Abstract
The P2X7 receptor participates in purinergic signaling, which may promote the progression of ADPKD. We examined the effects of a P2X7 receptor antagonist and a P2X7 receptor agonist on cyst development in a zebrafish model of polycystic kidney disease in which we knocked down pkd2 by morpholinos. We used live wt-1b pronephric-specific GFP-expressing zebrafish embryos to directly observe changes in the pronephros. Exposure of pkd2-morphant zebrafish to a P2X7 receptor antagonist (oxidized ATP [OxATP]) significantly reduced the frequency of the cystic phenotype compared with either exposure to a P2X7 receptor agonist (BzATP) or with no treatment (P < 0.01). Histology confirmed improvement of glomerular cysts in OxATP-treated pkd2 morphants. OxATP also reduced p-ERK activity and cell proliferation in pronephric kidneys in pkd2 morphants. Inhibition of P2X7 with an additional specific antagonist (A-438079), and through morpholino-mediated knockdown of p2rx7, confirmed these effects. In conclusion, blockade of the P2X7 receptor reduces cyst formation via ERK-dependent pathways in a zebrafish model of polycystic kidney disease, suggesting that P2X7 antagonists may have therapeutic potential in ADPKD.
Autosomal dominant polycystic kidney disease (ADPKD), caused by mutations in either PKD1 or PKD2, is the most common genetic kidney disease, characterized by progressive enlargement of cysts due to excessive fluid secretion and proliferation of renal tubular epithelial cells.1 It is one of the major causes of end stage renal disease and there is currently no proven treatment.
Multiple signaling pathways are involved in cyst formation and progression, and studies of these signaling pathways have led to potential treatments for ADPKD, such as vasopressin V2 receptor antagonists, mTOR inhibitors, and somatostatin analogues.1 A combination therapy targeting different pathways in cystogenesis may be required to maximize the treatment effects and limit their side effects.2 A potential treatment target that has not been completely explored is the purinergic receptor pathway regulated by adenosine 5′ triphosphate (ATP). Besides its classical role as an intracellular energy source, ATP has been recognized as an extracellular signaling molecule.3,4 It has been proposed that ATP release from cyst epithelial cells in response to stimuli such as mechanical deformation may contribute to cyst formation and enlargement by activating purinergic receptors.5 A previous study has shown that primary cultures of ADPKD epithelial cells release two- to fivefold more ATP under basal and hypotonicity challenge compared with non-ADPKD cells.6 In ADPKD cysts, ATP is present in significant quantities (up to 10 μM) sufficient to stimulate purinergic receptors.6 Thus, ATP release and signaling could contribute detrimentally to the gradual expansion of cyst fluid volume that is a hallmark of polycystic kidney disease.6,7 However, in vivo data are still lacking to support this hypothesis.
Two groups of purinergic receptors regulated by ATP (ATP-gating P2X receptors and ATP-sensing P2Y G-protein-coupled receptors) have been identified and their autocrine and paracrine functions have been reviewed.4,7 In kidneys, these receptors may regulate water and ion transport in different nephron segments.7 The P2X7 receptor is unique in its function in cytolytic pore formation and modulating cytokine release, proliferation, and apoptosis.8,9 The P2X7 receptor is only activated at high ATP concentrations, allowing Ca2+ and Na+ influx and K+ efflux, and thus is regarded as mediating a danger pathway in the face of cell injury.10 In addition, P2X7 receptor expression has been shown in dilating collecting ducts and cysts in human autosomal recessive polycystic kidney disease (ARPKD).8 P2X7-expressing collecting duct cysts dominate kidney histology of cpk/cpk mice from two weeks after birth until they die from uremia.11 However, the possibility that P2X7 receptors contribute to cyst formation of ADPKD has not been examined.
The zebrafish (Danio rerio) has become a popular model of various diseases not only for the study of gene functions but also for screening small molecules because of the ease of waterborne treatments.12 Knockdown of polycystic kidney disease 2 (pkd2) with morpholinos (MO) in zebrafish embryos led to the formation of kidney cysts,13–17 which is the first orthologous zebrafish model for polycystic kidney disease. Moreover, the zebrafish P2X receptor subunit genes, which are orthologs of mammalian P2X receptors, have been characterized.18 This in vivo model provides a unique opportunity to examine the hypothesis that blocking purinergic receptors may affect cyst development in ADPKD.
In this work, we evaluated whether P2X7 receptors contributed to cyst formation and progression in zebrafish knockdown of pkd2. We have examined the effects of oxidized ATP (OxATP), a P2X7 receptor antagonist, and BzATP, a P2X7 receptor agonist, on the development of cystic phenotype in the PKD2 zebrafish model. In addition, we have assessed whether knockdown of P2X7 receptors in pkd2 morphants may prevent the progression of cyst. From these observations, we describe the first in vivo evidence that P2X7 receptor inhibition can modify cyst formation in an ADPKD model.
