Abstract
Proper organization of the actin cytoskeleton is essential for the normal structure and function of podocytes. RhoA modulates actin dynamics but its role in podocyte biology is controversial. Here, we generated transgenic mice that express a constitutively active form of RhoA in a podocyte-specific and doxycycline-inducible manner. Induction of activated RhoA with doxycycline resulted in significant albuminuria. Furthermore, both the degree of albuminuria and the histologic changes in the glomerulus positively correlated with the level of constitutively active RhoA expression: low levels of expression associated with segmental foot-process effacement without changes observable by light microscopy, whereas higher levels of expression associated with both extensive foot-process effacement and histologic features of focal segmental glomerulosclerosis (FSGS). In addition, induction of activated RhoA markedly upregulated glomerular mRNA expression of fibronectin and collagen IA1, and the degree of upregulation positively correlated with the level of albuminuria. Withdrawal of doxycycline led to a decline in albuminuria toward basal levels in most mice, but heavy albuminuria persisted in some mice. Taken together, these data suggest that activation of RhoA in podocytes leads to albuminuria accompanied by a range of histologic changes characteristic of minimal change disease and FSGS in humans. Although most changes are reversible, severe and prolonged activation of RhoA may cause irreversible glomerulosclerosis.
Visceral glomerular epithelial cells (commonly known as podocytes) are highly differentiated cells of the kidney glomerulus, which have a critical role in the maintenance of glomerular permselectivity. Podocytes have complex morphology that is characterized by “foot-like” tertiary processes called “foot processes.” The intricate structure of foot processes is central to their function in blood filtration, and one of the earliest features of many glomerular diseases is the loss of foot processes (“foot process effacement”), which is accompanied by various degrees of albuminuria. Normal foot processes contain bundles of well organized actin filaments, which are disrupted into disorganized short filaments during foot process effacement.1 Therefore, regulation of the actin cytoskeleton is essential for podocyte morphology and function.1 However, how the actin cytoskeleton is disturbed in pathologic conditions is not completely understood.
Rho-family small GTPases are important mediators of actin cytoskeletal dynamics that facilitate changes in morphology, motility, adhesion, and malignant transformation.2 Although it is established that the actin cytoskeleton is central to podocyte function and that Rho-GTPases have pivotal roles in cytoskeletal regulation, the role of Rho-GTPases in podocytes has not been investigated extensively. Most of the studies performed to date have focused on RhoA. RhoA is a prototypical member of the Rho-family and is best known for its ability to induce focal adhesions and stress fibers in fibroblasts.3 It was reported that RhoA is upregulated upon differentiation in immortalized cultured mouse podocytes (MPs).4 Stability of RhoA protein was dependent on the podocyte protein, synaptopodin, whose stability in turn was dependent on its phosphorylation.4,5 The authors proposed that RhoA is important in facilitating podocyte motility during development or maintaining intact glomerular permeability.4,5 In contrast, a number of observations suggest a possibility that excessive activation of RhoA may be detrimental to podocyte health; in cultured MP, filamentous actin reorganization by mechanical stress was blocked by an inhibitor of Rho-kinase (downstream kinase of Rho).6 Inhibition of Rho-kinase also promoted process elongation in cultured MPs, implying that activation of the Rho pathway may be inhibitory to podocyte process formation.7,8 Furthermore, the Rho-kinase inhibitors Y27632 and fasudil were shown to inhibit renal injury and/or albuminuria in various rodent models.9–12 We also showed previously that RhoA activation induces cell contraction and loss of processes in MPs.13 Nonetheless, to date, there is no direct/conclusive evidence to show that activation of RhoA has a negative impact on podocyte morphology and/or function in vivo.
In the present study, we have investigated the causal link between RhoA activation in podocytes and proteinuria in vivo by establishing a transgenic mouse line that expresses constitutively active (CA)-RhoA in a podocyte-specific and doxycycline (Dox)-inducible manner. We demonstrate that weak activation of RhoA in podocytes induces foot process effacement and albuminuria, similar to minimal change disease. However, when RhoA activation is strong and sustained, it induces heavier albuminuria with histologic features similar to focal segmental glomerulosclerosis (FSGS), accompanied by upregulation of extracellular matrix genes. Thus, we provide direct evidence that activation of RhoA is detrimental to podocyte health in vivo, contributing to podocytopathy.
RESULTS
Establishment of Podocyte-specific, Inducible Expression of CA-RhoA in Mice
To test whether activation of RhoA in podocytes would cause albuminuria, we established a transgenic mouse line that expresses a CA mutant of RhoA in a podocyte-specific, Dox-inducible manner. First, we expressed CA-RhoA (Flag-tagged) under the control of a tetracycline-responsive element (TRE) and confirmed its inducibility in vitro (Figure 1A). We also verified that the addition of the amino-terminal triple Flag epitope tag did not affect the activity of CA-RhoA (Supplementary Table 1). Next, inducible CA-RhoA was expressed in mice (Figure 1B, Tg2). PCR-based genotyping confirmed seven founders, of which four founders (two females, two males) or their progenies were crossed with transgenic mice expressing a reverse tetracycline-controlled transcriptional activator (rtTA) under the control of the podocin promoter (Figure 1B, Tg1).14 Mice positive for both the CA-RhoA transgene and the podocin-rtTA transgene were identified by genotyping (hereafter referred to as “double transgenic”). Double transgenic mice were born at the expected Mendelian frequency, and there were no apparent differences in general appearance or health between double transgenic mice and their littermates for up to six months of age.
Figure 1.
