.
Results:
TAC was found to be significantly lower in Eales’ disease with active vasculitis (0.28 ± 0.09 mM, P<0.001), Eales’ disease with healed vasculitis (0.67 ± 0.09 mM), uveitis (0.46 ± 0.09 mM, P<0.001) and cataract (0.53 ± 0.1 mM, P=0.001) compared to the healthy controls, with a TAC level of 0.77 ± 0.09 mM. The TAC was found to correlate positively with vitamin E levels (P=0.05), GSH (P=0.02) but not with vitamin C, as seen in ED cases. In ED cases supplemented with vitamin E and C, there was a significant increase in the TAC level (P=0.02).
Interpretation & conclusions:
The TAC measurement provided a comprehensive assay for establishing a link between the antioxidant capacity and the risk of disease as well as monitoring antioxidant therapy. This method is a good substitute for assay of individual antioxidants as it clearly gives the status of the oxidative stress in the disease process.
Keywords: Antioxidant, cataract, Eales' disease, oxidants, uveitis
Cells and biological fluids in the human system have an array of protective antioxidant mechanisms, both for preventing the production of free radicals (O2•, OH•, ROO•, RO•, NO•) and for repairing oxidative damage1,2. Any imbalance due to an excess oxidant formation or lowered antioxidants leads to an oxidative stress damaging lipids, proteins and nucleic acids which leads to inflammation followed by tissue injury and cell death.
To assess the status of oxidative stress, it is usually necessary to estimate the oxidants markers and the antioxidant parameters. TBARS (Thiobarbituric acid reactive substances) are direct markers of oxidative stress. The antioxidant parameters that can be analyzed are the non-enzymatic ones like vitamin A, E and C, glutathione, apart from the enzymatic ones like catalase, superoxide dismutase (SOD) and glutathione peroxidase (GPx). To study all these parameters is tedious, time consuming and uneconomical. To replace the analysis of the various antioxidants with a single test, the total antioxidant capacity (TAC) assay is done in normal and pathological conditions3,4. According to the method by Koracevic et al5 TAC measures predominantly low molecular weight, chain breaking antioxidants (urate, ascorbate, bilirubin, albumin and thiols in the aqueous phase and α-tocopherol, carotenoids, and flavonoids in the lipid phase), excluding the contribution of antioxidant enzymes and metal binding proteins. The ability of the antioxidants to suppress the formation of TBARS by OH•, is called direct inhibition by antioxidants and TAC assay is a direct inhibition assay.
Oxidative stress is considered a major factor contributing to the pathogenesis of the Eales’ disease (ED) which is a relatively rare disease observed in the Indian subcontinent in healthy adult males6,7. ED is an idiopathic inflammatory venous occlusion that primarily affects the peripheral retina of adults8. Stages of ED broadly include stage of retinal phlebitis, stage of peripheral nonperfusion, and stage of retinal neovascularization7. Previous work in our laboratory has proved the role of free radicals in increasing the markers of lipid peroxidation namely, TBARS9,10, protein oxidation products as carbonyl content11, nitrotyrosine12 and DNA oxidative products as 8-hydroxy guanosine in ED13,14. Oxidative stress has also been reported in the platelets of Eales’ disease cases15,16. Uveitis is another inflammatory process involving the anterior segment of the eye. Inflammation of the uvea is termed iridocyclitis. In uveitis, metabolites of oxygen-free radicals generated by polymorphonuclear leukocytes and macrophages are believed to inflict the initial tissue damage in acute inflammation17. Studies have shown the involvement of superoxide, nitric oxide and peroxynitrite in experimental autoimmune uveitis18. While ED and uveitis involve acute and active periods of inflammation and oxidative stress, cataract is more of a chronic condition exhibiting oxidative stress with ageing. Cataract formation is one of the many destructive changes that can occur with overproduction of oxidants, possibly due to deficiency of an important protective antioxidant called glutathione. Glutathione occurs in high levels in the eye and helps clean up these free radicals. One theory posits that in the ageing eye, barriers develop that prevent glutathione and other protective antioxidants from reaching the nucleus in the lens, thus making it vulnerable to oxidation19,20.