RESULTS
Expression of P2X7 Receptors in the Zebrafish Pronephros
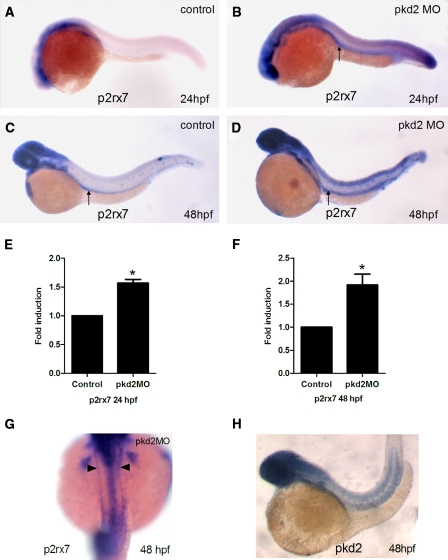
We chose to study the role of purinergic receptor signaling in a well-characterized zebrafish pkd2 model.13,15 The P2X7 receptor is unique in both its tissue localization and functional response, their activation requiring 10- to 100-fold higher concentrations of ATP than the P2X1 to 6 receptors.9 A comparative expression study of zebrafish P2X receptors has shown that the other P2X receptor family members are specifically localized to the nervous system, but the expression of P2X7 is more ubiquitous in other organs,19 suggesting a possibility of its localization in the zebrafish pronephros. First, we determined the mRNA expression of P2X7 receptors in pkd2 morphants with a specific focus on pronephros. In addition to diffuse brain and spinal cord staining, as illustrated in two previous studies,18,19 we found evidence of P2X7 receptor expression in the pronephric region (Figure 1). Notably, the p2rx7 mRNA expression is earlier (at 24hpf) and stronger in pkd2 morphants compared with controls (Figure 1 A-F), suggesting P2X7 receptors may be involved in the formation of cystic phenotype of pkd2 morphants. A dorsal view of the pkd2 morphant embryo confirmed the expression of P2X7 receptors in the two pronephric ducts (Figure 1 G). The pattern of p2rx7 mRNA expression was very similar to the pkd2 mRNA expression (Figure 1 H), suggesting a possible functional link between pkd2 and P2X7 receptors.
Figure 1.
Increased expression of p2rx7 mRNA in pkd2 morphants. Whole-mount in situ hybridization for p2rx7 mRNA in 24 hpf (A, B) and 48 hpf (C, D) zebrafish embryos. Note expression of p2rx7 in pronephric ducts (arrow) in wild-type siblings (C) and pkd2 morphants (B, D). Diffuse staining in brain and spinal cord was also noted. Quantitative real-time PCR showing p2rx7 mRNA upregulation in pkd2 morphants at 24 hpf (E) and 48 hpf (F) (n = 3 and 4, respectively). *P < 0.05. (G) Dorsal view of pkd2 morphant embryos showing localization of p2rx7 mRNA in pronephric ducts (arrowheads). (H) In situ hybridization showing a similar expression pattern of pkd2 to p2rx7 mRNA.
OxATP Inhibits Pronephric Cyst Formation in pkd2 Morphants
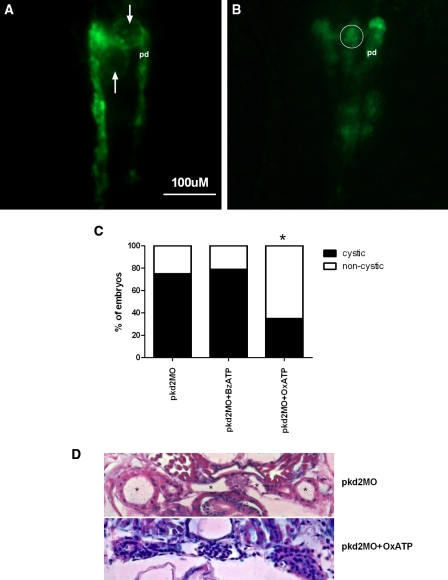
Pkd2 morphants showed a curly-tail phenotype and a cystic phenotype including glomerular cyst, pronephric duct dilation, and peritubular edema,14,15 which could be used as parameters for assessment of treatment effects on drug screening.20 First, we examined the effects of P2X7 receptor inhibition with OxATP and stimulation with BzATP. No significant phenotype was observed in wild-type embryos treated with OxATP (100μM) or BzATP (100μM). We observed the early changes in the pronephros (48 hpf) using wt-1b pronephric-specific GFP zebrafish embryos with morpholino-mediated knockdown of pkd221. Pkd2 morphant embryos developed cysts in the region of the glomerulus and tubules, which were directly observed under a fluorescence microscope (arrows in Figure 2 A, B). The frequency of cystic pronephros was 36/48 (75%) in pkd2 morphant embryos. Treatment of pkd2 morphant embryos with BzATP to stimulate the P2X7 receptor resulted in cysts in 41/52 (78.8%) fish, while inhibition of the receptor with OxATP decreased the frequency significantly (P < 0.01) to 18/52 (34.6%; Figure 2C). Histologic examination of H and E stained sections revealed profound glomerular cystic dilation and pronephric tubular dilation in pkd2 morphants at 5 dpf, whereas the cystic dilation was markedly improved in embryos incubated with OxATP (Figure 2D). The frequency of abdominal edema in pkd2 morphants at 5 dpf was significantly reduced in the OxATP-treated embryos (50.9 ± 7.1%, n = 87, from three independent experiments) as compared with the BzATP-treated embryos (92.7%±2.0, n = 82) or the untreated controls (75.9%±3.0, n = 81; P < 0.01; Supplemental Figure S1). These data support a role of OxATP in suppressing the early formation of pronephric cyst in pkd2 morphant embryos.
Figure 2.
Disruption of P2X7 receptor signaling attenuates cystic dilation in pkd2 morphants. The wt-1b pronephric-specific GFP zebrafish embryos were microinjected with a pkd2 ATG morpholino. Cystic phenotype was observed directly in lived embryos at 2 dpf. (A) Pronephric cysts (arrows) in a pkd2 morphant embryo (dorsal view, anterior to the top); pd, pronephric duct. (B) Restoration of normal glomerular structure (white circle) in a pkd2 morphant treated with OxATP. (C) The frequency of cystic phenotype in pkd2 morphants (n = 48) and those treated with 100μM BzATP (n = 52) or 100μM OxATP (n = 52). Data were pooled from three independent experiments. *P < 0.01. (D) Transverse sections of a pkd2 morphant at 5 dpf show profound glomerular and tubular dilations indicated by the mark * (upper panel). Minimal glomerular cyst and no tubular dilation in a pkd2 morphant incubated continuously with 100μM OxATP (lower panel).