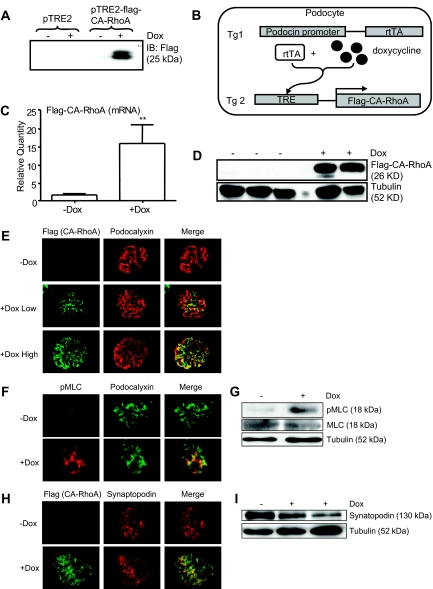
Establishment of podocyte-specific, Dox-inducible expression of CA-RhoA in mice. (A) Flag-tagged CA-RhoA was subcloned into the vector, pTRE2, and was transfected into HeLa cells stably expressing rtTA. Cells were treated with Dox (1 μg/ml) for 16 hours, and cell lysates were subjected to immunoblotting with anti-Flag antibody. Inducibility of CA-RhoA by Dox was confirmed. (B) Schematic representation of the strategy to establish the mouse line (see the Concise Methods section). (C through I) Double transgenic mice (2 to 3 months old) were treated with Dox (4 mg/ml in drinking water) or vehicle (−Dox) for 4 weeks. (C) Glomerular lysates were analyzed by real-time PCR for mRNA expression of the transgene. Flag-CA-RhoA mRNA was significantly induced by Dox (n = 8) versus −Dox (n = 9), **P < 0.001. (D) Glomerular lysates were analyzed by immunoblotting using anti-Flag antibody. Flag-CA-RhoA protein was induced by Dox. (E, F, and H) Kidney sections were immunostained for Flag (for CA-RhoA), pMLC, podocalyxin, and synaptopodin. Dox induced the expression of CA-RhoA in glomeruli, in a pattern consistent with the podocyte distribution. See Figure 3 for definition of low/high responders in (E). (G and I) Glomerular lysates were immunoblotted for pMLC, MLC, synaptopodin, and tubulin.
To assess the expression of the CA-RhoA transgene in vivo, double transgenic mice were treated with Dox for 4 weeks, and glomeruli were isolated. Expression of the transgene was confirmed both at the mRNA (Figure 1C) and protein (Figure 1D) level. Immunofluorescence staining demonstrated the expression of CA-RhoA in podocytes (Figure 1E); when the expression was high, the staining overlapped completely with that of the podocyte marker, podocalyxin, consistent with podocyte-specific expression, whereas the staining was segmental when the expression was low. No Flag staining was detected in vehicle-treated mice, indicating that expression of the transgene is not “leaky” in the absence of Dox. Phosphorylation of myosin light chain (MLC) at Ser19, a known downstream event of RhoA/Rho-kinase activation, was increased by Dox in the glomerulus and podocytes (Figure 1, F and G). In contrast, we did not observe marked changes in the level of synaptopodin expression (Figure 1, H and I). Densitometric analysis of immunoblots, normalized to tubulin, showed a trend of decrease of synaptopodin, but the difference was not statistically significant (−Dox: 1 ± 0, +Dox: 0.8 ± 0.3, arbitrary units, n = 4 and 6 mice, respectively). Taken together, the results confirm that CA-RhoA expression was induced by Dox specifically in podocytes and was functionally active.
Activation of RhoA in Podocytes Induces Albuminuria
We next studied the effect of Dox-induced CA-RhoA expression in podocytes on glomerular permselectivity. Typically, Dox treatment was started at the age of 8 to 12 weeks and continued for 4 weeks. Initially, double transgenic mice derived from the four founders were treated with Dox for 4 weeks and screened for albuminuria. Progeny of one female founder did not show obvious increase of the urine albumin-to-creatinine ratio (ACR), whereas the other three lines showed various degrees of increase. In subsequent studies, we analyzed offspring from one founder, which showed consistent increase of the urine ACR in response to Dox.
The baseline urine ACR was not different between double transgenic mice and littermate controls that lacked the CA-RhoA transgene and/or the rtTA transgene (not shown). When double transgenic mice were treated with vehicle alone for 4 weeks, the average urine ACR did not change significantly (before treatment: 71 ± 18 μg/mg, 4 weeks: 98 ± 20 μg/mg, n = 7). In contrast, when double transgenic mice were treated with Dox for 4 weeks, the urine ACR increased significantly (before treatment: 109 ± 26 μg/mg, 4 weeks: 3035 ± 783 μg/mg, n = 15, P < 0.001), although there was notable variation in the degree of albuminuria among mice (see below) (Figure 2A). Dox treatment of littermate control mice did not increase albuminuria (Figure 3A), demonstrating that albuminuria was not caused by a nonspecific toxicity of Dox on podocytes. Coomassie Brilliant Blue staining of the urine samples separated by SDS-PAGE also showed intense bands at the size of albumin (approximately 65 kD) in Dox-treated double transgenic mice, confirming impaired glomerular permeability against albumin (Figure 2B).
Figure 2.
