Abstract
Functional impairment of HDL may contribute to the excess cardiovascular mortality experienced by patients with renal disease, but the effect of advanced renal disease on the composition and function of HDL is not well understood. Here, we used mass spectrometry and biochemical analyses to study alterations in the proteome and lipid composition of HDL isolated from patients on maintenance hemodialysis. We identified a significant increase in the amount of acute phase protein serum amyloid A1, albumin, lipoprotein-associated phospholipase A2, and apoC-III composing uremic HDL. Furthermore, uremic HDL contained reduced phospholipid and increased triglyceride and lysophospholipid. With regard to function, these changes impaired the ability of uremic HDL to promote cholesterol efflux from macrophages. In summary, the altered composition of HDL in renal disease seems to inhibit its cardioprotective properties. Assessing HDL composition and function in renal disease may help identify patients at increased risk for cardiovascular disease.
Cardiovascular disease, stroke, and peripheral vascular disease are notorious problems in patients with chronic kidney disease.1 Cardiac mortality in dialysis patients aged 45 years or younger is more than 100-fold increased in comparison with the general population.2 Accelerated atherosclerosis is thought to be caused by increased inflammation, oxidative stress, and impaired triglyceride and HDL metabolisms.3,4 HDL is thought to protect against atherosclerosis by promoting reverse cholesterol transport and potentially through anti-oxidative and anti-inflammatory activities.5–9 More recent studies suggest that the HDL proteome is implicated in HDL functionality, identifying HDL-associated proteins involved in lipid metabolism, complement activation, growth factor, and proteolysis regulation.10–13 These multiple mechanisms of action make HDL a therapeutic target with great potential for the treatment of patients with atherosclerosis.
Low levels of HDL, such as found in renal patients, correlate with an increased risk of atherosclerotic vascular disease.14 However, more recent findings have suggested that the relationship between HDL and cardiovascular risk is more complex and extends beyond the levels of HDL in plasma.15 Notably, it was found that HDL particles may become dysfunctional or even proinflammatory in chronic and inflammatory diseases.16 Recent studies focused on the loss of antioxidant and anti-inflammatory effects of HDL in dialysis patients.17,18 The loss of anti-inflammatory properties in HDL of renal patients was shown to correlate with a higher 30-month adjusted hazard ratio for death in uremic patients.19 Moreover, oxidized plasma proteins in end-stage renal disease patients were shown to interfere with HDL clearance, thereby potentially contributing to the abnormal composition of HDL.20 However, data available regarding the effect of advanced renal disease on HDL functionality are limited, and the effects of renal disease on the first step of reverse cholesterol transport, the efflux of cellular cholesterol from macrophages to HDL, have not been established yet. This is of particular interest, because cholesterol efflux capacity from macrophages has a strong inverse association with the likelihood of coronary artery disease, independent of HDL cholesterol levels.21 Accordingly, to understand the role of HDL in chronic and inflammatory diseases, as end-stage renal disease, it is essential to analyze the protein and lipid composition of HDL to reflect its functional state. Therefore, this study was designed to test the hypothesis that renal disease modifies HDL composition, thereby altering anti-atherogenic properties of HDL.
In this explorative study, liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis was used to investigate the proteomic profile of HDL from 27 hemodialysis patients and 19 control subjects. The analysis revealed that HDL from hemodialysis patients carries a distinct protein and lipid cargo that is linked to decreased cholesterol efflux properties.
RESULTS
Characteristics of Study Subjects
HDL was isolated from end-stage renal disease patients on maintenance hemodialysis (n = 27) and healthy subjects (n = 19). The hemodialysis patient group consisted of both diabetic and nondiabetic individuals with and without lipid-lowering therapy. The control subjects showed no sign of renal insufficiency, were neither hyperlipidemic nor diabetic, and did not receive any lipid-lowering therapy. The clinical characteristics of the study groups are given in Table 1. The hemodialysis group showed significantly elevated plasma concentrations of creatinine, urea, and C-reactive protein (CRP), whereas plasma hemoglobin was reduced. Plasma lipid profiles of hemodialysis patients displayed characteristics of renal dyslipidemia, with significantly increased triglyceride and free-cholesterol levels. Triglyceride/HDL cholesterol ratios were increased in hemodialysis patients, whereas the apolipoprotein (apo) A-I/apoC-III ratios were decreased.
Table 1.
Clinical characteristics of study subjects
| Controls | HD | |
|---|---|---|
| n | 19 | 27 |
| Mean age (years) | 52.9 ± 12.3 | 61.9 ± 17.2 |
| Male/female | 9/11 | 15/12 |
| Statins | 0/19 | 6/27 |
| Diabetes mellitus | 0/19 | 10/27 |
| Plasma parameter | controls | HD |
| Creatinine (mg/dl) | 0.9 (0.9 to 1.1) | 6.9 (6.3 to 10.0)a |
| Urea (mg/dl) | 28 (25 to 34) | 116 (97 to 143)a |
| Hemoglobin (mg/dl) | 13.8 (13.1 to 14.1) | 11.5 (10.5 to 12.4)a |
| CRP (mg/L) | 1 (0 to 2) | 9 (3 to 19)a |
| Fibrinogen (mg/dl) | 307 (235 to 396) | 490 (411 to 618)a |
| Total cholesterol (mg/dl) | 182 (176 to 224) | 164 (126 to 199) |
| Free cholesterol (mg/dl) | 54 (49 to 62) | 54 (38 to 67) |
| Cholesterolester (mg/dl) | 101 (99 to 119) | 115 (88 to 134)b |
| Triglycerides (mg/dl) | 112 (83 to 169) | 147 (97 to 202) |
| HDL cholesterol (mg/dl) | 61 (45 to 72) | 43 (37 to 47)b |
| LDL cholesterol (mg/dl) | 109 (87 to 123) | 87 (62 to 127) |
| Phospholipids (mg/dl) | 270 (221 to 314) | 201 (185 to 239)b |
| Free fatty acids (μmol/L) | 310 (300 to 440) | 370 (240 to 560) |
| TG/HDL-C ratio | 2.5 (1.3 to 3.2) | 3.4 (2.3 to 5.0)b |
| ApoA-I/apoC-III ratio | 19.5 (16.3 to 25.7) | 12.8 (8.4 to 20.3)b |
| HDL lipid composition | ||
| total cholesterol (μg/mg protein) | 260 (235 to 295) | 228 (181 to 260)b |
| triglycerides (μg/mg protein) | 37 (23 to 47) | 74 (52 to 123)a |
| cholesterylester (μg/mg protein) | 196 (175 to 223) | 183 (160 to 214) |
| free cholesterol (μg/mg protein) | 62 (50 to 71) | 37 (28 to 56)b |
| phospholipids (μg/mg protein) | 442 (420 to 482) | 297 (235 to 371)a |
| lyso-PC (nmol/mg protein) | 8.8 (8.2 to 9.1) | 13.5 (11.6 to 20.9)a |
The results are given as medians with the interquartile range. TG, triglycerides; HDL-C, HDL-cholesterol.
aSignificance was accepted at P < 0.001 (Mann-Whitney U test).
bSignificance was accepted at P < 0.01 (Mann-Whitney U test).