OxATP Inhibits pERK Activation in pkd2 Morphants
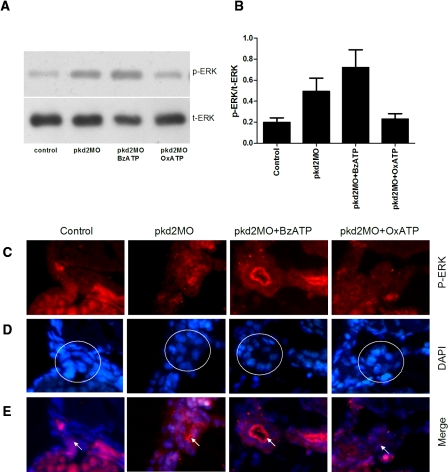
The ERK pathway has been implicated in mediating proliferation and fluid secretion in various PKD models.22 We therefore looked at the activation of the ERK pathway in the pkd2 morphants and the effect of OxATP treatment. We observed a 2.5-fold increase of p-ERK expression in pkd2 morphants by denstiometric analysis of Western blot, which was suppressed by 53% with OxATP treatment (Figure 3 A and B). Consistently, immunostaining for p-ERK showed increased expression in pronephric epithelial cells of pkd2 morphants that was suppressed by OxATP treatment (Figure 3 C–E), suggesting that pronephros contributed to the increased level of p-ERK in pkd2 morphants. Consistent with these results, stimulation of the P2X7 receptor with BzATP increased the level of p-ERK in the morphants (Figure 3 C–E). We also observed prominent p-ERK staining on the apical surface (brush border) of the pronephric ducts, suggesting that activation of p-ERK might have a role in mediating flow-induced mechanosensation and ion transport.23
Figure 3.
OxATP inhibits phospho-ERK1/2 activation in pkd2 morphants at 5dpf. (A) Western blot analysis with antibody for phospho-ERK1/2 (p-ERK) and total ERK1/2 (t-ERK) shows increased p-ERK expression in pkd2 morphants and reduced expression with 5-d of OxATP (100μM) incubation. (B) Denstiometric analysis shows that OxATP significantly reduced p-ERK/t-ERK expression (n = 4). (C) Cross-sections of immunostaining for p-ERK on anterior pronephric duct show increased expression of p-ERK in pkd2 morphants, which is suppressed by OxATP. (D) DAPI nuclear staining for the anterior pronephric ducts. The location of anterior pronephric ducts are circled with a white line. (E) Merged images of p-ERK and DAPI staining show p-ERK expression (arrows) in both nucleus and cytoplasm.
OxATP Reduces Cell Proliferation of Pronephric Ducts in pkd2 Morphants
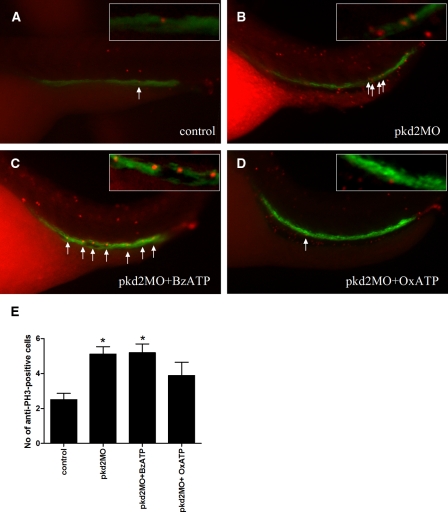
Increased cell proliferation has been reported in mammalian PKD models.24,25 We observed a twofold increase in anti-phospho-H3 staining cells in the pronephros of pkd2 morphants (5.1 ± 0.4) at 5 dpf compared with wild-type embryos (2.5 ± 0.4; P < 0.05; Figure 4). Increased proliferation was also observed in BzATP-treated embryos (5.2 ± 0.5; P < 0.05 versus wild-type controls). However, cell proliferation of pronephric ducts was reduced in OxATP-treated pkd2 morphants (3.9 ± 0.8; P > 0.05 versus wild-type controls; Figure 4 E). We also examined apoptosis using the TUNEL assay but there were too few apoptotic cells in the pronephros for meaningful comparison (data not shown).
Figure 4.
OxATP reduces cell proliferation of pronephric kidneys in pkd2 morphants. Embryos at 5 dpf were stained for anti-PH3 (red) and anti-α6F (green). Images were merged for counting proliferating cells (orange) in the pronephric kidneys. (A) A wild-type sibling at 5 dpf showing a proliferative cell in the pronephric kidney (arrow). (B) A pkd2 morphant with increased proliferating cells in the pronephric kidney (arrows). (C) A BzATP-treated pkd2 morphant showing multiple proliferating cells in the pronephric kidney (arrows). (D) Reduced proliferating cells in an OxATP-treated pkd2 morphant embryo (arrow). (E) Counts for anti-PH3 and anti-α6F positive cells in the pronephric kidneys. n = 16 to 26 for each group. * indicates significant different means compared with the sibling controls with P < 0.05 by Kruskal-Wallis test and Dunn's multiple comparison test.
IL-1 Beta mRNA Expression in pkd2 Morphants
Inflammatory cytokines have been proposed to promote cyst progression in ADPKD.26,27 Consistent with this, we observed a twofold increase of IL-1 beta mRNA expression in 5 dpf pkd2 morphants (Supplemental Figure S2). Strikingly, stimulation of purinergic receptors with BzATP led to a significant increase of IL-1 beta expression. However, the higher IL-1 beta expression in pkd2 morphants was not suppressed by OxATP. These results suggest that OxATP does not suppress cyst progression through an anti-inflammatory effect.