Induction of CA-RhoA by Dox causes albuminuria in mice. (A) Double transgenic mice were treated with Dox or vehicle for 4 weeks, and the urine ACR (μg/mg) was determined before and after the treatment. The ACR in Dox-treated mice (n = 15) was significantly higher than in vehicle-treated mice (n = 9) after 4 weeks of treatment. **P < 0.001 versus −Dox (4 weeks). (B) After 4 weeks of treatment with Dox, 3 μl of urine samples were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. Dox-treated mice showed intense albuminuria.
Figure 3.
The level of CA-RhoA expression correlates with the level of albuminuria. (A) Double transgenic mice (DT) and control littermates (Cntl) were treated with Dox as in Figure 2 for 4 weeks, and the urine ACR was determined at 2 and 4 weeks. The degree of Dox-induced albuminuria was highly variable among mice (see Figure 2A). We defined mice with urine ACR < 1000 μg/mg as low-responders (+Dox Low), and those with >1000 μg /mg as high responders (+Dox High). Both groups (n = 7 and 8) showed significantly higher ACR at 2 and 4 weeks of treatment, compared with vehicle-treated mice (−Dox, n = 9), *P < 0.05 and **<0.001. (B) Double transgenic mice were treated with Dox or vehicle for 4 weeks, and glomerular mRNA expression of the transgene was quantified by real-time PCR for the three groups. High responders (n = 4) showed significantly higher transgene expression, compared with low responders (n = 4) and vehicle-treated mice (n = 9). **P < 0.001 versus −Dox. (C) Urine ACR was plotted against transgene expression for individual mice. Positive correlation was seen (n = 17, R2 = 0.7762).
The Level of CA-RhoA Expression Correlates with the Level of Albuminuria
As described above, we noted a large variation in the degree of albuminuria among Dox-treated double transgenic mice (Figure 2A). For further analyses, we chose to separate these mice into two groups, i.e., “low responders” and “high responders”, by assigning a cutoff of the urine ACR at 1000 μg/mg. The average urine ACR for the high responders was more than 10-fold higher (6012 ± 537 μg/mg, n = 7), compared with the low responders (484 ± 50 μg/mg, n = 8, P < 0.001) (Figure 3A). When quantified by real-time PCR, mRNA expression of CA-RhoA in high responders was 4.4-fold higher than low responders (high responders: 20.5, n = 4 versus low responders: 4.6, n = 4, P < 0.05) (Figure 3B). ACR showed a positive correlation with the expression level of glomerular CA-RhoA (R2 = 0.7762) (Figure 3C), suggesting that the degree of albuminuria is likely to be dependent on the level of CA-RhoA expression in podocytes.
Albuminuria Induced by CA-RhoA Is, at Least Partially, Reversible
We next studied whether the albuminuria induced by CA-RhoA was reversible. Double transgenic mice were treated with Dox for 4 weeks, and the urine ACR was quantified for an additional 2 weeks after Dox withdrawal (total 6 weeks). In low responders, the urine ACR returned to the baseline level within 1 to 2 weeks of Dox withdrawal (456 ± 140 μg/mg at 4 weeks, 73 ± 24 μg/mg at 5 weeks, and 68 ± 8 μg/mg, at 6 weeks, n = 4) (Figure 4A). High responders displayed variable rates of recovery; some mice showed a slow return toward the baseline (mouse 1 and 2 in Figure 4B), whereas others showed sustained heavy albuminuria after the withdrawal of Dox (mice 3 and 4 in Figure 4B). On average, the urine ACR tended to decline after Dox withdrawal but remained high even 2 weeks after (7164 ± 274 μg/mg at 4 weeks, 7109 ± 1277 at 5 weeks, and 6312 ± 1553 μg/mg at 6 weeks, n = 4). Immunoblotting also showed the disappearance of the CA-RhoA protein at variable rates (Figure 4C). Mice with sustained albuminuria often had a high level of residual CA-RhoA expression even 2 weeks after the Dox withdrawal, as shown in mouse 3 in Figure 4B and lane 3 in Figure 4C.
Figure 4.
Albuminuria induced by CA-RhoA is, at least partially, reversible. Double transgenic mice were treated with Dox for 4 weeks, and then Dox was discontinued. Urine ACR was measured for 2 additional weeks after the withdrawal. (A) Urine ACR of low responders returned to baseline (n = 4). *P < 0.05. **P < 0.001 versus 0 weeks. (B) Time courses of four individual high responder mice are shown. High responders had variable rates of recovery; some mice showed a slow return toward the baseline (mice 1 and 2), whereas others showed sustained heavy albuminuria even at 2 weeks after Dox withdrawal (mice 3 and 4). On average, the urine ACR tended to decline after Dox withdrawal but remained high even after 2 weeks. (C) Glomerular lysates of high responders obtained 2 weeks after Dox withdrawal were analyzed by immunoblotting using anti-Flag antibody. In two of three mice (lanes 1 and 2), expression of Flag-CA-RhoA returned to minimal, whereas in one mouse (lane 3), intense expression was still detected. Lanes 1 to 3 correspond to mice 1 to 3 in Figure 4B. Lanes 4 to 6: double transgenic mice treated with vehicle for 6 weeks.