Since TAC measurement can provide a quick tool for establishing a link between oxidant stress and the severity of the disease as well as in monitoring of antioxidant therapy, the present study was aimed to estimate the serum total antioxidant capacity (TAC) and compare it with individual antioxidants such as glutathione (GSH), vitamin E and C apart from the oxidative stress marker TBARS in ocular inflammatory diseases such as Eales’ disease, in a more acute inflammatory condition as in uveitis as well as in cataract that is more chronic, in comparison to healthy controls.
Material & Methods
Subjects: Eales’ disease - After the approval for two years pilot study from the institutional research board, patients were proved clinically by ophthalmologists based on fundus examination using indirect ophthalmoscopy and by laboratory investigations13. The study was conducted at Sankara Nethralaya, a tertiary eye hospital in South India in the period of February 2005 - January 2007. ED was diagnosed on the basis of the following criteria: periphlebitis of the retina, neovascularization and venous insufficiency (stages Ia-IIIb) according to the classification of Saxena & Kumar21 and not associated with anterior uveitis, choroiditis, pars planitis or other retinal vascular diseases which mimic ED. Clinically, “active state” of the disease was associated with the inflammation, perivasculitis and venous insufficiency. Absence of inflammation, peripheral venous sheathing or sclerosis, with retinal ischaemia and occlusion of retinal capillaries, indicated the “inactive” (healed) state. Patients with ED and control groups were non-diabetic, non-obese, non-smoking and non-alcoholic. A clinical proforma, which was used in the study, followed the stipulated inclusion/exclusion criteria. The inclusion criteria were based on inflammation, primary phlebitis of the retina, neovascularization. The exclusion criteria included vitreous haemorrhage not associated with anterior uveitis, choroiditis, pars planitis, diabetes mellitus, cataract, glaucoma, optic atrophy, corneal opacities or macular degeneration. Patients who took vitamins, aspirin or persantine for three days prior to the test were excluded. Blood (10 ml) samples from 20 consecutive patients with ED (active and healed) were obtained after their written consent. The differential diagnosis of ED was made by performing routine haematological, biochemical and immunological tests as described earlier12.
Eales’ disease patients on vitamin supplements and the placebo group - Patients diagnosed with ED were randomized and separated into two groups namely the placebo (n=10) and the vitamin-supplemented (n=10). All patients were treated with oral Prednisone, 1.0 mg/kg body weight everyday for one week, and tapering by 10 mg per week for 6 to 8 wk as they were in active vasculitis stage on admission. 400 IU of vitamin E and 500 mg vitamin C were given daily as oral antioxidant supplements to 10 patients. Patients who received Kieselguhr, a form of silica that is completely excreted, formed the placebo group. Blood samples were collected for the analysis during their first visit: base line (0 wk) and 18th wk.
Uveitis cases - Blood samples obtained in the lab from 20 consecutive patients with uveitis were utilized for the study. Uveitis associated with primary periphlebitis of the retina, neovascularisation and vitreous haemorrhage, diabetes mellitus, cataract, glaucoma, optic atrophy, corneal opacities or macular degeneration were excluded. Patients who had taken vitamins, asprin, or persantine, 3 days prior to the test were also excluded from the study.
Cataract cases - Blood samples from patients with cataract (n=20) were utilized as a non-inflammatory ocular disease control. Cataracts associated with systemic hypertension, proliferative diabetic retinopathy, retinal haemorrhage, chronic bronchitis, bronchial asthma, RPE defect or any other ocular problems were excluded.
Healthy controls - Blood samples from normal healthy subjects (n=19) with no ocular or other systemic problems, who were volunteers from the institute were included in the study.
Serum total antioxidant capacity (TAC) assay: TAC estimation involved, Fe-EDTA complex formation that reacted with hydrogen peroxide by a Fenton type reaction, leading to the formation of hydroxyl radicals (•OH), a reactive oxygen species that degraded benzoate, resulting in the TBARS formation. Antioxidants present in the added serum causes suppression of the TBARS. The serum's capacity to suppress this TBARS formation was designated as the total antioxidant capacity of the serum. The fall in the absorbance was read spectrophotometrically at 535 nm. TAC was expressed as antioxidant activity (AOA) in mmol/l5.
Plasma TBARS: Malondialdehyde and similar compounds produced during peroxidation of lipids reacted with thiobarbituric acid to generate pink colored chromogen, with the absorbance read spectrophotometrically at 532 nm22.