P2X7 Knockdown Reduces Cystic Phenotype of pkd2 Morphants
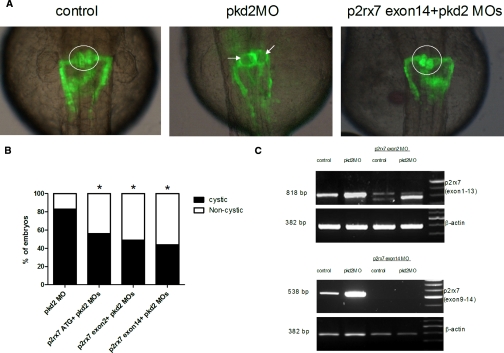
To further confirm if inhibition of P2X7 receptors is responsible for the beneficial effect of OxATP in pkd2 morphants, we designed three different morpholinos to specifically target p2rx7: on the ATG site that blocks translation and on the splicing sites at the intron 1/exon 2 and the intron 13/exon 14 boundaries. Exon 2 of p2rx7 contains the five positive charged amino acid residues important for nucleotide binding, and exon 14 of p2rx7 contains domains responsible for receptor trafficking and function.9 No significant cystic phenotype was observed in wild-type siblings or the embryos injected with p2rx7 morpholinos. Consistent with the effects of OxATP, p2rx7 knockdown (with any one MO) significantly reduced the frequency of cystic phenotype in pkd2 morphants (Figure 5 A). The frequency of cystic phenotype in pkd2 morphants was significantly reduced from 69/83 (83.1%) to 24/43 (55.8%) with p2rx7 ATG-MO (P < 0.01); 33/68 (48.5%) with p2rx7 exon 2 splice-MO (P < 0.01); and 31/71 (43.7%) with p2rx7 exon 14 splice-MO (P < 0.01; Figure 5 B, C). Similar to OxATP treatment, P2X7 knockdown reduced cell proliferation and p-ERK expression in pronephric ducts in pkd2 morphants (Figure 6).
Figure 5.
Coinjection of morpholinos against pkd2 and p2rx7 in zebrafish embryos inhibits the cystic phenotype. (A) Note the cystic dilation (arrow) of the pronephros in pkd2 morphants were rescued by p2rx7 knockdown. The glomerular structure is circled in white. (B) Reduced frequencies of cystic phenotype in pkd2 morphants with microinjection of morpholinos against p2rx7 at the ATG site or the splicing sites of exon 2 and exon 14. *P < 0.01. n = 43 to 83 in each groups. (C) RT- PCR from lysates of 48-hpf wild-type and pkd2 morphant embryos coinjected with the splicing-morpholinos targeted against p2rx7 exon 2 and exon 14, showing the splicing is blocked in the p2rx7 transcripts. P2rx7 was amplified using primers directed to exon 1 to 13 (818 bp) and exon 9 to 14 (538 bp).
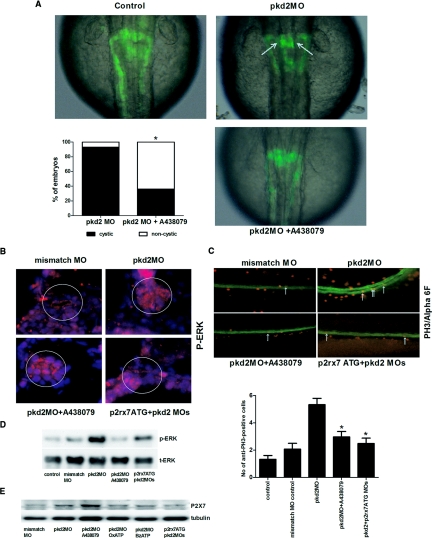
Figure 6.
A P2X7 specific antagonist A-438079 reduces the frequency of pronephric cyst formation in pkd2 morphants. (A) Overlays of transmission (gray) and fluorescence (green) images (dorsal view, anterior to the top) showing effects of A438079 (0.1μM) on pronephric cysts (arrows) in pkd2 morphant embryos at 2 dpf; comparative frequencies of cystic phenotype in pkd2 morphants at 2 dpf with (n = 27) and without A-438079 treatment (n = 42). Results were pooled from two independent experiments. *P < 0.01. (B) Merged images of p-ERK and DAPI staining showing decreased p-ERK expression in pronephric ducts (white circle) with A-438079 treatment and p2rx7 MO knockdown. (C) Counts for anti-PH3 and anti-α6F double positive cells (orange; arrows) in the pronephric ducts at 5 dpf. n = 26 to 29 for each group. *P < 0.05 compared with control. (D) Western blot showing increased p-ERK activities in pkd2 morphants and the effects of A438079 and p2rx7 MO knockdown. (E) Western blot showing increased expression of P2X7 protein in pkd2 morphants and the effects of treatments. Data shown are representative of two experiments with similar results.
A P2X7 Specific Antagonist A-438079 Significantly Reduces the Frequency of Pronephric Cyst Formation in pkd2 Morphants
We have tested a novel P2X7 specific antagonist,28 A-438079, to confirm the effect of pharmacologic inhibition of P2X7 on pkd2 morphants by OxATP. Consistently, A-438079 (0.1μM) showed similar effects to OxATP in reducing the frequency of cystic phenotype in pkd2 morphant embryos. The frequency of cystic phenotype was reduced from 25/27(92.6%) to 15/42 (35.7%; P < 0.01), confirming the beneficial effect of P2X7 blockade on pkd2 morphants (Figure 6 A). Similarly, p-ERK expression and cell proliferation of pronephric ducts were suppressed in A-438079-treated pkd2 morphants (Figure 6 B-D). Expression of P2X7 protein in pkd2 morphants was analyzed by Western blotting using a custom rabbit polyclonal antibody, and upregulation of P2X7 receptors in pkd2 morphants was confirmed (Figure 6 E). The expression of P2X7 receptors was not significantly changed by OxATP and BzATP (Figure 6 E), but it was slightly upregulated with A438079, suggesting a possible compensatory effect.
