Abstract
Pre-emptive kidney transplantation is considered the best available renal replacement therapy, but no guidelines exist to direct its timing during CKD progression. We used a national cohort of 19,471 first-time pre-emptive kidney transplant recipients between 1995–2009 to evaluate patterns and implications of transplant timing. Mean estimated GFR (eGFR) at the time of pre-emptive transplant increased significantly over time, from 9.2 ml/min/1.73m2 in 1995 to 13.8 ml/min/1.73m2 in 2009 (P<0.001). Patients with eGFR≥15 ml/min/1.73m2 represented an increasing proportion of pre-emptive transplant recipients, from 9% in 1995 to 35% in 2009; the trend for patients with eGFR≥10 was similar (30% to 72%). We did not detect statistically significant differences in patient survival or death-censored graft survival between strata of eGFR at the time of transplant, either in the full cohort or in subgroup analyses of patients who might theoretically benefit from earlier pre-emptive transplantation. In summary, pre-emptive kidney transplantation is occurring at increasing levels of native kidney function. Earlier transplantation does not appear to associate with patient or graft survival, suggesting that earlier pre-emptive transplantation may subject donors and recipients to premature operative risk and waste the native kidney function of recipients.
Pre-emptive kidney transplantation (PKT), or transplantation before the initiation of dialysis, is considered the optimal renal replacement therapy in advanced chronic kidney disease (CKD).1–4 PKT is associated with improved patient and graft survival,5,6 higher return-to-work rates,7 better quality of life,8 and lower long-term medical costs compared with transplantation after dialysis initiation.9 In addition, PKT has several theoretical advantages, most gained simply by dialysis avoidance: decreased incidence of catheter-associated infection, no requirement for permanent vascular access, and a reduction in dialysis-associated cardiovascular events, such as sudden cardiac death or accelerated progression of heart failure.10–13
Despite wide acceptance of the superiority of PKT, however, no guidelines exist for when in the course of CKD progression PKT should be performed. PKT too early in the course of renal decline may waste native kidney function and prematurely expose donors and recipients to operative and immunosuppressive risk associated with transplantation.14–18 Conversely, earlier restoration of renal function may slow cardiovascular progression, with early PKT potentially preventing CKD-associated cardiovascular morbidity and mortality.19 The goal of this study was to evaluate practice patterns in PKT timing as well as associations between PKT timing and graft and patient survival.
There were 19,471 first-time PKT recipients reported to the United Network for Organ Sharing (UNOS) between January 1, 1995 and December 31, 2009. The number of PKTs increased steadily over time (605 in 1995 and 2192 in 2009), with the majority of organs coming from living donors (11,554 living donor and 7917 deceased donor recipients). As a proportion of total living donor kidney transplants, PKT increased from 17.9% in 1995 to 32.1% in 2009. Use of PKT varied by cause of renal disease: In 2009, over 50% of living donor recipients with polycystic kidney disease (PKD) were transplanted pre-emptively, whereas only 25% of living donor transplants among diabetics were pre-emptive.
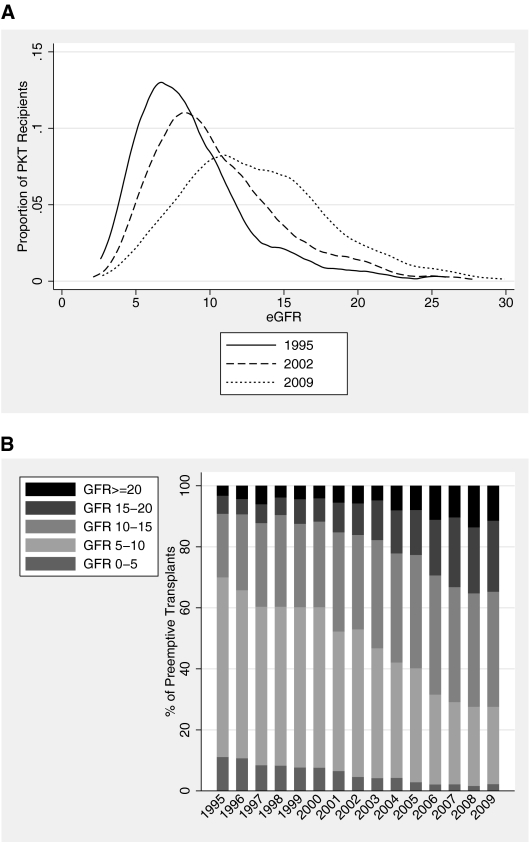
There was a strong trend toward earlier PKT over time (Figure 1). Among PKT recipients, mean pretransplant eGFR was 9.2 ml/min/1.73 m2 in 1995 and 13.8 in 2009 (P<0.001). Increasing numbers of PKT recipients were transplanted with higher native eGFR: The proportion of PKT recipients with eGFR≥15 (“early PKT”) increased from 9.2% in 1995 to 34.7% in 2009; the proportion with eGFR≥10 increased from 30.0% to 72.4%. Trends were comparable within deceased donor and living donor PKT recipients.