We assessed the effect of diabetes and lipid-lowering therapy on plasma and HDL parameters. In the hemodialysis patient group, only plasma triglyceride levels of those individuals who received statins were significantly decreased; all other parameters were not altered (Supplemental Table S1).
HDL from HD Patients Carries a Distinct Protein Cargo
Over the past years, proteomic studies have markedly extended the list of HDL-associated proteins.10–13 We sought to investigate possible alterations in the HDL proteome of end-stage renal disease patients undergoing regular hemodialysis. HDL was digested, and the resulting peptides were analyzed by tandem mass spectrometry.
LC-MS/MS analysis identified 35 proteins to be associated with HDL (Table 2). As expected, we found that the major protein components of HDL, from the hemodialysis group and the control group, were apolipoprotein (apo)A-I and apoA-II, accounting for about 50 and 10% of all detected peptides, respectively. Most of the major apolipoproteins (apoA-I, apoA-II, apoC-III, apoE, apoC-I, apoD, apoC-II, and apoM) as well as SAA1, SAA4, and albumin were detected in all samples from hemodialysis patients and controls, whereas antitrypsin, retinol-binding protein 4 (RBP4), transthyretin, apoA-VI, and further minor proteins were only detected in uremic HDL. ApoB was detected in four samples in trace amounts, always accompanied by apo(a) indicating the presence of lipoprotein(a) whose hydrated density overlaps with HDL (Table 2).
Table 2.
Identification of proteins in HDL isolated from hemodialysis patients (HD) and control subjects (control)
| Accession Number | Protein Name | HDL-derived Peptides |
P | Ratio | |||
|---|---|---|---|---|---|---|---|
| HD (n = 27) | Control (n = 19) | ||||||
| 178775 | ApoA-I | 331.3 | (27/27) | 416.2 | (19/19) | <0.001 | 0.8 |
| 4502149 | ApoA-II | 60.9 | (27/27) | 72.3 | (19/19) | <0.001 | 0.8 |
| 167887493 | ApoC-III | 46.5 | (27/27) | 33.5 | (19/19) | <0.001 | 1.4 |
| 119588814 | SAA1 | 45.6 | (27/27) | 5.6 | (17/19) | <0.001 | 8.2 |
| 10835095 | SAA4 | 44.9 | (27/27) | 37.9 | (19/19) | 0.001 | 1.2 |
| 4557325 | ApoE | 42.9 | (27/27) | 45.0 | (19/19) | 0.671 | 1.0 |
| 4502027 | Albumin | 40.8 | (27/27) | 13.5 | (19/19) | <0.001 | 3.0 |
| 4502157 | ApoC-I | 25.4 | (27/27) | 30.2 | (19/19) | 0.001 | 0.8 |
| 4502163 | ApoD | 17.2 | (27/27) | 16.2 | (19/19) | 0.300 | 1.1 |
| 32130518 | ApoC-II | 13.9 | (27/27) | 8.5 | (19/19) | 0.001 | 1.6 |
| 22091452 | ApoM | 8.0 | (27/27) | 11.8 | (19/19) | 0.002 | 0.7 |
| 105990532 | ApoB | 4.0 | (4/27) | — | (0/19) | 0.067 | — |
| 114062 | Apo(a) | 3.2 | (5/27) | 0.4 | (3/19) | 0.778 | — |
| 93163358 | ApoA-IV | 2.9 | (27/27) | — | (0/19) | <0.001 | — |
| 15080499 | Antitrypsin | 2.9 | (11/27) | — | (0/19) | <0.001 | — |
| 18088326 | RBP4 | 2.0 | (17/27) | — | (0/19) | <0.001 | — |
| 114318993 | Transthyretin | 1.0 | (14/27) | — | (0/19) | <0.001 | — |
| 6960317 | α-2 catenin | 1.0 | (14/27) | 0.6 | (3/19) | 0.002 | — |
| 298532 | Paraoxonase 1 | 0.8 | (8/27) | 0.4 | (3/19) | 0.329 | — |
| 34364645 | Igα | 0.7 | (5/27) | — | (0/19) | 0.067 | — |
| 45643462 | GIP 12 | 0.3 | (1/27) | — | (0/19) | — | — |
| 4557894 | Lysozyme | 0.3 | (1/27) | — | (0/19) | — | — |
| 194386720 | KIAA0590 | 0.3 | (4/27) | — | (0/19) | — | — |
| 7020972 | PHIP | 0.3 | (7/27) | — | (0/19) | — | — |
| 4503107 | Cystatin C | 0.3 | (5/27) | — | (0/19) | — | — |
| 194386720 | Unknown protein | 0.3 | (4/27) | — | (0/19) | — | — |
| 4505733 | Unknown protein | 0.3 | (7/27) | — | (0/19) | — | — |
| 146424184 | ApoC-IV | 0.2 | (5/27) | — | (0/19) | — | — |
| 179665 | Complement C3 | 0.1 | (1/27) | — | (0/19) | — | — |
| 4885179 | Defensin α3 | 0.1 | (1/27) | — | (0/19) | — | — |
| 119579599 | Haptoglobin | 0.1 | (1/27) | — | (0/19) | — | — |
| 181482 | Vit.D-BP | 0.1 | (2/27) | — | (0/19) | — | — |
| 4507261 | Statherin | 0.1 | (1/27) | — | (0/19) | — | — |
| 4505733 | Platelet factor 4 | — | (0/27) | 0.6 | (8/19) | — | — |
| 148745121 | ApoL-I | — | (0/27) | 0.2 | (1/19) | — | — |
| Mean total peptides/subject | 698 ± 61 | 693 ± 79 | |||||
| Lp-PLA2 mass (densitometry units) | 2.7 ± 1.3 | 1.00 ± 0.8 | <0.001 | ||||
HDL was isolated from 27 HD patients and 19 control subjects by one-step ultracentrifugation. The HDL proteome was analyzed on a LC-MS/MS system. The data were analyzed by searching the human NCBI nonredundant public database with Spectrum mill Rev. A0.03.03.078 (Agilent) and Mascot 2.2 (MatrixScience). The values shown represent the mean peptide count per analyzed subject with the corresponding number of patients where respective protein was identified. Statistical significance was calculated with a Mann-Whitney U test. Lp-PLA2 mass associated with HDL was determined by Western blot and densitometric quantification. GIP12, growth-inhibiting protein 12; Vit.D-BP, vitamin D-binding protein; PHIP, PH interacting protein. —, not detected.
Many lines of evidence suggest that spectral counting is a valid tool for assessing overall protein abundance.22,23 After statistical analysis, we identified nine proteins to be significantly altered in hemodialysis patients (P < 0.001). Strikingly, we observed on average three- and eight-fold increases in albumin and SAA1 content of uremic HDL, respectively, making them the major HDL-associated proteins (Table 2).
We were not able to assess the content of lipoprotein-associated phospholipase A2 (Lp-PLA2) (alternatively named platelet-activating factor acetylhydrolase) with LC-MS/MS. Lp-PLA2 is an important phospholipase that has been recently associated with an enhanced risk of coronary artery disease, stroke, and mortality.24 Therefore, we performed immunoblot analysis, revealing a 2.8-fold increased Lp-PLA2 content in uremic HDL (Table 2).