Degree of Foot Process Effacement Correlates with the Level of Albuminuria
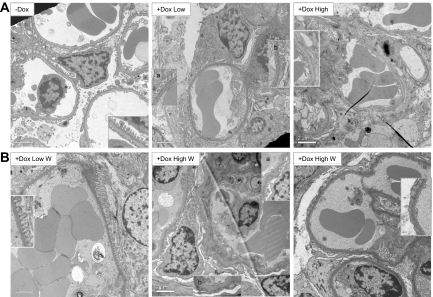
We next studied podocyte ultrastructure by electron microscopy. In low responders, segmental foot process effacement was observed, affecting approximately 30 to 40% of the total area (Figure 5A, middle). In contrast, high responders showed extensive foot process effacement (Figure 5A, right). When Dox was withdrawn (W), low responders regained normal foot process structure after 2 weeks (Figure 5B, left). In contrast, recovery of the high responders was variable, similar to the recovery of albuminuria; mice with a clear trend of declining albuminuria showed partial restoration of normal foot processes (Figure 5B, middle), whereas mice with a sustained high level of albuminuria showed extensive foot process effacement even 2 weeks after Dox withdrawal (Figure 5B, right). These results indicate that when the level of CA-RhoA expression is low, albuminuria and foot process effacement are reversible. When the level of CA-RhoA expression is high, albuminuria and foot process effacement do not normalize within 2 weeks after Dox withdrawal, although there were some signs of reversibility such as declining urine ACR and focal restoration of normal foot processes. Whether the high responders recover completely at later time points could not be concluded in the current study.
Figure 5.
Foot process effacement caused by CA-RhoA is reversible. Transmission electron micrographs are shown. (A) Double transgenic mice were treated with Dox or vehicle (−Dox) for 4 weeks. Vehicle-treated mice (left) showed normal podocyte ultrastructure (foot process effacement <10%). Low responders (middle) showed a mixture of areas with normal foot processes (inset a) and foot process effacement (inset b). High responders (right) showed more extensive effacement. (B) Double transgenic mice were treated with Dox for 4 weeks and then Dox was withdrawn (W). Kidneys were harvested 2 weeks later. Low responders (left) showed normal foot processes 2 weeks after Dox withdrawal. High responders, which had a trend of declining albuminuria (middle), showed partial restoration of foot processes (inset a), whereas other areas remained fused (inset b). High responders with sustained heavy albuminuria (right) showed persistent extensive foot process effacement even at 2 weeks after Dox withdrawal. Magnification, ×6000, Scale bars = 2 μm.
High Level and Sustained CA-RhoA Expression Causes FSGS
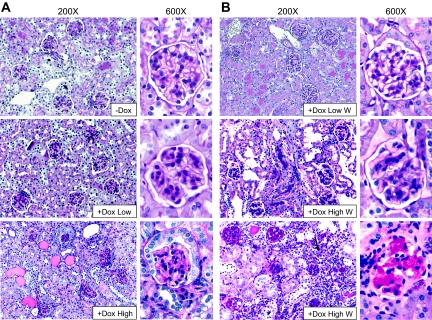
We next studied histologic changes by light microscopy. Periodic acid-Schiff (PAS) staining of the low responders appeared normal (Figure 6A, middle). In contrast, high responders showed various degrees of glomerulosclerosis; although there were many glomeruli that showed minimal sclerosis, other glomeruli showed segmental or total sclerosis, and a few glomeruli were obliterated (Figure 6A, bottom). These results suggest that a low level of RhoA activation in podocytes causes foot process effacement and mild albuminuria without discernible abnormalities by light microscopy, reminiscent of human minimal change disease, whereas a high level of RhoA activation leads to heavier albuminuria and histologic changes similar to FSGS. We also studied kidney histology after Dox withdrawal. PAS staining of the low responders remained normal (Figure 6B, top). Histology of the high responders after Dox withdrawal was variable; a mouse that showed a clear trend for recovery (urine ACR dropped from 6941 μg/mg at 4 weeks to 2618 μg/mg at 6 weeks) had minimal sclerosis by PAS staining (Figure 6B, middle). By contrast, a mouse that showed a sustained heavy albuminuria (urine ACR: 6509 μg/mg at 4 weeks and 7663 μg/mg at 6 weeks) had FSGS. Interstitial leukocyte infiltration was also noted, most likely in response to heavy albuminuria (Figure 6B, bottom). Quantification of histologic changes is shown in Supplementary Figure 1. It is noteworthy that the mouse shown in Figure 6B, middle, still showed distinct foot process effacement by electron microscopy (Figure 5B, middle), mimicking the histologic features of minimal change disease in humans. Whether the mice that showed clear histologic changes of FSGS (as in Figure 6B, bottom) would eventually recover after Dox withdrawal could not be concluded from the current study.
Figure 6.
High level of CA-RhoA expression induces FSGS. PAS staining of kidney sections is shown. (A) Double transgenic mice were treated with Dox or vehicle (−Dox) for 4 weeks. Glomeruli of low responders (middle) showed no abnormality and were not significantly different from vehicle-treated mice (top). Glomeruli of high responders (bottom) showed various degree of sclerosis. Note that cuboidal parietal glomerular epithelial cells (bottom) are often seen even in normal male mice. (B) Double transgenic mice were treated with Dox for 4 weeks, and then Dox was withdrawn (W). Kidneys were harvested 2 weeks later. Low responders (+Dox Low W, top) consistently showed normal kidney histology. Many high responders had a trend of declining albuminuria after withdrawal. PAS staining of these mice showed minimal sclerosis (+Dox High W, middle). Other high responders with sustained heavy albuminuria showed typical FSGS with interstitial leukocyte infiltration (arrow) (+Dox High W, bottom). Magnification, ×200 and ×600.