Figure 1.
Recipient native eGFR at PKT. (A) Distributions of eGFR in 1995, 2002, and 2009 are illustrated by kernel density plots. (B) Distribution of eGFR categories among PKT recipients, 1995–2009.
Among patients undergoing PKT, those transplanted more recently, those with diabetes or tubulointerstitial diseases, and those with poor functional status were more likely to do so at eGFR≥15 (adjusted relative rate [aRR] 1.12, 1.52, 1.30, and 1.14, respectively; Table 1). Patients with PKD, women, African Americans, and other nonwhites were less likely to undergo early PKT (aRR 0.77, 0.87, 0.90, and 0.78, respectively). Age was negatively associated with early PKT (9% less likely to undergo early PKT with every increasing decade) until the age of 50, after which there was no significant association. The association between some of these factors and early PKT changed significantly over time, as measured by interaction term analysis. Over time, diabetics became less likely to receive early PKT (6% smaller yearly increase than nondiabetics, P<0.001), whereas patients with PKD became more likely (3% greater yearly increase than non-PKD patients, P = 0.023). Similarly, a greater proportion of women underwent early PKT in recent years (3% greater yearly increase than men, P = 0.003).
Table 1.
Characteristics associated with early PKT (pretransplant eGFR ≥15), 1995–2009
| Characteristic | Adjusted Relative Rate (95% CI) | P Value |
|---|---|---|
| Transplant year | 1.12 (1.11–1.13) | <0.001 |
| Age (per decade, <50 yr)* | 0.91 (0.87–0.95) | <0.001 |
| Age (per decade, ≥50 yr)* | 1.04 (0.98–1.09) | 0.2 |
| Female gender | 0.87 (0.82–0.93) | <0.001 |
| Race | ||
| Caucasian | reference | |
| African American | 0.90 (0.81–1.00) | 0.04 |
| other | 0.78 (0.70–0.86) | <0.001 |
| Cause of ESRD | ||
| glomerulonephritis | reference | |
| diabetes | 1.52 (1.39–1.66) | <0.001 |
| polycystic kidney disease | 0.77 (0.70–0.86) | <0.001 |
| hypertension | 1.07 (0.97–1.19) | 0.2 |
| renovascular | 0.96 (0.77–1.19) | 0.7 |
| congenital | 0.94 (0.76–1.17) | 0.6 |
| tubulointerstitial | 1.30 (1.15–1.46) | <0.001 |
| neoplasm | 1.02 (0.53–1.96) | 0.9 |
| other | 1.13 (1.01–1.27) | 0.04 |
| Poor functional status** | 1.14 (1.04–1.25) | 0.006 |
| Blood type | ||
| A | reference | |
| B | 0.86 (0.77–0.95) | 0.004 |
| AB | 1.07 (0.94–1.21) | 0.3 |
| O | 0.95 (0.89–1.01) | 0.1 |
| Private insurance | 1.00 (0.93–1.08) | 0.9 |
| Living donor | 1.03 (0.97–1.11) | 0.3 |
| Donor age | 1.00 (1.00–1.00) | 0.6 |
* Age was modeled as a spline with a knot at 50 yr after bivariate exploration with the outcome.