P2X7 is Expressed in the Primary Cilia in Human Kidney Cells
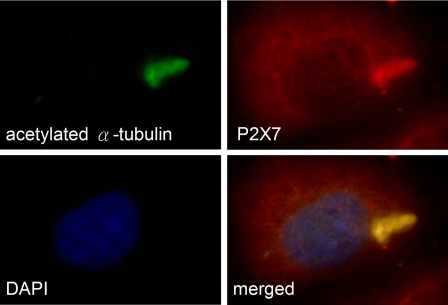
Primary cilium signaling defects is one of the pathogenic mechanisms of cyst formation in PKD.1 Therefore, we asked whether P2X7 receptors are located in the primary cilia of renal tubular epithelial cells. We performed a double immunocytochemistry for P2X7 and acetyl-α-tubulin in the human kidney epithelial cells (HK-2), and the result showed the expression of P2X7 receptors was indeed localize in the primary cilia (Figure 7), suggesting that P2X7 receptors may participate in the mechanosensory signaling in regulating cyst formation.
Figure 7.
P2X7 receptors are expressed in the primary cilium of cultured human proximal tubular cells (HK-2). Cilia were stained with anti-acetylated-α-tubulin antibodies (green). P2X7 were stained with anti-P2X7 antibodies (red). Nuclei were stained with DAPI (blue). P2X7 (red) and acetylated-α-tubulin (green) are colocalized, as shown in the merged image (yellow).
DISCUSSION
To explore whether inhibiting P2X7 receptors reduces cyst formation in ADPKD, we hypothesized that blocking P2X7 receptors would be effective in reducing renal cyst formation in a zebrafish model of PKD2. We showed that the cystic dilation of pronephros in pkd2 knockdown embryos was prevented by pharmacologic inhibition of P2X7 receptors with OxATP and A-438079. Similarly, knockdown of P2X7 receptors suppressed the cystic phenotype of pkd2 morphants. Our results suggest important roles for P2X7 receptors in modifying cyst formation in ADPKD.
One mechanism by which the P2X7 receptor blockade reduces cyst formation may be through inhibiting P2X7 receptor-mediated ERK activation. Activation of ERK by P2X7 receptors has been demonstrated in various epithelial cells, such as rat pancreas.29 P2X7 receptors are also present in the rat lacrimal gland, where stimulation with BzATP increases ERK 1/2 activation.30 P2X7 receptors are also involved in fluid flow-induced ERK activation in osteoblasts.31 Furthermore, ERK activation has an important role in determining the rate of cyst enlargement in ADPKD through its actions to stimulate cellular proliferation and transepithelial solute and fluid secretion.32–35 Consistent with such a role, we found activation of ERK pathway and increased tubular cell proliferation in the pronephric kidneys of pkd2 morphants, which was suppressed by blockade of P2X7 receptors with OxATP. Although originally thought as a death receptor, a trophic role of P2X7 receptor has recently been shown.10 Our results are consistent with a role of P2X7 in ERK activation and cell proliferation, which may contribute to cyst development and progression in ADPKD.
Reduced fluid flow or obstruction of pronephric duct (without apparent ciliary abnormality) has been shown in pkd2 morphants.14,15 With P2X7 receptor blockade, we observed a significant reduction of pronephric cystic dilation and peritubular edema. This suggests that P2X7 receptor blockade can reduce the ATP stimulation of salt and water fluxes into the lumen of the pronephric ducts. Whether this process was driven by calcium and/or the cystic fibrosis transmembrane-conductance-regulator (CFTR), as proposed previously, is worthy of further studies.7,36 Interestingly, we have found evidence of P2X7 expression at the primary cilia in cultured tubular epithelial cells, suggesting that P2X7 receptors may play a role in the defective cilium signaling in PKD. How P2X7 interact with PKD2 in the cilium signaling is still unclear. Since PKD2 has been shown to interact with many other membrane proteins,1 we speculate that a compensatory upregulation of P2X7 to overcome the deficiency of PKD2 may trigger an injury pathway leading to more severe cyst formation. The link between PKD2 deficiency and P2X7 overexpression warrants further study.
Our finding that P2X7 receptor blockade may suppress cyst formation is consistent with a previous in vitro study using MDCK-derived cyst cultures.37 Removal of ATP with apyrase and the use of nonspecific P2 receptor inhibitors reduced the growth of cysts by blocking cAMP-dependent activation of the ERK pathway,37 indicating a beneficial effect of blocking purinergic signaling pathways on cyst formation. In the same study, a nonselective P2X receptor inhibitor, Coomassie brilliant blue G, seemed ineffective in reducing cyst growth,37 but P2X7 specific antagonists were not examined in that study.
In a model of autosomal recessive kidney disease (ARPKD) using suspension culture of dissociated cpk/cpk kidneys cells, BzATP was found to reduce the number but not the size of cysts through an as-yet-undefined mechanism not related to proliferation and apoptosis,8 which is contrary to our results. These different results may be due to differences in the polycystic kidney models used (ADPKD versus ARPKD), the mechanisms of cyst development (zebrafish pronephric cyst versus 3-dimensional cyst culture), the efficacy of P2 receptor antagonists, or the tissue expression of P2 receptor subtypes.5,11,37
Zebrafish has been used as a model for screening drug candidates for different diseases.38 A recent study has validated that chemical modifiers identified by zebrafish screening assays could be applicable to a mouse PKD model.39,40 Our results suggest that P2X7 receptor antagonists may hold promise as a potential therapy for ADPKD. P2X7 antagonists have provided promising results in clinical trials for the treatment of rheumatoid arthritis, and these data raise hopes that new analgesic and anti-inflammatory treatments that target P2X receptors will be available.41 In animal models of spinal cord injury42 and experimental glomerulonephritis,43 P2X7 receptor antagonists have been shown to be effective. Interestingly, as the P2X7 receptor may enhance nociceptive transmission associated with microglial activation and secretion of IL-1 beta in the dorsal horn, a CNS-penetrant P2X7 receptor antagonists is beneficial for the treatment of persistent pain,44 which might improve pain management in ADPKD patients if proven in further studies.