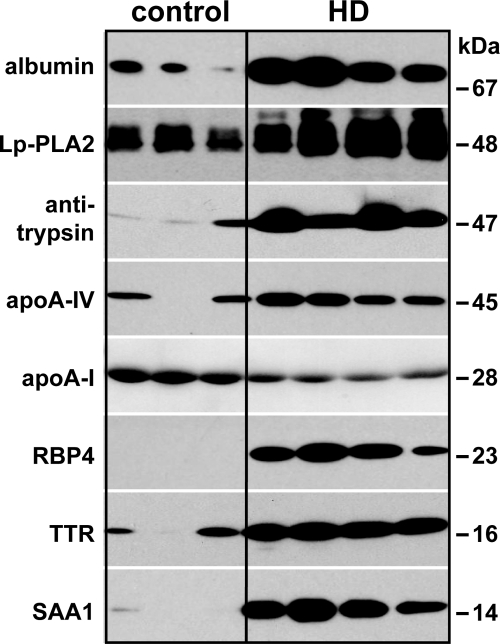
To validate the proteomic results, further immunoblot analysis was performed choosing significantly enriched proteins (Table 2). Protein enrichment could be confirmed by appearance of specific bands at 67 kD for albumin, 48 kD for Lp-PLA2, 47 kD for antitrypsin, 45 kD for apoA-VI, 23 kD for RBP4, 16 kD for transthyretin, and 14 kD for SAA1 on uremic HDL (Figure 1). The immunoblot reflected a similar enrichment for the respective proteins as observed by LC-MS/MS analysis (Table 2). In contrast to the LC-MS/MS analysis, immunoblot analysis was more sensitive and detected even small amounts of antitrypsin, transthyretin, and apoA-IV on control HDL. Interestingly, RBP4 was only detected in uremic HDL, indicating a specific enrichment.
Figure 1.
Immunoblot of HDL-associated proteins confirms LC-MS/MS results. To confirm the results obtained by LC-MS/MS, HDL isolated from hemodialysis patients and control subjects was subjected to immunoblot analysis. HDL-associated proteins were separated by SDS-PAGE, transferred to PVDF membranes, and probed using specific antibodies. Molecular mass is indicated on the right.
Chronic kidney disease is associated with an inflammatory response caused by the dialysis procedure, increased oxidative stress, and dyslipidemia.25 The hemodialysis group exhibited a significant increase in the plasma inflammatory markers CRP and fibrinogen (Table 1). As expected, the HDL-associated acute-phase protein SAA1 correlated with the plasma concentration of CRP and fibrinogen in hemodialysis patients (Supplemental Table 2). Interestingly, HDL-associated albumin did not correlate with plasma CRP or fibrinogen levels and did not correlate with HDL-associated SAA1 or plasma levels of albumin in hemodialysis patients (Supplemental Table 2). Moreover, apoC-III accumulation in HDL showed no correlation with HDL-associated albumin or SAA1 (Supplemental Table 2) but correlated with plasma apoC-III (not shown) and creatinine (Supplemental Table 2). Therefore, our data indicate different underlying mechanisms resulting in the enrichment of HDL with albumin, apoC-III, or SAA1.
However, further data analysis revealed a strong positive correlation between HDL-associated albumin and apoA-VI, antitrypsin, and RBP4 (Supplemental Table 3). In line with this observation, a recent study observed that plasma albumin binds several plasma proteins including apoA-VI, antitrypsin, and RBP4,26 raising the possibility that albumin carries proteins onto HDL.
Cholesterol Acceptor Capability of Uremic HDL Is Decreased
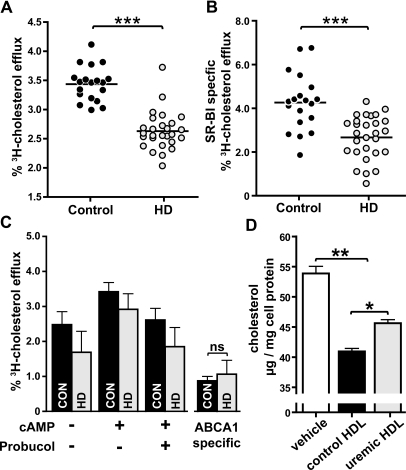
Cholesterol efflux capacity from macrophages has a strong inverse association with the likelihood of coronary artery disease, independent of HDL cholesterol levels.21 We observed that the capability of uremic HDL to promote cholesterol efflux from lipid-laden macrophages was significantly reduced at 2 hours (Figure 2A) and after prolonged exposure of 20 hours (Supplemental Figure 1) in comparison with control HDL. Importantly, neither diabetes nor lipid-lowering therapy significantly altered cholesterol acceptor capacity (Supplemental Figure 2) or proteomic composition of uremic HDL (Supplemental Table 4).
Figure 2.
Uremia impairs cholesterol efflux capability of HDL. HDL from 27 hemodialysis patients (HD) and 19 control subjects (CON) were examined for their ability to efflux [3H]cholesterol from TO-901317-stimulated lipid-loaded RAW267 macrophages. (A) [3H]cholesterol-labeled cells were incubated with HDL (50 μg/ml) for 2 hours at 37°C. Cholesterol efflux is expressed as radioactivity in the medium relative to total radioactivity in medium and cells. The values shown represent the means of three individual experiments performed in duplicates. (B) To determine SR-BI-mediated efflux, [3H]cholesterol-labeled ldlA7[SR-BI] cells and control ldlA7 cells were incubated with HDL (100 μg/ml) for 2 hours at 37°C. The difference in efflux between control and SR-BI-overexpressing cells was taken as a measure of SR-BI-mediated efflux. The values shown represent the means of two individual experiments performed in triplicates. (C) To assess ABCA1-specific efflux, [3H]cholesterol RAW267 macrophages were stimulated with cAMP and incubated in the presence of 20 μg/ml HDL for 3 hours. ABCA1-specific efflux to HDL was determined by subtracting cholesterol efflux in presence of probucol (ABCA1 inhibitor) from efflux in absence of the inhibitor. (D) To assess net cholesterol flux, RAW267 macrophages were cholesterol-loaded as described in the Concise Methods section. Cellular cholesterol flux was initiated by exposure of cells to serum-free medium with or without HDL (100 μg/ml) for 14 hours. Afterwards cellular lipids were extracted, and total cholesterol mass/mg cell protein was determined. For ABCA1-specific efflux (C) and net cholesterol flux (D), pooled HDL fractions of HD patients and controls were used. The results represent the means of triplicate determinations ± SD of two experiments. Statistical analysis was performed by t test for two groups and with ANOVA for more than two groups. Significances were accepted at *P < 0.05, **P < 0.01, and ***P < 0.001.
Cholesterol efflux is mediated by scavenger receptor class B, type 1 (SR-BI) and ATP-binding cassette transporter A1 (ABCA1) in human monocyte-derived macrophages.27 Therefore, we investigated whether SR-BI- and/or ABCA1-mediated cholesterol efflux to uremic HDL was impaired. For that purpose, we used SR-BI-overexpressing Chinese hamster ovary cells and cAMP-stimulated macrophages to specifically up-regulate ABCA1. The induction of ABCA1 was confirmed by cholesterol efflux studies with lipid-free apoA-I (Supplemental Figure 3). The difference in efflux between control and SR-BI-overexpressing cells was taken as a measure of SR-BI-mediated efflux. ABCA1-specific efflux was determined by subtracting cholesterol efflux in presence of probucol (ABCA1 inhibitor) from efflux in absence of the inhibitor. We observed that SR-BI-mediated cholesterol efflux to uremic HDL was significantly reduced compared with control HDL (Figure 2B), but ABCA1-induced efflux to uremic and control HDL did not differ significantly (Figure 2C).