** Poor functional status reflected any impairment in functional status at transplantation or registration. The 4.5% of recipients missing functional status were assumed to be 100% functional.
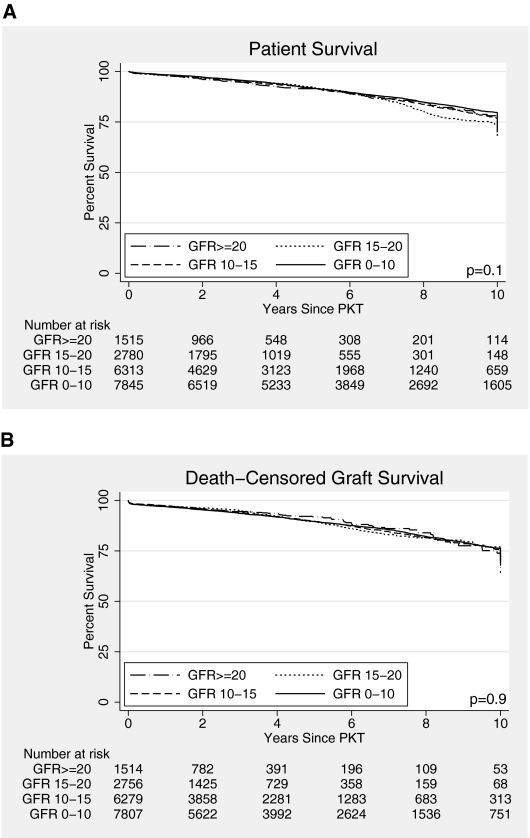
There were no differences in unadjusted patient or graft survival by pre-PKT eGFR (Figure 2), and a possible trend toward worse propensity score-adjusted patient survival associated with higher pretransplant eGFR (Table 2). Results comparing early (eGFR≥15) with later PKT were similar by various methods of accounting for confounding and treatment selection bias: propensity score-adjusted analyses (adjusted hazard ratio [aHR] for death, 1.10, 95% CI 0.98 to 1.24, P = 0.1; aHR for death-censored graft loss, 0.96, 95% CI 0.84 to 1.08, P = 0.5), multivariate Cox proportional hazards analyses (live donors: aHR for death, 0.89, 95% CI 0.74 to 1.08, P = 0.2; aHR for graft loss, 1.01, 95% CI 0.84 to 1.21, P = 0.9; deceased donors: aHR for death, 1.16, 95% CI 1.00 to 1.35, P = 0.1; aHR for graft loss, 0.99, 95% CI 0.83 to 1.19, P = 0.9), and instrumental variable analyses (adjusted odds ratio [aOR] for death, 1.01, 95% CI 0.91 to 1.13, P = 0.8; aOR for graft loss 0.98, 95% CI 0.87 to 1.11, P = 0.8). Similarly, no statistically significant differences were seen when limiting the analyses to subgroups of recipients that might theoretically benefit from early PKT (diabetics, recipients over 65, those with poor functional status, and men; Supplemental Figure 1).
Figure 2.
Kaplan-Meier estimates of (A) patient survival, and (B) death-censored graft survival in PKT recipients, by pretransplant eGFR.
Table 2.
PKT recipient and graft survival associated with pretransplant eGFR*
| eGFR at PKT | Adjusted HR of Death | Adjusted HR of Death-Censored Graft Loss |
|---|---|---|
| eGFR <10 | reference | reference |
| eGFR 10–15 | 1.10 (95% CI 0.99–1.21, P = 0.07) | 0.97 (95% CI 0.88–1.08, P = 0.6) |
| eGFR 15–20 | 1.16 (95% CI 1.00–1.34, P = 0.05) | 0.95 (95% CI 0.81–1.11, P = 0.5) |
| eGFR ≥20 | 1.12 (95% CI 0.93–1.34, P = 0.2) | 0.94 (95% CI 0.77–1.15, P = 0.5) |
*Propensity score-adjusted.