Although our study has shown promising effects of P2X7 receptor blockade in the PKD2 zebrafish model, further studies on other animal models are required to confirm the results. Animal studies on different models of PKD, such as PKD1 or ARPKD, are also needed to clarify the effects. Effects of other purinergic receptor antagonists on other P2X or P2Y receptors may need further studies. However, the current study provides the first in vivo evidence for a beneficial effect of P2X7 receptor blockade in ADPKD.
Conclusion
This study demonstrates that inhibition of P2X7 receptors can reduce cystic phenotype in pkd2 knockdown zebrafish. Our studies provide support for the hypothesis that modifying purinergic-signaling pathways may affect cyst formation in PKD. Our results further imply that purinergic receptor blockade could be a potential therapeutic modality for ADPKD.
CONCISE METHODS
Fish Maintenance
Zebrafish and embryos were maintained by standard methods.45 Embryos were staged according to hours post fertilization (hpf). The transgenic line wt1b:: GFP with pronephros specific GFP expression21 was kindly provided by Dr. Christoph Englert (Fritz Lipmann Institute, Jena, Germany). The study was approved by the Institutional Animal Care and Use Committee of Chang Gung University.
Morpholino Injection
One- to two-cell stage embryos were microinjected with 0.125 mM antisense morpholino oligos (MO) at the amount of approximately 4.45 ng per embryo (Gene Tools, Philomath, OR). For knockdown of pkd2, we used pkd2 ATG-MO (5′-AGGACGAACGCGACTGGAGCTCATC-3′) as described previously.13 The pkd2 morphants showed consistent cystic dilation of pronephros and severe edema by 5 d postfertilization suitable for drug screening. For knockdown of P2X7 receptors (p2rx7), an ATG-MO (5′-AGAGGTTCAGCAAAACGCAAGGCAT-3′) designed by Gene Tools, and two MOs against splicing sites at the intron/exon boundaries of p2rx7 exon2 (5′-CATGATACTGGAAAGTAAAGACATT-3′) and p2rx7 exon14 (5′-TCATTCTGAGAACAGACATGAGGTA-3′) were used at a concentration of 0.25 mM. The knockdown efficacy of the p2rx7 splice-MOs was checked by reverse transcriptase PCR. A morpholino standard control oligo (5′-CCTCTTACCTCAGTTACAATTTATA-3′), pkd2 5-mismatch MO (5′-AGCACCAACCCGACTGCACCTCATC-3′), p2rx7 exon2 5-mismatch MO (5′-TCATTCTGAGAACAGACATGAGGTA-3′) and p2rx7 exon14 5-mismatch MO (5′-CATGATACTGGAAAGTAAAGACATT-3′) were applied as negative controls.
Drug Treatment
A purinergic P2X7 receptor agonist BzATP (3′-O-(4-benzoylbenzoyl)-ATP) and a P2X7 receptor blocker, oxidized ATP, were added directly into E3 medium (5mM NaCl, 0.17mM KCl, 0.4mM CaCl2, and 0.16mM MgSO4) in 6-cm Petri dishes. The final concentration of each molecule was 100 μM. Pkd2 morphants embryos were incubated from 4 hpf (after microinjection) till 5 dpf at 28.5 °C in the dark.12 OxATP and BzATP were purchased from Sigma. We have chosen OxATP initially because all of the effects of P2X7 receptors, including the ATP-mediated cytotoxicity, can be abrogated by this antagonist.46 Another highly selective P2X7 receptor antagonist (A-438079, Tocris Bioscience, Bristol, UK) was used at a concentration of 0.1 μM to confirm the results.28
Direct Observation of Pronephric Cyst in Live Embryos
The wt-1b:: GFP transgenic fish line21 was microinjected with pkd2 MO for direct observation of the cystic phenotype. At 48 hpf, embryos were anesthetized with tricaine (0.2 mg/ml) and oriented in 5% methyl cellulose (Sigma). The presence of pronephric cysts was determined using fluorescence microscope.
Histology
Zebrafish embryos were fixed in 4% paraformaldehyde in Phosphate Buffered Saline (PBS) overnight at 4 °C, embedded in glycolmethacrylate (JB-4; Polyscience), and cut at 4 μm. Slides were stained with Hematoxylin and Eosin.
Immunohistochemistry
Embryos were fixed overnight in 4% paraformaldehyde at 4 °C. Immunostaining for phospho-ERK (extracellular signal regulated kinases) was performed according to published protocols.47 An antibody to phospho-ERK 1/2 (1: 100) was purchased from Cell Signaling. Immunostaining was performed in whole-mount embryos and then embedded in OTC medium and cryosectioned. Sections were mounted in Vectashield (Vector Laboratories) with DAPI.
Double staining was performed in whole-mount embryos using an anti- phospho-H3 antibody (1:100, Upstate) to label proliferating cells and an anti-NA/K ATPase α-1 subunit (α6F) antibody (1:200, Developmental Studies Hybridoma Bank) to label pronephric epithelial cells. Secondary antibodies used were Alexa Fluor 594 goat anti-rabbit IgG and Alexa Fluor 488 goat anti-mouse IgG (1:500, Molecular Probes). Images for anti-phospho-H3 and anti-α6F staining were taken and merged for counting proliferating cells in the pronephric kidneys.
Whole-mount In Situ Hybridization
Embryos were fixed overnight in 4% paraformaldehyde at 4 °C. Whole-mount in situ hybridization was performed according to published protocols.48 The digoxigenin-labeled antisense probe for P2X7 receptors was prepared from a pCRII-TOPO-p2rx7 construct (a kind gift from Dr Lior Appelbaum, Stanford University) using the most 3′ 600 bp fragment of p2rx7.19
Western Blot Analysis
Proteins extracted from whole embryos were analyzed by Western blotting following the standard method.49 Antibodies used include anti-phospho-ERK1/2 (1:1000, Cell Signaling), anti-total ERK1/2 (1:1000, Santa Cruz),47 anti-tubulin (clone DM1a, Sigma), and a custom-made rabbit polyclonal antibody raised against a N-terminal peptide (EKGFSDQHSHGVQTGAC) of zebrafish P2X7 receptor (Kelowna, Taiwan).