Cholesterol efflux is bi-directional, and measurement of cholesterol efflux alone does not indicate net cholesterol flux from cells. Therefore, we measured the cholesterol content of lipid-laden macrophages after a 20-hour exposure to control and uremic HDL. We observed that control HDL reduced intracellular cholesterol approximately 20%, whereas uremic HDL was significantly less potent in mediating net cholesterol flux (Figure 2D).
SAA1, Albumin, ApoC-III, and Triglyceride Content of HDL Correlate with Impaired Cholesterol Efflux Potential
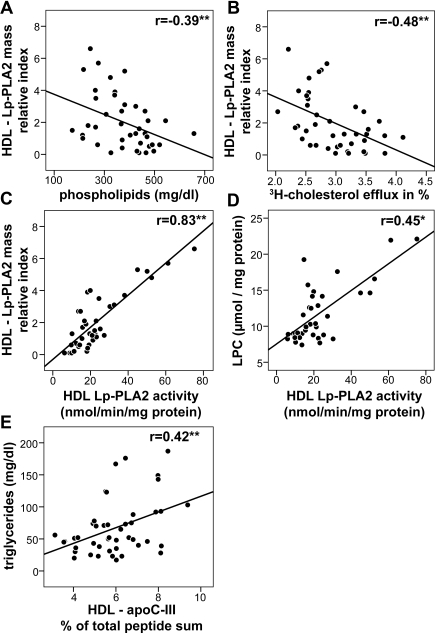
Prompted by the observation that the cholesterol efflux capability of uremic HDL was significantly reduced, we assessed whether the relative concentration of major proteins and lipids in uremic HDL correlates with the impaired ability to efflux cholesterol.
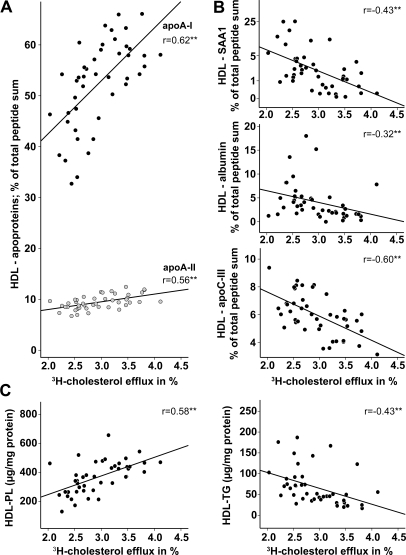
As expected, a strong positive correlation of HDL-associated apoA-I, apoA-II (Figure 3A), and phospholipids (Figure 3C) with the cholesterol efflux potential of uremic and control HDL was observed. Notably, apoA-I and apoA-II are also positively correlated with the phospholipid content of HDL, which is in agreement with the high phospholipid binding capability of apoA-I/A-II (Supplemental Figure 4). In contrast, the HDL proteins: SAA1, albumin, and apoC-III (Figure 3B), and HDL triglyceride content (Figure 3C) negatively correlated with the cholesterol efflux capability of HDL.
Figure 3.
Determinants of cholesterol efflux potential of HDL. To determine factors influencing HDL cholesterol efflux potential, proteomic data were correlated with [3H]cholesterol efflux capability of HDL preparations from patients and controls. (A) Positive correlation of apoA-I and apoA-II with [3H]cholesterol efflux. (B) Negative correlation of HDL-SAA1, HDL-albumin, and HDL-apoC-III with [3H]cholesterol efflux. (C) Correlations of phospholipid and triglyceride content of HDL with the [3H]cholesterol efflux. Pearson's correlation coefficients are noted for each plot. **P < 0.01. PL, phospholipids; TG, triglycerides.
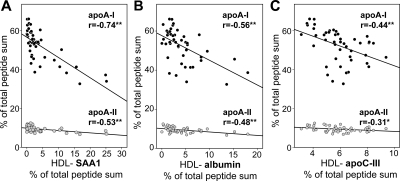
SAA1, Albumin, and ApoC-III Replace Apolipoproteins A-I and A-II
It is well known that in circulation SAA associates with HDL particles, causing HDL remodeling with displacement of apoA-I.28–30 To gain further insight into the relationship between protein composition and cholesterol efflux potential of HDL, a detailed correlation analysis was performed. An inverse correlation of HDL-associated apoproteins A-I and A-II with SAA1 was observed (Figure 4A). Interestingly, albumin and apoC-III also inversely correlated with apoA-I and apoA-II content of HDL (Figure 4B and 4C).
Figure 4.
SAA1, albumin, and apoC-III replace the HDL apolipoproteins apoA-I and apoA-II. ApoA-I and apoA-II content of HDL from patients and controls was correlated with HDL-SAA1 (A), HDL-albumin (B), and HDL-apoC-III (C) content. Pearson's correlation coefficients are noted for each plot. **P < 0.01; *P < 0.05.
ApoC-III Is Linked to a High-Triglyceride Content, Whereas Lp-PLA2 Negatively Correlates with HDL-associated Phospholipids
Changes in the lipid composition of HDL directly influence the ability of the lipoprotein to bind and retain cholesterol.31,32 Cholesterol efflux was shown to be directly proportional to the amount of phospholipids in reconstituted HDL particles.31 In line with these observations, we found a strong correlation between the phospholipid content of HDL and its cholesterol efflux capability (Figure 3C). We next assessed whether the Lp-PLA2 content of HDL correlates with the phospholipid content of HDL. HDL Lp-PLA2 mass showed a negative correlation with HDL phospholipids (Figure 5A) and cholesterol efflux capability (Figure 5B). Quantification of HDL-associated Lp-PLA2 activity revealed a strong correlation with HDL-Lp-PLA2 mass (Figure 5C). We also measured lysophospholipid content of HDL and observed that Lp-PLA2 activity significantly correlates with lysophospholipid content of HDL, indicating that Lp-PLA2 hydrolyzes phospholipids in HDL (Figure 5D). The levels of apoC-III, a known inhibitor of lipoprotein lipase and an important regulator of triglyceride metabolism,33 were reported to be altered in renal disease.34 In good agreement, we observed an increased apoC-III and triglyceride content in uremic HDL (Table 1) correlating with each other (Figure 5E). Therefore, the known risk factors apoC-III and Lp-PLA2 may be directly involved in rendering HDL dysfunctional.
Figure 5.
Increased content of Lp-PLA2 correlates with reduced HDL-phospholipid content and cholesterol efflux capability. (A) Negative correlation between the HDL-Lp-PLA2 mass and HDL phospholipids. (B) Negative correlation between HDL-Lp-PLA2 mass and HDL-mediated [3H]cholesterol efflux capability. (C) Positive correlation between HDL-Lp-PLA2 mass and HDL-Lp-PLA2 activity. (D) Positive correlation of HDL-Lp-PLA2 activity and lyso-PC content of HDL. (E) Positive correlation of HDL-apoC-III with HDL triglycerides. Pearson's correlation coefficients are noted for each plot. *P < 0.05; **P < 0.01.