This large national observational study identifies a dramatic trend toward PKT earlier in the course of CKD. Among PKT recipients, patients receiving PKT at eGFR≥15 were more often Caucasian, male, diabetic, with worse functional status. However, even after adjustment for these differences and potential treatment selection bias, early PKT was not correlated with improved patient or graft survival. Early PKT may introduce risks of transplantation and “start the clock” on allograft survival unnecessarily early.
The trend toward early PKT contrasts with published suggestions that late PKT may be preferable. An older study of 4046 PKT recipients found no association between pretransplant eGFR≥15 and PKT outcomes, including graft survival, patient survival, and 6-mo post-transplant eGFR.18 A two-center study of 671 PKT recipients looked at additional categories of pretransplant eGFR and found no benefit with earlier PKT.17 In our study, using the largest cohort of PKT recipients to date, spanning a 15-yr time period, and using multiple analytic methods to minimize bias, we were also unable to demonstrate a benefit of early PKT, even in populations that intuitively might benefit from early restoration of renal function (e.g., those with a greater burden of comorbid disease or a higher risk of cardiovascular events).
Given the consistent lack of benefit, the rationale driving earlier PKT is unclear. It may be an unintended consequence of the push to “transplant first.”4 Estimating rates of kidney function decline is difficult,20–23 and current estimates may stem from historical rates that are now inaccurate given improvements in CKD care. Earlier PKT may be pursued to avoid unanticipated acceleration of CKD progression, dialysis requirement, and the morbidity associated with vascular access creation. Nationally, dialysis initiation is occurring earlier, which, in turn, forces earlier PKT.24 Alternatively, transplant timing may be unduly driven by donor factors. Approving a suitable living donor can be a lengthy process, and programs may be reluctant to delay transplantation once a donor is cleared. Early PKT may be pursued out of a concern that a donor may become unable to donate, or to accommodate a donor's schedule.
The implications of early PKT may be worrisome. The parallel trend in early-start dialysis has been associated with worse or no different outcomes when compared with later dialysis initiation.24–26 Early intervention, whether dialysis or transplantation, increases costs and significantly changes a patient's life, with required adherence to new medication or treatments. In living donor PKT, both recipient and donor may undergo potentially premature operative risk. Given the enormity of the organ shortage and the independence of pretransplant eGFR and graft survival, premature transplantation may waste allograft life of a precious resource.
There are several important limitations in this study. First and foremost, the data are observational. There may be treatment selection bias, where recipients undergo early PKT for reasons correlated with survival. For example, sicker patients may be referred more often for early PKT. However, we used several analytic methods designed to minimize this bias, including propensity score adjustment and instrumental variable analysis, an approach that may more closely approximate results of randomized controlled trials.27 Furthermore, results are similar within deceased donor PKT recipients, a group in which treatment selection bias should be reduced because of unpredictable organ availability.
Second, starting the “survival clock” at PKT requires that a patient survive and remain off dialysis until transplantation. As a result, the late PKT group may be healthier: They were able to survive longer CKD progression without development of uremia. Additionally, measuring survival from the time of PKT does not credit late PKT recipients with this period of CKD progression, nor does it account for those would-be late PKT recipients who died before transplant. A better method to compare early versus late PKT timing might be to measure survival from the time potential PKT recipients reach a given eGFR, although this would not be possible using transplant registry data.
Finally, kidney function at PKT was determined using only one value of nonstandardized serum creatinine, and we assumed the patient was in steady-state. More accurate estimations of native kidney function, such as measured GFR, may improve estimations of relative risk.
In summary, we report that PKT is being performed earlier, with more patients transplanted at higher levels of native kidney function. There was no demonstrated survival advantage to PKT at higher levels of eGFR, with results robust to three methods of accounting for potential bias in observational studies. Until better data are available, providers and transplant programs should strive to individualize transplant timing decisions to avoid PKT too early in the course of CKD.