Serum vitamin E: This method was based on the reduction of ferric to ferrous ions by tocopherols, which formed a red complex with α,α’-dipyridyl. Tocopherols and carotenes were first extracted into xylene and the intensity of colour was read at 460 nm to measure the carotenes. A correction was made for this after adding FeCl3 colour intensity and read at 520 nm23.
Plasma vitamin C: The ascorbic acid in the plasma was oxidized by Cu (II) to form dehydroascorbic acid, which reacted with acidic 2, 4-dinitrophenylhydrazine to form a red bis-hydrazine, which was measured at 520 nm24.
Plasma glutathione: Plasma GSH was measured by fluorimetry using o-phthalaldehyde as a fluorescent reagent at pH 8.0 with excitation at 350 nm and emission at 420 nm25.
Statistical analysis: All values were expressed as mean ± SD. Statistical significance of the data was determined by Student's ‘t’ test to compare means. Differences were considered significant at P<0.05. Pearson's correlation coefficient test was employed to assess the relationship between TAC levels with vitamin E, C and GSH, P<0.05 was considered significant. Statistical analysis was done using SPSS software version 14.0 (Ilinois, USA).
Results
Age distribution and gender ratio for the ED group was 30 ± 7 yr, M:10, F: 0 in cases with active vasculitis and 36 ± 10 yr, M:10, F:0 for healed vasculitis and for placebo group and the vitamin supplemented group was found to be 27 ± 7 yr (M:10, F: 0) and 31± 9 yr (M:10, F:0) respectively. The cataract cases had a mean age of 58 ± 10 yr with M: 12, F: 8, while in the uveitis group it was 45 ± 13 yr with M: 15 and F: 5 and healthy controls were 42 ± 6 yr with M: 17, F:2.
Total antioxidant capacity in the normal healthy subjects was found to be 0.77 ± 0.09 mM. (range 0.61 to 0.91 mM) This was significantly reduced to 0.28 ± 0.09 mM (P<0.001) in the active stages of ED cases and 0.67 ± 0.09 mM in healed vasculitis. The ED cases also showed a significant decrease in the levels of the individual small molecule antioxidants namely vitamin C (P<0.001), vitamin E (P<0.001) and GSH (P<0.01). ED active cases also showed a significant increase (P<0.001) in the TBARS levels compared to controls. In healed cases of ED, the AO levels, except GSH were significantly higher than the active cases. This was reflected in the TAC which was also significantly (P<0.001) elevated (Table I). The TAC was found to correlate positively with vitamin E (P<0.05 in active ED; P<0.001 in healed ED), GSH (P=0.02 in active ED and P<0.05 in healed ED) but not with vitamin C, as seen in both the healthy subjects and in ED cases (Fig. 1). However, negative correlation of TAC with TBARS was observed only in the healthy controls data not shown.
Table I.
Comparison of TAC and individual antioxidant parameters between healthy controls and Eales’ disease
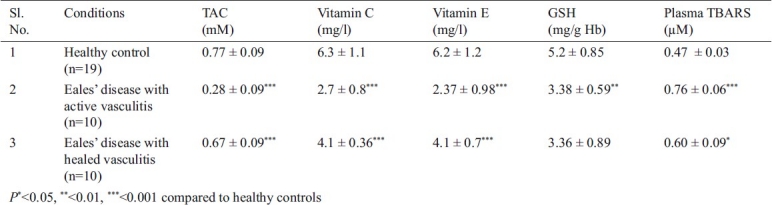
Fig. 1.
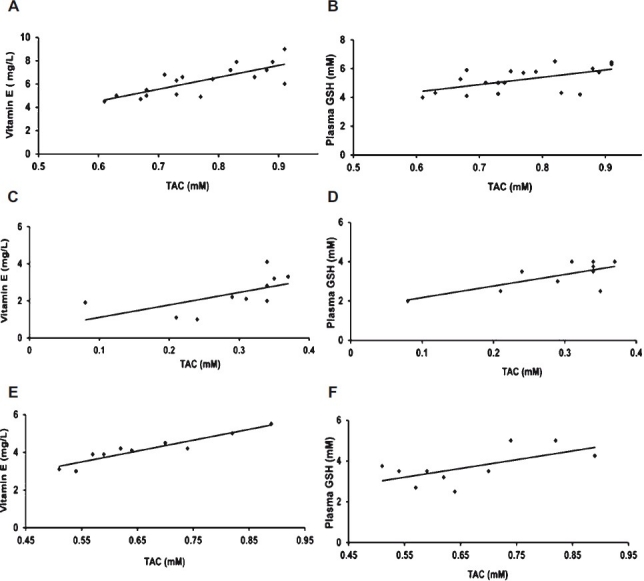
Correlation between TAC, vitamin E & GSH in Eales’ disease and healthy control. A: A positive correlation between TAC & vitamin E in healthy control (P<0.001). B: A positive correlation between TAC & GSH in healthy control (P=0.01). C: A positive correlation between TAC & vitamin E in active Eales’ disease (P=0.05). D: A positive correlation between TAC & GSH in active Eales’ disease (P=0.02). E: A positive correlation between TAC & vitamin E in healed Eales’ disease (P<0.001). F: A positive correlation between TAC & GSH in healed Eales’ disease (P=0.05).