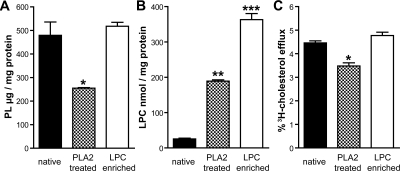
Loss of HDL-associated Phospholipids Impairs Cholesterol Efflux Capacity
Next, we tested whether reducing phospholipids to levels observed in uremic HDL lowers cholesterol efflux capacity. Phospholipase treatment effectively reduced the phospholipid content of HDL (Figure 6A) paralleled by an increased lysophospholipid content (Figure 6B). To assess the effect of lysophospholipids on cholesterol efflux capacity, control HDL was enriched with 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (lyso-PC) (Figure 6B). Importantly, phospholipase-mediated hydrolysis of phospholipids reduced cholesterol efflux capacity, whereas lyso-PC enrichment did not (Figure 6C).
Figure 6.
PLA2 treatment reduces cholesterol efflux capacity of HDL. Control HDL was PLA2-treated or enriched with lyso-PC (LPC) as described in the Concise Methods section and analyzed for their phospholipid content (A), lysophospholipid content (B), and [3H]cholesterol efflux capability (C). Statistical analysis was performed by ANOVA. Significances were accepted at *P < 0.05, **P < 0.01, and ***P < 0.001.
DISCUSSION
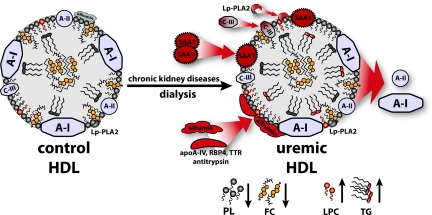
This study provides evidence that HDL of patients suffering from renal disease carries a unique proteome and lipid composition linked to an impaired cholesterol efflux capacity. Our findings raise the possibility that dysfunctional HDL contributes to accelerated atherosclerosis in end-stage renal patients. We applied LC-MS/MS, immunoblot technique, and lipid analysis to determine alterations of the HDL proteome and lipid composition in end-stage renal disease patients on regular hemodialysis. Several proteins, including highly-abundant proteins like SAA1, albumin, apoC-III and slightly-abundant proteins like antitrypsin, apoA-VI, RBP4, transthyretin, and Lp-PLA2, were identified to be significantly enriched in uremic HDL (Figure 7). SAA1 and albumin content of uremic HDL showed eight- and three-fold increases, respectively, making them the major HDL-associated proteins. Notably, SAA1 accumulation correlated with plasma inflammatory markers CRP and fibrinogen, whereas albumin, apoC-III, and Lp-PLA2 did not. Moreover, HDL-associated apoC-III significantly correlated with plasma creatinine, but not with SAA1, albumin, or Lp-PLA2, suggesting that different mechanisms mediate their association to HDL. However, further data analysis revealed a strong positive correlation between HDL-associated albumin and apoA-VI, antitrypsin, transthyretin, and RBP4. These data indicate that similar mechanisms trigger HDL enrichment with these proteins or that albumin itself carries proteins onto HDL. In line with this finding, a recent study observed that plasma albumin binds several plasma proteins including apoA-VI, antitrypsin, and RBP4.26
Figure 7.
Schematic illustration of HDL remodeling in uremic patients. HDL from uremic hemodialysis patients exhibits various alterations in the proteome and lipid composition that are linked to impaired cholesterol acceptor capacity. Uremic HDL is significantly enriched in SAA1, albumin, and apoC-III, as well as in the low-abundant HDL-associated proteins apoA-IV, RBP4, transthyretin (TTR), antitrypsin, and Lp-PLA2. These proteomic alterations were accompanied by the loss of apoA-I and apoA-II. In addition, uremia and dialysis lead to profound alterations in HDL-lipid composition reflected as a decrease in phospholipids (PL) and free cholesterol (FC) and an increase in triglyceride (TG) and lyso-PC (LPC) content.
A major finding of this study is that enrichment of HDL with SAA1, albumin, apoC-III, and Lp-PLA2 correlated with the altered metabolic properties of HDL. We observed that the reduced cellular cholesterol efflux capability of uremic HDL is linked to a depletion of HDL-associated apoA-I, apoA-II, and phopholipids, all factors that are known to increase the cholesterol acceptor capability of HDL.35 The shedding of predominantly apoA-I is not surprising, because apoA-I is the least lipophilic of the HDL apolipoproteins. Our data indicate that in addition to SAA1, which is known to displace apoA-I from HDL,28,29 albumin and apoC-III are capable of replacing apolipoproteins on HDL. The substitution of apoA-I and apoA-II negatively correlated with the phospholipid content of HDL, in agreement with the high phospholipid binding capacity of apoA-I and apoA-II. We conclude that the reduced phospholipid content of uremic HDL is the main contributor to the low cholesterol efflux capacity, because phospholipase treatment of control HDL (which results in a HDL-phospholipid content similar to that of uremic HDL) decreased efflux capacity to about the same extent as observed with uremic HDL. In line with our observation, it was recently demonstrated that alterations in phospholipid content and not the presence of SAA in human HDL impaired the ability of acute phase HDL to promote cholesterol efflux.36 In plasma, HDL undergoes a series of changes that are mediated by cholesteryl ester transfer protein (CETP) and phospholipid transfer protein (PLTP). CETP mediates the exchange of HDL-associated cholesteryl esters with triglycerides from apoB-containing lipoproteins, whereas PLTP transfers phospholipids and free cholesterol from triglyceride-rich lipoproteins to HDL. However, most studies have shown no significant change in plasma CETP or PLTP concentrations or activities in patients with end-stage renal disease on hemodialysis,14 ruling out a major effect of these enzymes on lipid alterations observed in uremic HDL.
A recent study demonstrated that chronic kidney disease patients of stages 3 and 4 are characterized by an increase in plasma Lp-PLA2 activity.37 A novel finding in this study was that Lp-PLA2, a novel risk factor that is involved in the development of atherosclerosis, was 2.8-fold increased in uremic HDL. Interestingly, Lp-PLA2 content of HDL negatively correlated with phospholipid levels of HDL, indicating that Lp-PLA2 might hydrolyze phospholipids in HDL. In line with this observation, we found that HDL associated Lp-PLA2 activity, and lyso-PC content significantly correlated with HDL-associated Lp-PLA2 mass. In circulation Lp-PLA2 normally travels mainly with LDL, but about 20% of Lp-PLA2 is associated with HDL.38 It has to be noted that HDL isolation methods on the basis of prolonged ultracentrifugation may result in the dissociation and redistribution of Lp-PLA2 among lipoproteins. In particular, Lp-PLA2 associated with small dense LDL appears to shift by ultracentrifugation.39 We isolated HDL in a one-step rapid ultracentrifugation protocol and did not detect apoB-100 (major apolipoprotein of LDL) in isolated HDL. Therefore, we can exclude a major contribution of small dense LDL-associated Lp-PLA2 in our HDL fraction. A recent meta-analysis reported that Lp-PLA2 levels are positively correlated with an increased risk of developing coronary heart disease and stroke,24 and therefore therapies targeting Lp-PLA2 in plasma and atherosclerotic plaque are now being developed. The observed increased content of Lp-PLA2 in uremic HDL raises the possibility that HDL-associated Lp-PLA2 might also contribute to increased atherosclerosis in renal patients.