CONCISE METHODS
Study Population
A national cohort of 152,731 first-time, kidney-only adult transplant recipients between January 1, 1995, and December 31, 2009, was identified through the UNOS registry. PKT recipients (n = 19,471) were defined by the absence of exposure to dialysis before kidney transplantation. Ascertainment of death was supplemented by linkage to the Social Security Death Master File.
Definitions
Estimated GFR (eGFR) was calculated using the four-variable Modification of Diet in Renal Disease (MDRD) equation, using serum creatinine at the time of transplantation as supplied to UNOS. Pretransplant serum creatinine was available for 95.9% of living donor PKT recipients and 92.4% of deceased donor PKT recipients over the study period. Early PKT was defined as PKT performed when native eGFR was ≥15 ml/min/1.73 m2.
Statistical Analysis
Associations with early PKT were estimated using a generalized linear model, as described previously.28 To determine if the early PKT trends were different among different patient phenotypes, interactions with year of transplant were tested with five factors specified a priori: age, gender, cause of ESRD, donor type, and functional status.
Accounting for Potential Bias
Outcomes associated with early PKT were estimated in three ways: propensity score-adjusted Cox proportional hazards regression, multivariate Cox proportional hazards regression, and instrumental variable analysis. Propensity scores were derived using random forest regression, a machine learning regression tree technique designed to account for all possible levels of multi-interaction;29–31 individual propensity scores for early PKT were categorized into quartiles and included in a survival model of all PKT recipients. To test the sensitivity of our inferences to the method of propensity score derivation, the analysis was repeated using propensity scores derived from simple multivariate logistic regression, and no inferential difference was noted. Covariates in the multivariate survival analyses included transplant year as well as all variables specified in standard Scientific Registry for Transplant Recipients (SRTR) outcome models for 3-yr postkidney transplant patient survival;32 the model was specified separately for deceased donor and living donor recipients to remain consistent with the SRTR models. Finally, instrumental variable analysis was performed with the binary outcomes of 3-yr post-PKT mortality and death-censored graft loss, using regional rate of early PKT as the instrumental variable and adjusting for covariates listed in Table 1. All analyses were performed using STATA 11.0/MP for Linux (College Station, TX) except for the random forest regression, which was implemented using R 2.7.2 (The R Foundation for Statistical Computing, Vienna, Austria).
DISCLOSURES
None.
Acknowledgments
We report an analysis of data collected by the Organ Procurement and Transplantation Network (OPTN). The OPTN is supported by Health Resources and Services Administration contract 234-2005-370011C. The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. This work was supported in part by a National Institutes of Health National Research Service Award Institutional Research Training Grant (T32 DK 007732 – 15) awarded to Morgan Grams. Part of this work was submitted in abstract form to the American Transplant Congress and will be delivered as an oral presentation on May 2, 2011, in Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at www.jasn.org.
REFERENCES
- 1. Kallab S, Bassil N, Esposito L, Cardeau-Desangles I, Rostaing L, Kamar N: Indications for and barriers to preemptive kidney transplantation: A review. Transplant Proc 42: 782–784, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Yoo SW, Kwon OJ, Kang CM: Preemptive living-donor renal transplantation: Outcome and clinical advantages. Transplant Proc 41: 117–120, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Davis CL: Preemptive transplantation and the transplant first initiative. Curr Opin Nephrol Hypertens 19: 592–597, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Abecassis M, Bartlett ST, Collins AJ, Davis CL, Delmonico FL, Friedewald JJ, Hays R, Howard A, Jones E, Leichtman AB, Merion RM, Metzger RA, Pradel F, Schweitzer EJ, Velez RL, Gaston RS: Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol 3: 471–480, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mange KC, Joffe MM, Feldman HI: Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 344: 726–731, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT: Preemptive kidney transplantation: The advantage and the advantaged. J Am Soc Nephrol 13: 1358–1364, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Katz SM, Kerman RH, Golden D, Grevel J, Camel S, Lewis RM, Van Buren CT, Kahan BD: Preemptive transplantation–an analysis of benefits and hazards in 85 cases. Transplantation 51: 351–355, 1991 [PubMed] [Google Scholar]