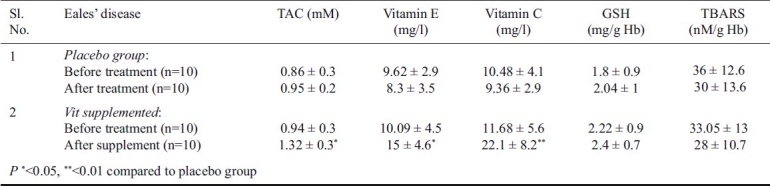
There was a significant increase in the vitamin C (P<0.01) and E levels (P<0.05) on the supplement of the same which was reflected in the TAC values that was significantly increased (P<0.05). There was no change in TAC as there was no significant change in the individual antioxidants E and C in the placebo group. A decrease, though not significant, was seen in TBARS in the supplemented group. However, no change in the GSH levels was observed in the placebo and supplement groups (Table II). Probably, a longer supplement duration and larger sample size was warranted.
Table II.
TAC, antioxidants and the TBARS levels in Eales’ disease before and after vitamin E and vitamin C supplement
The TAC value was the least in the ED in active form followed by uveitis with a TAC level of 0.46 ± 0.09 mM (P<0.001), cataract with a TAC level of 0.53 ± 0.1 mM (P<0.001) compared to the control group (Fig. 2A). Correspondingly, the vitamin C and E levels were found to be significantly lower in these conditions (Fig. 2B,2C). GSH was found to be lowered significantly in the active ED (P=0.002), Uveitis (P=0.01) and cataract (P<0.001) compared to the control (Fig. 2D). The TBARS levels increased significantly in the active ED, uveitis and cataract respectively (Fig. E)
Fig. 2 A-E.
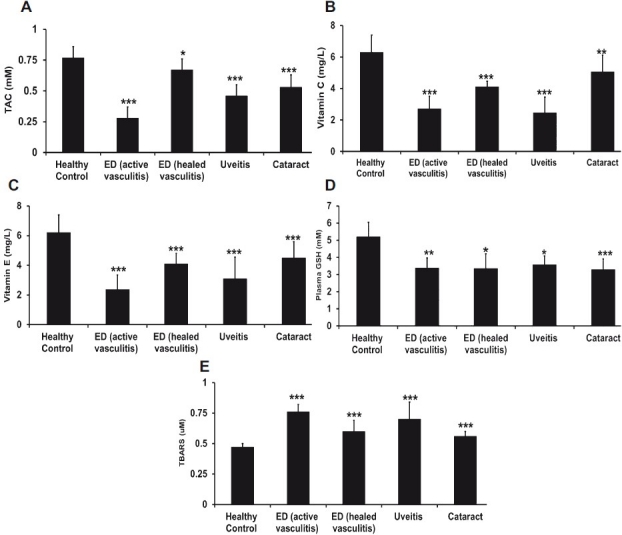
Comparison of various antioxidant and oxidant parameters such as TAC, vitamin E, vitamin C, GSH among disease and healthy controls. Statistical signifi cance (P<0.05) is comparison between control (n=19) and Eales’ disease (n=20), uveitis (n=20), cataract (n=20). P value, *<0.05, **<0.01, ***<0.001.
A significant positive correlation was seen between the TAC and GSH levels in uveitis (P=0.004) and cataract (P<0.004). Similarly, the TAC vs vitamin E levels in uveitis showed a significant positive correlation (P<0.004). This was, however, not significant in cataract (Fig. 3). This was probably because the vitamin E deficiency was more prominent in uveitis than in cataract.
Fig. 3.