A further notable finding in this study was that increased apoC-III content of uremic HDL was coupled to an increased content of HDL associated triglycerides and correlated with plasma creatinine levels. This clearly indicates that apoC-III metabolism is altered in renal disease. Most importantly, both HDL-associated apoC-III and triglyceride content negatively correlated with cholesterol efflux potential of HDL. ApoC-III is a small apolipoprotein that is synthesized mainly in the liver and circulates in the plasma in association with apoB-containing lipoproteins and HDL. ApoC-III inhibits lipoprotein lipase and hepatic lipase and is thought to inhibit hepatic uptake of triglyceride-rich particles as well.40 In case-control studies, plasma concentrations of apoC-III-containing lipoproteins were reported to be strong independent risk factors for cardiovascular disease.41 Moreover, recent experimental studies have also shown that apoC-III may be a crucial link between renal dyslipidemia and increased atherosclerosis.42
Recent advances in proteomic analysis because of the use of high-throughput and high-content analysis has paved the way for clinical proteomics. The proteomic alterations in uremic HDL identified in this study could therefore offer basis for (1) assessment of HDL functionality and (2) identification of humans at increased risk of cardiovascular disease. In summary, we here report that renal disease impairs the cholesterol acceptor potential of HDL. The functional impairment is linked to a unique protein cargo in uremic HDL accompanied by a decreased phospholipid and increased triglyceride content.
CONCISE METHODS
Blood Collection
Blood was taken from hemodialysis patients before the dialysis session and from age-matched control subjects at the time of routine laboratory investigations in agreement with the Ethical Committee of the Medical University of Graz. Blood (5 ml) was collected in standard sterile polystyrene vacuum tubes with 5 mmol/L EDTA.
Isolation of HDL
HDL was isolated by an improved one-step density gradient ultracentrifugation method.43 Plasma was separated by centrifugation at 400 × g for 15 minutes at 8°C, and the density was adjusted with potassium bromide to 1.24 g/L. To optimize lipoprotein separation, long centrifuge tubes (16 × 76 mm; Beckman) were used. A density gradient was generated by layering the density-adjusted plasma (1.24 g/ml) underneath a NaCl-density solution (1.063 g/ml). The samples were centrifuged at 694,000 × g for 4 hours, and HDL was collected, desalted by gel filtration on Sephadex PD-10 columns (Amersham Biosciences, Uppsala, Sweden), and either directly used or stored at −70°C for further analysis.
Determination of Plasma and HDL Lipid Composition
The levels of total cholesterol, nonesterified cholesterol, triglycerides, phospholipids (Diasys, Holzheim, Germany), lysophosphatidylcholine (Cosmo Bio Co. Ltd., Tokyo, Japan), and nonesterified fatty acids (Wako Chemicals, Neuss, Germany) were measured enzymatically. LDL cholesterol was calculated according to the Friedewald equation described previously44 using HDL cholesterol values measured in the supernatant of the phosphotungstic precipitation.
Apolipoprotein Determination by Immunoturbidimetry
ApoA-I, apoA-II, apoB, apoC-II, apoC-III, apoE (Greiner, Flacht, Germany), and lipoprotein(a) (Wako Chemicals, Neuss, Germany) were determined by immunoturbidimetry. All of the lipoprotein analyses were performed on an Olympus AU640 analyzer (Olympus Diagnostika, Hamburg, Germany).
SDS-PAGE and Western Blotting
Antibodies for apoA-VI (ab72395), albumin (ab83465), antitrypsin (ab90158), transthyretin (ab16006), SAA1 (ab81483), and RBP4 (ab64194) were purchased from Abcam (Cambridge, UK). HDL-associated proteins (1 μg for apoA-I; 2.5 μg for albumin; 5 μg for antitrypsin, RBP4, and SAA1; and 10 μg for transthyretin and apoA-VI) were separated by SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes, and probed using specific antibodies. SDS-PAGE for protein separation and protein transfer to PVDF membranes for Western blotting were performed as described previously.29
Lp-PLA2 Mass and Activity
To assess HDL-associated Lp-PLA2 mass, HDL (10 μg) was separated by SDS-PAGE, blotted, and probed with Lp-PLA2 antibody (Cayman Europe, Talinn, Estonia) as described above. Western blots were densitometrically analyzed, and the Lp-PLA2 content was calculated as a relative index to the average control population content. Lp-PLA2 activity was measured with a commercially available photometric assay (Cayman Europe) with 2-thio platelet activating factor as the Lp-PLA2 substrate.
Cellular Cholesterol Efflux Assays
Cholesterol efflux assay was performed as described previously, with minor modifications.46 RAW267 macrophages maintained in DMEM plus 10% FBS were plated on 24-well plates. The cells were labeled for 24 hours with [3H]cholesterol (1 μCi/ml) in medium supplemented with 5% FBS and 50 μg/ml carbamylated LDL. For the last 14 hours of incubation, the cells were stimulated with the LXR agonist TO-901317 (2 μmol/L). After labeling, the cells were rinsed, equilibrated in serum-free medium with 0.2% BSA for 2 hours, rinsed again, and incubated with 50 μg/ml HDL for 2 hours at 37°C to determine the [3H]cholesterol efflux.
SR-BI-specific efflux was assessed using ldlA7 and ldlA7[SR-BI] cells (kindly provided by Dr. Monty Krieger, Massachusetts Institute of Technology, Boston, MA). The cells were loaded with [3H]cholesterol (1 μCi/ml) for 24 hours and equilibrated in serum-free media with 0.2% BSA for 2 hours, and cellular cholesterol efflux was assessed in response to 100 μg/ml HDL for 2 hours at 37°C.
To determine ABCA1 specific efflux, [3H]cholesterol-loaded (1 μCi/ml, 24 hours) cAMP-stimulated (300 μmol/L, 14 hours) RAW macrophages were equilibrated in serum-free medium with 0.2% BSA for 2 hours in the presence or absence of 20 μmol/L probucol (ABCA1 inhibitor). The cells were incubated with 20 μg/ml HDL or apoA-I, and [3H]cholesterol efflux was assessed. ABCA1-specific efflux was determined by subtracting efflux in the presence of probucol from efflux in absence of the inhibitor.
For all of the efflux experiments, supernatants and cells were separately collected, and radioactivity was measured by liquid scintillation counting. Cholesterol efflux to the acceptors was measured in aliquots of medium and expressed as radioactivity in the medium relative to total radioactivity in medium and cells. Specific cholesterol efflux was calculated by subtracting cholesterol efflux in the absence of HDL from efflux in the presence of HDL.