- 8. Shrestha A, Shrestha A, Basarab-Horwath C, McKane W, Shrestha B, Raftery A: Quality of life following live donor renal transplantation: A single centre experience. Ann Transplant 15: 5–10, 2010 [PubMed] [Google Scholar]
- 9. John AG, Rao M, Jacob CK: Preemptive live-related renal transplantation. Transplantation 66: 204–209, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Meier-Kriesche HU, Port FK, Ojo AO, Rudich SM, Hanson JA, Cibrik DM, Leichtman AB, Kaplan B: Effect of waiting time on renal transplant outcome. Kidney Int 58: 1311–1317, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Meier-Kriesche HU, Schold JD: The impact of pretransplant dialysis on outcomes in renal transplantation. Semin Dial 18: 499–504, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Wali RK, Wang GS, Gottlieb SS, Bellumkonda L, Hansalia R, Ramos E, Drachenberg C, Papadimitriou J, Brisco MA, Blahut S, Fink JC, Fisher ML, Bartlett ST, Weir MR: Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol 45: 1051–1060, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Wang AY, Lam CW, Chan IH, Wang M, Lui SF, Sanderson JE: Sudden cardiac death in end-stage renal disease patients: A 5-year prospective analysis. Hypertension 56: 210–216, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Cosio FG, Pesavento TE, Osei K, Henry ML, Ferguson RM: Post-transplant diabetes mellitus: Increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int 59: 732–737, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Snyder JJ, Israni AK, Peng Y, Zhang L, Simon TA, Kasiske BL: Rates of first infection following kidney transplant in the United States. Kidney Int 75: 317–326, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Akkina SK, Connaire JJ, Snyder JJ, Matas AJ, Kasiske BL: Earlier is not necessarily better in preemptive kidney transplantation. Am J Transplant 8: 2071–2076, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Ishani A, Ibrahim HN, Gilbertson D, Collins AJ: The impact of residual renal function on graft and patient survival rates in recipients of preemptive renal transplants. Am J Kidney Dis 42: 1275–1282, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B: Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant 4: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Anderson S, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kaysen GA, Kusek JW, Nayfield SG, Schmader K, Tian Y, Ashworth JR, Clayton CP, Parker RP, Tarver ED, Woolard NF, High KP, workshop participants: Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol 20: 1199–1209, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Alves TP, Wang X, Wright JT, Jr, Appel LJ, Greene T, Norris K, Lewis J: AASK Collaborative Research Group: Rate of ESRD exceeds mortality among African Americans with hypertensive nephrosclerosis. J Am Soc Nephrol 21: 1361–1369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barbour SJ, Er L, Djurdjev O, Karim M, Levin A: Differences in progression of CKD and mortality amongst Caucasian, Oriental Asian and South Asian CKD patients. Nephrol Dial Transplant 25: 3663–3672, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Conway B, Webster A, Ramsay G, Morgan N, Neary J, Whitworth C, Harty J: Predicting mortality and uptake of renal replacement therapy in patients with stage 4 chronic kidney disease. Nephrol Dial Transplant 24: 1930–1937, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Rosansky SJ, Clark WF, Eggers P, Glassock RJ: Initiation of dialysis at higher GFRs: Is the apparent rising tide of early dialysis harmful or helpful? Kidney Int 76: 257–261, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF: Early start of hemodialysis may be harmful. Arch Intern Med 171: 396–403, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA: IDEAL Study: A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 363: 609–619, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ: Analysis of observational studies in the presence of treatment selection bias: Effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA 297: 278–285, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zou G: A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 159: 702–706, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Maindonald J, Braun J: Data Analysis and Graphics Using R. Cambridge, England: Cambridge University Press; 2003 [Google Scholar]
- 30. Breiman L: Random forests. Mach Learning 45: 5–32, 2001 [Google Scholar]
- 31. Lee BK, Lessler J, Stuart EA: Improving propensity score weighting using machine learning. Stat Med 29: 337–346, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arbor Research Collaborative for Health: Scientific Registry of Transplant Recipients, 2010. http://www.ustransplant.org Accessed December 10, 2010