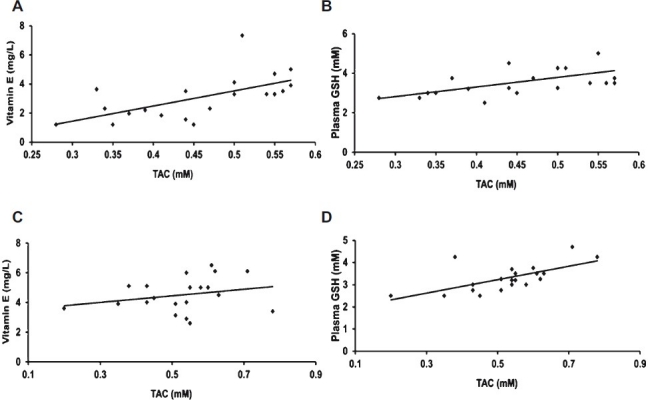
Correlation between TAC, vitamin E & GSH in uveitis and cataract. A&B: A significant (P=0.004) positive correlation between TAC, vitamin E & GSH in uveitis. C&D: A significant (P=0.004) positive correlation between TAC, vitamin E & GSH in cataract.
Discussion
Our previous studies and those of others9–16 have documented an increase in oxidative stress markers with decreased antioxidant levels as estimated in erythrocytes, vitreous, platelets and monocytes in ED cases9–14. In addition to regular anti-inflammatory steroids, when the ED cases were supplemented with antioxidants namely vitamins E and C, the ratio of Fe3+/Fe2+ and TBARS lowered significantly, with a desirable clinical trend shifting from active state of the disease to the healed state26. In addition to ED, in all the disease status in which the TAC was estimated, there was a significant lowering of TAC while the TBARS increased significantly. In Eales’ disease with active vasculitis, TBARS increased significantly by 48 per cent compared to control; whereas in healed vasculitis TBARS increased by 28 per cent. Similarly, the TBARS in uveitis increased by 48 per cent, while in cataract it increased by 20 per cent. TBARS correlated with the disease severity in the order of ED, uveitis and cataract. In the same order, TAC was found to be decreased. There was a 70 per cent decrease of TAC in Eales’ disease with active vasculitis compared to healthy controls while in healed vasculitis it was only 10 per cent. This showed that the active stage of vasculitis was severe with less antioxidant levels than in healed vasculitis. Therefore, TAC can be used as a good prognostic marker. In uveitis, TAC decreased by 40 per cent, while in cataract there was a 30 per cent decrease. Thus TAC seems to correlate with the disease severity in the order of ED, uveitis and cataract. This study also showed that with the vitamin supplements in ED cases, TAC was significantly improved.
TAC estimation has been done in many studies including diabetes mellitus4, stroke27 and liver transplantation28. It has also been used as an early biomarker in HIV-1 infected patients to help monitor and optimize antioxidant therapy in such patients29. In contrast to lowered TAC in these conditions, elevated serum TAC level reflected clinical severity as in sepsis30. TAC in breast milk was reported to reflect the maternal TAC status which was found to be lowered in the mature milk compared to colostrums during the course of lactation31. Thus TAC seems to be a good marker of the oxidative stress implicated in a variety of pathological events in the ocular diseases apart from ED, uveitis, cataract such as in central retinal vein occlusion32, and glaucoma33. Thus, the total antioxidant capacity considers the cumulative of all the small molecule antioxidants present in plasma and body fluids.
Biological fluids contain numerous compounds with chain breaking antioxidant activity, including uric acid, ascorbic acid, bilirubin, and thiols in the aqueous phase and α-tocopherol, carotenoids, and flavanoids in the lipid phase. It is thought that the co-operation of antioxidants in human serum provides greater protection against attack by free radicals than any of the sole antioxidant. Though TAC did not indicate the role of enzymatic antioxidants, the levels correlated with TBARS indicating the major role of small molecule antioxidants. A low total antioxidant capacity could be indicative of oxidative stress or increased susceptibility to oxidative damage. Since the ROS removal rate is mostly controlled by a variety of low molecular weight antioxidants, the concept of a single test that can reflect TAC can be indicative5. This method is also rapid, reliable and practical for the routine measurement in serum.
Acknowledgments
This work was supported by grant from Vision Research Foundation, Chennai, Tamil Nadu.