Immunocytochemistry
HK-2 cells (immortalized human proximal tubular cell line, American Type Culture Collection, Manassas, VA) were grown in 8-well culture chamber slide for 8 d. Slides were washed in PBS, fixed in 4% paraformaldehyde, blocked with 1% BSA, and incubated with primary antibody, P2X7 (1:100, APR-008; Alomone) and acetylated-α-tubulin (1:100, clone 6-11-B1; Sigma) overnight at 4 °C. After washes in PBS, the cells were incubated with the secondary antibody Alexa Fluor 594 donkey anti-rabbit IgG and Alexa Fluor 488 goat anti-mouse IgG (1:300, Molecular Probes) for 30 min at room temperature. DAPI was used to counterstain nuclei.
Real-time PCR
Embryos from each treatment group (n = 15 to 30) were mixed and grinded in liquid nitrogen. RNA-Bee isolation kit (TEL-TEST, Inc., Friendswood, TX) was used for total RNA extraction. First strand cDNA synthesis kit for RT-PCR (AMV; Roche Diagnostics, Germany) was used for cDNA synthesis. Real-time PCR was performed with the ABI PRISM 7700 sequence detection system (Applied Biosystems) in the presence of SYBR Green. PCR primers for zebrafish IL-1β were 5′-CAT TTG CAG GCC GTC ACA-3′(forward) and 5′-GGA CAT GCT GAA GCG CAC TT-3′(reverse).50 PCR primers for p2rx7 were 5′-CTG CCG AGA CCT TAC AGG AG-3′ (forward) and 5′-AAA GCG TTC TCC AGG ACA GA-3′ (reverse). Zebrafish ß-actin was used as internal control. Reaction was performed in triplicate for each sample.
Statistical Analyses
Values are expressed as means ± SEM. Comparison between groups was performed by ANOVA followed by Dunnett multiple comparison test. Values that were not normally distributed were analyzed by Kruskal-Wallis test followed by Dunn multiple comparison test. The comparison of binary variables was performed by Fisher's exact t test or Chi-square test. P < 0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
We are grateful to Christoph Englert for the gift of wt-1b GFP zebrafish line; Lior Appelbaum for the p2rx7 plasmid; Thomas T. Chen for helpful discussions; Chia-Hsien Fan for help with in situ hybridization; Yi-Ching Ko, Ya-Tzu Lin, Hsiang-Chi Huang and Chiung-Tseng Huang for their excellent technical assistance; and Paul J. Scotting for critical reading of the manuscript. This work was supported by grants from the National Science Council and Chang Gang Medical Research Projects, Taiwan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at www.jasn.org.
REFERENCES
- 1. Torres VE, Harris PC: Autosomal dominant polycystic kidney disease: The last 3 years. Kidney Int 76: 149–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang MY, Ong AC: Autosomal dominant polycystic kidney disease: Recent advances in pathogenesis and treatment. Nephron Physiol 108: 1–7, 2008 [DOI] [PubMed] [Google Scholar]
- 3. James G, Butt AM: Adenosine 5′ triphosphate evoked mobilization of intracellular calcium in central nervous system white matter of adult mouse optic nerve. Neurosci Lett 268: 53–56, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Khakh BS, North RA: P2X receptors as cell-surface ATP sensors in health and disease. Nature 442: 527–532, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL: Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol 296: F1464–1476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM: ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J Am Soc Nephrol 10: 218–229, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Hovater MB, Olteanu D, Welty EA, Schwiebert EM: Purinergic signaling in the lumen of a normal nephron and in remodeled PKD encapsulated cysts. Purinergic Signal 4: 109–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hillman KA, Woolf AS, Johnson TM, Wade A, Unwin RJ, Winyard PJ: The P2X7 ATP receptor modulates renal cyst development in vitro. Biochem Biophys Res Commun 322: 434–439, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Lopez-Castejon G, Young MT, Meseguer J, Surprenant A, Mulero V: Characterization of ATP-gated P2X7 receptors in fish provides new insights into the mechanism of release of the leaderless cytokine interleukin-1 beta. Mol Immunol 44: 1286–1299, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Monif M, Reid CA, Powell KL, Smart ML, Williams DA: The P2X7 receptor drives microglial activation and proliferation: A trophic role for P2X7R pore. J Neurosci 29: 3781–3791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hillman KA, Johnson TM, Winyard PJ, Burnstock G, Unwin RJ, Woolf AS: P2X(7) receptors are expressed during mouse nephrogenesis and in collecting duct cysts of the cpk/cpk mouse. Exp Nephrol 10: 34–42, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Murphey RD, Zon LI: Small molecule screening in the zebrafish. Methods 39: 255–261, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N: A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131: 4085–4093, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Sullivan-Brown J, Schottenfeld J, Okabe N, Hostetter CL, Serluca FC, Thiberge SY, Burdine RD: Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants. Dev Biol 314: 261–275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Obara T, Mangos S, Liu Y, Zhao J, Wiessner S, Kramer-Zucker AG, Olale F, Schier AF, Drummond IA: Polycystin-2 immunolocalization and function in zebrafish. J Am Soc Nephrol 17: 2706–2718, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schottenfeld J, Sullivan-Brown J, Burdine RD: Zebrafish curly up encodes a pkd2 ortholog that restricts left-side-specific expression of southpaw. Development 134: 1605–1615, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Bisgrove BW, Snarr BS, Emrazian A, Yost HJ: Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer's vesicle are required for specification of the zebrafish left-right axis. Dev Biol 287: 274–288, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Kucenas S, Li Z, Cox JA, Egan TM, Voigt MM: Molecular characterization of the zebrafish P2X receptor subunit gene family. Neuroscience 121: 935–945, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Appelbaum L, Skariah G, Mourrain P, Mignot E: Comparative expression of p2x receptors and ecto-nucleoside triphosphate diphosphohydrolase 3 in hypocretin and sensory neurons in zebrafish. Brain Res 1174: 66–75, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Cao Y, Semanchik N, Lee SH, Somlo S, Barbano PE, Coifman R, Sun