Activation of RhoA Causes Loss of Processes and Cell Contraction in Cultured Mouse Podocytes
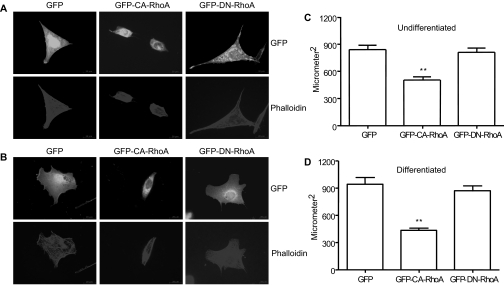
The above results indicate that the morphologic changes of podocytes induced by a low level of CA-RhoA expression, and possibly even by a high level of expression, are reversible. This implies that CA-RhoA is modulating actin dynamics. Therefore, we next studied the effect of CA-RhoA on the morphology of cultured mouse podocytes. Transient transfection of CA-RhoA, but not green fluorescent protein (GFP) alone or dominant negative (DN)-RhoA, caused the loss of cellular processes and cell contraction in undifferentiated mouse podocytes (Figure 7A), consistent with our previous results.13 Loss of cellular processes/lamellipodia induced by CA-RhoA was even more apparent in differentiated cells (Figure 7B). When cell size was quantified, CA-RhoA significantly reduced cell size in both undifferentiated (839 ± 246 μm2 for GFP versus 501 ± 197 μm2 for CA-RhoA, n = 30, P < 0.001, 40% decrease) and differentiated cells (941 ± 332 μm2 for GFP versus 432 ± 102 μm2 for CA-RhoA, n = 30, P < 0.001, 54% decrease) (Figure 7, C and D). These in vitro results support the hypothesis that CA-RhoA causes reversible actin cytoskeletal remodeling in vivo, which is likely to contribute to foot process retraction. Consistent with this notion, cell contraction induced by CA-RhoA was reversible in cultured rat glomerular epithelial cells (Supplementary Figure 2).
Figure 7.
Activation of RhoA causes loss of processes and cell contraction in cultured mouse podocytes. Mouse podocytes, either undifferentiated or differentiated for 10 days, were transfected with pEGFP, GFP-CA-RhoA, or GFP-Dominant Negative (DN)-RhoA. After 24 hours, cells were permeabilized and stained for phalloidin. (A and B) Immunofluorescence for GFP and phalloidin are shown for undifferentiated cells (A) and differentiated cells (B). Magnification, ×600. Cells transfected with CA-RhoA showed significant cell contraction. (C and D) Cell size was quantified as in the Concise Methods section. (C) Undifferentiated cells. n = 30. (D) Differentiated cells, n = 20, **P < 0.001 versus GFP.
Podocyte CA-RhoA Increases the Expression of Fibronectin In Vivo and In Vitro
As shown in Figure 6, high and sustained expression of RhoA induced histologic changes similar to FSGS in humans. We next studied whether the expression of CA-RhoA in podocytes affects gene regulation, which could explain the pathomechanisms of the development of glomerulosclerosis. First, we examined the expression of major extracellular matrices and podocyte-associated genes in the glomerulus of double transgenic mice treated with Dox or vehicle alone. Real-time PCR revealed that mRNA levels of nephrin, podocin, synaptopodin, α-actinin-4, Wt1, and laminin were unchanged in Dox-treated mice, compared with vehicle-treated mice, although there was a downward trend for nephrin, podocin, and synaptopodin (Figure 8A). In contrast, expression of fibronectin and collagen IA1 mRNA was markedly increased in Dox-treated mice (n = 8, P < 0.001) (Figure 8A). Upregulation of fibronectin mRNA was significant in both high responders and low responders (P < 0.001 and P < 0.05 versus −Dox, respectively, n = 8), but high responders showed markedly higher upregulation (approximately 21-fold), compared with low responders (about threefold) (Figure 8B). Upregulation of fibronectin protein was also confirmed by immunofluorescence staining, mainly in glomeruli (Figure 8C).
Figure 8.
CA-RhoA upregulates fibronectin in vivo. (A) Double transgenic mice were treated with Dox or vehicle (−Dox) for 4 weeks. (A) Glomerular mRNA expression of the indicated genes was analyzed by real-time PCR. Only fibronectin and collagen IA1 were significantly upregulated. n = 8, **P < 0.001 versus vehicle-treated (n = 9). (B) Quantification of fibronectin mRNA by real-time PCR is shown according to the level of the urine ACR. Both high (n = 4) and low responders (n = 4) showed a significant upregulation of fibronectin, compared with vehicle-treated mice (n = 9). **P < 0.001 and *P < 0.05 versus −Dox. (C). Immunofluorescence staining of fibronectin is shown. Dox-treated mice (high responder, left) showed increased expression of fibronectin in glomeruli, compared with vehicle-treated mice (right). Magnification, ×100 (top) and ×600 (bottom). Scale bars = 20 μm.
We next studied if CA-RhoA upregulates fibronectin by transactivation of the gene. When the reporter construct for the fibronectin promoter was transfected in HEK293T cells, promoter activity was increased by approximately fivefold by CA-RhoA, and this increase was abolished by the Rho-kinase inhibitor, Y27632 (Figure 9A). We also examined whether RhoA activation leads to fibronectin protein upregulation. We used a subclone of rat glomerular epithelial cells that expresses CA-RhoA in a ponasterone A-inducible manner.15 When these cells were stimulated with ponasterone A, fibronectin protein was induced concomitant with the induction of CA-RhoA (Figure 9B). These results suggest that, in addition to actin cytoskeletal modulation, CA-RhoA stimulates gene transcription of fibronectin in podocytes, contributing to the development of glomerulosclerosis.
Figure 9.