PLA2 Treatment and Lysophospholipid Enrichment of HDL
HDL was incubated in the presence of 500 ng/ml phospholipase A2 (Cayman Europe) for 16 hours to hydrolyze HDL-associated phospholipids. After incubation, phospholipase A2 was removed by density gradient ultracentrifugation of HDL as described above. To generate lyso-PC-enriched HDL, 0.65 mmol of lyso-PC (Avanti Polar Lipids) dissolved in chloroform/methanol (1:1, vol/vol) was placed in a glass tube, and the solvent was evaporated with nitrogen. Dried lyso-PC was resuspended in PBS by vortexing for 3 minutes, added to 1 mg/ml HDL drop-wise, and incubated for 2 hours at 37°C. Unbound lyso-PC was removed by gel filtration, and lyso-PC content was analyzed as described above.
Net Cholesterol Efflux from Lipid-laden Macrophages
RAW267 macrophages maintained in DMEM plus 10% FBS were plated on 6-well plates. The cells were loaded with cholesterol by incubating with aggregated LDL (100 μg/ml) in DMEM plus 5% FBS in the presence of 100 nM PMA for 48 hours. The cellular cholesterol mass of untreated control cells was 11.4 ± 2.3 μg total cholesterol/mg cell protein and 53.9 ± 2.0 total cholesterol/mg cell protein for cholesterol loaded cells. After cholesterol loading, the cells were washed twice and equilibrated in serum-free medium with 0.2% BSA for 8 hours. The medium was removed, and cholesterol efflux was initiated by the addition of HDL (100 μg/ml) for 14 hours. At the end of the experiment, the cells were washed three times, and the lipids were extracted with 2 ml of hexane/isopropyl alcohol (3:2, vol/vol) for 1 hour at 4°C. The lipids were dried under nitrogen and resuspended in 200 μl 0.5% Triton X-100 in chloroform. Chloroform was evaporated under nitrogen, and the lipids were resuspended in 100 μl of PBS for 15 minutes at 37°C for cholesterol quantification as described above.
LC-MS/MS Analysis
For tryptic digest, 50 μg of protein of HDL preparations were precipitated with 3 volumes of acetone at −20°C overnight, solubilized in 30 μl (6 mol/L) ammonium guanidinium hydrochloride, reduced with 34 μl of (10 mmol/L) DTT for 20 minutes by shaking at 550 rpm at 56°C and alkylated with 8 μl of (55 mmol/L) iodoacetamide for 15 minutes by shaking at 550 rpm at room temperature. Protein was digested by adding 1 μg of modified trypsin (Promega, Mannheim, Germany) and left for overnight shaking at 550 rpm at 37°C. Completeness of digests was controlled by analyzing 5-μg aliquots by SDS-PAGE. The resulting peptide solution was acidified by adding 1.6 μl of 5% formic acid and diluted in solvent A (see below) to a theoretical final concentration of 50 ng/μl. The samples (40 μl) were separated by nano-HPLC on an Agilent 1200 system equipped with a Zorbax enrichment column (300SB-C18, 5 μm, 5 × 0.3 mm) and a Zorbax nanocolumn (300SB-C18, 3.5 μm, 150 × 0.075 mm). The samples were injected and concentrated on the enrichment column for 6 minutes using 0.1% formic acid as an isocratic solvent at a flow rate of 20 μl/min. The column was then switched into the nanoflow circuit, where the sample was loaded for 6 minutes on the nanocolumn at a flow rate of 300 nL/min and separated using the following gradient: solvent A: water, 0.3% formic acid; solvent B: acetonitril/water 80/20, 0.3% formic acid; 0 to 10 minutes: 10% solvent B; 10 to 120 minutes 10 to 60% solvent B, 120 to 122 minutes 60 to 95% solvent B, 122 to 130 minutes 95% solvent B, 130 to 132 minutes 95 to 10% solvent B, 132 to 140 minutes reequilibration at 10% B. The sample was ionized in the nanospray source equipped with nanospray tips (PicoTipTM stock number FS360-75 to 15-D-20, coating: 1P-4P, 15 ± 1 μm emitter; New Objective) and analyzed in a LTQ-FT mass spectrometer (Thermo Scientific, Waltham, MA) in positive ion mode by alternating full scan MS (m/z 200 to 2000) in the ion cyclotron resonance cell and MS/MS by collision-induced dissociation of the five most intense peaks in the ion trap with dynamic exclusion enabled.
LC-MS/MS Data Analysis and Statistical Analysis
LC-MS/MS data were analyzed by searching the human NCBI nonredundant public database with Spectrum mill Rev. A.03.03.078 (Agilent, Vienna, Austria) and Mascot 2.2 (MatrixScience, London, UK). Detailed settings: enzyme: trypsin, maximum missed cleavage sides: 2, N terminus: hydrogen, C terminus: free acid, search mode: homology search, maximum precursor charge: 3; precursor mass tolerance: ±0.05 Da, product mass tolerance: ±0.7 Da; acceptance parameters were two or more identified peptides after automatic validation (Mascot: P < 0.05, FDR <5%; Spectrum Mill: for precursor charge of 2: score threshold is 6.0, %SPI threshold is 60.0, Fwd-Rev score threshold is 2.0 and rank 1 to 2 score threshold is 2.0, for precursor charge of 1: score threshold is 6.0, %SPI threshold is 70.0, Fwd-Rev score threshold is 2.0 and rank 1 to 2 score threshold is 2.0, for precursor charge of 3: score threshold is 8.0, %SPI threshold is 70.0, Fwd-Rev score threshold is 2.0 and rank 1 to 2 score threshold is 2.0).
Differences in plasma and HDL parameters between hemodialysis and control group were analyzed using a Mann-Whitney U test. Two-way ANOVA was used to assess for differences in plasma and HDL parameters between the control group and four hemodialysis subgroups (with/without diabetes mellitus, with/without lipid lowering therapy).
Changes in HDL proteome were evaluated from spectral counts (e.g. the number of MS/MS spectra assigned to a protein) of automatically validated proteins. The SD of spectral counts was below 10% between duplicates. The Shapiro-Wilk test (at the level of 10%) was used to assess data normality. Because proteomic data markedly violate the assumption of normality, the Mann-Whitney U test was used for analysis of differences.
Correlations between HDL mediated [3H]cholesterol efflux, HDL phospholipids, HDL triglycerides, HDL-Lp-PLA2, and proteomic data were determined using Pearson product-moment estimates. Significance was accepted at probability levels of P < 0.05 and P < 0.01. Statistical analyses were performed with PASW Statistics, version 18.
Disclosures
None.