Z: Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc Natl Acad Sci USA, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perner B, Englert C, Bollig F: The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev Biol 309: 87–96, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Omori S, Hida M, Fujita H, Takahashi H, Tanimura S, Kohno M, Awazu M: Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J Am Soc Nephrol 17: 1604–1614, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Flores D, Battini L, Gusella GL, Rohatgi R: Fluid shear stress induces renal epithelial gene expression through polycystin-2-dependent trafficking of extracellular tegulated kinase. Nephron Physiol 117: 27–36, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang MY, Parker E, Ibrahim S, Shortland JR, Nahas ME, Haylor JL, Ong AC: Haploinsufficiency of Pkd2 is associated with increased tubular cell proliferation and interstitial fibrosis in two murine Pkd2 models. Nephrol Dial Transplant 21: 2078–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Spirli C, Okolicsanyi S, Fiorotto R, Fabris L, Cadamuro M, Lecchi S, Tian X, Somlo S, Strazzabosco M: Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology 51: 1778–1788, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gardner KD, Jr, Burnside JS, Elzinga LW, Locksley RM: Cytokines in fluids from polycystic kidneys. Kidney Int 39: 718–724, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Li X, Magenheimer BS, Xia S, Johnson T, Wallace DP, Calvet JP, Li R: A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat Med 14: 863–868, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang XF, Shieh CC, Wismer CT, Zhu CZ, Gauvin DM, Fabiyi AC, Honore P, Gregg RJ, Kort ME, Nelson DW, Carroll WA, Marsh K, Faltynek CR, Jarvis MF: P2X7-related modulation of pathological nociception in rats. Neuroscience 146: 1817–1828, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Amstrup J, Novak I: P2X7 receptor activates extracellular signal-regulated kinases ERK1 and ERK2 independently of Ca2+ influx. Biochem J 374: 51–61, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hodges RR, Vrouvlianis J, Shatos MA, Dartt DA: Characterization of P2X7 purinergic receptors and their function in rat lacrimal gland. Invest Ophthalmol Vis Sci 50: 5681–5689, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okumura H, Shiba D, Kubo T, Yokoyama T: P2X7 receptor as sensitive flow sensor for ERK activation in osteoblasts. Biochem Biophys Res Commun 372: 486–490, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, Rome LA, Sullivan LP, Grantham JJ: cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int 57: 1460–1471, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Okumura Y, Sugiyama N, Tanimura S, Nishida M, Hamaoka K, Kohno M, Yokoyama T: ERK regulates renal cell proliferation and renal cyst expansion in inv mutant mice. Acta Histochem Cytochem 42: 39–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park EY, Sung YH, Yang MH, Noh JY, Park SY, Lee TY, Yook YJ, Yoo KH, Roh KJ, Kim I, Hwang YH, Oh GT, Seong JK, Ahn C, Lee HW, Park JH: Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice. J Biol Chem 284: 7214–7222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parker E, Newby LJ, Sharpe CC, Rossetti S, Streets AJ, Harris PC, O'Hare MJ, Ong AC: Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int 72: 157–165, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hillman KA, Burnstock G, Unwin RJ: The P2X7 ATP receptor in the kidney: a matter of life or death? Nephron Exp Nephrol 101: e24–30, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Turner CM, King BF, Srai KS, Unwin RJ: Antagonism of endogenous putative P2Y receptors reduces the growth of MDCK-derived cysts cultured in vitro. Am J Physiol Renal Physiol 292: F15–25, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Ou HC, Santos F, Raible DW, Simon JA, Rubel EW: Drug screening for hearing loss: Using the zebrafish lateral line to screen for drugs that prevent and cause hearing loss. Drug Discov Today 15: 265–271, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cao Y, Semanchik N, Lee SH, Somlo S, Barbano PE, Coifman R, Sun Z: Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc Natl Acad Sci USA 106: 21819–21824, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mangos S, Lam PY, Zhao A, Liu Y, Mudumana S, Vasilyev A, Liu A, Drummond IA: The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis Model Mech 3: 354–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Young MT: P2X receptors: dawn of the post-structure era. Trends Biochem Sci 35: 83–90, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M: P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med 10: 821–827, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Taylor SR, Turner CM, Elliott JI, McDaid J, Hewitt R, Smith J, Pickering MC, Whitehouse DL, Cook HT, Burnstock G, Pusey CD, Unwin RJ, Tam FW: P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20: 1275–1281, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, Malcangio M: P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci 30: 573–582, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Westerfield M: The Zebrafish Book. Eugene, OR: University of Oregon; 2000 [Google Scholar]
- 46. Moayeri M, Wickliffe KE, Wiggins JF, Leppla SH: Oxidized ATP protection against anthrax lethal toxin. Infect Immun 74: 3707–3714, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Krens SF, He S, Lamers GE, Meijer AH, Bakkers J, Schmidt T, Spaink HP, Snaar-Jagalska BE: Distinct functions for ERK1 and ERK2 in cell migration processes during zebrafish gastrulation. Dev Biol 319: 370–383, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Thisse C, Thisse B: High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3: 59–69, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Santoriello C, Deflorian G, Pezzimenti F, Kawakami K, Lanfrancone L, d'Adda di Fagagna F, Mione M: Expression of H-RASV12 in a zebrafish model of Costello syndrome causes cellular senescence in adult proliferating cells. Dis Model Mech 2: 56–67, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rojo I, de Ilarduya OM, Estonba A, Pardo MA: Innate immune gene expression in individual zebrafish after Listonella anguillarum inoculation. Fish Shellfish Immunol 23: 1285–1293, 2007 [DOI] [PubMed] [Google Scholar]