CA-RhoA transactivates the fibronectin gene in vitro. (A) The reporter construct, p1900FN-luc, was transfected into HEK293T with CA-RhoA or empty vector, and cell lysates were analyzed for luciferase activity after 24 hours. Rho kinase inhibitor Y27632 (10 μM) was added overnight before the harvest. n = 6 in each group, **P < 0.001 versus empty vector. (B) Cell lysates were analyzed by immunoblotting using anti-fibronectin or anti-RhoA antibody. Rat visceral glomerular epithelial cells, which express CA-RhoA in an inducible manner, were stimulated with ponasterone A or vehicle (ethanol) overnight. Fibronectin protein was induced concurrently with CA-RhoA.
DISCUSSION
Although the importance of the actin cytoskeleton in podocytes is indisputable, the precise role of RhoA in podocytes has not been clearly defined. RhoA is considered as an important player for the normal development and morphology of podocytes.2 However, several reports including ours imply that activation of RhoA in podocytes may contribute to proteinuria and/or renal dysfunction in rodent models9–13,15 In the current study, we have definitively demonstrated that activation of RhoA in podocytes causes deranged cell morphology and albuminuria in adult mice. Observed phenotype was dose dependent and higher expression of CA-RhoA resulted in more dramatic morphologic changes and heavier albuminuria.
In our studies, expression of CA-RhoA led to albuminuria in 100% of mice; however, we noted that the albuminuric response was quite variable, which led us to classify mice as either low responders or high responders. Except for the initial pilot experiments, all of the experiments were done using the progeny of one founder for the CA-RhoA transgene, and breeding was set up so that hemizygosity was maintained for both the CA-RhoA and rtTA transgenes. Therefore, gene dosing or different gene integration site is not likely to explain the variability. Similar to other studies using inducible transgenic mice, the mice used in the current study had a mixed background of C3H, C57Bl/6, and FVB/N, thus potential contribution of genetic background cannot be excluded. Nonetheless, differences in severity of the phenotype in several other transgenic models of glomerular disease have been documented previously. Eremina et al. demonstrated that inducible expression of VEGF in adult podocytes led to pronounced proteinuria in all treated mice, although only nine of 62 mutant mice could be classified as comparable high responders.16 Furthermore, Michaud et al. reported that only eight of 18 mice developed significant proteinuria in mutant α-actinin-4 transgenic mice.17 This variability could not be correlated with transgene copy number and the distribution of proteinuric versus nonproteinuric mice did not change after backcrossing into pure C57Bl/6 and C3H for five generations. Similarly, Krall et al. reported that in transgenic mice expressing podocyte-specific wild-type or mutant TRPC6, the percentage of albuminuric mice ranged from 23 to 45% in each founder line.18 Such phenotypic variability is also observed in human glomerular diseases. Interestingly, we found that the level of CA-RhoA expression at both the mRNA (Figure 3, B and C) and protein (Figure 1E) level positively correlated with disease severity, and a similar trend was recently reported by Kurbegovic et al. in Pkd1 transgenic mice.19 Our findings are thus consistent with the variable degrees of phenotypic expression observed in both human and mouse glomerular diseases.
Pathologic processes in podocytes appeared to be distinct between low responders and high responders. In low responders, histologic abnormality was obvious only by electron microscopy and not by light microscopy, analogous to human minimal change disease (Figures 5 and 6). Segmental foot process effacement seen in the low responders, unlike diffuse effacement seen in human minimal change disease, is likely to be explained by the focal segmental nature of CA-RhoA expression when the expression level was low (not shown). These changes were reversible and are most likely explained by the cytoskeletal action of RhoA, as seen in Figure 7 and Supplementary Figure 2. In contrast to low responders, high responders showed clear histologic changes by light microscopy, characterized by increased glomerular extracellular matrices and FSGS, whereas electron microscopy showed extensive foot process effacement (Figures 5 and 6). There was a positive correlation between the level of CA-RhoA expression, the degree of albuminuria (Figure 3C), and the extent of foot process effacement (Figure 5). Therefore, the degree of RhoA activation appears to dictate the level of podocyte pathology. In high responders, RhoA also upregulated extracellular matrices in addition to its impact on the cytoskeleton (Figure 8). These results highlight that distinct podocyte pathologies can be induced by different levels of activation of one molecule (i.e., RhoA). In this sense, the findings are compatible with the notion that minimal change disease and FSGS may be a continuum of the same disease spectrum in that they are caused by the same mechanism but manifest with different severity. It is also noteworthy that synaptopodin expression tended to be lower in Dox-treated mice both at the protein and mRNA level, although the differences were not significant (Figures 1I and 8A). Because synaptopodin is importance for podocyte function, its downregulation may also contribute to podocyte pathology.4,5
Although both albuminuria and histologic changes in low responders were reversible after Dox withdrawal, in the current studies, we were not able to determine whether podocyte damage and FSGS observed in high responders were completely reversible. The rate of recovery of the urine ACR was variable, although there was a general trend of decline (Figure 4B). Histology at 2 weeks after Dox withdrawal was also variable; some mice showed partial restoration of foot processes and normal PAS staining, whereas others showed persistent extensive foot process effacement and typical FSGS (Figure 5 and 6). We noted that when a mouse had sustained high expression of CA-RhoA protein even after Dox withdrawal, both the urine ACR and glomerular histology remained abnormal. Therefore, we suspect that some of the irreversibility we observed up to 2 weeks after Dox withdrawal was caused by residual expression of CA-RhoA protein when the expression level was very high. Alternatively, it is possible that high and sustained level of CA-RhoA causes more significant damage and permanent scarring of the glomerulus. Additional systematic studies, which correlate glomerular expression of CA-RhoA mRNA/protein, urine ACR, and histology after Dox withdrawal for an extended period, would be required to determine the reversibility in high responders.