Acknowledgments
M.H., D.E., and V.B. were funded by the Ph.D. Program Molecular Medicine of the Medical University of Graz. This work was supported by the Austrian Science Fund FWF (Grants P21004-B02, P-22521-B18, and P22976-B18).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Stenvinkel P: Chronic kidney disease: A public health priority and harbinger of premature cardiovascular disease. J Intern Med 268: 456–467, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM: The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62: 1524–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Stenvinkel P: Interactions between inflammation, oxidative stress, and endothelial dysfunction in end-stage renal disease. J Ren Nutr 13: 144–148, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM: Antiinflammatory properties of HDL. Circ Res 95: 764–772, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Tall AR: Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med 263: 256–273, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Duffy D, Rader DJ: Update on strategies to increase HDL quantity and function. Nat Rev Cardiol 6: 455–463, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Linsel-Nitschke P, Tall AR: HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat Rev Drug Discov 4: 193–205, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Kaysen GA: Lipid and lipoprotein metabolism in chronic kidney disease. J Ren Nutr 19: 73–77, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW: Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 117: 746–756, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rezaee F, Casetta B, Levels JH, Speijer D, Meijers JC: Proteomic analysis of high-density lipoprotein. Proteomics 6: 721–730, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Karlsson H, Leanderson P, Tagesson C, Lindahl M: Lipoproteomics II: Mapping of proteins in high-density lipoprotein using two-dimensional gel electrophoresis and mass spectrometry. Proteomics 5: 1431–1445, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A: Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: Relevance to antioxidative function. Arterioscler Thromb Vasc Biol 29: 870–876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaziri ND, Navab M, Fogelman AM: HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol 6: 287–296, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Sviridov D, Mukhamedova N, Remaley AT, Chin-Dusting J, Nestel P: Antiatherogenic functionality of high density lipoprotein: How much versus how good. J Atheroscler Thromb 15: 52–62, 2008 [DOI] [PubMed] [Google Scholar]
- 16. McGillicuddy FC, de la Llera MM, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP: Inflammation impairs reverse cholesterol transport in vivo. Circulation 119: 1135–1145, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jurek A, Turyna B, Kubit P, Klein A: The ability of HDL to inhibit VCAM-1 expression and oxidized LDL uptake is impaired in renal patients. Clin Biochem 41: 1015–1018, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M: In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int 76: 437–444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalantar-Zadeh K, Kopple JD, Kamranpour N, Fogelman AM, Navab M: HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int 72: 1149–1156, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Marsche G, Frank S, Hrzenjak A, Holzer M, Dirnberger S, Wadsack C, Scharnagl H, Stojakovic T, Heinemann A, Oettl K: Plasma-advanced oxidation protein products are potent high-density lipoprotein receptor antagonists in vivo. Circ Res 104: 750–757, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khera AV, Cuchel M, Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ: Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364: 127–135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Washburn MP, Ulaszek RR, Yates JR, 3rd: Reproducibility of quantitative proteomic analyses of complex biological mixtures by multidimensional protein identification technology. Anal Chem 75: 5054–5061, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR, Resing KA, Ahn NG: Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics 4: 1487–1502, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Thompson A, Gao P, Orfei L, Watson S, Di AE, Kaptoge S, Ballantyne C, Cannon CP, Criqui M, Cushman M, Hofman A, Packard C, Thompson SG, Collins R, Danesh J: Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: Collaborative analysis of 32 prospective studies. Lancet 375: 1536–1544, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saland JM, Ginsberg HN: Lipoprotein metabolism in chronic renal insufficiency. Pediatr Nephrol 22: 1095–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Gundry RL, Fu Q, Jelinek CA, Van Eyk JE, Cotter RJ: Investigation of an albumin-enriched fraction of human serum and its albuminome. Proteomics Clin Appl 1: 73–88, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larrede S, Quinn CM, Jessup W, Frisdal E, Olivier M, Hsieh V, Kim MJ, Van EM, Couvert P, Carrie A, Giral P, Chapman MJ, Guerin M, Le GW: Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler Thromb Vasc Biol 29: 1930–1936, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC: Serum amyloid A-containing human high density lipoprotein 3: Density, size, and apolipoprotein composition. J Biol Chem 261: 9644–9651, 1986 [PubMed] [Google Scholar]
- 29. Artl A, Marsche G, Lestavel S, Sattler W, Malle E: Role of serum amyloid A during metabolism of acute-phase HDL by macrophages. Arterioscler Thromb Vasc Biol 20: 763–772, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Jahangiri A, de Beer MC, Noffsinger V, Tannock LR, Ramaiah C, Webb NR, van der Westhuyzen DR, de Beer FC: HDL remodeling during the acute phase response. Arterioscler Thromb Vasc Biol 29: 261–267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao Y, Sparks DL, Marcel YL: Specific phospholipid association with apolipoprotein A-I stimulates cholesterol efflux from human fibroblasts: Studies with reconstituted sonicated lipoproteins. J Biol Chem 271: 25145–25151, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Rothblat GH, Phillips MC: High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol 21: 229–238, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jong MC, Hofker MH, Havekes LM: Role of ApoCs in lipoprotein metabolism: Functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler Thromb Vasc Biol 19: 472–484, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Hirano T, Sakaue T, Misaki A, Murayama S, Takahashi T, Okada K, Takeuchi H, Yoshino G, Adachi M: Very low-density lipoprotein-apoprotein CI is increased in diabetic nephropathy: Comparison with apoprotein CIII. Kidney Int 63: 2171–2177, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Sankaranarayanan S, Oram JF, Asztalos BF, Vaughan AM, Lund-Katz S, Adorni MP, Phillips MC, Rothblat GH: Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J Lipid Res 50: 275–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Beer MC, Ji A, Jahangiri A, Vaughan AM, de Beer FC, van der Westhuyzen DR, Webb NR: ATP binding cassette G1-dependent cholesterol efflux during inflammation. J Lipid Res 52: 345–353, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Papavasiliou EC, Gouva C, Siamopoulos KC, Tselepis AD: PAF-acetylhydrolase activity in plasma of patients with chronic kidney disease: Effect of long-term therapy with erythropoietin. Nephrol Dial Transplant 21: 1270–1277, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Zalewski A, Macphee C: Role of lipoprotein-associated phospholipase A2 in atherosclerosis: Biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol 25: 923–931, 2005 [DOI] [PubMed] [Google Scholar]
- 39. McCall MR, La BM, Forte TM, Krauss RM, Takanami Y, Tribble DL: Dissociable and nondissociable forms of platelet-activating factor acetylhydrolase in human plasma LDL: Implications for LDL oxidative susceptibility. Biochim Biophys Acta 1437: 23–36, 1999 [DOI] [PubMed] [Google Scholar]
- 40. von EA, Holz H, Sandkamp M, Weng W, Funke H, Assmann G: Apolipoprotein C-III (Lys58-Glu): Identification of an apolipoprotein C-III variant in a family with hyperalphalipoproteinemia. J Clin Invest 87: 1724–1731, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ooi EM, Barrett PH, Chan DC, Watts GF: Apolipoprotein C-III: Understanding an emerging cardiovascular risk factor. Clin Sci 114: 611–624, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Bobik A: Apolipoprotein CIII and atherosclerosis: Beyond effects on lipid metabolism. Circulation 118: 702–704, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Holzer M, Gauster M, Pfeifer T, Wadsack C, Fauler G, Stiegler P, Koefeler H, Beubler E, Schuligoi R, Heinemann A, Marsche G: Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxid Redox Signal 14: 2337–2346, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 45. Marsche G, Heller R, Fauler G, Kovacevic A, Nuszkowski A, Graier W, Sattler W, Malle E: 2-Chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arterioscler Thromb Vasc Biol 24: 2302–2306, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Marsche G, Frank S, Raynes JG, Kozarsky KF, Sattler W, Malle E: The lipidation status of acute-phase protein serum amyloid A determines cholesterol mobilization via scavenger receptor class B, type I. Biochem J 402: 117–124, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]