Footnotes
Conflict of Interest: The authors have no existing competing financial interests with respect to publication of this work.
References
- 1.Reiter RJ. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. 1995;9:526–33. [PubMed] [Google Scholar]
- 2.Halliwell B. Antioxidants in hyuman health and disease. Annu Rev Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 3.Dasgupta A, Malhotra D, Levy H, Marcadis D, Blackwell W, Johnston D. Decreased total antioxidant capacity but normal lipid hydroperoxide concentrations in sera of critically ill patients. Life Sci. 1997;60:335–40. doi: 10.1016/s0024-3205(96)00634-0. [DOI] [PubMed] [Google Scholar]
- 4.Ceriello A, Bortolotti N, Pirisi M, Crescentini A, Tonutti L, Motz E, et al. Total plasma antioxidant capacity predicts thrombosis-prone status in NIDDM patients. Diabetes Care. 1997;20:1589–93. doi: 10.2337/diacare.20.10.1589. [DOI] [PubMed] [Google Scholar]
- 5.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–61. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das T, Biswas J, Kumar A, Nagpal PN, Namperumalsamy P, Patnaik B, et al. Eales’ disease. Indian J Ophthalmol. 1994;42:3–18. [PubMed] [Google Scholar]
- 7.Angayarkanni N, Selvi R, Pukhraj R, Biswas J, Bhavesh SJ, Tombran-Tink J. Ratio of the vitreous vascular endothelial growth factor and pigment epithelial-derived factor in Eales’ disease. J Ocul Biol Dis Inform. 2009;2:20–8. doi: 10.1007/s12177-009-9017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura SJ, Carriker FR, Hogen MJ. Retinal vasculitis with intra ocular hemorrhage. Classification and results of special studies. Arch Opthalmol. 1956;56:361–7. doi: 10.1001/archopht.1956.00930040369005. [DOI] [PubMed] [Google Scholar]
- 9.Bhooma V, Sulochana KN, Biswas J, Ramakrishnan S. Eales’ disease: accumulation of reactive oxygen intermediates and lipid peroxides and decrease of antioxidants causing inflammation, neovascularization and retinal damage. Curr Eye Res. 1997;16:91–5. doi: 10.1076/ceyr.16.2.91.5096. [DOI] [PubMed] [Google Scholar]
- 10.Sulochana KN, Biswas J, Ramakrishnan S. Eales’ disease: increased oxidation and peroxidation products of membrane constitutents chiefly lipids and decreased antioxidant enzymes and reduced glutathione in vitreous. Curr Eye Res. 1999;19:254–9. doi: 10.1076/ceyr.19.3.254.5312. [DOI] [PubMed] [Google Scholar]
- 11.Rajesh M, Sulochana KN, Coral K, Punitham R, Biswas J, Babu K, et al. Determination of carbonyl groups content in plasma proteins as a useful marker to assess the impairment in antioxidant defense in patients with Eales’ disease. Indian J Ophthalmol. 2004;52:139–44. [PubMed] [Google Scholar]
- 12.Rajesh M, Sulochana KN, Punitham R, Biswas J, Lakshmi S, Ramakrishnan S. Involvement of oxidative and nitrosative stress in promoting retinal vasculitis in patients with Eales’ disease. Clin Biochem. 2003;36:377–85. doi: 10.1016/s0009-9120(03)00058-4. [DOI] [PubMed] [Google Scholar]
- 13.Rajesh M, Ramesh A, Ravi PE, Balakrishnamurthy P, Coral K, Punitham R, et al. Accumulation of 8-hydroxydeoxyguanosine and its relationship with antioxidant parameters in patients with Eales′disease: implications for antioxidant therapy. Curr Eye Res. 2003;27:103–10. doi: 10.1076/ceyr.27.2.103.15951. [DOI] [PubMed] [Google Scholar]
- 14.Rajesh M, Sulochana KN, Ramakrishnan S, Biswas J, Manoharan PT. Iron chelation abrogates excessive formation of hydroxyl radicals and lipid peroxidation products in monocytes of patients with Eales’ disease: direct evidence using electron spin resonance spectroscopy. Curr Eye Res. 2004;28:399–407. doi: 10.1080/02713680490503723. [DOI] [PubMed] [Google Scholar]
- 15.Saxena S, Srivastava P, Kumar D, Khanna VK, Seth PK. Decreased platelet membrane fluidity in retinal periphlebitis in Eales’ disease. Ocul Immunol Inflamm. 2006;14:113–6. doi: 10.1080/09273940600557043. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava P, Saxena S, Khanna VK, Kumar D, Nath R, Seth PK. Raised platelet thiobarbituric acid-reacting substances in proliferative Eales’ disease. Indian J Ophthalmol. 2000;48:307–9. [PubMed] [Google Scholar]