There is limited information in the literature regarding the role of RhoA in fibronectin gene regulation. Expression of CA-RhoA in renal cancer cells resulted in upregulation of fibronectin protein.20 Krepinsky and colleagues showed that, in glomerular mesangial cells, RhoA mediates upregulation of fibronectin by TGF-β,21 mechanical stretch,22 and high glucose.11 Although precise mechanisms were not studied in these reports, the transcription factor activator protein-1 (AP-1), was implicated in glucose-induced fibronectin upregulation.11 RhoA has been shown to induce and/or activate several transcription factors including serum responsive factor,23 c-jun,24,25 GATA-4,26 nuclear factor kappa B,27,28 and myocyte enhancer factor-2.29 It will be interesting in future investigations to elucidate which transcription factors mediate RhoA-induced transactivation of the fibronectin gene in podocytes.
In summary, activation of RhoA in podocytes in mice causes heavy proteinuria and FSGS. Partial reversibility of the changes upon removal of Dox provides a proof for the causal relationship. We propose that RhoA activation is likely to contribute to the pathogenesis of certain forms of FSGS and that RhoA may be a potential therapeutic target.
CONCISE METHODS
Mice
First, a transgenic mouse line was generated, which expresses flag-tagged CA-RhoA under the cytomegalovirus minimal promoter and TRE. Full-length mouse RhoA with a constitutively active mutation (L63) was generated by PCR using pRK5-L63RhoA15 as a template with the following primers: forward, 5-cgggatccaccatggattacaaggatgacgacgataaggattacaaggatgacgacgataaggattacaaggatgacgacgataagccggctgccatccggaagaaac-3′, reverse, 5′-aaggaaaaaagcggccgctcacaagataaggcacccagatt-3′. The forward primer contains three flag tags and a BamHI site (underline). The reverse primer contains an NotI site (underline) and a single nucleotide mutation (underline) to convert the human amino acid sequence to the mouse sequence (otherwise, the human and mouse amino acid sequences are identical). PCR product was subcloned between the BamHI site and the NotI site in pTRE2 (Clontech, New York, New York). Inducibility of this construct was verified by transiently transfecting it into HeLa cells stably expressing rtTA and treating the cells with Dox (Figure 1A). A fragment containing the promoter region, coding sequence, and polyA sequence was microinjected into C3H/C57BL6 mouse embryos. Embryos were then surgically transferred to the oviduct of pseudopregnant C3H-recipient female mice. Genotyping of the resultant pups was done by extracting tail DNA followed by PCR. The forward primer, 5′-tccaccatggattacaagga-3′, is based on the DNA sequence within the three flag tags. The reverse primer, 5′-gctttccatccacctcgata-3′, is in the third exon (PCR product 235 bp). This line (flag-CA-RhoA) was crossed with transgenic mice expressing the rtTA under the control of the podocyte-specific podocin (NPHS2) promoter (provided by Dr. Jeffrey B. Kopp, National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland14). Primers used for genotyping of podocin-Cre were described previously.14 Breeding was set up so that the hemizygosity would be maintained for both transgenes. Dox was administered in drinking water at the concentration of 4 mg/ml. The studies were approved by the McGill University Animal Care Committee.
Cell Culture
Immortalized mouse podocytes stably expressing rat nephrin (MP-nephrin) were described previously.13 Culture of HEK293T was described previously.30 Transient transfection of MP-nephrin and HEK293T was performed using Lipofectamine 2000 (Invitrogen). Immunofluorescence staining for MP-nephrin was performed as described previously.13
Immunoblotting
Immunoblotting was performed as described previously.31
Quantification of Albuminuria
Mouse urine albumin concentration was quantified using an ELISA kit (Bethyl Laboratories, Montgomery, Texas). Urine creatinine concentration was measured using a colorimetric assay kit (Cayman Chemical, Ann Arbor, Michigan). Excretion of albumin was expressed as the ACR (μg/mg).
Luciferase Assay
HEK293T were cultured in a 24-well plate at 105 cells/well. On the following day, 200 ng of the plasmid encoding CA-RhoA or empty vector was cotransfected with 100 ng of pGLF1900 FN-luc and 5 ng of pRL-TK (Promega, Madison, Wisconsin). Cells were harvested after 24 hours, and the luminescence was quantified using Dual Luciferase Assay System (Promega).
Immunofluorescence Staining of Kidney Sections
Kidney fragments were snap-frozen in isopentane (−80°C). Six-micrometer sections were fixed with ice-cold acetone for 10 minutes, followed by paraformaldehyde at 4°C for 5 minutes. Incubations with the first and second antibodies were at 4°C overnight and at 22°C for 60 minutes, respectively. Microscopy details are provided in Supplementary Methods.
Real-time PCR
Real-time PCR was performed as described in Supplementary materials.
Statistical Analysis
Data are expressed as means ± SEM. The t statistic was used to determine significant differences between two groups. P < 0.05 was considered significant.
Disclosures
None.
Acknowledgments
This study was supported by a grant from the Canadian Institutes of Health Research (CIHR) (MOP-53335 to T.T.). T.T. holds a scholarship from the Fond de la recherche en santé du Québec. N.J. is the recipient of a New Investigator Award from the Kidney Research Scientist Core Education and National Training (KRESCENT) Program and is supported by a New Investigator Research Grant from SickKids Foundation and CIHR. The authors thank Dr. Andrew Herzenberg for evaluating electron micrographs.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Burridge K, Wennerberg K: Rho and Rac take center stage. Cell 116: 167–179, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Ridley AJ, Hall A: The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70: 389–399, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P: Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8: 485–491, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, Endlich K: Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Gao SY, Li CY, Chen J, Pan L, Saito S, Terashita T, Saito K, Miyawaki K, Shigemoto K, Mominoki K, Matsuda S, Kobayashi N: Rho-ROCK signal pathway regulates microtubule-based process formation of cultured podocytes—Inhibition of ROCK promoted process elongation. Nephron Exp Nephrol 97: e49–e61, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Kobayashi N, Gao SY, Chen J, Saito K, Miyawaki K, Li CY, Pan L, Saito S, Terashita T, Matsuda S: Process formation of the renal glomerular podocyte: Is there common molecular machinery for processes of podocytes and neurons? Anat Sci Int 79: 1–10, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Gojo A, Utsunomiya K, Taniguchi K, Yokota T, Ishizawa S, Kanazawa Y, Kurata H, Tajima N: The Rho-kinase inhibitor, fasudil, attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. Eur J Pharmacol 568: 242–247, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Kanda T, Wakino S, Hayashi K, Homma K, Ozawa Y, Saruta T: Effect of fasudil on Rho-kinase and nephropathy in subtotally nephrectomized spontaneously hypertensive rats. Kidney Int 64: 2009–2019, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Peng F, Wu D, Gao B, Ingram AJ, Zhang B, Chorneyko K, McKenzie R, Krepinsky JC: RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes 57: 1683–1692, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Shibata S, Nagase M, Fujita T: Fluvastatin ameliorates podocyte injury in proteinuric rats via modulation of excessive Rho signaling. J Am Soc Nephrol 17: 754–764, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Attias O, Jiang R, Aoudjit L, Kawachi H, Takano T: Rac1 contributes to actin organization in glomerular podocytes. Nephron Exp Nephrol 114: e93–e106, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB: Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol 14: 1998–2003, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Zhang H, Cybulsky AV, Aoudjit L, Zhu J, Li H, Lamarche-Vane N, Takano T: Role of Rho-GTPases in complement-mediated glomerular epithelial cell injury. Am J Physiol Renal Physiol 293: F148–F156, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Michaud JL, Lemieux LI, Dube M, Vanderhyden BC, Robertson SJ, Kennedy CR: Focal and segmental glomerulosclerosis in mice with podocyte-specific expression of mutant alpha-actinin-4. J Am Soc Nephrol 14: 1200–1211, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Krall P, Canales CP, Kairath P, Carmona-Mora P, Molina J, Carpio JD, Ruiz P, Mezzano SA, Li J, Wei C, Reiser J, Young JI, Walz K: Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS One 5: e12859, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurbegovic A, Cote O, Couillard M, Ward CJ, Harris PC, Trudel M: Pkd1 transgenic mice: Adult model of polycystic kidney disease with extrarenal and renal phenotypes. Hum Mol Genet 19: 1174–1189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feijoo-Cuaresma M, Mendez F, Maqueda A, Esteban MA, Naranjo-Suarez S, Castellanos MC, del Cerro MH, Vazquez SN, Garcia-Pardo A, Landazuri MO, Calzada MJ: Inadequate activation of the GTPase RhoA contributes to the lack of fibronectin matrix assembly in von Hippel-Lindau protein-defective renal cancer cells. J Biol Chem 283: 24982–24990, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peng F, Zhang B, Wu D, Ingram AJ, Gao B, Krepinsky JC: TGFbeta-induced RhoA activation and fibronectin production in mesangial cells require caveolae. Am J Physiol Renal Physiol 295: F153–F164, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krepinsky J: Mechanical stretch-induced signal transduction in cultured mesangial cells. Methods Mol Biol 466: 205–221, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Hill CS, Wynne J, Treisman R: The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell 81: 1159–1170, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Marinissen MJ, Chiariello M, Tanos T, Bernard O, Narumiya S, Gutkind JS: The small GTP-binding protein RhoA regulates c-jun by a ROCK-JNK signaling axis. Mol Cell 14: 29–41, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Marinissen MJ, Chiariello M, Gutkind JS: Regulation of gene expression by the small GTPase Rho through the ERK6 (p38 gamma) MAP kinase pathway. Genes Dev 15: 535–553, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Charron F, Tsimiklis G, Arcand M, Robitaille L, Liang Q, Molkentin JD, Meloche S, Nemer M: Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev 15: 2702–2719, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anwar KN, Fazal F, Malik AB, Rahman A: RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65. J Immunol 173: 6965–6972, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Benitah SA, Valeron PF, Lacal JC: ROCK and nuclear factor-kappaB-dependent activation of cyclooxygenase-2 by Rho GTPases: Effects on tumor growth and therapeutic consequences. Mol Biol Cell 14: 3041–3054, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takano H, Komuro I, Oka T, Shiojima I, Hiroi Y, Mizuno T, Yazaki Y: The Rho family G proteins play a critical role in muscle differentiation. Mol Cell Biol 18: 1580–1589, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li H, Zhu J, Aoudjit L, Latreille M, Kawachi H, Larose L, Takano T: Rat nephrin modulates cell morphology via the adaptor protein Nck. Biochem Biophys Res Commun 349: 310–316, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Li H, Lemay S, Aoudjit L, Kawachi H, Takano T: SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol 15: 3006–3015, 2004 [DOI] [PubMed] [Google Scholar]