- 17.Rao NA, Romero JL, Fernandez MA, Sevanian A, Marak GE., Jr Effect of iron chelation on severity of ocular inflammation in an animal model. Arch Ophthalmol. 1986;104:1369–71. doi: 10.1001/archopht.1986.01050210123038. [DOI] [PubMed] [Google Scholar]
- 18.Wu GS, Zhang J, Rao NA. Peroxynitrite and oxidative damage in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 1997;38:1333–9. [PubMed] [Google Scholar]
- 19.Varma SD, Hegde K, Henein M. Oxidative damage to mouse lens in culture. Protective effect of pyruvate. Biochim Biophys Acta. 2003;1621:246–52. doi: 10.1016/s0304-4165(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 20.Giblin FJ. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther. 2000;16:121–35. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- 21.Saxena S, Kumar D. A new staging system for idiopathic retinal periphlebitis. Eur J Ophthalmol. 2004;14:236–9. doi: 10.1177/112067210401400308. [DOI] [PubMed] [Google Scholar]
- 22.Ledwozyw A, Michalak J, Stepien A, Kadziolka A. The relationship between plasma triglycerides, cholesterol, total lipids, and lipid peroxidation products during human atherosclerosis. Clin Chim Acta. 1986;155:275–83. doi: 10.1016/0009-8981(86)90247-0. [DOI] [PubMed] [Google Scholar]
- 23.Baker H, Frank O. Determination of viltamin E. In: Baker H, Frank O, editors. Clinical vitaminology: methods and interpretation. New York: Wiley; 1968. pp. 172–6. [Google Scholar]
- 24.Carl A, Edward B, Tietz AR. Text book of clinical chemistry. 2nd ed. Pennsylvania: W.B. Saunders Co; 1994. pp. 1313–4. [Google Scholar]
- 25.Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74:214–26. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 26.Selvi R, Angayarkanni N, Bharathselvi M, Sivaramakrishna R, Anisha T, Jyotirmoy B, et al. Increase in Fe3+/Fe2+ ratio and iron-induced oxidative stress in Eales disease and presence of ferrous iron in circulating transferrin. Curr Eye Res. 2007;32:677–83. doi: 10.1080/02713680701486402. [DOI] [PubMed] [Google Scholar]
- 27.Gariballa SE, Hutchin TP, Sinclair AJ. Antioxidant capacity after acute ischaemic stroke. QJM. 2002;95:685–90. doi: 10.1093/qjmed/95.10.685. [DOI] [PubMed] [Google Scholar]
- 28.Thorat VN, Suryakar AN, Naik P, Tiwale BM. Total antioxidant capacity and lipid peroxidation in liver transplantation. Indian J Clin Biochem. 2009;24:102–4. doi: 10.1007/s12291-009-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suresh DR, Annam V, Pratibha K, Prasad BV. Total antioxidant capacity - a novel early bio-chemical marker of oxidative stress in HIV infected individuals. J Biomed Sci. 2009;16:61–4. doi: 10.1186/1423-0127-16-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chuang CC, Shiesh SC, Chi CH, Tu YF, Hor LI, Shieh CC, et al. Serum total antioxidant capacity reflects severity of illness in patients with severe sepsis. Crit Care. 2006;10:R36. doi: 10.1186/cc4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarban A, Taheri F, Chahkandi T, Sharifzadeh G, Khorashadizadeh M. Antioxidant and radical scavenging activity of human colostrum, transitional and mature milk. J Clin Biochem Nutr. 2009;45:150–4. doi: 10.3164/jcbn.08-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angayarkanni N, Barathi S, Seethalakshmi T, Punitham R, Sivaramakrishna R, Suganeswari G, et al. Serum PON1 arylesterase activity in relation to hyperhomocysteinaemia and oxidative stress in young adult central retinal venous occlusion patients. Eye (Lond) 2008;22:969–74. doi: 10.1038/sj.eye.6703062. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137:62–9. doi: 10.1016/s0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]


